Abstract
Leukocyte migration is critically important during all protective and pathological immune and inflammatory responses. Chemokines play fundamental roles in this process, and chemokine concentration gradients stimulate the directional migration of leukocytes. The formation and regulation of these gradients is poorly understood. These are complex processes that depend on the specific properties of each chemokine and interactions between physical, biological and biochemical processes, including production, diffusion, advection, scavenging, post-translational modification, and extracellular matrix (ECM) binding. While some of these mechanisms have been investigated in isolation or limited combinations, more integrative research is required to provide a quantitative knowledge base that explains how chemokine gradients are established and maintained, and how cells respond to, and modify, these gradients.
Introduction
An effective immune system requires the functions of a diverse array of leukocytes (white blood cells). The correct localization of these cells is critical, and this is largely governed by a family of secreted proteins called chemokines. Chemokine-directed leukocyte migration controls the development and homeostasis of the immune system, and plays a key role in all protective immune and inflammatory responses. It also contributes to the development and progression of many diseases, including cardiovascular disease, autoimmunity, chronic inflammation and cancer. Moreover, cancer cells can exploit chemokine-directed migration to facilitate their metastatic spread. Understanding the mechanisms that regulate chemokine function therefore has substantial implications in health and disease. Leukocytes sense chemokines via G-protein coupled ‘conventional’ chemokine receptors (cCKRs). There are more than 40 chemokines, each signaling through one or more of 18 cCKRs [1], and this complexity is required to robustly regulate the diverse leukocyte populations of the immune system [1]. The chemokines are split into four subfamilies (CC, CXC, CX3C and XC) based on the precise arrangement of conserved cysteine residues in the mature protein, with the CC and CXC families being by far the largest with 28 and 16 members, respectively. This subdivision largely aligns with receptor binding: CC chemokines operate primarily through CC chemokine receptors (CCRs), CXC chemokine through CXC chemokine receptors (CXCRs), and so on. Chemokines are named according to a standardized nomenclature in which the subfamily name is followed by the letter ‘L’ (for ligand), and then a number indicating when the gene encoding that chemokine was identified. Thus, CCL21 is a CC chemokine whose gene was the 21st CC chemokine gene to be characterized.
Chemokines direct leukocyte extravasation from blood and lymph, and control the migratory behavior and positioning of leukocytes within tissues. The spatial distribution of chemokines is therefore critical for their correct functioning. In some contexts, chemokines form concentration gradients that stimulate directional leukocyte migration. These gradients depend on numerous integrated biological and physical processes. First, chemokine is secreted: the type and quantity depends on the identity of the secreting cell and the many environmental signals it receives and integrates. These signals can include physical parameters: for example, flow-induced wall shear stress upregulates expression of CCL21 by lymph node fibroblastic reticular cells (FRCs) [2] and lymphatic endothelial cells (LECs) [3], while stretch can upregulate expression and release of pro-inflammatory chemokines in alveolar epithelium [4]. Chemokine movement through interstitial spaces occurs through diffusion and advection, and this is profoundly affected by chemokine/ECM interactions [5–7]. Some chemokines bind strongly to ECM components, while others exhibit little or no affinity. These processes will not only shape interstitial gradients, but will also regulate the quantity of chemokine that enters the lymphatic vasculature with the tissue fluid. This is important because lymph-borne chemokines can form flow-regulated intralymphatic gradients [8,9], modify chemokine gradients in downstream lymph nodes (LNs) [10], and reach high endothelial venules to directly control leukocyte recruitment into LNs [11,12]. Further gradient modulation and regulation involves chemokine removal by leukocytes (via cCKRs and non-receptor mediated mechanisms (e.g. pinocytosis) [13–15]), and by specialized chemokine scavengers called ‘atypical’ chemokine receptors (ACKRs) that are primarily expressed by stromal cells [16]. Migratory cells can also cleave chemokines to dramatically alter their ECM-binding properties [5], and chemokine-driven cCKR regulation means that exposure to chemokine can alter a cell’s subsequent migratory properties. Importantly, each chemokine has a unique set of properties that will influence its distribution and therefore the nature of the concentration gradients it can form. Therefore, a complex combination of physical and biological factors determines how chemokine gradients are generated, maintained and regulated, and, importantly, how they direct the migration and interstitial positioning of leukocytes.
The chemokine biology research literature is vast, and it is not the intent of this review to provide extensive coverage of all background material. Rather, we focus on a subset of those studies that incorporated some aspect of transport mechanisms in the analysis of chemokine gradients and cell actions. The chemokine axis that includes CCL21 and CCL19 has been characterized more extensively than most others, and thus will constitute much of the material of this review. To put these studies in context, we include reference to other key experimental studies even though the importance of transport phenomena may not have been recognized or included in the analysis of the results.
Quantitative Approaches to Characterizing Chemokine/Cell Systems
A quantitative knowledge base of chemokine gradients, and the ability to modulate them, requires a tightly integrated, synergistic approach in which experimental and modeling approaches evolve in an interdependent manner. The majority of chemokine research to date comes from biological experiments involving individual chemokine-receptor axes, with some exploration of receptors that bind to multiple ligands. Modeling approaches thus far have similarly taken a reductionist approach. Even under these conditions, evidence of complex behaviors has emerged.
One well-studied example of interstitial chemokine gradients involves those generated by LECs. These cells, which line lymphatic vessels (LVs), form a key microanatomical barrier and play critical roles in regulating the migration, localization and departure of interstitial leukocytes by producing distinct subsets of chemokines and ACKRs which, under homeostatic and inflammatory conditions, create interstitial gradients around LVs. Figure 1 shows the key physical and biological processes that are likely to shape these gradients. The best-studied LEC-derived chemokine is CCL21, which, by interacting with its receptor CCR7, guides dendritic cells (DCs) towards, and into, LVs [17,18]. Interstitial LEC-derived CCL21 gradients can also be exploited by invading cancer cells which up-regulate CCR7 to aid their dissemination to draining LNs. Due to CCL21’s remarkably high affinity for ECM, it has been possible to directly visualize LEC-derived CCL21 gradients in skin, and DC migration along these gradients have been tracked in ear skin explants [17]. There also appears to be a gradient of CCL21 along the inner surface of initial lymphatics that guides DCs crawling towards collecting LVs [8]. DC migratory behavior was disrupted in explanted tissue, suggesting an important role for lymph flow in maintaining the CCL21 gradient, although, to our knowledge, it is not known if this gradient consists of bound and/or unbound CCL21. In addition to CCL21, LECs have the capacity to release many other chemokines, constitutively or in response to inflammatory stimuli. This includes CCL2, which, through its receptor CCR2, controls interstitial macrophage positioning [19]. In mice, CCL2 and CCL21 carry an extended C-terminus that interacts strongly with ECM to contribute to interstitial gradient formation. DCs can cleave off the C-terminus of CCL21 to create a version with much lower ECM affinity that resembles CCL19, the other CCR7 ligand [5]. CCL2 is susceptible to similar post-translational modification (RJB Nibbs, unpublished). CCL2 is scavenged by leukocytes using CCR2, ACKR2, or non-receptor-mediated processes [13,15]. Moreover, LECs express ACKRs that play critical roles in regulating interstitial leukocyte migration [10,20–22]. ACKR2, which scavenges CCL2 and other pro-inflammatory chemokines, regulates interstitial macrophage localization, which in turn controls LV density in the skin [19] and branching morphogenesis in the developing breast [23]. ACKR4, which scavenges CCL19 and CCL21, helps form CCL21 gradients in LNs that guide DC trafficking out of the subcapsular sinus (SCS) [10]. ACKR4 deficiency also disrupts DC departure from inflamed skin because loss of scavenging causes CCL19 dysregulation, which interferes with CCL21 gradient sensing by CCR7+ DCs [22].
Figure 1. Factors influencing LEC-derived chemokine gradients.
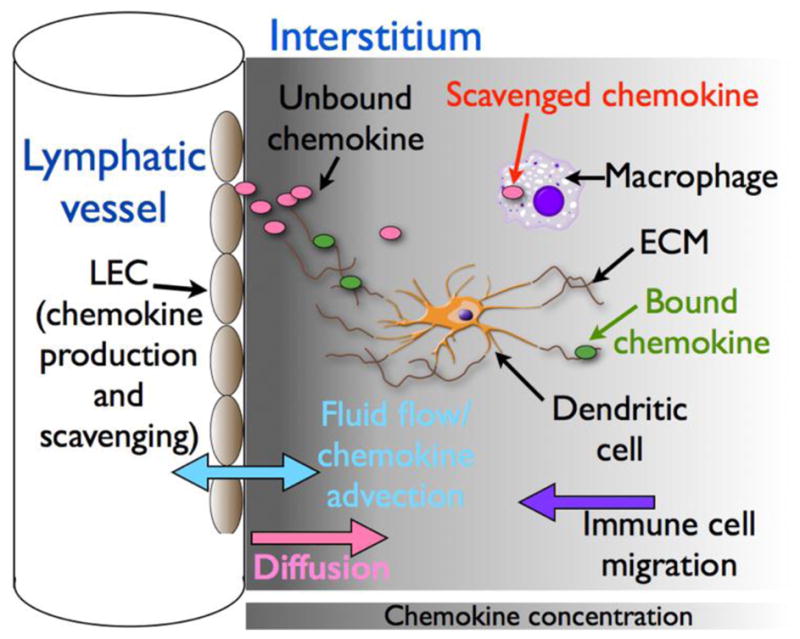
Depending on environmental conditions, LECs release distinct subsets of chemokines (pink and green ovals represent unbound and ECM-bound chemokine, respectively). The distribution of these chemokines within the adjacent tissue (indicated by the grey shading) is likely to be influenced by many physical and biological factors including production rate, diffusion, fluid flow, the nature of the ECM, the chemokine’s ECM binding properties, and chemokine scavenging/uptake mediated by ACKRs, cCKRs and pinocytosis by resident tissue cells, such as macrophages and LECs themselves. The ensuing gradients will direct the migration of responsive cell types, such as dendritic cells, which can then modify the gradients by, for example, chemokine scavenging and/or cleavage.
Diffusion and interstitial fluid flow modulate the gradients through multiple direct and indirect mechanisms. Using Boyden chambers with LEC layers cultured onto the bottom surface, Shields et al [24] showed that CCL21-driven migration of tumor cells through Matrigel was enhanced by the presence of interstitial flow (Peclet number = 0.02). Accompanying mathematical modeling indicated that this was in part due to autologous chemokine gradients resulting from expression of proteases by tumor cells that liberate ECM-bound CCL21. Fluid flow toward the LEC monolayer (which also modifies the paracrine signaling gradient) washes more proteases downstream, which skews the concentration of liberated chemokine. Fleury et al [25] more generally characterized autologous chemotactic protein gradients mathematically. However, due to absence of specific experimental information, modeling parameters associated with protease and ECM degradation, as well as protein liberation, were assumed to be of similar order of magnitude to the diffusion and advection terms in the governing advection-reaction-diffusion equations. Under these assumptions, autologously generated gradients due to liberation of matrix-bound protein were found to be several times larger than gradients due to autologous secretion in the presence of flow.
Competitive binding of CCL21 and CCL19 to CCR7 was explored in a microfluidic chamber model system that limited transport to diffusion only, but allowed competing gradients (equal in magnitude; opposite in direction) to be established across 3D cultures of dendritic cells [26,27]. Migration was preferentially in the direction of higher CCL21 concentration, even when ECM binding was inhibited, although it is worth noting that chemokines were fluorescently-labeled in this study in a way that may interfere with binding to ECM, CCR7 or both. Because CCL21 binds readily to several ECM components, the presence of binding would have created a different gradient shape, and in vivo the bound concentrations are likely to be several orders of magnitude higher than unbound. The relative importance of unbound vs bound chemokine gradients in vivo is not known, and measuring in vivo unbound chemokine concentrations is currently not possible, but disruption of the bound gradients alters DC behavior in mouse ear experiments [17].
In other experiments using microfluidic chambers in which CCL21 gradients were established by micropatterning, haptotactic DC migration was found to be stronger when the concentration of immobilized CCL21 was increased, even when the gradient was kept constant [28]. The same group demonstrated that unbound gradients of CCL19 (or CCL21 lacking its C-terminus) can steer haptokinetic DC migration stimulated by full-length immobilized CCL21 [5], and that a ‘medium’, but not a ‘low’, concentration gradient of unbound CCL19 can interfere with CCL21-directed haptotactic DC migration [29]. In addition, it has recently been reported that DCs migrate poorly in stable gradients of soluble CCL19 created in microfluidic chambers, irrespective of their steepness, but showed prolonged responses in gradients in which the CCL19 concentration was progressively rising over time [30]. These elegant studies illustrate the many potential complexities present in the system.
There is a rich history of general mathematical exploration of reaction-diffusion systems, dating back at least to the landmark papers of Turing [31], and Keller & Segal [32,33]. The coupled system of partial differential equations governing concentration and density of the chemo-attractant and migratory cells, respectively, can give rise to instabilities that result in dynamic behaviors and spatio-temporal pattern formation, even in the presence of steady state input conditions. Recently, Lee et al [34] explored such behaviors in a system of three coupled components (antigen, chemokine and cells). At higher values of the parameter representing the sensitivity of the cells’ migratory behavior to the chemokine gradient, the equilibrium state was found to be asymptotically unstable. This resulted in both spatial and temporal oscillations in cell density. Potential pattern formation and “waves” of cell populations indicated by this result could have implications for how cells respond to chemokine gradients in vivo, and that the precise nature of leukocyte migration may vary with time. Future experiments should therefore be attentive to potential for time dependent cell behaviors.
The addition of multiple cell types to experimental and mathematical model systems also reveals interesting behaviors in signaling systems that bear some similarity to chemokines. Oyler-Yaniv et al [35] showed that the spatial extent of IL-2 signaling in the spleen and lymph nodes depends on the absolute and relative densities of producers and consumers. Their combined in vitro and mathematical approach (with support from in vivo observations) demonstrated the importance of analyzing populations of interacting cells, and provided important insight into previous seemingly conflicting experimental results.
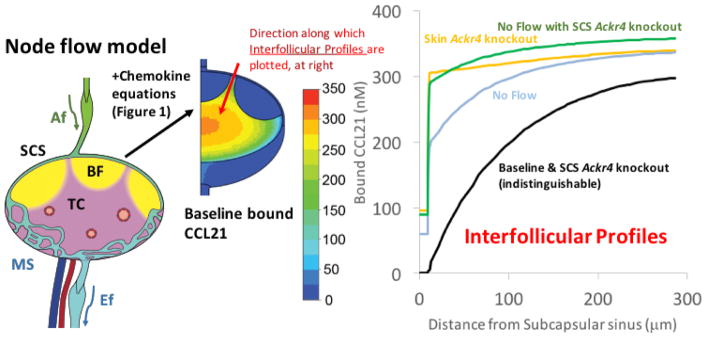
We have recently used a mathematical approach to simulate CCL19/CCL21 distribution, and CCR7 occupancy in skin-draining LNs (SLNs) [36]. This study incorporated a previous model of lymph flow patterns in SLNs [37] and used literature-based parameter estimates for the relevant biological processes. Notably, the model recapitulated CCL21 gradients observed experimentally in SLNs in the interfollicular regions and at the boundary of the T cell area and B cell follicle [10,38] (Figure 2). The results further indicated that the absence of ACKR4 from SLN alone (where it is expressed only by LECs on the SCS ceiling) is not sufficient to cause the disruption of interfollicular CCL21 gradients seen in Ackr4-deficient mice [10]. However, gradients in the model could be disrupted when CCL21 was added to afferent lymph, mimicking loss of ACKR4-mediated scavenging in skin [22]. The model predicted that ACKR4 in skin, rather than SLN, could be responsible for maintaining intranodal CCL21 gradients under normal lymph flow conditions, while ACKR4 in the SLN might only play a role when lymph flow is reduced, a phenomenon that might occur when the upstream tissue becomes inflamed. These results highlight that a combination of physical processes and biological factors is likely to be responsible for maintaining intranodal gradients of CCL21. Inclusion of leukocyte-mediated gradient modification may further alter these findings.
Figure 2. Predicted CCL21 concentrations in skin-draining lymph nodes, and the influence of ACKR4 and/or lymph flow.
The image on the left shows a stylized lymph node showing T cell area (TC, pink), B cell follicles (BF, yellow), afferent lymphatics (Af, green), medullary sinuses (MS, light blue) and efferent lymphatics (Ef, light blue). Mathematical models incorporating multiple physical and biological parameters lead to the baseline CCL21 distribution pattern shown in the middle of the figure. The color key shows the chemokine concentrations. The line graph on the right shows predicted interfollicular CCL21 gradients from the ceiling of the subcapsular sinus (SCS) into the T cell area (along the red arrow shown, 300μm into the T cell area) under the following scenarios: (i) Baseline: Ackr4 and lymph flow intact (black line); (ii) SCS Ackr4 knockout: deletion of Ackr4 from LECs lining the SCS ceiling (identical to baseline so also represented by black line); (iii) Skin Ackr4 knockout: deletion of Ackr4 in the skin (mimicked by inclusion of CCL21 in afferent lymph) (orange line); (iv) No Flow: cessation of lymph flow (light blue line), or (v) No Flow with SCS Ackr4 knockout: no lymph flow plus deletion of Ackr4 from LECs lining the SCS ceiling (green line). Adapted from Jafarnejad et al. [36].
Conclusions
The studies outlined above provide some indications of how effective manipulation of leukocyte or metastatic cell migration requires an in-depth understanding of chemokine systems. However, the complex behaviors observed, using even reductionist approaches, point to the need for a more profound and integrated understanding of the key biological and physical parameters. Interstitial transport is clearly important, but is difficult to quantify and control in vivo. Tissues clearly exhibit inhomogeneity and anisotropy, neither of which has been incorporated into any chemokine transport study, to our knowledge. Additionally, chemokine binding to different ECM components requires further characterization. Further, the relative importance of unbound and bound chemokines in eliciting chemotaxis and haptotaxis needs to be explored for different cell types. There is also clearly a need to characterize more thoroughly other chemokine axes besides CCL21/CCL19. The degree of importance of the basic system components (diffusion, advection, ECM binding, receptor dynamics, migratory response) will depend on the chemokine(s), ECM and responding cells present. The effects on leukocyte behavior summarized here for the CCL21/CCL19 axis might not be directly applicable to other chemokines. However, computational modeling incorporating appropriate chemokine-specific properties will allow gradients of other chemokines to be explored in silico: this could lead to the formulation of new hypotheses about the control leukocyte migration in vivo, and how this migration could be manipulated therapeutically. A good place to start would be with additional LEC-derived chemokines, such as CCL2, or other chemokines involved in leukocyte trafficking in lymph nodes, such as CXCL13, a critical chemokine involved in B cell migration in follicles.
While much is known about which cell types produce chemokines, the conditions that regulate production are not yet precisely defined, and the impact of physical processes is poorly understood. For example, as mentioned earlier, flow-induced wall shear stress upregulates CCL21 production by at least two cell types, but these cells are subjected to both dynamic flow and substrate stretching in vivo. Incorporation of these mechanical factors into mathematical models will require not only more information from experiments to identify system parameters, but also carefully guided approaches that recognize the importance of fully dynamic behaviors which may have physiologic and pathologic implications.
Future chemokine system research will lead to a deeper understanding of critical immunological processes that contribute to protection from infection, facilitate responses to vaccination, and drive pathology in a broad spectrum of diseases. Further evolution of knowledge in this field will come from a combined, synergistic interaction of in vitro, in vivo and mathematical approaches. The implications in health and disease are therefore substantial, and new immunoengineered therapies involving chemokine gradient manipulation might be a real possibility in the future.
Highlights.
Chemokine concentration gradients stimulate directional leukocyte migration.
Multiple physical and biological factors shape chemokine gradients
Integrating these factors provides new insights into gradient form and function
Acknowledgments
This work was supported by the Royal Society, the Royal Academy of Engineering, the Sir Leon Bagrit Trust, National Institutes of Health Grant U01-HL-123420, and Wellcome Trust Collaborative Award 206284/Z/17/Z. The U.K. Medical Research Council funds work in R.J.B.N.’s laboratory.
Abbreviations
- ACKR
atypical chemokine receptor
- cCKR
conventional chemokine receptor
- CCR
CC chemokine receptor
- CXCR
CXC chemokine receptor
- DC
dendritic cell
- ECM
extracellular matrix
- FRC
fibroblastic reticular cell
- LEC
lymphatic endothelial cell
- LN
lymph node
- LV
lymphatic vessel
- SCS
subcapsular sinus
- SLN
skin-draining lymph node
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
*Papers of special interest:
**Papers of outstanding interest:
- 1.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J Immunol. 2009;183:4273–4283. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- 3.Pisano M, Triacca V, Barbee KA, Swartz MA. An in vitro model of the tumor-lymphatic microenvironment with simultaneous transendothelial and luminal flows reveals mechanisms of flow enhanced invasion. Integr Biol (Camb) 2015;7:525–533. doi: 10.1039/c5ib00085h. [DOI] [PubMed] [Google Scholar]
- 4.Rentzsch I, Santos CL, Huhle R, Ferreira JMC, Koch T, Schnabel C, Koch E, Pelosi P, Rocco PRM, Gama de Abreu M. Variable stretch reduces the pro-inflammatory response of alveolar epithelial cells. PLoS One. 2017;12:e0182369. doi: 10.1371/journal.pone.0182369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Forster R, Lutz MB, Sorokin L, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. This work was the first to show that DCs cleave the positively-charged C-terminal extension off CCL21 to release an truncated active form of the chemokine with high diffusivity. [DOI] [PubMed] [Google Scholar]
- 6.Patel DD, Koopmann W, Imai T, Whichard LP, Yoshie O, Krangel MS. Chemokines have diverse abilities to form solid phase gradients. Clin Immunol. 2001;99:43–52. doi: 10.1006/clim.2000.4997. [DOI] [PubMed] [Google Scholar]
- 7.de Paz JL, Moseman EA, Noti C, Polito L, von Andrian UH, Seeberger PH. Profiling heparin-chemokine interactions using synthetic tools. ACS Chem Biol. 2007;2:735–744. doi: 10.1021/cb700159m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo E, Teijeira A, Vaahtomeri K, Willrodt AH, Bloch JS, Nitschke M, Santambrogio L, Kerjaschki D, Sixt M, Halin C. Intralymphatic CCL21 Promotes Tissue Egress of Dendritic Cells through Afferent Lymphatic Vessels. Cell Rep. 2016;14:1723–1734. doi: 10.1016/j.celrep.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 9.Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, Angeli V, Shakhar G. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, Eller K, Chan L, Lucas B, Novitzky-Basso I, Nakamura K, et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol. 2014;15:623–630. doi: 10.1038/ni.2889. This was the first study to precisely determine the location of ACKR4-expressing cells in lymph nodes, and demonstrate that, by scavenging chemokines, ACKR4 regulates CCL21 gradients in interfollicular regions of lymph nodes to facilitate the migration of DCs from the subcapsular sinus towards the T cell area. [DOI] [PubMed] [Google Scholar]
- 11.Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH, Brandtzaeg P, Haraldsen G. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001;193:1105–1112. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansell CA, Schiering C, Kinstrie R, Ford L, Bordon Y, McInnes IB, Goodyear CS, Nibbs RJ. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood. 2011;117:5413–5424. doi: 10.1182/blood-2010-11-317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford LB, Cerovic V, Milling SW, Graham GJ, Hansell CA, Nibbs RJ. Characterization of conventional and atypical receptors for the chemokine CCL2 on mouse leukocytes. J Immunol. 2014;193:400–411. doi: 10.4049/jimmunol.1303236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nibbs RJ, Graham GJ. Immune regulation by atypical chemokine receptors. Nat Rev Immunol. 2013;13:815–829. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- 17*.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. A key study that was the first to visualise CCL21 gradients in skin in situ, and demonstrate that DCs are guided by these gradients to lymphatic vessels. [DOI] [PubMed] [Google Scholar]
- 18.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 19.Lee KM, Danuser R, Stein JV, Graham D, Nibbs RJ, Graham GJ. The chemokine receptors ACKR2 and CCR2 reciprocally regulate lymphatic vessel density. Embo j. 2014;33:2564–2580. doi: 10.15252/embj.201488887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 22.Bryce SA, Wilson RA, Tiplady EM, Asquith DL, Bromley SK, Luster AD, Graham GJ, Nibbs RJ. ACKR4 on Stromal Cells Scavenges CCL19 To Enable CCR7-Dependent Trafficking of APCs from Inflamed Skin to Lymph Nodes. J Immunol. 2016;196:3341–3353. doi: 10.4049/jimmunol.1501542. [DOI] [PubMed] [Google Scholar]
- 23.Wilson GJ, Hewit KD, Pallas KJ, Cairney CJ, Lee KM, Hansell CA, Stein T, Graham GJ. Atypical chemokine receptor ACKR2 controls branching morphogenesis in the developing mammary gland. Development. 2017;144:74–82. doi: 10.1242/dev.139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Fleury ME, Boardman KC, Swartz MA. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys J. 2006;91:113–121. doi: 10.1529/biophysj.105.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haessler U, Kalinin Y, Swartz MA, Wu M. An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed Microdevices. 2009;11:827–835. doi: 10.1007/s10544-009-9299-3. [DOI] [PubMed] [Google Scholar]
- 27**.Haessler U, Pisano M, Wu M, Swartz MA. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc Natl Acad Sci U S A. 2011;108:5614–5619. doi: 10.1073/pnas.1014920108. Investigated the migration of DCs in response to competing gradients of CCL19 and CCL21. Equal but opposite gradients were established using diffusion across a microfluidic chamber. Cells migrated in the direction of the CCL21 gradient even when matrix binding was inhibited. This was the first demonstration of DC migration in response to oppositely directed gradients of CCL19 and CCL21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz J, Bierbaum V, Vaahtomeri K, Hauschild R, Brown M, de Vries I, Leithner A, Reversat A, Merrin J, Tarrant T, et al. Dendritic Cells Interpret Haptotactic Chemokine Gradients in a Manner Governed by Signal-to-Noise Ratio and Dependent on GRK6. Curr Biol. 2017;27:1314–1325. doi: 10.1016/j.cub.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz J, Bierbaum V, Merrin J, Frank T, Hauschild R, Bollenbach T, Tay S, Sixt M, Mehling M. A microfluidic device for measuring cell migration towards substrate-bound and soluble chemokine gradients. Sci Rep. 2016;6:36440. doi: 10.1038/srep36440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrie Aronin CE, Zhao YM, Yoon JS, Morgan NY, Prustel T, Germain RN, Meier-Schellersheim M. Migrating Myeloid Cells Sense Temporal Dynamics of Chemoattractant Concentrations. Immunity. 2017;47:862–874. e863. doi: 10.1016/j.immuni.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turing AM. The chemical basis of morphogenesis 1953. Bull Math Biol. 1990;52:153–197. doi: 10.1007/BF02459572. discussion 119–152. [DOI] [PubMed] [Google Scholar]
- 32.Keller EF, Segal LA. Initiation of Slime Mold Aggregation Viewed as an Instability. J Theor Biol. 1970;26:399–415. doi: 10.1016/0022-5193(70)90092-5. [DOI] [PubMed] [Google Scholar]
- 33.Keller EF, Segal LA. Model for Chemotaxis. J Theor Biol. 1971;30:225–234. doi: 10.1016/0022-5193(71)90050-6. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Kim SW, Oh Y, Hwang HJ. Mathematical modeling and its analysis for instability of the immune system induced by chemotaxis. J Math Biol. 2017;75:1101–1131. doi: 10.1007/s00285-017-1108-7. [DOI] [PubMed] [Google Scholar]
- 35**.Oyler-Yaniv A, Oyler-Yaniv J, Whitlock BM, Liu Z, Germain RN, Huse M, Altan-Bonnet G, Krichevsky O. A Tunable Diffusion-Consumption Mechanism of Cytokine Propagation Enables Plasticity in Cell-to-Cell Communication in the Immune System. Immunity. 2017;46:609–620. doi: 10.1016/j.immuni.2017.03.011. Quantified the spatial extent of cytokine communication between producing and responding cell types in dense tissues. Demonstrated that the distance over which cytokine gradients act depends on the local cell densities, and thus the importance of analyzing cell populations rather than just single cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Jafarnejad M, Zawieja DC, Brook BS, Nibbs RJB, Moore JE., Jr A Novel Computational Model Predicts Key Regulators of Chemokine Gradient Formation in Lymph Nodes and Site-Specific Roles for CCL19 and ACKR4. J Immunol. 2017;199:2291–2304. doi: 10.4049/jimmunol.1700377. This study reported the development of a novel computational model of chemokine gradients in lymph nodes that incorporated multiple biological and physical parameters, including lymph flow. It was used to indicate, for the first time, that lymph flow is likely to be important in shaping interfollicular CCL21 gradients, and that the disruption of these gradients observed in Ackr4-deficient lymph nodes [10] is probably due to a failure to scavenge CCL21 in skin rather than the lymph node. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jafarnejad M, Woodruff MC, Zawieja DC, Carroll MC, Moore JE., Jr Modeling Lymph Flow and Fluid Exchange with Blood Vessels in Lymph Nodes. Lymphat Res Biol. 2015;13:234–247. doi: 10.1089/lrb.2015.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Lee S, Kim SW, Oh Y, Hwang HJ. Mathematical modeling and its analysis for instability of the immune system induced by chemotaxis. J Math Biol. 2017 doi: 10.1007/s00285-017-1108-7. Demonstrated that mathematical instabilities can result for certain parameter values when cells are directed to migrate along the gradient of a single chemoattractant in the presence of a single antigen. This was the first study to demonstrate instabilities in this particular mathematical system. [DOI] [PubMed] [Google Scholar]