Abstract
Direct-acting antiviral agents (DAAs) are very effective therapy for chronic hepatitis C infection, and have revolutionized the treatment of hepatitis C in kidney allograft recipients. Although well tolerated in general, rare renal complications have been reported. We describe a case of recurrent membranous nephropathy and acute cellular rejection in a kidney allograft recipient after DAA (ledipasvir/sofosbuvir) therapy, whose allograft function had been stable for more than 30 years. The patient presented with nephrotic range proteinuria with stable creatinine. The kidney allograft biopsy revealed recurrent membranous nephropathy with fine granular deposits of IgG1/IgG4 codominance and positive phospholipase A2 receptor (PLA2R) staining. The patient was treated with pulse steroid and rituximab, leading to a decrease in proteinuria. As DAAs are more frequently used, physicians should be aware of immune-related renal complications.
Introduction
Nearly 10% of kidney transplant recipients in the US and Europe have chronic hepatitis C virus (HCV) infection, which has been associated with higher graft loss and mortality rates (1). Interferon-and ribavirin-free direct-acting antiviral (DAA) regimens are highly effective in achieving a sustained virologic response (SVR) and have dramatically changed the therapeutic approach to chronic HCV infection in transplant patients (2, 3).
We report a patient who experienced acute cell-mediated rejection and concomitant recurrence of membranous nephropathy 30 years post transplant, after ledipasvir/sofosbuvir therapy.
Case Report
A 67-year-old Caucasian male presented with a new-onset proteinuria post-transplant. He was initially diagnosed with idiopathic membranous nephropathy (MN) at the age of 31, which manifested as severe nephrotic syndrome, and he rapidly progressed to kidney failure. A living-related kidney transplant from his fully HLA-matched sibling resulted in abrupt recurrence of MN 3 months post-transplant and early graft failure. Since his subsequent second transplant from a deceased donor at the age of 36, his kidney function had been stable (Cre 1.2 mg/dL) and without proteinuria for 30 years post-transplantation (Figure 1). Immune suppression was minimized to only cyclosporine 50 mg daily (trough level ~30 ng/mL) 22 years post-transplantation, due to steroid-associated vertebral compression fractures and multiple cutaneous squamous cell cancers.
Figure 1:
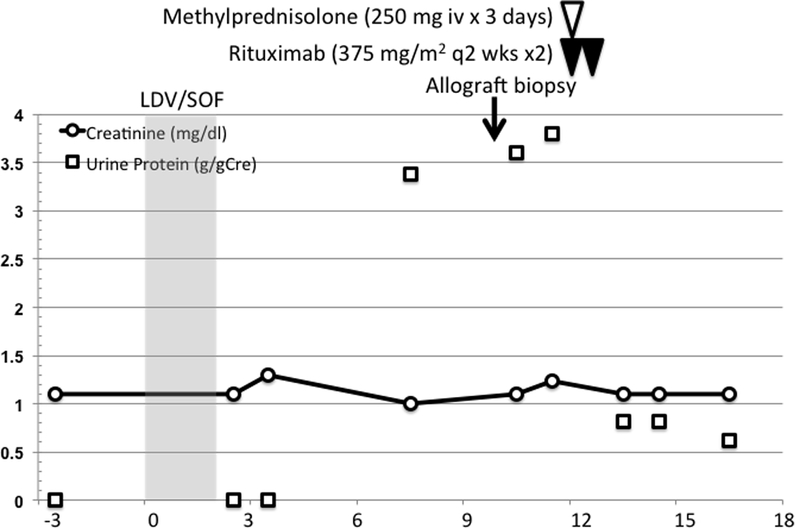
Timeline of the patient’s clinical course. X-axis: months after initiation of DAA ledipasvir/sofosbuvir (LDV/SOF) treatment. The trend of urine protein (g/gCre) and serum creatinine (mg/dl) was shown in dotted and solid line, respectively. Allograft biopsy was performed 10 months after initiation of DAAs, and patient was treated with steroid pulse and two doses of rituximab infusion, 2 weeks apart.
The patient became HCV positive (genotype 1a) following a transfusion after the first transplant. Before treatment, there was mild elevation of aminotransferase levels (AST 48 U/L, ALT 82 U/L), albumin 3.5 g/dL, total bilirubin 0.7 mg/dL, direct bilirubin 0.2 mg/dL, platelets 204 ×103/mL, hepatitis C viral load was 1–2 million IU/mL, without evidence of liver cirrhosis by ultrasound. Neither liver biopsy nor non-invasive assessment (e.g. fibroscan) was performed prior to the initiation of treatment. Urinary protein was negative 3 months prior to the initiation of DAA (Figure 1). Ledipasvir/sofosbuvir treatment was initiated, and SVR was achieved 12 weeks after the end of 8 weeks of treatment. Seven months after the initiation of DAA, the patient presented with slight ankle edema and nephrotic range proteinuria (4.2 g/24 hours, serum albumin 3.6 g/dL, total cholesterol 169 mg/dL) with preserved renal allograft function (Cre 1.1 mg/dL, eGFR 62.6 ml/min/1.73 m2, Figure 1). There was no reported clinical event suggesting infection during the treatment. Urinalysis was negative for WBCs or RBCs. Donor-specific antibody (DSA) was not detected using single antigen beads (SAB) testing, and circulating anti-phospholipase A2 receptor (PLA2R) antibody undetectable. Cryoglobulins were undetectable, rheumatoid factor was negative, and complement levels normal. A kidney allograft biopsy was performed 2 months later, due to the persistence of proteinuria.
The allograft kidney biopsy showed a total of 36 glomeruli, one of which was globally sclerosed and none showed segmental sclerosis, crescents or hypercellularity. The glomeruli showed minimal alteration with slight thickening of the basement membranes. There was mild interstitial inflammation and focal tubulitis, consistent with borderline acute cellular rejection without significant peritubular capillaritis (Figure 2A-B). There was minimal interstitial fibrosis and tubular atrophy (<10% of the cortex). The immunofluorescence microscopy study revealed fine granular global IgG deposition along the glomerular capillary walls (Figure 2C) with IgG1 and IgG4 co-dominance (Figure 2G-J), and positive PLA2R staining of the deposits (Figure 2D). The ultrastructural study revealed mildly thickened glomerular basement membranes with segmental (often large) subepithelial electron-dense deposits without well-formed lateral spikes (Stage I of IV (Ehrenreich and Churg)), indicative of an early-stage recurrent MN (Figure 2K).
Figure 2.
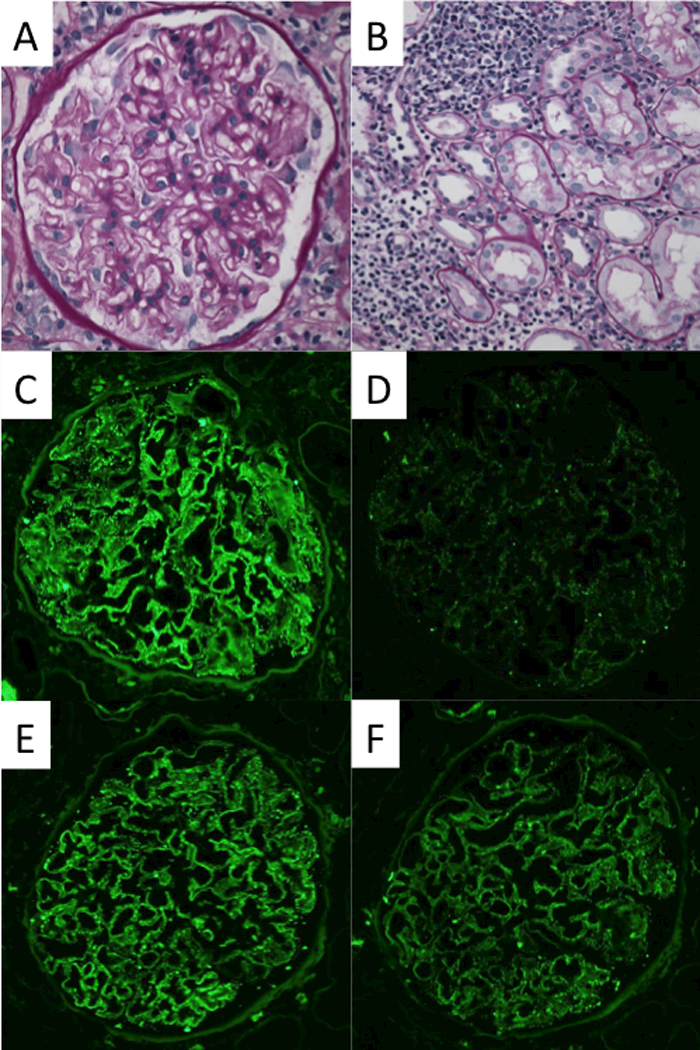
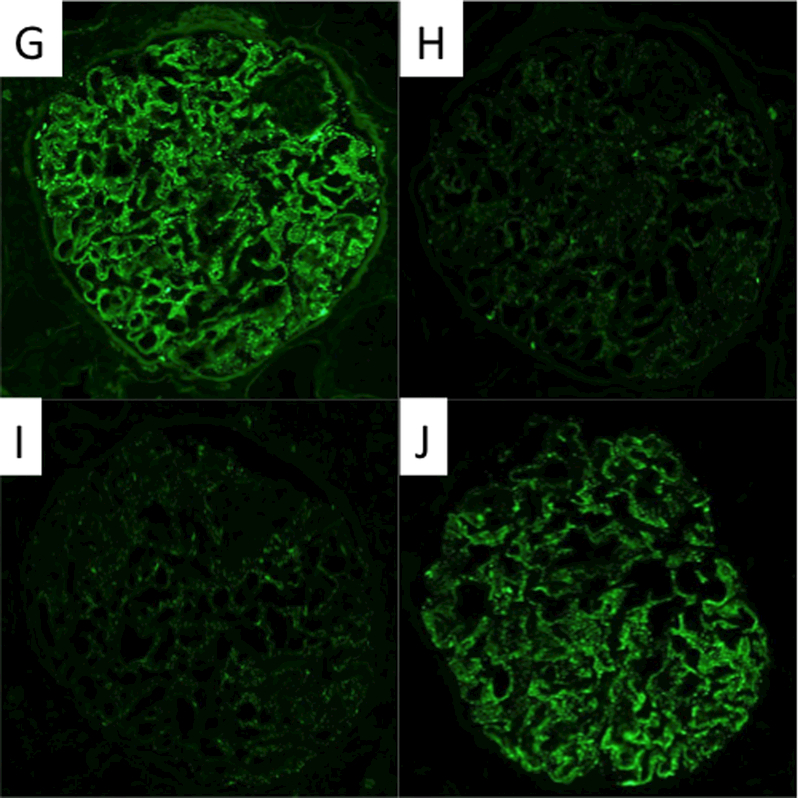
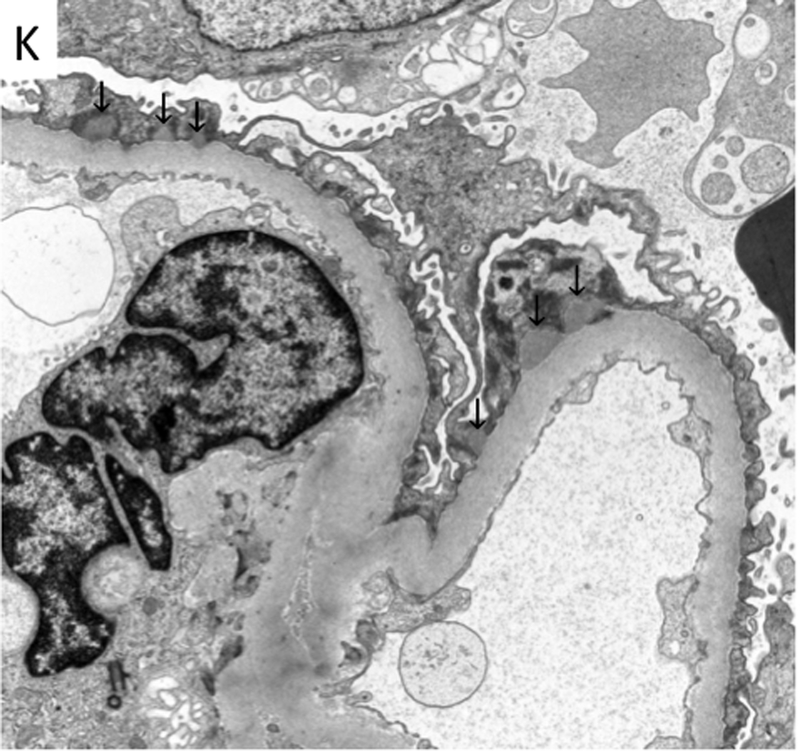
Morphological features of the kidney allograft biopsy. The light microscopy reveals minimal alteration in the glomerulus with slight thickening of the basement membranes (a), mild interstitial inflammation, and mild tubulitis (b) (Banff i1, t1, v0). (c)-(j) Immunofluorescence microscopy reveals fine granular deposits along glomerular basement membranes reactive for antibodies specific for immunoglobulin heavy chain IgG (4+) (c), PLA2R(1+) (d), immunoglobulin light chain kappa(3+) (e) and lambda(3+) (f), and IgG subclasses gamma1 (3+) (g), gamma2 (1+) (h), gamma3 (1+) (i), and gamma4 (3+) (j), respectively. (k) Electron microscopy reveals fine granular subepithelial electron-dense deposits (indicated by arrows) with no separation from each other by basement membrane “spikes” (stage I). Mesangial or subendothelial deposits are not seen.
The patient was treated with a steroid pulse (methylprednisolone 250 mg i.v. daily for three doses) for acute rejection, and rituximab (375 mg/m2 every 2 weeks for two doses) for recurrent membranous nephropathy (4). The dose of cyclosporine was increased to achieve trough level of 60–100 ng/ml. This led to a decrease in proteinuria to 0.8 g/gCre 4 weeks after the treatment, and the patient maintained a low-level of proteinuria 0.6–0.8 g/gCre for 6 months after the treatment (Figure 1) with stable allograft function (Cre 1.1 mg/dL, eGFR 65.1 ml/min/1.73 m2). Hepatitis C viral load in plasma was negative by PCR throughout the course.
Discussion
Here, we reported a case of recurrent membranous nephropathy and possible acute cellular rejection in a long-time survivor of a kidney allograft recipient who received DAA therapy.
Chronic HCV infection affects more than 185 million people around the world, with an increased risk of cirrhosis and hepatocellular carcinoma (HCC). The prevalence of the infection in end-stage kidney disease population and kidney transplant recipients is significantly higher compared with the general population (5), and it is associated with worse allograft outcome (1). However, the management of chronic HCV infections in kidney allograft recipients has been challenging, as conventional interferon-based regimens were not routinely recommended because of the concern for increased risk of acute rejection (6). Direct-acting antiviral agents (DAAs) dramatically changed the treatment of chronic HCV infection in transplant recipients, as it has been shown to be safe and efficient in this population (3).
Though DAAs are generally well tolerated, our patient developed recurrent membranous nephropathy 7 months after treatment with DAA therapy. Sise et al. recently reported 3 cases of acute immune complex glomerulonephritis with a lupus-like pattern six to nine months after sofosbuvir treatment (7). Two of the reported cases were in non-transplant setting and one was in a post-liver transplant recipient (Table 1). In addition, Hogan et al. reported three cases of new-onset nephrotic range proteinuria in the context of DAA treatment post-kidney transplant. In these cases, the allograft biopsy at 2–3 months after initiation of DAA revealed focal segmental glomerulosclerosis (FSGS) (8). Furthermore, the Transplanting Hepatitis C Kidneys into Negative Kidney Recipients [THINKER] trial group reported one case of FSGS after elbasvir/grazoprevir treatment in a patient with a history of IgA nephropathy before transplantation (9) (Table 1). In our case, the timing of the recurrence suggested that DAA treatment played a role in development of the recurrence. Recurrence of MN post-transplantation is relatively common (40–50%) (10), however, it is most frequently seen in the first year post-transplant, rarely occurs 10 years post-transplant but it is still possible that MN can recur decades later (4, 11).
Table 1:
Cases of glomerulonephropathy after DAA therapy
| Age, Sex, Ethnicity |
HCV Genoty pe |
Medical History |
Baseline Parameters |
DAA (treatment duration) |
SV R1 2 |
Renal Presentation | Kidney Biopsy | Reference |
|---|---|---|---|---|---|---|---|---|
| Non-transplant | ||||||||
| 51F, Asian | 3A | Cirrhosis | Cre 0.72 | SOF/RBV (24 wks) |
Yes | Rash, 9 months post DAA initiation, AKI, Cre 1.65, hematuria |
Acute immune complex glomerulonephritis with a lupus-like pattern |
Sise et al. KI reports 2016 |
| 48 F, Hispanic |
1 | Cirrhosis, ITP, SLE |
Cre 0.95 | SOF/SIM (9 wks) |
Yes | Rash, AKI, Cre 1.45, proteinuria, hematuria |
Severe active and chronic immune complex-mediated glomerulonephritis |
Sise et al. KI reports 2016 |
| Transplant | ||||||||
| 56M, Caucasian |
1b | Liver transplant, cirrhosis in allograft |
Cre 1.4–1.7 | SOF/RBV (24 wks) |
Yes | Joint pain, AKI, 2 wks post DAA, Cre 2.6, hematuria, trace protainuria |
Acive immune complex-mediated glomerulonephritis with a lupus-like pattern of immune complex deposition. |
Sise et al. KI reports 2016 |
| 62M, Caucasian |
1a | Liver transplant, DM |
Cre 1.0, trace albuminuria |
LDV/SOF/RBV (12 wks) |
Yes | Acute nephrotic syndrome 10 wks post DAA, AKI, ESRD |
FSGS | Hogan et al. Hepatology 2017 |
| 62M, African American |
1, 1a (donor) |
Kidney transplant, HTN |
Cre 1.4, UPCR 0.9 g/gCre |
LDV/SOF/RBV (12 wks) |
Yes | Cre 1.5 mg/dl, UPCR 4.1 g/gCre, 10 wks post DAA |
FSGS, collapsing variant |
Hogan et al. Hepatology 2017 |
| 61F, Nigerian |
4, 1a (donor) |
Kidney transplant, HTN |
Cre 1.0, UPCR 0.1 g/gCre |
LDV/SOF (12 wks) |
Yes | Cre 0.9 mg/dl, Protein 2.2 g/24 hour, 12 wks post DAA |
Mesangial sclerosis, diffuse podocytopathy (early FSGS) |
Hogan et al. Hepatology 2017 |
| 39M, Hispanic |
1a | Kidney transplant |
UPCR 9 g/day | SOF/SIM (12 wks) |
Yes | UPCR 5–10 g/day | Collapsing glomerulopathy |
Lin et al. PlosOne 2016 |
| 61M, Caucasian |
1a | Liver and kidney transplant |
UPCR 1.9 g/day |
SOF/SIM (12 wks) |
Yes | UPCR 2–3 g/day | FSGS, collapsing features |
Lin et al. PlosOne 2016 |
| Not Described |
1 (donor) recipie nt negativ e |
Kidney transplant, IgA nephropath y |
(Received kidney transplant from HCV viremic donor) |
EBR/GZR (12 wks) |
Yes | Protein 2 g/24 hour | FSGS | Goldberg et al. NEJM 2017 |
AKI: acute kidney injury, DAA: direct-acting antiviral agent, EBR: elbasvir, DM: diabetes, FSGS: focal segmental glomerulosclerosis, GZR: grazoprevir, HCV: hepatitis C virus, HTN: hypertension, ITP: idiopathic thrombocytopenic purpura, LDV: Ledipasvir
MPGN: membranoproliferative glomerulonephritis, RBV: Ribavirin, SIM: Simeprevir, SLE: systemic lupus erythematosus, SOF: Sofosbuvir, SVR: Sustained virologic response, UPCR: urine protein-creatinine ratio
In addition to the recurrent membranous nephropathy, our patient appears to have had an acute cellular rejection episode. Previous studies reported only isolated cases of acute rejection after DAA treatment: no rejection was observed in the cohorts of Kamar et al (12). (n=25), Sawinski et al (13). (n=20), or Colombo et al (3). (n=114); Fernandez et al (14). reported one case of antibody-mediated rejection and three cases of acute cell-mediated rejection (n=103) and Bhamidimarri et al (15). reported three cases of antibody-mediated rejection. Acute cell-mediated rejection is uncommon after 10 years post-transplant (16) and considering in this case the recipient is 30 years post-transplantation, there is a possibility that a change in effective cyclosporine level might have triggered cell-mediated rejection, especially as this patient was on a single immunosuppressive agent. Clinical trials have reported a minimal interaction between cyclosporine and hepatitis C DAAs, however we cannot totally exclude the possibility that ledipasvir/sofosbuvir lowered the cyclosporine level, as a documented trough level of cyclosporine is unavailable at the relevant time point (14).
In addition, we speculate that DAA therapy might have restored adaptive and innate immune responses that had been suppressed due to chronic viral infection, resulting in acute cellular rejection and recurrent autoimmune disease, in the setting of minimized immunosuppression. While dysregulation of the cellular immune response in chronic HCV infection has been extensively studied (17), the mechanisms of this process during DAA therapy have just started to be elucidated (18, 19). Clinically, autoimmune phenomena are well-described complications of IFN-based HCV treatment (20). Additionally, kidney allograft recipients with HCV who received IFN-α therapy have been shown to have a higher incidence of acute cellular and humoral rejection compared to others not exposed to IFN-α therapy (21). In DAA therapy, successful eradication of HCV is associated with decrease in endogenous type II and III IFN response and recovery in type I IFN response in the liver (22). It is possible that the restored endogenous type I interferon response might have enhanced the autoimmune response and further investigation is needed to support this hypothesis.
As a single case report, our observation has several limitations, including limited available clinical data: such as liver fibrosis status pre-DAA treatment, protein measurements between the end of DAA treatment and the diagnosis of recurrent MN. In addition, given the long time gap between DAA treatment and onset of symptom, it might be difficult to prove direct causality. However, reported cases of the DAA-associated FSGS and lupus nephritis occurred 6–9 months after treatment initiation and our case did not deviate largely from previously described cases. Moreover, as a long-term survivor following kidney transplantation (more than 30 years), recurrence of membranous nephropathy in allograft is extremely uncommon after 10 years and was not expected, as is acute cellular rejection. Also, we did not find another trigger other than DAA, such as an infection to explain the recurrence of the glomerulopathy and the focal inflammatory process in the allograft.
In summary, this case, together with other reported cases of immune complex-mediated glomerulonephritis (7) and FSGS following DAA treatment, suggests immune modulation after DAA. As DAAs will be increasingly used for well-defined benefit to cure HCV infection, clinicians should be aware of potential immune-related complications.
Acknowledgement:
We thank Dr. Michifumi Yamashita (Ceders-Sinai Medical Center, CA, USA) for fruitful discussions and suggestions. N.M. is supported by National Institutes of Health (T32 DK7527).
Footnotes
Disclosure:
The authors of this manuscript have no conflicts of interest to disclose.
Author contributions:
NM wrote the manuscript, contributed to patient care, concept, and data interpretation. YD performed pathology analysis and interpretation, wrote the manuscript. AKC and DJC contributed to patient clinical care and critically revised the manuscript. HGR introduced the concept, supervised histology analysis and interpretation, and critically revised the manuscript.
References:
- 1.Fabrizi F, Martin P, Dixit V, Bunnapradist S, and Dulai G. Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant 2005; 5(6): 1452–1461. [DOI] [PubMed] [Google Scholar]
- 2.Kwo PY, Mantry PS, Coakley E et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med 2014; 371(25): 2375–2382. [DOI] [PubMed] [Google Scholar]
- 3.Colombo M, Aghemo A, Liu H et al. Treatment With Ledipasvir-Sofosbuvir for 12 or 24 Weeks in Kidney Transplant Recipients With Chronic Hepatitis C Virus Genotype 1 or 4 Infection: A Randomized Trial. Ann Intern Med 2017; 166(2): 109–117. [DOI] [PubMed] [Google Scholar]
- 4.Grupper A, Cornell LD, Fervenza FC, Beck LH Jr., Lorenz E, and Cosio FG. Recurrent Membranous Nephropathy After Kidney Transplantation: Treatment and Long-Term Implications. Transplantation 2015. [DOI] [PubMed] [Google Scholar]
- 5.Terrault NA and Adey DB. The kidney transplant recipient with hepatitis C infection: pre- and posttransplantation treatment. Clin J Am Soc Nephrol 2007; 2(3): 563–575. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global O. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008(109): S1–99. [DOI] [PubMed] [Google Scholar]
- 7.Sise ME, Wisocky J, Rosales IA et al. Lupus-like Immune Complex-mediated Glomerulonephritis in Patients with Hepatitis C Virus Infection Treated with Oral, Interferon-free, Direct-acting Antiviral Therapy. Kidney Int Rep 2016; 1(3): 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan JJ, Lim MA, Palmer MB, Bloom RD, Chung RT, and Sise ME. Development of proteinuria and focal segmental glomerulosclerosis during direct-acting antiviral therapy for hepatitis C virus infection. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg DS, Abt PL, Blumberg EA et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med 2017; 376(24): 2394–2395. [DOI] [PubMed] [Google Scholar]
- 10.Cosio FG and Cattran DC. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney international 2017; 91(2): 304–314. [DOI] [PubMed] [Google Scholar]
- 11.Dabade TS, Grande JP, Norby SM, Fervenza FC, and Cosio FG. Recurrent idiopathic membranous nephropathy after kidney transplantation: a surveillance biopsy study. Am J Transplant 2008; 8(6): 1318–1322. [DOI] [PubMed] [Google Scholar]
- 12.Kamar N, Marion O, Rostaing L et al. Efficacy and Safety of Sofosbuvir-Based Antiviral Therapy to Treat Hepatitis C Virus Infection After Kidney Transplantation. Am J Transplant 2016; 16(5): 1474–1479. [DOI] [PubMed] [Google Scholar]
- 13.Sawinski D, Kaur N, Ajeti A et al. Successful Treatment of Hepatitis C in Renal Transplant Recipients With Direct-Acting Antiviral Agents. Am J Transplant 2016; 16(5): 1588–1595. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez I, Munoz-Gomez R, Pascasio JM et al. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol 2016. [DOI] [PubMed] [Google Scholar]
- 15.Bhamidimarri KR, Ladino M, Pedraza F et al. Transplantation of kidneys from hepatitis C-positive donors into hepatitis C virus-infected recipients followed by early initiation of direct acting antiviral therapy: a single-center retrospective study. Transplant international : official journal of the European Society for Organ Transplantation 2017. [DOI] [PubMed] [Google Scholar]
- 16.Halloran PF, Chang J, Famulski K et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. Journal of the American Society of Nephrology : JASN 2015; 26(7): 1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SH and Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity 2014; 40(1): 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin B, Hennecke N, Lohmann V et al. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol 2014; 61(3): 538–543. [DOI] [PubMed] [Google Scholar]
- 19.Serti E, Chepa-Lotrea X, Kim YJ et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology 2015; 149(1): 190–200 e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson LE, Widman D, Dikman SH, and Gorevic PD. Autoimmune disease complicating antiviral therapy for hepatitis C virus infection. Semin Arthritis Rheum 2002; 32(3): 163–173. [DOI] [PubMed] [Google Scholar]
- 21.Baid S, Tolkoff-Rubin N, Saidman S et al. Acute humoral rejection in hepatitis C-infected renal transplant recipients receiving antiviral therapy. Am J Transplant 2003; 3(1): 74–78. [DOI] [PubMed] [Google Scholar]
- 22.Meissner EG, Wu D, Osinusi A et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest 2014; 124(8): 3352–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]


