Abstract
Introduction:
Placebo and nocebo effects form part of all therapeutic environments and play a significant role in the effectiveness of treatment outcomes. Patient expectancies drive these phenomena, which can be shaped through contextual factors including verbal suggestions, conditioning, and social observation.
Objectives:
This review seeks to identify the biopsychosocial factors of the patient– practitioner interaction that play a role in the development of placebo and nocebo effects, as well as the anthropological elements of the biodynamic process of relating that are meaningful in the development of expectancies.
Methods:
We conducted a narrative review of frameworks of the placebo and nocebo effect, including the impact of expectancies and interpersonal relationships in the context of healing and the clinical setting.
Results:
Expectancies leading to placebo and nocebo effects can be modified by macro and micro factors, such as culture and society, as well as individual psychobiological traits, respectively. The developmental sociobiological adaptations that form and consolidate mindsets and meaningful contexts play an important role in shaping patient expectancies, as well as patients’ conscious and subconscious reactions to signs and actions taking place within the clinical environment. Practitioner characteristics, like empathy, friendliness, and competence, favor the formation of positive expectancies. Caring and warm patient–practitioner interactions can enhance the therapeutic value of clinical encounters when patients’ positive expectancies are actively encouraged and engaged.
Conclusion:
A patient-centered approach rooted in demonstrating care and empathy can positively enhance a patient’s experience within the clinical environment and activate psychosociobiological adaptations associated with the placebo phenomenon. Pain patients could particularly benefit from non-invasive approaches for improving treatment effectiveness and quality-of-life.
1. BACKGROUND
The placebo and nocebo effects have fascinated scholars for decades due to the powerful and significant impact they have on different experimental and health outcomes. Numerous and rigorous research has found that placebo and nocebo effects are associated to multiple eliciting factors, such as verbal suggestions, social observation, conditioning and prior experience, individual personality traits, and genetic variants (Blasini, Corsi, Klinger, & Colloca, 2017; Colagiuri, Schenk, Kessler, Dorsey, & Colloca, 2015; Colloca, 2014; Corsi & Colloca, 2017). These different aspects interact with each other and modulate the expectancies that individuals may have regarding a treatment, intervention, or practitioner. If this combination of factors leads to a positive expectancy, it will likely result in a beneficial or improved outcome through the placebo effect, whereas negative expectancies could induce harmful outcomes due to the activation of the nocebo effect. Overall, we can state that these phenomena are associated to cognitive–affective factors that can trigger a top-down neural and biochemical modulation of different physiological processes, thus influencing pain (Benedetti, Pollo, & Colloca, 2007; Colloca & Grillon, 2014; Petersen et al., 2014; Price, Craggs, Nicholas Verne, Perlstein, & Robinson, 2007), fatigue (Shaibani, Frisaldi, & Benedetti, 2017), Parkinson’s disease (Frisaldi et al., 2017; Udupa & Fox, 2015), immunity (Tekampe et al., 2017; Wendt, Albring, & Schedlowski, 2014), irritable bowel syndrome (Ballou et al., 2017; Kaptchuk et al., 2010), and the effectiveness of medications (Ader et al., 2010; Bingel et al., 2011; Colloca, Enck, & DeGrazia, 2016), among others. While ethical considerations have always been of concern due to an association of the effectiveness of placebos with deception, newer research has found that placebo effects can occur even when an individual is aware of receiving or having received a placebo intervention (Ballou et al., 2017; Carvalho et al., 2016; Colloca, Pine, Ernst, Miller, & Grillon, 2016; Kaptchuk et al., 2010; Locher et al., 2017).
Although the attention to the placebo and nocebo continues to increase as more is discovered about their intricate origin and nature, these are not phenomena that have only recently appeared in the modern human experience of health and illness. Researchers have suggested that these mechanisms are part of adaptations favored through sociobiological evolution (Colloca, 2014). Some of the earliest reports of placebos date back to the 1500s, when a “trick trial” was done to disprove exorcisms in face of rising Protestant skepticism around these events and the Catholic Church. In this trial, ordinary water was placed in a religious flask and was given to a girl who was deemed to be possessed by the devil; an event which should have caused the girl to go into contortions typically described during exorcisms. However, in other occasions, true holy water was covertly given to the girl, and no effects occurred (Kaptchuk, Kerr, & Zanger, 2009). In 1955, Dr. Henry K. Beecher, an anesthesiologist, published in the Journal of the American Medical Association that placebos accounted for an average of approximately 35% of therapeutic effectiveness across a variety of conditions ranging from headache and nausea, to post-operative pain and anxiety, based on 15 studies that included a total of 1082 patients (Beecher, 1955). In particular, Beecher pointed out how much of a drug’s action can be related to the individual processing of “suffering” rather than direct effects on the original ailments requiring medication (Beecher, 1955).
Placebo and nocebo effects do not solely need the administration of an inert substance (i.e., a placebo) for them to occur. Contextual factors, which include the patient–practitioner interaction, social observation of others, and environmental cues, can add to the stimuli of these effects. In other words, the occurrence and magnitude of the placebo or nocebo effect can be modulated through the individual positive or negative psychosocial perceptions resulting from the contexts in which they occur (Colloca & Benedetti, 2005; Colloca, Lopiano, Lanotte, & Benedetti, 2004; Di Blasi, Harkness, Ernst, Georgiou, & Kleijnen, 2001; Di Blasi & Reilly, 2005).
Inevitably, the clinical setting is accompanied by a myriad of messages carried through symbols, signs, words, and actions that convey meaning to each patient. These carriers of information lead to the changes in expectancy that can cause placebo and nocebo effects. This article explores important biopsychosocial aspects of these phenomena, namely, through a characterization of anthropological and contextual elements involved in the patient–practitioner relationship. Further, we discuss how to pragmatically approach these aspects within the clinical environment. Finally, we provide questions that can serve as potential avenues of research to overcome challenges arising from the complexity of systemic, sociocultural, and psychobiological factors involved in the context of health and the placebo effect.
2. THE PATIENT–PRACTITIONER INTERACTION: A SOCIOBIOLOGICAL APPROACH
The interaction between a patient and a healthcare provider has the potential to influence clinical outcomes through the continual psychosocial process of relating (Adler, 2007). Relating represents a set of complex interactions in which the engaged parties reciprocally effect the behavior, as well as the experience, of the other (Adler, 2007). Given that no psychosocial influence occurs without a precedent and subsequent psychophysio-logical response, this continual process may also significantly impact factors relevant to clinical and health outcomes. The patient–practitioner interaction can be then described as an emergent, reciprocal, and self-organizing process that creates feedback loops leading to the mutual regulation of “biopsychosocial responses” within the medical context (Adler, 2007).
Adler and colleagues supported the theory that the placebo effect is fundamentally rooted in the evolutionary human necessity for groups and systems (Adler & Hammett, 1973). The complex and progressive evolutionary events that led to human development as a social animal, such as the need for prolonged care of infants postpartum when compared to other mammals and the need for adequate integration and communication of individual physical and external environments, have played a vital role in the ability of humans to form complex psychosocial matrices. These psychosocial aspects of the human experience are intrinsically and essentially connected to proper psychophysical function.
As described by Adler and others, social interactions continue to actively modify neural structures (Greenough, 1992; Kandel, 1979) and circuits (Kandel & Squire, 2000) that impact neurobiological, neuroendocrine, and affective responses within the context of social attachment throughout the lifespan (Adler, 2007; Insel, 1997; Nelso & Panksepp, 1998; Young, Wang, & Insel, 1998). Interpersonal interactions and relationships have the ability to influence and regulate physiological function (Adler, 2007; Hofer, 1981, 1984). An important aspect of this mutual co-regulation is the concept of affect attunement. This can be understood as a “secure, affectional bond” (Adler, 2007) leading to a level of autonomic synchronization that promotes feelings of satisfaction and calmness that are similar in nature to what is encountered between a caregiver and an infant (Adler, 2007; Faude, Jones, & Robins, 1996). Affect attunement, which is likely not under complete conscious control due to its instant and continuous characteristics, can play a significant role in creating feelings of empathy in the person who is functioning primarily in the role of caregiving (Adler, 2007; Murray, 1979).
Importantly, empirical evidence suggests that empathy can be considered a neurobiological response, as demonstrated by a study with positron emission tomography (PET) in which empathy of distress was significantly associated with specific neural network activation (Adler, 2007; Shamay-Tsoory et al., 2005).
A recently published study supports the importance of physician characteristics during interpersonal interactions within an experimental medical setting. Investigators found that perceived physician warmth and competence, along with positive expectations of treatment, boosted placebo responses that ameliorated skin allergic reactions resulting from a histamine skin prick with the subsequent application of a cream with no active ingredients (Howe, Goyer, & Crum, 2017). Although these physician characteristics relate more to perceived likeability and credibility rather than empathy itself, they form part of the contextual psychosocial factors that help establish rapport with patients (see also Sussex, 2018).
The distress and suffering that an illness or disease can produce in a patient likely result in the activation of the sympathetic nervous system, primarily the hypothalamic–pituitary–adrenal axis (HPA). Anxiety can play a strongly influential role in the process of illness, and thus, this period may be characterized by a vulnerability in which the patient may either intentionally or inadvertently seek relief and psychobiological security by bonding with a caregiver figure, in this case the physician or health provider (Adler, 2007; Benedetti, Lanotte, Lopiano, & Colloca, 2007; Colloca & Benedetti, 2007). This vulnerability could likely increase the opportunity for the application of clinical empathy to create a positive psychobiological enhancement during the clinical encounter, and thus potentially improve both psychological and physiological outcomes. The skillful engagement of emotional resonance and affect attunement by a physician in the form of clinical empathy can help regulate the patient’s autonomic arousal and decrease allostatic load (McEwen, 1998), thus promoting physiological homeostasis (Adler, 2007; Carter, 1998; Porges, 1998).
Although the process of empathy can be variable and driven by many subjective factors, the concept of clinical empathy is characterized by certain elements that allow for the consideration of it as a “clinical procedure” (Adler, 2007). Adler presents three primary features that support this idea. First, the engagement of clinical empathy in the context of disease and relief of suffering requires a specific medical interaction, and thus clinical empathy would have a medical indication. Second, the use of clinical empathy would require a set of interpersonal skills that depend upon active “emotional labor” by the physician or health provider (Adler, 2007; Larson & Yao, 2005). Finally, clinical empathy is a purposeful process attempting to attain specific outcomes within the clinical context that will result in the improvement of health through the engagement of psychobiological mechanisms (Adler, 2007).
It is important to note that by deeply immersing into an empathic experience with their patients, physicians may experience a high degree of emotional vulnerability that could potentially lead to emotional imbalance (Brody, 1997). Given that current burn out rates among physicians in the United States are estimated to be over 50% (Shanafelt, Dyrbye, & West, 2017; Shanafelt et al., 2015), it is critical to engage many entities, leaders, medical educators, and organizations within the healthcare system to address this issue that not only affects physicians, but by extension, can also affect the health outcomes of their patients. Building emotional resilience among physicians can counteract the burden that proactive empathy and emotional attunement may cause (Brody, 1997; Coulehan, 1995).
3. INTERPERSONAL HEALING
Research has suggested that physician expectations and beliefs influence their patients’ clinical outcomes. A study by Gracely, Dubner, Deeter, and Wolskee (1985) compared postoperative pain after dental surgery in a double-blind setting. Patients were told that they would be administered fentanyl (opioid), naloxone (opioid antagonist), or a placebo. A total of 60 patients were divided into a placebo–naloxone (PN) group and a placebo– naloxone–fentanyl group (PNF). Doctors were told that patients would be receiving one of the substances corresponding to each group (i.e., placebo or naloxone only for PN group; placebo, naloxone or fentanyl for PNF group). Neither patients nor doctors knew which of the substances were given at the time of administration. Results showed that pain levels in the placebo arm of the PNF group were significantly lower when compared to the placebo PN group. The specific results regarding naloxone administration were not discussed (Gracely et al., 1985). This study suggested that doctors’ expectations, and likely, subtle behaviors of the clinician administering the drug, might have had an effect in pain levels. These effects could be associated to the way that physicians communicate and express their own personal biases while engaging in their interpersonal rapport, and thus can influence patients’ perceptions of the information provided by the physician in terms of treatment, health and disease outcomes, as well as the perceptions of the physician characteristics themselves. Thus, it was concluded that “clinical analgesia depends not only on the physiological action of the treatment administered but also on the expectations of both the patient and clinician” (Gracely et al., 1985).
Miller and colleagues proposed the consideration of placebo effects as a form of interpersonal healing (Miller, Colloca, & Kaptchuk, 2009). The authors describe how modern biomedicine, primarily focused in patho-physiological states of disease, has paid much less attention to the concept of illness and how the medical encounter can help in the “relief of suffering,” suffering being described as the “illness component of disease and injury.” Treating an illness can be supported and encouraged through the patient–physician relationship, rather than solely by administering specific treatments (Miller et al., 2009). Although placebo effects include changes in measurements within subjective and objective domains, of which the latter have been confirmed by brain-imaging, biochemical and pharmacological studies (Colloca, Benedetti, & Porro, 2008; Faria, Fredrikson, & Furmark, 2008; Miller et al., 2009), there is little quality evidence linking mitigation of symptoms with actual changes in the pathophysiology of diseases.
The placebo effect can then be considered as a general concept through which multiple mechanisms and pathways implicated in relief and coping can be engaged and established during the patient–clinician interaction as well as during the patient’s experience of therapeutic outcomes. These pathways are varied, and can be psychologically related to expectancy mechanisms, and neurobiologically related to the activation of endogenous opioid, dopamine, endocannabinoid, oxytocin, vasopressin and serotonin systems, and the inactivation of cholecystokinin (Benedetti, 2009; Miller et al., 2009).
Importantly, Miller et al. describe certain aspects related to the concept of interpersonal healing that differ from natural and technological healing, the latter two referring to the natural progressions of disease and illness, and biomedical intervention, respectively (Miller et al., 2009). Specifically, it is mentioned how some level of a patient’s attention and alertness is required for interpersonal healing and the mechanisms of the placebo effect to occur (Miller et al., 2009). This concept has been demonstrated by Colloca and colleagues, in which a paradigm of open and hidden administration of medication showed improved outcomes in pain, bradykinesia, and anxiety in patients who were aware of receiving an active medication through direct interaction with a health practitioner along with suggestions of benefit, when compared to those patients who received the same pharmacological medication through an automated pump (Colloca et al., 2004). Additionally, shifting a patient’s attention away from a focus on noxious or other negative stimuli can help in reducing fear and other factors that influence the placebo and nocebo phenomenon, as well as patient wellbeing, and can thus help mitigate the symptoms of illness (Miller et al., 2009).
4. SIGNS AND MEANING
As demonstrated by the open-hidden paradigm, interactions and communications serve as a vehicle for facilitating both placebo and nocebo effects. Miller and Colloca applied the theory of signs developed by American philosopher Charles Sanders Peirce, named semiotics, as a way to bridge the neurobiological, psychological and cultural components of the placebo effect with the concept of meaning (Colloca et al., 2011; Miller & Colloca, 2010). Peirce developed the theory of signs to form a systematic comprehension of the phenomenon of logic, communication, and learning (Peirce, 1940). Within this context, Peirce defined signs as “something which stands to somebody for something” (Peirce, 1940). Peirce’s theory described the consequences and influences of signs as a relationship between three main components: (1) the sign vehicle “carrying” or conveying an object; (2) the significance of the object represented by the sign vehicle; and (3) the idea within the mind of the interpreter about the sign vehicle and associated object. In these circumstances, the interpreter consciously and subconsciously interprets the information provided by the sign.
Furthermore, Peirce categorized signs into three primary types: icons, indexes and symbols. Icons provide meaning to signs through observable likeness between the sign and the represented object, and can include, for example, diagrams and pictures (Miller & Colloca, 2010; Peirce, 1940). Icons convey information through resemblance.
Indexes, on the other hand, represent a direct association between a sign and the object, which also encompass the memories and senses of the interpreter. The use of conditioned and unconditioned stimuli to create conditioned responses, namely, classical conditioning, falls within this category. The recognition of, and response to, an index sign is analogous to the conditioned response brought by a conditioned stimulus (Colloca et al., 2011; Miller & Colloca, 2010).
Finally, Pierce defines symbols as signs that associate a general concept by virtue of conventional rules and the conceptualizations and understandings of the interpreter. This can include, for example, verbal communication (Colloca et al., 2011; Miller & Colloca, 2010; Peirce, 1904). Within a clinical context, a symbol can be the verbal language coming from a physician stating “this will help your pain” prior to the injection of a solution, which will be interpreted by the patient as a symbol meaning that pain relief should be expected (Colloca et al., 2011; Miller & Colloca, 2010). As noted by Kirsch (1997) these verbal suggestions and symbols can engage placebo effects through the manipulation of individual expectancies, which include aspects of conditioned responses (Kirsch, 1997; Miller & Colloca, 2010).
Based on this theory, signs give information about objects to an interpreter, and these signs, whether icons, indexes or symbols, or indexes, serve as a contextual vehicle for the placebo effect in the clinical setting. For the patient, the interpreted signs can function as therapeutic agents for responses that have been learned through the continuous processing of what signs, within the clinical setting, convey. This interpretation by the patient can be shaped by both emotion and cognition and can cause both positive or negative effects on the patient’s health or outcome, namely, a placebo or a nocebo effect (Colloca & Miller, 2011; Miller & Colloca, 2010). As a result, the semiotics model helps bridge the linguistic, sociocultural and neurobiological elements of the placebo phenomenon within the dynamics of learning, meaning, and contextual factors.
An important aspect in the way signs convey information relates to the meaning that is developed with continuous exposure to these signs. Daniel Moerman, a well-known anthropologist, developed the concept and role of “meaning” within health and illness as a framework for understanding the placebo phenomenon. In fact, Moerman proposed replacing the vernacular of placebo response—to meaning response (Moerman, 2002), as he believed that changing the terminology was important for mitigating negative historical connotations around the term “placebo” (i.e., the placebo effect being thought of as a “nuisance variable”). His proposal allowed for a focus on what he believed is a key part of the healing and disease process: the meaning of an illness. Moerman and Jonas co-authored an article (2002) that defined the meaning response as the “physiologic or psychological effects of meaning in the origins or treatment of illness” (Moerman & Jonas, 2002).
For example, a British study of 835 women frequently using analgesics for headaches evaluated the effects of branding in both aspirin and placebo. Investigators found that aspirin provided in a branded box worked better than its unbranded counterpart, and that the branded placebo worked better than the unbranded placebo, although the former did not surpass the effectiveness of the unbranded aspirin (Branthwaite & Cooper, 1981; Moerman & Jonas, 2002). From a total of 435 headaches that were reported by those receiving branded placebo, a significant 64% reported symptom improvement after only 1 hour following medication administration, while from the 410 headaches reported by the unbranded placebo group, only a 45% reported improvement at the same time point (Branthwaite & Cooper, 1981).
It is important to consider the high variability that can exist in individual meaning due to terminology and conceptual differences originating from social and cross-cultural factors. As outlined by Moerman and Jonas (2002), “…1) meaning has biological consequence and 2) meanings vary across cultures, [thus] we can anticipate that biology will differ in different places, not because of genetics but because of these entangled ideas” (Moerman & Jonas, 2002).
Although the individual concept of meaning varies, meaning itself is a fundamental construct shaped by social contexts and processes, perceptions, and identities (Fife, 2005). Contextual meaning goes beyond an individual’s self-meaning, which is more closely associated to identity, by encompassing specific circumstantial factors related to an individual’s perception of the responses of others in association to changes brought by an event (Fife, 2005). In the context of illness or a treatment, different meanings lead to distinct adaptive responses, as well as varying levels of stress responses. Thus, since these fundamental cognitions shape the individual interpretation and classification of events and have the potential to influence both behavior and physiology, it would be plausible to consider that within a clinical context these cognitive and mentalizing processes may engage psychosocial elements which in turn, engage placebo and nocebo effects. Whether these effects are positive or negative depends upon the context and perspectives associated with circumstantial factors surrounding the individual, the clinical setting, and treatments (Moerman & Jonas, 2002).
Some point to limitations of the meaning framework when it comes to explaining the placebo phenomenon within certain contexts. Specifically, it relies on the perception of psychosocially-driven “symbolic meaning,” and thus may potentially fail to account for the observed phenomenon of some conditioned placebo effects that occur independently of conscious perception or meaning (Miller et al., 2009). Further, placebo effects have been experimentally elicited through conditioning in animals such as mice and rats, in which the influence of symbolic meaning is expected to be, for the most part, absent (Keller, Akintola, & Colloca, 2018). In summary, although meaning itself could be understood as a human characterization, it undoubtedly plays a role in the placebo and nocebo phenomenon by influencing learning and shaping expectancies, as meaningful symbols also form part of conditioned stimuli and responses.
5. CONNECTING THE DOTS: A CLINICAL APPROACH
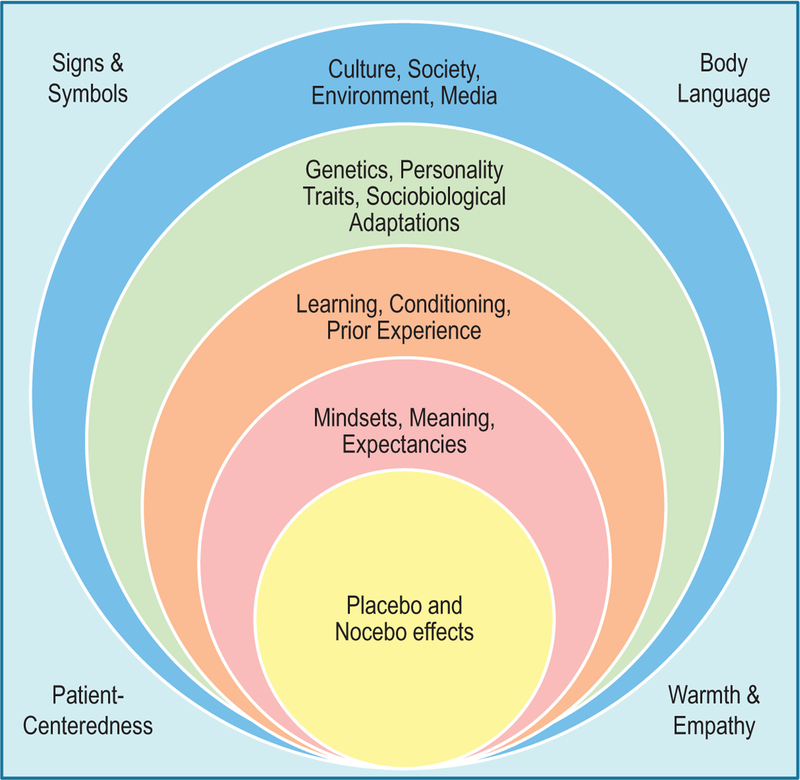
During a period of illness, patients often find themselves in a vulnerable and stressful position. In this case, the simple behavior of going to see a healthcare practitioner creates a context of healing that forms part of the therapeutic process (Adler, 2002). The physician himself/herself becomes a sign that conveys a meaning, whether positive or negative, depending on the context upon which prior and current interactions occur. Establishing a positive therapeutic alliance with patients is crucial during the clinical encounter not only to promote placebo-induced relief of symptoms, but to preserve and enhance treatment efficacy and effectiveness (Fig. 1).
Fig. 1.
Sociobiological and behavioral organization of placebo and nocebo effects. Like a socio-ecological model of human behavior and development, placebo and nocebo effects are preceded and modeled by many sociopsychobiological factors that influence patients’ expectancies. On a macro scale, the environment, society, and culture play a very important role as they become one of the first filters of reality and day-to-day experience of human beings. Societal and cultural factors influence how an individual perceives disease, well-being, treatments, and even the figure of the physician. Since they can directly affect how a person is able to identify, cope, and function with illnesses, even those originating from genetic traits, psychobiological factors were located a level under the sociocultural tier. Placebo and nocebo effects are the product of a dynamic relational process with external forces that provide meaning and context to health, disease, the patient–practitioner relationship, and the therapeutic process. These meaningful contexts are influenced by an individual’s priming and experience, and help shape the formation of expectancies, mindsets and the relevance of signs and symbols. At the center of all of these multi-layered factors lie the mechanisms of the placebo and nocebo effect. At the periphery, we find external vehicles and facilitators of messages that convey important information within the patient–practitioner interaction.
For a patient–practitioner relationship to be therapeutic in itself, it would need to be perceived by patients as a caring, non-judgmental, and supportive interaction that provides relief during a period of high stress (Kornhaber, Walsh, Duff, & Walker, 2016). Characteristics of such alliances include friendliness, empathy, warmth, and genuine involvement and interest. Being purposeful with body language and facial expressions, active listening, showing concern, receptiveness, and responsiveness can strengthen the therapeutic relationship with a patient and set a tone for an empowering alliance. Non-verbal communication is of vital importance during the patient–practitioner interaction, as it is estimated that the meaning of a message can be divided into 7% of content, 38% tone of voice, and 55% body language (Mehrabian, 1971). A recent review found that there is substantial evidence that positive therapeutic relationships between patients and providers are correlated with improvements in quality of life, anxiety and depression levels, as well as patient satisfaction and treatment adherence (Kornhaber et al., 2016). A positive doctor–patient relationship can also help decrease the quantity of medication used, and therefore, experienced side effects. A commentary published in the New England Journal of Medicine suggested the application of placebos in the treatment for pediatric migraine in order to reduce side effects (Jackson, 2017). On the other hand, negative patient–practitioner relationships are characterized by psychological distress and the onset of negative feelings of invalidation and dehumanization (Kornhaber et al., 2016).
Interactions that hold a business-like character and that create doubt and uncertainty around the practitioner’s skills and competence could lead to nocebo effects or to a reduction of placebo effects (Howe et al., 2017). HPA axis activation, anxiety, and the activity of the cholecystokinin system have been associated to the occurrence of nocebo effects (Benedetti, Amanzio, Casadio, Oliaro, & Maggi, 1997; Benedetti, Amanzio, Vighetti, & Asteggiano, 2006; Blasini et al., 2017). Nocebo effects are strong enough to decrease the efficacy of remifentanil, a short-acting and very potent opioid (Bingel et al., 2011). Therefore, preventing interactions that are uncaring, judgmental and insensitive, which send a message of invalidation and lack of warmth, while at the same time creating a safe and positive environment for patients to begin internalizing their own process of healing, can prevent the nocebo effect while promoting the activation of placebo mechanisms.
This “healing environment” can be concretely achieved through different approaches aiming to promote the formation and consolidation of positive expectancies. These positive expectancies do not refer to a use of illusory or deceptive information. Rather, positive expectancies can begin to be established through a clear identification of patient beliefs, concerns, and goals (also, see Darnall & Colloca, 2018). This patient-centered approach can help identify patient mindsets, which are views or constructs that orient the development of associations, convictions, and dispositions about situations and experiences. Mindsets can be formed and shaped by different factors, including culture, socialization processes, spirituality and religion, and media exposure (Crum & Zuckerman, 2017). These factors help direct decision-making, behaviors, and attitudes, and can function as a shortcut for mental processes and behaviors. Changes toward the establishment and modification of positive mindsets have been shown to provoke physiological changes in immunity (Howe et al., 2017), as well as in multiple hormonal and cardiovascular factors including cortisol (Crum, Salovey, & Achor, 2013) and hemoglobin A1C (Crum & Zuckerman, 2017). Researchers have identified two main subtypes of mindsets that are particularly influential in the context of the patient–practitioner relationship: mindsets regarding treatment efficacy and individual capacity to change (Crum & Zuckerman, 2017). When strategically approached, perhaps through the application of questioning and motivational interviewing skills, identifying patient mindsets may serve as an efficient way for evaluating a patient’s stance on different aspects of the clinical interaction and setting, including previous experiences and expectations regarding treatment plans and other outcomes.
In addition to the practitioner’s expression of certain psychosocial characteristics within their role, other actions can be taken to enhance the placebo and prevent the nocebo effect. For instance, a practitioner could avoid over-emphasizing negative information regarding a treatment by balancing it with information about the positive effects in a truthful and ethical manner that preserves patient autonomy (Colloca, 2017; Klinger, Blasini, Schmitz, & Colloca, 2017). Some research has suggested that providing patients with a rationale within an honest and positive interaction (Ballou et al., 2017; Kaptchuk et al., 2010), and explaining the mechanisms of action of a therapy (Colloca, 2017), boost placebo effects and prevent the formation of the nocebo effect.
It is imperative to consider the importance that cultural differences may have in the clinical setting, particularly in the identification of signs and cues within the patient–practitioner interaction. As stated by Dr. Moerman, “Much of our knowledge of the world is not an elicitation of what ‘is,’ but rather it is a construction laid atop the world of experience” (Moerman, 2002). Differences in the meaning of cultural signs and socialization lead to different psychosocial adaptations and subsequent physiologic responses to events related to health and illness. This occurs because these social processes form perceptions tailored to the culture and environment in which a person may be developing. Although this adds a degree of complexity to the practitioner who is part of the bi-cultural patient–practitioner relationship, training which focus on building linguistic and cultural competency skills can help overcome some of these challenges.
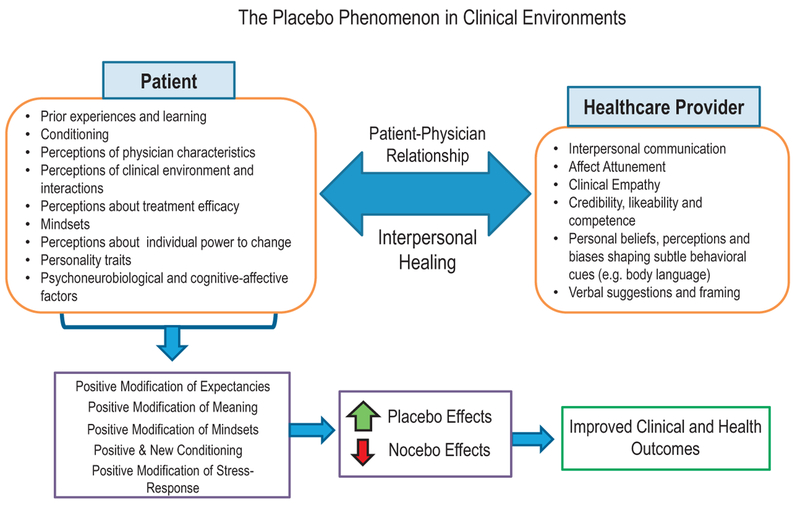
In summary, these described characteristics of a therapeutic interpersonal relationship harness the underlying mechanisms of the placebo effect via sociobiological modulation of physiologic responses. In the era of patient-centered outcomes in healthcare, physicians can be seen as “therapeutic agents” that can exert an effect through mechanisms associated with the placebo effect as a tool to help facilitate health and healing (Wampold, 2018). As stated in Kaptchuk’s article, “supportive and attentive health care legitimately creates a therapeutic bias” in patients toward hope and an experience of relief (Fig. 2; Kaptchuk & Miller, 2015).
Fig. 2.
The patient–practitioner interaction as a vehicle for the dynamic exchange of sociobiological information in the context of health and illness. A patient-centered process of relating sets the foundation for interpersonal healing to occur, and in doing so, it has the power to positively modify the expectancies that could influence a patient’s health and clinical outcomes. This dyadic concept of the placebo phenomenon encompasses how a practitioner establishes a context of healing and well-being. The placebo and nocebo phenomena can be provoked and prevented, respectively, through a positive therapeutic alliance.
6. CONCLUSION
Medicine is a field that has been inherently built through rituals and symbols such as the act of going to a doctor or healer, writing prescriptions, and administering medication (Kaptchuk, 2011). As part of a larger contextual process of relating, the placebo effect can be implemented to help enhance treatments. Empathy, positive interpersonal relationships, cultural competence and sensitivity, and personalized interactions according to identified meaningful mindsets and conceptions, can become part of the therapeutic target as ways to improve clinical outcomes. Active engagement from physicians and health care providers can help patients positively reframe their mindsets, expectations, and expectancies in ways that activate both conscious and subconscious biological and coping mechanisms that can reduce symptom severity, promote patient satisfaction, and improve the natural physiological process of healing.
An attitude of caring, respect, and patient empowerment through meaningful patient-centered discussions within the patient–practitioner interaction can serve as a fundamental basis for engaging the placebo effect and preventing the nocebo effect in any treatment that is decided upon (Moerman, 2002). More research should be directed into how the characteristics of the patient–practitioner relationship help to improve health outcomes, for both objective and subjective measurements.
Although most of these concepts are not entirely new to the healthcare community, as health coaches and similar practitioners strategically utilize these during their interventions to promote and improve behavioral change, adherence, and lifestyle modifications (Wolever et al., 2013), they may lack practicality in clinical settings where there is limited time to spend with a patient or when there is insufficient communication between the various practitioners a patient may see. Many questions remain, as there are no specific guidelines that a practitioner can follow that will necessarily work exactly the same with each different patient. These questions include: Is there a specific order and method that can enhance the efficiency of the application of therapeutic interpersonal relationships in a system with limited time and resources? Could we specifically enhance the innate phenomenon of the placebo effect without the need of placebo interventions? How can these results differ across communities and cultures?
Despite these challenges, understanding the process of relating, as well as the bi-directional components that continually and dynamically feed into it, can help individual practitioners pragmatically and routinely engage these innate human mechanisms in an ethically compliant manner for the short- and long-term benefit of their patients. In the field of pain management, where clinically effective and lasting responses that protect a patient’s quality of life and function can be so difficult at times, having an awareness of the potential benefits of positive patient–practitioner interactions can be a tool for producing substantial positive influences which could make a considerable difference in the lives of pain patients.
ACKNOWLEDGMENTS
This research was supported by the University of Maryland, Baltimore (L.C.) and the National Institute of Dental and Craniofacial Research (NIDCR, R01DE025946, L.C.).
REFERENCES
- Ader R, Mercurio MG, Walton J, James D, Davis M, Ojha V, et al. (2010). Conditioned pharmacotherapeutic effects: A preliminary study. Psychosomatic Medicine, 72(2), 192–197. 10.1097/PSY.0b013e3181cbd38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler HM (2002). The sociophysiology of caring in the doctor-patient relationship. Journal of General Internal Medicine, 17(11), 874–881. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12406360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler HM (2007). Toward a biopsychosocial understanding of the patient-physician relationship: An emerging dialogue. Journal of General Internal Medicine, 22(2), 280–285. 10.1007/sll606-006-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler HM, & Hammett VB (1973). The doctor-patient relationship revisited. An analysis of the placebo effect. Annals of Internal Medicine, 78(4), 595–598. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4694043. [DOI] [PubMed] [Google Scholar]
- Ballou S, Kaptchuk TJ, Hirsch W, Nee J, Iturrino J, Hall KT, et al. (2017). Open-label versus double-blind placebo treatment in irritable bowel syndrome: Study protocol for a randomized controlled trial. Trials, 18(1), 234 10.1186/sl3063-017-1964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher HK (1955). The powerful placebo. Journal of the American Medical Association, 159(17), 1602 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- Benedetti F (2009). Placebo effects: Understanding the mechanisms in health and disease. Oxford University Press. [Google Scholar]
- Benedetti F, Amanzio M, Casadio C, Oliaro A, & Maggi G (1997). Blockade of nocebo hyperalgesia by the cholecystokinin antagonist proglumide. Pain, 71(2), 135–140. 10.1016/S0304-3959(97)03346-0. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, & Asteggiano G (2006). The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. Journal of Neuroscience, 26(46), 12014–12022. 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Lanotte M, Lopiano L, & Colloca L (2007). When words are painful: Unraveling the mechanisms of the nocebo effect. Neuroscience, 147, 260–271. 10.1016/j.neuroscience.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, & Colloca L (2007). Opioid-mediated placebo responses boost pain endurance and physical performance: Is it doping in sport competitions? Journal of Neuroscience, 27(44), 11934–11939. 10.1523/JNEUROSCI.3330-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, et al. (2011). The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Science Translational Medicine, 3(70), 70ral4 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Blasini M, Corsi N, Klinger R, & Colloca L (2017). Nocebo and pain: An overview of the psychoneurobiological mechanisms. Pain Reports, 2, 1–9. 10.1097/PR9.0000000000000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branthwaite A, & Cooper P (1981). Analgesic effects of branding in treatment of headaches. British Medical Journal (Clinical Research Ed.), 282(6276), 1576–1578. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6786566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H (1997). Placebo response, sustained partnership, and emotional resilience in practice. The Journal of the American Board of Family Practice, 10(1), 72–74. [PubMed] [Google Scholar]
- Carter CS (1998). Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology, 23(8), 779–818. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9924738. [DOI] [PubMed] [Google Scholar]
- Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, & Kirsch I (2016). Open-label placebo treatment in chronic low back pain: A randomized controlled trial. Pain, 157(12), 2766–2772. https://doi.Org/10.1097/j.pain.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colagiuri B, Schenk LA, Kessler MD, Dorsey SG, & Colloca L (2015). The placebo effect: From concepts to genes. Neuroscience, 307, 171–190. 10.1016/j.neuroscience.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L (2014). Placebo, nocebo, and learning mechanisms pp. (17–35). Berlin, Heidelberg: Springer, 10.1007/978-3-662-44519-8_2. [DOI] [PubMed] [Google Scholar]
- Colloca L (2017). Tell me the truth and i will not be harmed: Informed consents and nocebo effects. The American Journal of Bioethics: AJOB, 17(6), 46–48. 10.1080/15265161.2017.1314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, & Benedetti F (2005). Placebos and painkillers: Is mind as real as matter? Nature Reviews. Neuroscience, 6(7), 545–552. 10.1038/nrnl705. [DOI] [PubMed] [Google Scholar]
- Colloca L, & Benedetti F (2007). Nocebo hyperalgesia: How anxiety is turned into pain. Current Opinion in Anaesthesiology, 20(5), 435–439. 10.1097/ACO.0b013e3282b972fb. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F, & Porro CA (2008). Experimental designs and brain mapping approaches for studying the placebo analgesic effect In European Journal of Applied Physiology: Vol. 102 Heidelberg: Springer-Verlag; Retrieved from http://www.academia.edu/23744290/Experimental_designs_and_brain_mapping_approaches_for_studying_the_ placebo_analgesic_effect. [DOI] [PubMed] [Google Scholar]
- Colloca L, Enck P, & DeGrazia D (2016). Relieving pain using dose-extending placebos. Pain, 157, 1 https://doi.Org/10.1097/j.pain.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, & Grillon C (2014). Understanding placebo and nocebo responses for pain management. Current Pain and Headache Reports, 18(6), 419 10.1007/si1916-014-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Lopiano L, Lanotte M, & Benedetti F (2004). Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. The Lancet. Neurology, 3(11), 679–684. 10.1016/S1474-4422(04)00908-l. [DOI] [PubMed] [Google Scholar]
- Colloca L, Miller FG, Harrington A, Miller FG, Colloca L, Peirce C, et al. (2011). How placebo responses are formed: A learning perspective. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1572), 1859–1869. 10.1098/rstb.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Pine DS, Ernst M, Miller FG, & Grillon C (2016). Vasopressin boosts placebo analgesic effects in women: A randomized trial. Biological Psychiatry, 7.9(10), 794–802. https://doi.Org/10.1016/j.biopsych.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi N, & Colloca L (2017). Placebo and nocebo effects: The advantage of measuring expectations and psychological factors. Frontiers in Psychology, 8, 308 10.3389/fpsyg.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulehan JL (1995). Tenderness and steadiness: Emotions in medical practice. Literature and Medicine, 14(2), 222–236. [DOI] [PubMed] [Google Scholar]
- Crum AJ, Salovey P, & Achor S (2013). Rethinking stress: The role of mindsets in determining the stress response. Journal of Personality and Social Psychology, 104(4), 716–733. 10.1037/a0031201. [DOI] [PubMed] [Google Scholar]
- Crum A, & Zuckerman B (2017). Changing mindsets to enhance treatment effectiveness. JAMA, 317(20), 2063 10.1001/jama.2017.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall BD, & Colloca L (2018). Optimizing placebo and minimizing nocebo to reduce pain, catastrophizing, and opioid use: A review of the science and an evidence-informed clinical toolkit In Colloca L (Ed.), Neurobiology of the placebo effect, part II, International review of neurobiology. Vol. 139 (pp. 129–157). New York: Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Blasi Z, Harkness E, Ernst E, Georgiou A, & Kleijnen J (2001). Influence of context effects on health outcomes: A systematic review. The Lancet (London, England), 357(9258), 757–762. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11253970. [DOI] [PubMed] [Google Scholar]
- Di Blasi Z, & Reilly D (2005). Placebos in medicine: Medical paradoxes need disentangling. BMJ (Clinical Research Ed.), 330(7481), 45 10.1136/bmj.330.7481.45-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria V, Fredrikson M, & Furmark T (2008). Imaging the placebo response: A neurofunctional review. European Neuropsychopharmacology, 18(7), 473–485. 10.1016/j.euroneuro.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Fife BL (2005). The role of constructed meaning in adaptation to the onset of life-threatening illness. Social Science & Medicine, 61(10), 2132–2143. https://doi.Org/10.1016/j.socscimed.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Frisaldi E, Carlino E, Zibetti M, Barbiani D, Dematteis F, Lanotte M, et al. (2017). The placebo effect on bradykinesia in Parkinson’s disease with and without prior drug conditioning. Movement Disorders, 32, 1474–1478. 10.1002/mds.27142. [DOI] [PubMed] [Google Scholar]
- Faude J, Jones C, & Robins M (1996). The affective life of infants: empirical and theoretical foundations In Nathanson D (Ed.), Knowing feeling, affect, script, and psycho-therapy (pp. 219–256). New York: WW Norton & Co. [Google Scholar]
- Gracely R, Dubner R, Deeter W, & Wolskee P (1985). Clinicians’ expectations influence placebo analgesia. The Lancet, 325, 43 10.1016/S0140-6736(85)90984-5. [DOI] [PubMed] [Google Scholar]
- Greenough W, & Black J (1992). Induction of brain structure by experience: substrates for cognitive development In Gunnar M & Nelson C (Eds.), Minnesota symposia on child psychology: developmental neuroscience, Vol. 24 Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Hofer MA (1981). The roots of human behavior: An introduction to the psychobiology of early development. Myron A. Hofer. W. H. Freeman & Co., San Francisco, xiii + 331 pp. Cloth, $22.50. Paper, $11.50. Developmental Psychobiology, 15(1), 89–91. 10.1002/dev.420150113. [DOI] [Google Scholar]
- Hofer MA (1984). Relationships as regulators: A psychobiologic perspective on bereavement. Psychosomatic Medicine, 46(3), 183–197. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6739679. [DOI] [PubMed] [Google Scholar]
- Howe LC, Goyer JP, & Crum AJ (2017). Harnessing the placebo effect: Exploring the influence of physician characteristics on placebo response. Health Psychology, 36, 1074–1082. 10.1037/hea0000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T (1997). A neurological basis of social attachment. American Journal of Psychiatry, 154, 726–735. [DOI] [PubMed] [Google Scholar]
- Jackson JL (2017). Pediatric migraine headache—Still searching for effective treatments. New EnglandJournal of Medicine, 376(2), 169–170. 10.1056/NEJMel614628. [DOI] [PubMed] [Google Scholar]
- Kandel ER (1979). Psychotherapy and the single synapse: The impact of psychiatric thought on neurobiologic research. The New England Journal of Medicine, 301(19), 1028–1037. [DOI] [PubMed] [Google Scholar]
- Kandel ER, & Squire LR (2000). Neuroscience: Breaking down scientific barriers to the study of brain and mind. Science, 290(5494), 1113–1120. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ (2011). Placebo studies and ritual theory: A comparative analysis of Navajo, acupuncture and biomedical healing. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 366(1572), 1849–1858. 10.1098/rstb.2010.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, et al. (2010). Placebos without deception: A randomized controlled trial in irritable bowel syndrome. PLoS One, 5(12), el5591 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, Kerr CE, & Zanger A (2009). Placebo controls, exorcisms, and the devil. The Lancet (London, England), 374(9697), 1234–1235. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19839101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, & Miller FG (2015). Placebo effects in medicine. New England Journal of Medicine, 373(1), 8–9. 10.1056/NEJMpl504023. [DOI] [PubMed] [Google Scholar]
- Keller A, Akintola T, & Colloca L (2018). Placebo analgesia in rodents: Current and future research. International Review of Neuwbiology, 138, 1–15. 10.1016/bs.im.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I (1997). Response expectancy theory and application: A decennial review. Applied and Preventive Psychology, 6(2), 69–79. 10.1016/S0962-1849(05)80012-5. [DOI] [Google Scholar]
- Klinger R, Blasini M, Schmitz J, & Colloca L (2017). Nocebo effects in clinical studies: Hints for pain therapy. Pain Reports, 2(2), e586 10.1097/PR9.0000000000000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhaber R, Walsh K, Duff J, & Walker K (2016). Enhancing adult therapeutic interpersonal relationships in the acute health care setting: An integrative review. Journal of Multidisciplinary Healthcare, 9, 537–546. 10.2147/JMDH.S116957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, & Yao X (2005). Clinical empathy as emotional labor in the patient-physician relationship. JAMA, 293(9), 1100 https://doi.Org/10.1001/jama.293.9.1100. [DOI] [PubMed] [Google Scholar]
- Locher C, Nascimento AF, Kirsch I, Kossowsky J, Meyer A, & Gaab J (2017). Is the rationale more important than deception? A randomized controlled trial of openlabel placebo analgesia. Pain, 158, 2320–2328. https://doi.Org/10.1097/j.pain.0000000000001012. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9629234. [DOI] [PubMed] [Google Scholar]
- Mehrabian A (1971). Silent messages. Belmont, CA: Wadsworth. [Google Scholar]
- Miller FG, & Colloca L (2010). Semiotics and the placebo effect. Perspectives in Biology and Medicine, 53(4), 509–516. 10.1353/pbm.2010.0004. [DOI] [PubMed] [Google Scholar]
- Miller FG, Colloca L, & Kaptchuk TJ (2009). The placebo effect: Illness and interpersonal healing. Perspectives in Biology and Medicine, 52(4), 518–539. 10.1353/pbm.0.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman D (2002). Meaning, medicine, and the “placebo effect”. New York: Cambridge University Press. [Google Scholar]
- Moerman DE, & Jonas WB (2002). Deconstructing the placebo effect and finding the meaning response. Annals of Internal Medicine, 136(6), 471–476. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11900500. [DOI] [PubMed] [Google Scholar]
- Murray AD (1979). Infant crying as an elicitor of parental behavior: An examination of two models. Psychological Bulletin, 86(1), 191–215. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/377352. [PubMed] [Google Scholar]
- Nelso E, & Panksepp J (1998). Brain substrates of infant—mother attachment: Contribu-tions of opioids, oxytocin, and norepinephrine. Neuroscience & Biobehavioral Reviews, 22(3), 437–452. [DOI] [PubMed] [Google Scholar]
- Petersen GL, Finnerup NB, Colloca L, Amanzio M, Price DD, Jensen TS, et al. (2014). The magnitude ofnocebo effects in pain: A meta-analysis. Pain, 155, 1426–1434. 10.1016/j.pain.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce C (1904). New elements In Project TPE (Ed.), The essential Peirce. (Vol. 2, pp. 300–324). Bloomington: Indiana University Press. [Google Scholar]
- Peirce C (1940). Logic as semiotic: The theory of signs In Bucheler J (Ed.), Philosophical writings of Peirce (pp. 98–119). New York: Dover. [Google Scholar]
- Porges SW (1998). Love: An emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology, 23(8), 837–861. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9924740. [DOI] [PubMed] [Google Scholar]
- Price DD, Craggs J, Nicholas Verne G, Perlstein WM, & Robinson ME (2007). Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain, 127(1), 63–72. 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Shaibani A, Frisaldi E, & Benedetti F (2017). Placebo response in pain, fatigue, and performance: Possible implications for neuromuscular disorders. Muscle & Nerve, 56(3), 358–367. 10.1002/mus.25635. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Lester H, Chisin R, Israel O, Bar-Shalom R, Peretz A, et al. (2005). The neural correlates of understanding the other’s distress: A positron emission tomography investigation of accurate empathy. Neuroimage, 27(2), 468–472. 10.1016/j.neuroimage.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Dyrbye LN, & West CP (2017). Addressing physician burnout. The Journal of the American Medical Association, 317(9), 901–902. 10.1001/jama.2017.0076. [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Hasan O, Dyrbye LN, et al. (2015). Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clinic Proceedings, 90(12), 1600–1613. 10.1016/j.mayocp.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Sussex R (2018). Describing placebo phenomena in medicine: A linguistic approach In Colloca L (Ed.), Neumbiology of the placebo effect, part II, International review of neurobiology. Vol. 139 (pp. 49–83). New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Tekampe J, Van Middendorp H, Meeuwis SH, Van Leusden JWR, Pacheco-López G, Hermus ARMM, et al. (2017). Conditioning immune and endocrine parameters in humans: A systematic review. Psychotherapy and Psychosomatics, 86, 99–107. 10.1159/000449470. [DOI] [PubMed] [Google Scholar]
- Udupa K, & Fox SH (2015). Placebo effect in Parkinson’s disease: Harnessing the mind in the treatment of PD. Movement Disorders, 30(6), 786 10.1002/mds.26222. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, & Insel TR (1998). Neuroendocrine bases of monogamy. Trends in Neurosciences, 21(2), 71–75. [DOI] [PubMed] [Google Scholar]
- Wampold BE (2018). The therapeutic value of the relationship for placebo effects and other healing practices In Colloca L (Ed.), Neurobiology of the placebo effect, part II, International review of neumbiology: Vol. 139 (pp. 191–210). New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Wendt L, Albring A, & Schedlowski M (2014). Learned placebo responses in neuroendocrine and immune functions pp. (159–181). Berlin, Heidelberg: Springer, 10.1007/978-3-662-44519-8_10. [DOI] [PubMed] [Google Scholar]
- Wolever RQ, Simmons LA, Sforzo GA, Dill D, Kaye M, Bechard EM, et al. (2013). A systematic review of the literature on health and wellness coaching: Defining a key behavioral intervention in healthcare. Global Advances in Health and Medicine, 2(4), 38–57. 10.7453/gahmj.2013.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER READING
- Jensen KB, Petrovic P, Kerr CE, Kirsch I, Raicek J, Cheetham A, et al. (2014). Sharing pain and relief: Neural correlates of physicians during treatment of patients. Molecular Psychiatry, 19(3), 392–398. 10.1038/mp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]