Abstract
Bath salts, or synthetic cathinones, have cocaine-like or amphetamine-like properties and induce psychoactive effects via their capacity to modulate serotonin (5-HT) and dopamine (DA). Structurally distinct synthetic cathinones are continuously being generated to skirt existing drug laws. One example of these modified compounds is cathinone phthalimide (CP), which has already appeared on the global market. The lack of toxicological studies on the effects of CP on monoaminergic systems led to the development of the present study in order to generate an acute toxicity profile for CP, and to clarify whether it primarily affects both dopamine and serotonin, like the synthetic cathinones mephedrone and methylone, or primarily affects dopamine, like 3, 4-methylenedioxypyrovalerone (MDPV). For the first time, the toxicity profile of CP (10µM - 1000µM) is reported. In pheochromocytoma cells, exposure to CP induced cell death, and altered mitochondrial function, as well as intracellular DA and 5-HT levels; at the same time, reduced glutathione (GSH) levels remained unaffected. This seems to indicate that CP functions like mephedrone or methylone. The role of CP metabolites, the effect of CP induced hyperthermia on neurotoxicity, and its ability to traverse the blood-brain barrier warrant further consideration.
Keywords: bath salt, cathinone phthalimide, dopamine, serotonin, methylone, mephedrone
1. Introduction
Cathinone is a β-keto analogue of amphetamine [22] that is found in the khat plant Catha edulis, which is native to East Africa and Southern Arabia [43]. In the United States, it is currently classified as a Schedule I controlled substance under the Controlled Substances Act [1]. Synthetic cathinones are a broad category of drugs commonly present in illicit “bath salt” products, which are typically marketed as inexpensive, and sometimes legal, substitutes for stimulants like methamphetamine (METH) and cocaine [33, 46]. Cathinones typically exert their psychostimulant effects via interactions with monoamine transporters, either by cocaine-like prevention of reuptake of neurotransmitters like dopamine (DA) and serotonin (5-HT) from the extracellular space back into the cytoplasm of neurons [9], or by amphetamine-like stimulation of efflux of DA and 5-HT [9, 36].
Because of the ability to modulate DA and 5-HT, synthetic cathinones have cocaine-like or amphetamine-like properties and therefore induce psychoactive effects [36]. Bath salts, specifically mephedrone, first appeared as a legal product in convenience stores in the early 2000s [6]. While mephedrone was more popular in Europe, 3, 4-methylenedioxypyrovalerone (MDPV) has been the most prevalent bath salt constituent in the United States. In 2011, the DEA temporarily scheduled possession and sale of mephedrone, MDPV, and methylone for 1 year pending additional evaluation [3]. In 2012, the Synthetic Drug Abuse Prevention Act permanently placed several synthetic cathinones into Schedule I of the Controlled Substances Act [35], including mephedrone and MDPV. Methylone was added to the Schedule I list in 2013 [2].
With increasing restrictions on recreational drug use, underground chemists have begun making new synthetic cathinones, in an attempt to subvert current legislation. Synthetic cathinones are being generated that are legal under existing drug laws and intended as alternatives to cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and METH [43]. These compounds are appealing to drug users not only because of their legality, but also their low cost, and because standard drug tests for cocaine, MDMA, and other amphetamines will not trigger a positive result from synthetic cathinones [6, 9]. One example of these modified compounds is cathinone phthalimide (CP), also called α-phthalimidopropiophenone or 2-(1-oxo-1-phenylpropan-2-yl)isoindole-1,3-dione, which has already been identified globally as a substance intended for recreational use [43]. For example, Neorganics, an Israel-based internet company, currently offers products containing CP [12]. One large problem with the drugs that produce “legal highs” is that users often assume they are safe because they are not illegal. Further complicating matters is the fact that companies distributing these products frequently change the active constituents of their brand-name products [14, 36]. In addition, synthetic cathinones are often found in drugs sold as “Molly,” slang for pure MDMA [6]. Some users seek Molly to avoid the additives routinely found in MDMA pills sold as Ecstasy, all the while still exposing themselves to the same risks [18, 24]. Therefore, the number of drug users exposed to synthetic cathinones is likely higher than reported, as is the frequency of exposure.
It is also known that synthetic cathinones are a complex group of compounds and their biological activity cannot be inferred from existing data, but rather should be determined on a case-by-case basis [9, 17]. Due to the lack of toxicological studies about the effects of CP on the dopaminergic system, the goal of the present study was to generate a basic toxicity profile for CP and to clarify whether it primarily affects dopamine and serotonin, like mephedrone and methylone [10, 13, 23, 46], or primarily affects dopamine, like MDPV [11, 26, 38].
2. Materials and Methods
2.1. Chemicals
Pheochromocytoma cells (PC12) were obtained from American Type Culture Collection (Manassas, VA). Cathinone phthalimide was graciously gifted by Kenner C. Rice from the National Institutes on Drug Abuse (NIDA). Cytotoxicity detection kits (LDH) were purchased from Roche Applied Sciences (Mannheim, Germany). RPMI1640 was purchased from Cellgro (Manassas, VA). Serum was purchased from Atlanta Biologicals (Flowery Branch, GA). Total Glutathione Detection Kit was purchased from Assay Designs (Farmingdale, NY). Pierce® BCA protein assay kit was obtained from Thermo Scientific (Waltham, MA). All remaining media supplements and reagents were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture
PC12 cells were grown at 37°C and 5% CO2 in complete media consisting of RPMI1640 supplemented with 10% horse serum, 5% fetal bovine serum, and 1% penicillin/streptomycin. Upon ~70% confluence, typically 5 days, cells were exposed to various concentrations (10µM to 1000µM) of CP for 24hrs.
2.3. Cell death
Lactate dehydrogenase (LDH) release, from the cytoplasm of damaged cells an indicator of membrane integrity and cell death [27], was measured according to manufacturer specifications. Briefly, after cells were treated with 10µM-1000µM CP for 24hrs, 100uL of media was incubated with 100µL of LDH substrate mixture in a 96 well plate. After 15 minutes on an incubated shaker, 50µL of stop solution was added to each well and the absorbance measured at 490nm with a reference wavelength of 650nm. Data were analyzed and presented as percent of control cells receiving only media. Three separate experiments were conducted with three replicates of each dose per experiment.
2.4. Mitochondrial function
The mitochondrial function of the PC12 cells was determined using previously described methods[28]. Briefly, cells were treated with 10µM-1000µM CP for 24hrs. Mitochondrial dehydrogenase-induced cleavage of 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT) to generate a water-soluble orange colored formazan derivative was then measured [37]. Fresh XTT reagent (30µL at 0.2 mg/mL) in the presence of 25µM phenazine methosulfate (PMS) was added to each well and the plate incubated for 2 hours at 37°C. Formazan production was measured at an absorbance wavelength of 450 nm with a reference wavelength of 650 nm. Data were analyzed and presented as percent of control cells receiving only media. Three separate experiments were conducted with three replicates of each dose per experiment.
2.5. Glutathione production
The level of reduced glutathione (GSH) in cell lysates was determined per manufacturer’s specifications. Briefly, after cells in 12 well plates were treated with 10µM-1000µM CP for 24hrs, cells were washed in cold PBS and 500µL of cold 5% (w/v) metaphosphoric acid was added per well. Wells were scraped thoroughly and contents transferred to a 1.5mL Eppendorf tube. Suspensions were sonicated for 30sec and stored on ice for 5min. Samples were then centrifuged at 18,828 X g for 5min at 4°C. Samples were then diluted with assay buffer and reaction mixture added. Absorbance was read at 405nm after 10min. Three separate experiments were conducted with each sample analyzed in triplicate and the average used in analysis.
2.6. High performance liquid chromatography (HPLC) with Electrochemical Detection (ED)
Intracellular concentrations of 5-HT and DA were quantified using a previously described modified HPLC-ECD method [4, 5]. Briefly, cells in 12 well plates were treated for 24 hours with 10µM −1000µM CP. After media removal, 500µL of ice cold extraction buffer, consisting of 0.2 N perchloric acid and the internal standard 3,4-dihydroxybenzylamine (DHBA), was added to each well. Using a cell lifter, cells were removed from each well and placed in 1.5mL tube and sonicated. Sonicates were then centrifuged at 4°C (23,143 X g, 10 min), and 150uL of supernatant was transferred to a 2mL Costar Spin-X centrifuge tube with 0.45µm filter and centrifuged at 4°C (865 X g, 3min). Twenty-five microliters of the filtered supernatants were injected directly into a HPLC-ECD system for analysis. Each sample was injected three times and the result averaged. Data were adjusted by the internal standard and normalized using protein concentration. Three separate experiments were conducted with each sample analyzed in triplicate and the average used in analysis.
2.7. Statistical analysis
Data were analyzed using one way ANOVA followed by Tukey post-hoc analysis in GraphPad Prism 6. The data are presented as means ± SEMs with alpha set at 0.05.
3. Results
3.1. Cathinone phthalimide induces cell death
High doses of CP increased extracellular levels of LDH, indicating compromised cell membranes and cell death (Fig. 1). For the 10µM, 100µM, and 1000µM dose, LDH levels were increased by 18%, 44%, and 321% over control levels, respectively (p < 0.0001, F = 460.5, DF = 60). Additionally, the 10µM and 100µM doses resulted in LDH levels that are significantly different from each other (p < 0.05, F = 460.5, DF = 60) and the 1000µM dose is significantly different from both the10µM and 100µM doses (p < 0.0001, F = 460.5, DF = 60).
Figure 1 -.
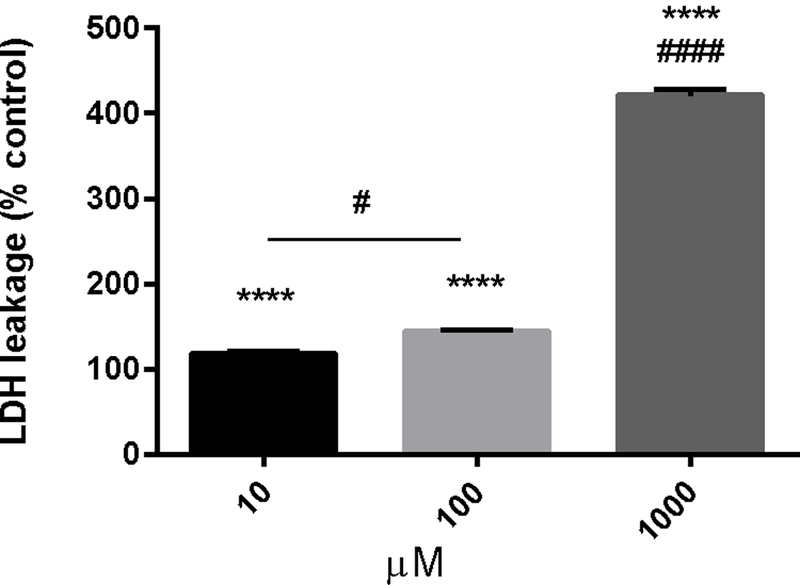
Cathinone phthalimide decreases membrane integrity, indicating increased cell death. PC12 cells were treated with increasing concentrations of cathinone phthalimide (10µM-1000µM) for 24hr. Data represent mean values ± SEM from 3 independent experiments conducted with three replicates of each treatment. **** p < 0.0001 vs control. In addtion, the 10µM and 100µM doses result in LDH levels that are significantly different from each other, # p < 0.05. The 1000µM dose is also significantly different from all other doses, #### p < 0.0001.
3.2. Cathinone phthalimide alters mitochondrial function
CP slightly increased mitochondrial function at the 10µM and 100µM doses, by 10% and 26%, respectively (p < 0.0001, F = 474.4, DF = 107). On the other hand, mitochondrial function was decreased at 1000µM dose by 91% (p < 0.0001, F = 474.4, DF = 107). Additionally, effect of each dose on mitochondrial function is significantly different from the effect of other doses (p < 0.0001, F = 474.4, DF = 107) (Fig. 2).
Figure 2 -.
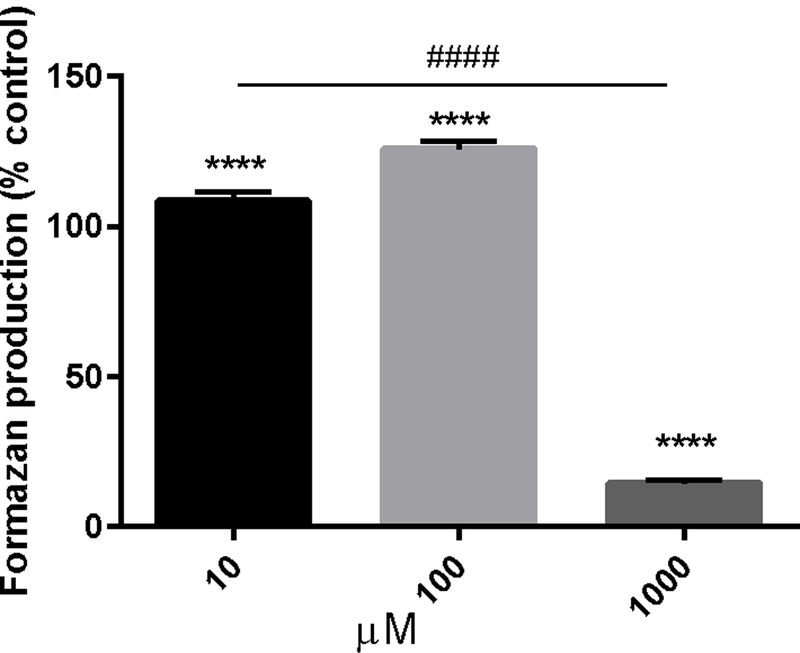
Cathinone phthalimide alters mitochondrial function. PC12 cells were treated with increasing concentrations of cathinone phthalimide (10µM-1000µM) for 24hr. Formazan production was taken as an index of mitochondrial function. Data represent mean values ± SEM from 3 independent experiments conducted with three replicates of each treatment. **** p < 0.0001 vs control. Response from all doses is also significanlty different from one another, #### p < 0.0001.
3.3. Cathinone phthalimide does not alter levels of reduced glutathione
Although reduced GSH levels produced after exposure to 10µM and 100µM were significantly different from one another (p < 0.01, F = 8.553 and DF = 12), no resulting levels were significantly different when compared to control (Fig. 3).
Figure 3-.
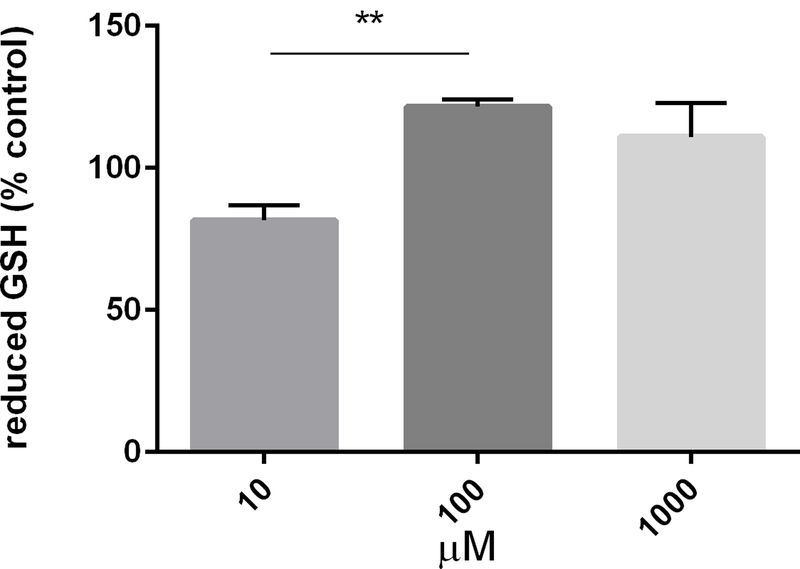
Cathinone phthalimide does not alter levels of reduced glutathione (GSH). PC12 cells were treated with increasing concentrations of cathinone phthalimide (10µM-1000µM) for 24hr. Reduced GSH levels were measured. No significant differences compared to control were noted, however levels were significantly different when comparing 10µM and 100µM doses, ** p < 0.01. Data represented as % control ± SEM from 3 independent experiments, with each sample analyzed in triplicate and the average used in analysis.
3.4. Cathinone phthalimide alters dopamine and serotonin levels
Intracellular DA levels were significantly different compared to control (179.6ng/mg protein) at 10µM (350.6ng/mg protein, p < 0.0001, F = 131.9, DF = 12), 100µM CP (253.3 ng/mg protein, p < 0.001, F = 131.9, DF = 12), and 1000µM CP (18ng/mg protein, p < 0.0001, F = 131.9, DF = 12) (Fig. 4a). Levels 10µM and 100µM were significantly different (p < 0.001, F = 131.9, DF = 12). The response at 1000µM was also significantly different from that of 10µM and (p < 0.0001, F = 131.9, DF = 12). At the same time, 10µM and 100µM CP resulted in significantly elevated 5-HT levels (8.1 and 11.2ng/mg protein respectively) compared to control (5.1ng/mg protein; Fig. 4b) (p < 0.05, F = 10.78, DF = 8) and the 1000µM dose (p <0.05 and p <0.001 respectively, F = 10.78, DF = 8). The 1000µM dose caused 5-HT levels to drop to 2.3 ng/mg protein, but this effect was not statistically different from control.
Figure 4-.
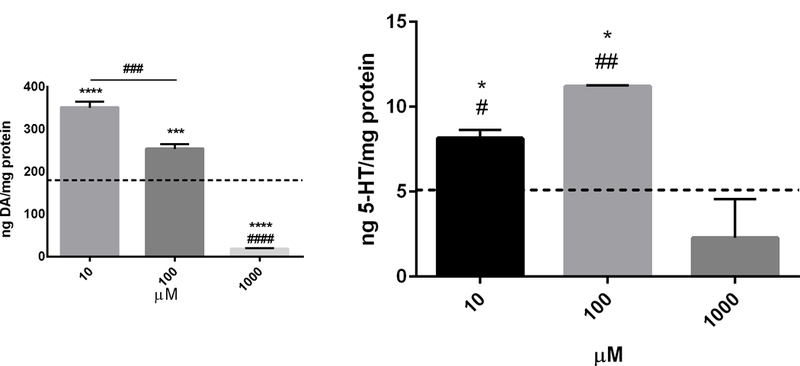
Cathinone phthalimide alters dopamine and serotonin levels. PC12 cells were treated with increasing concentrations of cathinone phthalimide (10µM-1000µM) for 24hr. Data were normalized to sample protein. Data are represented as mean ng neurotransmitter (NT) per mg protein ± SEM from 3 independent experiments, with each sample analyzed in triplicate and the average used in analysis. A) Intracellular dopmaine (DA) levels were determined from cell lysates. *** p < 0.001, and **** p < 0.0001 vs control. The 10µM and 100µM doses resulted in significanlty different dopamine levels, ### p < 0.001. Dopamine levels at the 1000µM dose are significanlty different from all other doses, #### p < 0.0001. B) . Intracellular levels of 5-HT were determined from cell lysates. * p < 0.05 vs control. # p <0.05, ## p <0.001 from 1000µM dose. The dashed lines represent control levels of the respective NTs: DA, 179.6ng/mg protein; 5-HT, 5.1 ng/mg protein.
4. Discussion
Recreational use of bath salts can lead to a variety of symptoms, depending on dose, including: enhanced mood, increased alertness, mild agitation, severe psychosis, hyperthermia, tachycardia, hyponatremia and death. The only treatment currently available for patients with exposure to synthetic cathinones is supportive therapy [36]. A recent epeidemic of adverse events associated with use of the synthetic cathinone alpha-pyrrolidinopentiophenone ( α-PVP) in Florida highlights the risk of these substances in human users [30] and there is a high likelihood that a similar incident will occur in the near future as underground chemists continue to create synthetic cathinones that evade the law.
The present study reports for the first time, an acute toxicity profile for cathinone phthalimide (CP) after 24hrs in vitro. This exposure time is similar to those used for other in vitro studies involving bath salts [38, 39, 44]. In PC12 cells, CP induced a slight but significant increase in cell death at 10µM and 100µM and drastically increases cell death at 1000µM (Fig. 1). The increase in mitochondrial function seen at lower doses (Fig. 2) is most likely attributable to cell survival mechanisms with the surviving cells functioning at higher capacity to protect themselves from exposure-induced cell death; the inability to compensate is clearly seen at the 1000µM dose. Glutathione exists in both it’s reduced from (GSH) and an oxidized dimer (GSSG), with the former being the largest fraction (~98%) [7], protecting against free radical damage by acting as a scavenger of hydroxyl radicals and superoxide [7, 40]. Although CP does not affect GSH levels (Fig. 3) under these conditions, it is possible that other mechanisms associated with oxidative stress may be involved. Additional studies further investigating the role of oxidative stress in CP toxicity are needed to clarify its influence. Intracellular levels of DA increased and then fell at 1000µM (Fig. 4a), and similar effects are seen with 5-HT but the drop at the higher dose was not statistically significant (Fig. 4b). The drop in DA and 5-HT levels at 1000µM may be the result of cell death (Fig. 1) and decreased mitochondrial function (Fig. 2). Together, the data may suggest that CP functions similar to mephedrone or methylone [10, 13, 23, 46], although whether any of the changes in neurotrasmitter levels are caused by their stimulated efflux or a block in their reuptake requires further investigation. However, recent research has indicated that some bath salts (MDPV) stimulate DA efflux [41]. A similar mechanism may be elicited by CP, which could account for the decrease in intracellular DA at 1000µM. Therefore, further studies are warranted to clarify the exact mechanism of action of CP.
Possible metabolites of CP are not presently known, but it is plausible that if present, active metabolites may also induce toxicity, thereby potentiating the effect of CP at lower concentrations. However, it is not likely that the preparation used in the present study generated any metabolites. Even though metabolites of CP have not been investigated to date, it is reasonable investigate their presence and impact on toxicity as MDMA metabolites are reported to induce toxicity at lower concentrations than MDMA alone [8, 16, 39]. Because of the structural similarity between CP and MDMA, potential effects of CP metabolites cannot be ruled out and also warrant further investigation.
With the establishment of an in vitro toxicity profile, how this relates to physiological levels of CP and other drugs of abuse found in abusers is important. Previous reports in PC12 indicate that MDMA, cocaine, and amphetamine diminish cell viability, but at much high doses (2–2.5mM, 1mM,and 2mM respectively) [20, 29, 31, 34] than seen with CP in the present study (10µM). Additionally, much higher doses of cocaine or amphetamine (50µM and 3mM respectively) are reported to alter intracellular DA levels [19, 34], whereas CP induces a response at 10µM. The bath salt MDPV in SH-SY5Y cells [38] also exerts toxic effects at lower concentrations (250µM); however, this concentration is still higher than what is found in the blood of drug users [25, 38, 47]. Nevertheless, this does not exclude the physiological relevance of previous studies on bath salts or the present study on CP if the influence of hyperthermia is taken into consideration. It is known that MDPV [15, 25, 32, 38] and cathinone [21, 42] induce hyperthermia, which plays an important role in potentiating neurotoxicity. One study reported the effects of MDPV on hepatotoxicity, with an increase in toxicity at 40.5°C compared to 37°C, indicating that temperature is a key factor in the toxic effects of the drug [45]. As hyperthermia is induced by cathinone, one may expect the same effect with CP and infer that in the case of elevated temperature, the presently reported toxic effects may be observed at even lower concentrations than reported here (perhaps those well within the physiological range). As there is no report of CP levels in drug abusers, the closest approximation would be other bath salts, such as MDPV, which is found at approximately 0.4µg/mL (1.45µM) postmortem in brain tissue [47]. In any event, further investigation is warranted to confirm whether hyperthermia potentiates CP toxicity.
Another point to consider is that of polarity. Because the addition of new ketals compared with the parent cathinone, it is possible that CP would be less polar and might therefore exhibit greater permeability through the blood-brain barrier, thereby increasing its availability in the brain and its psychoactive potency [43]. Further studies are warranted to compare its ability to pass the blood-brain barrier and to determine its ability to impair blood-brain barrier function, especially given that the bath salt MDPV has been found to induce toxicity in the blood-brain barrier at doses lower than that of METH and MDMA [39].
5. Conclusions
In summary, the findings from this study indicate for the first time that CP can alter monoaminergic systems and such effects occur at lower concentrations than reported for the traditionally abused psychostimulants MDMA, cocaine, amphetamine, or MDPV, indicating it is more potent in this in vitro model. Additional investigations are required to determine the mechanism(s) involved.
Acknowledgements
This research was supported by the National Center for Toxicological Research E0745201 and by an appointment (H. R-H) to the Research Participation Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcoholism and Alcohol Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Food and Drug Administration, the National Institute on Drug Abuse, the National Institute of Alcohol Abuse and Alcoholism, or the National Institutes of Health.
Abbreviations:
- METH
methamphetamine
- DA
dopamine
- 5-HT
serotonin
- MDPV
3, 4-methylenedioxypyrovalerone
- CP
cathinone phthalimide
- PMS
phenazine methosulfate
- LDH
Lactate dehydrogenase
- GSH
glutathione
- XTT
2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide
References
- [1].Schedules of Controlled Substances: Placement of Cathinone and 2,5-Dimethoxy-4-ethylamphetamine Into Schedule I In: D.O.J. Drug Enforcement Administration; (Ed.), 21 CFR Part 1308, Vol. 58, January 14, 1993, pp. 4316–4318. [Google Scholar]
- [2].Schedules of Controlled Substances: Placement of Methylone Into Schedule I. 21 CFR Part 1308, Vol. 78, April 12, 2013, pp. 21818–21825. [Google Scholar]
- [3].Schedules of Controlled Substances: Temporary Placement of Three Synthetic Cathinones Into Schedule I. In: D.O.J. Drug Enforcement Administration; (Ed.), 21 CFR Part 1308, Vol. 76, Federal Registrar, October 21, 2011, pp. 65371–65375. [PubMed] [Google Scholar]
- [4].Ali SF, David SN, Newport GD, Cadet JL, Slikker W Jr., MPTP-induced oxidative stress and neurotoxicity are age-dependent: evidence from measures of reactive oxygen species and striatal dopamine levels, Synapse 18 (1994) 27–34. [DOI] [PubMed] [Google Scholar]
- [5].Ali SF, Newport GD, Holson RR, Slikker W Jr., Bowyer JF, Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice, Brain Res 658 (1994) 33–38. [DOI] [PubMed] [Google Scholar]
- [6].Alliance DP, Fact Sheet: Synthetic Cathinones June 2016, pp. 1–5.
- [7].Bains JS, Shaw CA, Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death, Brain Res Brain Res Rev 25 (1997) 335–358. [DOI] [PubMed] [Google Scholar]
- [8].Barbosa DJ, Capela JP, Silva R, Vilas-Boas V, Ferreira LM, Branco PS, Fernandes E, Bastos Mde L, Carvalho F, The mixture of “ecstasy” and its metabolites is toxic to human SH-SY5Y differentiated cells at in vivo relevant concentrations, Arch Toxicol 88 (2014) 455–473. [DOI] [PubMed] [Google Scholar]
- [9].Baumann MH, Awash in a sea of ‘bath salts’: implications for biomedical research and public health, Addiction 109 (2014) 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baumann MH, Ayestas MA Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV, The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue, Neuropsychopharmacology 37 (2012) 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW, Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products, Neuropsychopharmacology 38 (2013) 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG, Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone, Forensic Sci Int 197 (2010) 59–66. [DOI] [PubMed] [Google Scholar]
- [13].Cozzi NV, Sievert MK, Shulgin AT, Jacob P 3rd, Ruoho AE, Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines, Eur J Pharmacol 381 (1999) 63–69. [DOI] [PubMed] [Google Scholar]
- [14].Davies S, Wood DM, Smith G, Button J, Ramsey J, Archer R, Holt DW, Dargan PI, Purchasing ‘legal highs’ on the Internet--is there consistency in what you get?, QJM 103 (2010) 489–493. [DOI] [PubMed] [Google Scholar]
- [15].Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC, In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity, Neuropsychopharmacology 38 (2013) 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ferreira PS, Nogueira TB, Costa VM, Branco PS, Ferreira LM, Fernandes E, Bastos ML, Meisel A, Carvalho F, Capela JP, Neurotoxicity of “ecstasy” and its metabolites in human dopaminergic differentiated SH-SY5Y cells, Toxicol Lett 216 (2013) 159–170. [DOI] [PubMed] [Google Scholar]
- [17].Glennon RA, Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention, Adv Pharmacol 69 (2014) 581–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Griffen D, Black N, DiCarlo P, 9 things everyone should know about the drug Molly. CNN Investigations, November 2, 2015.
- [19].Imam SZ, Duhart HM, Skinner JT, Ali SF, Cocaine induces a differential dose-dependent alteration in the expression profile of immediate early genes, transcription factors, and caspases in PC12 cells: a possible mechanism of neurotoxic damage in cocaine addiction, Ann N Y Acad Sci 1053 (2005) 482–490. [DOI] [PubMed] [Google Scholar]
- [20].Kaizaki A, Tanaka S, Tsujikawa K, Numazawa S, Yoshida T, Recreational drugs, 3,4-Methylenedioxymethamphetamine(MDMA), 3,4-methylenedioxyamphetamine (MDA) and diphenylprolinol, inhibit neurite outgrowth in PC12 cells, J Toxicol Sci 35 (2010) 375–381. [DOI] [PubMed] [Google Scholar]
- [21].Kalix P, Hyperthermic response to (−)-cathinone, an alkaloid of Catha edulis (khat), J Pharm Pharmacol 32 (1980) 662–663. [DOI] [PubMed] [Google Scholar]
- [22].Kalix P, Khan I, Khat: an amphetamine-like plant material, Bull World Health Organ 62 (1984) 681–686. [PMC free article] [PubMed] [Google Scholar]
- [23].Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T, Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats, Br J Pharmacol 164 (2011) 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kent JL, The Truth About Molly. High Times, August 05, 2014.
- [25].Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Doyon S, Chi P, Fowler DR, Methylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literature, J Forensic Sci 58 (2013) 1654–1659. [DOI] [PubMed] [Google Scholar]
- [26].Kolanos R, Solis E Jr., Sakloth F, De Felice LJ, Glennon RA, “Deconstruction” of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter, ACS Chem Neurosci 4 (2013) 1524–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Korzeniewski C, Callewaert DM, An enzyme-release assay for natural cytotoxicity, J Immunol Methods 64 (1983) 313–320. [DOI] [PubMed] [Google Scholar]
- [28].Lantz SM, Cuevas E, Robinson BL, Paule MG, Ali SF, Imam SZ, The Role of Harmane and Norharmane in In Vitro Dopaminergic Function, Journal of Drug and Alcohol Addiction 4 (2015) 8. [Google Scholar]
- [29].Lepsch LB, Munhoz CD, Kawamoto EM, Yshii LM, Lima LS, Curi-Boaventura MF, Salgado TM, Curi R, Planeta CS, Scavone C, Cocaine induces cell death and activates the transcription nuclear factor kappa-B in PC12 cells, Mol Brain 2 (2009) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McFadden C, Nadi A, Connor T, “Devil’s Drug:” Flakka is driving Florida Insane. NBC NEWS, 2015.
- [31].Milhazes N, Cunha-Oliveira T, Martins P, Garrido J, Oliveira C, Rego AC, Borges F, Synthesis and cytotoxic profile of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its metabolites on undifferentiated PC12 cells: A putative structure-toxicity relationship, Chem Res Toxicol 19 (2006) 1294–1304. [DOI] [PubMed] [Google Scholar]
- [32].Murray BL, Murphy CM, Beuhler MC, Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV), J Med Toxicol 8 (2012) 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].NIDA, Drug Facts: Synthetic Cathinones (“Bath Salts”) www.drugabuse.gov, January 2016, p. 4.
- [34].Oliveira MT, Rego AC, Morgadinho MT, Macedo TR, Oliveira CR, Toxic effects of opioid and stimulant drugs on undifferentiated PC12 cells, Ann N Y Acad Sci 965 (2002) 487–496. [DOI] [PubMed] [Google Scholar]
- [35].Portman R, S. 3190 To Amend the Controlled Substances Act to Place Sythetic Drugs in Schedule I. United States Publishing Office, May 16, 2012.
- [36].Prosser JM, Nelson LS, The toxicology of bath salts: a review of synthetic cathinones, J Med Toxicol 8 (2012) 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL, An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT, J Immunol Methods 142 (1991) 257–265. [DOI] [PubMed] [Google Scholar]
- [38].Rosas-Hernandez H, Cuevas E, Lantz SM, Imam SZ, Rice KC, Gannon BM, Fantegrossi WE, Paule MG, Ali SF, 3,4-methylenedioxypyrovalerone (MDPV) Induces Cytotoxic Effects on Human Dompinergic SH-SY5Y Cells, JDAR 5 (2016) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rosas-Hernandez H, Cuevas E, Lantz SM, Rice KC, Gannon BM, Fantegrossi WE, Gonzalez C, Paule MG, Ali SF, Methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxypyrovalerone (MDPV) induce differential cytotoxic effects in bovine brain microvessel endothelial cells, Neurosci Lett 629 (2016) 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schulz JB, Lindenau J, Seyfried J, Dichgans J, Glutathione, oxidative stress and neurodegeneration, Eur J Biochem 267 (2000) 4904–4911. [DOI] [PubMed] [Google Scholar]
- [41].Shekar A, Aguilar JI, Galli G, Cozzi NV, Brandt SD, Ruoho AE, Baumann MH, Matthies HJ, Galli A, Atypical dopamine efflux caused by 3,4-methylenedioxypyrovalerone (MDPV) via the human dopamine transporter, J Chem Neuroanat (2017). [DOI] [PMC free article] [PubMed]
- [42].Shortall SE, Green AR, Swift KM, Fone KC, King MV, Differential effects of cathinone compounds and MDMA on body temperature in the rat, and pharmacological characterization of mephedrone-induced hypothermia, Br J Pharmacol 168 (2013) 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Smolianitski E, Wolf E, Almog J, Proactive forensic science: a novel class of cathinone precursors, Forensic Sci Int 242 (2014) 219–227. [DOI] [PubMed] [Google Scholar]
- [44].Valente MJ, Araujo AM, Bastos Mde L, Fernandes E, Carvalho F, Guedes de Pinho P, Carvalho M, Editor’s Highlight: Characterization of Hepatotoxicity Mechanisms Triggered by Designer Cathinone Drugs (beta-Keto Amphetamines), Toxicol Sci 153 (2016) 89–102. [DOI] [PubMed] [Google Scholar]
- [45].Valente MJ, Araujo AM, Silva R, Bastos Mde L, Carvalho F, Guedes de Pinho P, Carvalho M, 3,4-Methylenedioxypyrovalerone (MDPV): in vitro mechanisms of hepatotoxicity under normothermic and hyperthermic conditions, Arch Toxicol 90 (2016) 1959–1973. [DOI] [PubMed] [Google Scholar]
- [46].Watterson LR, Olive MF, Synthetic cathinones and their rewarding and reinforcing effects in rodents, Adv Neurosci (Hindawi) 2014 (2014) 209875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP, Postmortem tissue distribution of MDPV following lethal intoxication by “bath salts”, J Anal Toxicol 37 (2013) 182–185. [DOI] [PubMed] [Google Scholar]


