Abstract
Dried plum has unique anabolic effects on bone, but the responsible bioactive components have remained unclear. This study investigated components of dried plum with potential osteoprotective activity utilizing aged, osteopenic Sprague Dawley rats fed diets supplemented with a crude polyphenol extract, potassium, vitamin K or their combination. Whole body and femoral bone mineral density were restored with the polyphenol and combination treatments to a similar extent as the dried fruit. The combination treatment reversed trabecular bone loss in the spine and cortical bone in the femur mid-diaphysis in a similar manner. Biomarkers of bone resorption were reduced by the polyphenol and combination treatments. The polyphenol extract accounted for most of the anabolic effect of dried plum on bone. This study is the first to show the bioactive components in dried plum responsible for restoring bone in vivo.
Keywords: dried plum, polyphenols, bone, estrogen deficiency, aging
Graphical abstract
1. Introduction
More than 50 million Americans over the age of 50 are affected by osteoporosis and approximately 70% are women (Burge et al., 2007). Bone loss in females is frequently attributed to the decline of estrogen at menopause, but there is evidence that loss of trabecular bone, especially in the spine, begins as early as the 3rd decade of life, long before menopause (Cenci et al., 2003; Macdonald, Nishiyama, Kang, Hanley, & Boyd, 2011; Riggs et al., 2004; Riggs et al., 2008; Roggia et al., 2001; Weitzmann, Roggia, Toraldo, Weitzmann, & Pacifici, 2002). Whether age-related or estrogen deficiency-induced bone loss, the underlying mechanisms involve alterations in oxidative stress and the immune response (Almeida et al., 2007; Chung et al., 2009). Under inflammatory conditions, activated immune cells (e.g., macrophages and neutrophils) produce reactive species (Nandi et al., 2015). The accumulation of free radicals, which is synonymous with oxidative stress, increases osteoblast and osteocyte apoptosis, and enhances osteoclast activity (Almeida et al., 2007; Baek et al., 2010). These alterations result in a decrease in bone formation and an increase in bone resorption, ultimately negatively affecting bone quality. Likewise, free radicals can activate inflammatory signaling pathways, resulting in an increase in circulating pro-inflammatory cytokines (interleukin-1 beta or IL-1β, tumor necrosis factor alpha or TNF-α, and IL-6) known to induce bone loss (Chung et al., 2009; Roggia et al., 2001). Thus, foods or dietary supplements rich in compounds that have the capacity to scavenge free radicals and downregulate inflammatory signaling are considered promising interventions for preventing bone loss and promoting long-term skeletal health.
Dried plums are rich in phytochemicals with antioxidant and anti-inflammatory properties and the incorporation of this fruit into the diet has been shown to not only prevent and but also to reverse bone loss due to aging and gonadal hormone deficiency (Bu et al., 2007; Halloran et al., 2010; Hooshmand et al., 2011; Hooshmand et al., 2015; Kim, Chun, Kim, Moon, & Lee, 2003; Rendina et al., 2013; Smith, Bu, et al., 2014; Smith, Graef, et al., 2014). Clinical trials have demonstrated that supplementing the diet with dried plum results in a higher bone mineral density (BMD) in postmenopausal women (Arjmandi et al., 2002) and more recently in older osteopenic women (Hooshmand et al., 2016). In pre-clinical studies, dried plum consumption has prevented or reversed the deterioration in bone density, trabecular and cortical bone microarchitecture and biomechanical properties, and favorably altered biomarkers of bone metabolism in male and female animal models of aging and gonadal hormonal deficiency (Bu et al., 2007; Halloran et al., 2010; Rendina et al., 2013; Rendina et al., 2012; Smith, Bu, et al., 2014; Smith, Graef, et al., 2014). The mechanisms by which dried plum improves bone health have been reported to include both suppression of bone resorption and upregulation of bone formation (Hooshmand et al., 2016; Rendina et al., 2013; Smith, Bu, et al., 2014). For example, dried plum consumption resulted in a reduction in serum tartrate-resistant acid phosphatase isoform 5b (TRAP-5b) (Hooshmand et al., 2016) and urinary excretion of deoxypyridinoline crosslinks (Dpd), which are indicators of osteoclast activity (Franklin et al., 2006; Rendina et al., 2013; Smith, Bu, et al., 2014). Additionally, in vivo and in vitro studies have revealed that dried plum upregulates runt-related transcription factor 2 (Runx2), the master regulator of osteoblast differentiation which is enhanced by bone morphogenetic protein (BMP) signaling. While progress has been made as to the mechanisms by which dried plum improves bone health, it is still unclear which bioactive components within the fruit are exerting these osteoprotective effects.
Much of the focus in determining the bioactive components of dried plum has been on the fruit’s polyphenols. Dried plums are a rich source of polyphenolic compounds, containing ~1200 mg of polyphenols per 100 g of fresh fruit (Wu et al., 2004). Of the polyphenols in dried plum, hydroxycinnamic acids are the most abundant and make up ~98% of the total polyphenolic compounds that have been identified (Donovan, Meyer, & Waterhouse, 1998; Nakatani et al., 2000). Flavonoids, including the flavonol, rutin, and the anthocyanin, cyaniding-3-rutinoside, account for ~2% of the polyphenolic compounds in dried plums. Of the hydroxycinnamic acids, neochlorogenic acid (3-O-caffeoylquinnic acid) is most abundant, accounting for ~65% of the total phenolic acids. It should be noted that the process of drying the fruit is detrimental to the flavonoid content, with the cyanidin-3-rutinoside content especially decreased, while the hydroxycinnamic acids are more resistant to the effects of heat (Piga, Del Caro, & Corda, 2003). Other hydroxycinnamates present include chlorogenic acid (5-O-caffeoylquinnic acid) and cryptochlorogenic acid (4-O-caffeoylquinnic acid), as well as caffeic acid and p-coumaric acid (Nakatani et al., 2000; Rothwell et al., 2013). Chlorogenic acid isomers and caffeic acid have been shown to have high free radical scavenging capacities, potentially indicating a major bioactive role in vivo (Nakatani et al., 2000). Our laboratory has reported that treatment with an ethanol extract of total polyphenolic compounds from dried plum suppresses osteoclast differentiation and activity and increases osteoblast differentiation and activity under normal and inflammatory conditions in commercially available cell lines (Bu, Hunt, & Smith, 2009; Bu et al., 2008). However, the ability of polyphenolic compounds extracted from dried plum to mediate the fruit’s effects on bone in an animal model of osteoporosis has not been investigated.
Aside from the polyphenolic acid composition, dried plums are a rich source of potassium (732 mg/100 g) and vitamin K (59.5 μg/100 g), both of which can affect bone metabolism (USDA, 2011). Epidemiological studies have shown that potassium intake is correlated with higher BMD in adult men (Whiting, Boyle, Thompson, Mirwald, & Faulkner, 2002), and bone resorption is increased and collagen synthesis is decreased in vitro when potassium availability is low (Bushinsky, Riordon, Chan, & Krieger, 1997). Postmenopausal women on a potassium citrate supplement (4–8 g/d), experienced a reduction in urinary acid excretion, and biochemical markers of bone resorption (Gregory et al., 2015; Marangella et al., 2004). The reduction in bone resorption that occurs with potassium supplementation has been attributed to the mineral’s ability to neutralize the acidic environment that is favorable for osteoclast activity (Bushinsky et al., 1997; Marangella et al., 2004). Vitamin K, on the other hand, is required for the gamma-carboxylation of osteocalcin and matrix gla protein, both of which play an important role in regulating bone mineralization (Bugel, 2008). Low dietary consumption of vitamin K is associated with reduced BMD in postmenopausal women (Booth et al., 2003) and an increased risk of fracture in elderly men and women (Booth et al., 2000; Feskanich et al., 1999). Vitamin K supplementation (500 – 1000 μg/d phylloquinone) has been found to reduce urinary calcium excretion and increase serum under-carboxylated osteocalcin; however, supplementation with vitamin K alone has not been found to improve bone density (Binkley, Krueger, Engelke, Foley, & Suttie, 2000; Booth et al., 2008; Knapen, Hamulyak, & Vermeer, 1989).
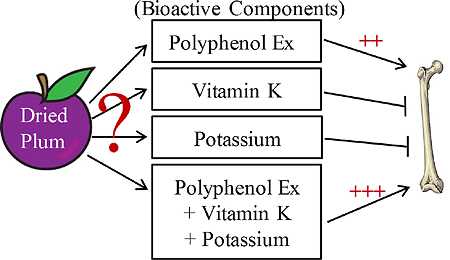
It is evident that there are several bioactive components within dried plum that could be responsible for altering bone metabolism, including potassium (Bushinsky et al., 1997), vitamin K (Bugel, 2008), and polyphenolic compounds (Bu et al., 2009; Bu et al., 2008). While each of these candidate components is known to have positive effects on bone metabolism, it is unclear whether a single bioactive component provides the unique bone protective properties observed with dried plum supplementation, or whether dried plum provides an optimal combination of these components. Therefore, the purpose of this study was to examine the efficacy of an extract of polyphenolic compounds, potassium, and vitamin K, as well as their combination, to improve bone quality in aged, estrogen-deficient animal model.
2. Materials and Methods
2.1 Preparation and Characterization of the Polyphenol Extract
Extraction of total polyphenols from the dried plum (DP) powder (‘Improved French’ provided by California Dried Plum Board, Sacramento, CA, USA) has been described previously (Bu et al., 2008). Briefly, 10 g of DP powder was added to 100 mL of 80% ethanol and sonicated for 20 minutes under nitrogen gas to reduce oxidation. The solution was then vacuum filtered and the remaining powder was rinsed with 80% ethanol (50 ml) and sonication was repeated. Following the second sonication, the solution was again vacuum filtered and the solution containing the polyphenols was concentrated using a roto-evaporator. This extraction protocol was repeated until enough polyphenolic extract was produced to formulate the treatment diets and complete any dietary analyses. Total polyphenolic content was quantified using the Folin-Ciocalteu assay (Singleton, Orthofer, & Lamuela-Raventos, 1999). The 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, p-Coumaric acid, caffeic acid, and rutin content of the extract was quantified using HPLC by a commercial lab (BioProfile Testing Laboratories, LLC, St. Paul, MN). Composition of this crude dried plum polyphenolic extract is shown in Table 1. Macronutrient, calcium, phosphorus and potassium content of dried plum powder and the crude polyphenol extract were assessed by NP Analytical Laboratories (St. Louis, MO, USA). Vitamin K concentrations in DP powder and the polyphenol extract were assessed by Covance Laboratories Inc. (Madison, WI, USA).
Table 1.
Composition of dried plum polyphenol extract
| Per kg diet | |
|---|---|
| Carbohydrates (g) | 118.7 |
| Fats (g) | 2.9 |
| Proteins (g) | 6.1 |
| Micronutrients | |
| Vitamin K (μg) | 98.4 |
| Calcium (mg) | 24.8 |
| Phosphorus (mg) | 131.4 |
| Potassium (g) | 1.3 |
| Phenolics | |
| Gallic Acid (mg) | 206.1 |
| 3-O-caffeoylquinic Acid (mg) | 163 |
| 4-O-caffeoylquinic Acid (mg) | 89.8 |
| 5-O-caffeoylquinic Acid (mg) | 4.6 |
| p-Coumaric Acid (mg) | 8.2 |
| Caffeic Acid (mg) | 3.2 |
| Rutin (mg) | 5.6 |
| Total Phenolics (mg) | 480.5 |
2.2 Animal Care and Experimental Design
Nine month-old female Sprague Dawley® (Hsd:Sprague Dawley® SD®) rats (n=93) were purchased from Harlan Laboratories (Indianapolis, IN, USA). Animals were housed in an environmentally-controlled facility with a 12 hour light/dark cycle at 20 °C, and acclimated for 7–10 days with free access to semi-purified AIN-93M (control) diet and reverse osmosis (RO)-purified water. Following acclimation, the animals were anesthetized with a ketamine/xylazine cocktail (70 mg/kg ketamine and 7 mg/kg xylazine) and baseline whole body dual-energy X-ray absorptiometry (DXA; QDR 4500-A Elite, Hologic, Waltham, MA, USA) scans were performed. Following bilateral ovariectomy (OVX) or sham surgery, all groups were maintained on a semi-purified AIN-93M diet for 2 months to allow for bone loss. Osteopenia in the OVX groups was confirmed by a second whole body DXA scan performed prior to initiating dietary treatments.
Next, the 3-month treatment phase of the study began, with animals fed diets supplemented with polyphenols, potassium citrate (Sigma, St. Louis, MO, USA), vitamin K1 (Sigma, St. Louis, MO, USA) or a combination of the three to match that provided in the 25% (w/w) dried plum diet. The seven treatment groups consisted of the Sham-control diet (Sham; n=12 rats/group); OVX-Control diet (OVX-CON; n=14 rats/group); OVX-Control diet supplemented with 25% (w/w) dried plum (OVX-DP; n=13 rats/group) or a comparable dose of polyphenols (135 g extract/kg; n=14 rats/group) from dried plum (OVX-PP), potassium (20.3 g/kg; OVX-K+;n=13 rats/group), vitamin K (4.13 g/kg; OVX-Vit K; n=14 rats/group), or with the combination of DP polyphenol extract, potassium, and vitamin K (OVX-Combo; n=13 rats/group). It should be noted that the polyphenol extract was analyzed for potassium (1.3 g/kg) and vitamin K (98.4 μg/kg), and the amount of additional potassium and vitamin K added to the dietary treatment for the OVX-Combo group was adjusted accordingly. The rationale for the dose of dried plum was based on our previous reports that 25% dried plum was most efficacious in restoring bone lost due to ovarian hormone deficiency (Deyhim, Stoecker, Brusewitz, Devareddy, & Arjmandi, 2005; Rendina et al., 2012; Smith, Bu, et al., 2014). All diets were formulated based on the AIN-93M diet and provided similar total energy, macronutrient, fiber, calcium and phosphorus content.
Throughout the study, the OVX groups were match fed to the mean intake of the sham group to control for food intake and the animals had free access to reverse osmosis water. In addition, weekly body weights were recorded. After 3 months of treatment, the animals were placed in metabolic cages and 12-hour urine samples were collected. Two days later, the animals were anesthetized with the ketamine/xylazine cocktail and a final whole body DXA scan was performed. The anesthetized animals were then euthanized by exsanguination via the descending aorta. The uterus was collected and weighed to ensure OVX and to assess estrogenic effect of treatments. Bone specimens (i.e. femur and spine) for structural analysis were collected and stored at 4°C until the time of analyses. For gene expression analyses related to bone formation, one excised femur was flushed of bone marrow with ice cold phosphate-buffered saline (PBS) and then flash frozen and stored in liquid nitrogen until the time of analysis. Ethical care and treatment of animals were strictly followed according to the guidelines of the Institution Animal Care and Use Committee at Oklahoma State University.
2.3 Whole Body, Vertebral, and Femoral Bone Densitometry
Whole body DXA scans were completed to assess bone mineral area (BMA), bone mineral content (BMC), bone mineral density (BMD), and body composition (i.e. lean mass and fat mass) using the Regional High Resolution software package (Hologic, Waltham, MA) designed for small animal studies. Excised femur and spine specimens were also scanned and a region of interest (ROI) was identified (i.e., 4th lumbar vertebra).
2.4 Microcomputed Tomography Analyses
To assess the effect of potential bioactive components in dried plum on bone microarchitectural properties, the 4th lumbar vertebra, distal femur metaphysis, and femur mid-diaphysis were scanned by x-ray microcomputed tomography (microCT; SCANCO μCT40 scanner, SCANCO Medical, Brüttisellen, Switzerland). Trabecular bone was assessed in the distal femur metaphysis and 4th lumbar vertebral body. These scans were performed at a resolution of 1024 × 1024 pixels. In the distal femur metaphysis, the volume of interest (VOI) included 150 cross sectional slices beginning 0.40 mm proximal to the distal growth plate. The VOI in the vertebra was defined as ~300 cross sectional slices of secondary spongiosa between the dorsal and caudal growth plates. Mineralized tissue in the distal femur metaphysis was determined at a threshold of 290 mg hydroxyapatite/cm3 and filtered with a sigma of 0.7 and support of 1.0. A threshold of 380 and a sigma of 1.2 and support of 2.0 were used to determine mineralized tissue of the vertebra. Trabecular bone parameters evaluated included bone volume expressed per unit of total volume (BV/TV), trabecular number (TbN), trabecular thickness (TbTh), trabecular separation (TbSp), connectivity density (ConnDens) and structure model index (SMI).
Cortical bone properties were examined within a 0.5 mm VOI in the femur mid-diaphysis. Cortical bone porosity, thickness and area and the medullary area were determined. The images were analyzed at a threshold of 290, a sigma of 0.7 and support of 1.0.
2.5 Biochemical Assessment of Bone Resorption
Based on our previous work (Bu et al., 2007; Franklin et al., 2006; Smith, Bu, et al., 2014), urinary Dpd excretion has been shown to be a sensitive indicator of bone resorption in gonadal hormone deficiency models. Therefore, we assessed urinary Dpd using a commercially available kit (Quidel Biosystems, Mountain View, CA, USA). The assay was performed following the manufacturer’s recommendations with intra- and inter-assay coefficient of variations of 4–8% and 3–5%, respectively. To account for differences in hydration status, and therefore concentration of metabolites, urinary creatinine was measured using an ACE clinical analyzer (Alfa Wassermann, West Caldwell, NJ, USA), and Dpd data was expressed relative to creatinine.
2.6 Gene Expression Analysis of Bone Formation
Based on previous data suggesting dried plum upregulated BMP signaling in vivo (Smith, Bu, et al., 2014), the gene expression of osteogenic Bmp2 and Bmp4 was assessed in the distal femur metaphysis. The femur metaphysis (n=6 samples/group) was pulverized in a liquid nitrogen-containing Freezer/Mill (Spex 6770 Freezer/Mill, Metuchen, NJ, USA) and total cellular RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA), following the protocol provided by the manufacturer. The concentration and quality of the RNA were confirmed using optical density (OD) measures at 260 and 280 nm (NanoDrop Spectrophotometer, Rockland, DE, USA) and agarose gel electrophoresis. DNase I-treated total RNA (2 μg) was reverse transcribed using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Expression of target genes was assessed with real-time reverse transcription polymerase chain reaction (qRT-PCR; Applied Biosystems 7900HT Fast Real-Time PCR System, Foster City, CA, USA) of 50 ng of cDNA using SYBR green technologies (Invitrogen, Carlsbad, CA, USA). Primers specific to rat Bmp2 (forward: 5′-ggaaaacttcccgacgcttct-3′, and reverse 5′-cctgcatttgttcccgaaaa-3′) and Bmp4 (forward: 5′-ttatgaggttatgaagccccca-3′, and reverse 5′-gctcacatcgaaagtttcccac-3′) were used to amplify gene-specific sequences. Abundance of amplified gene-specific sequences was normalized to the reference gene Rpl19 to determine relative abundance of each target gene.
2.7 Statistical Analyses
Statistical analyses were completed using SAS Version 9.41 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were calculated and the Shapiro-Wilk test for normality performed to determine if the data were normally distributed. If normal distribution was confirmed, one-way ANOVA was used to compare between treatment groups. If the data were not normally distributed, as with the qRT-PCR data, the data were log transformed prior to statistical analysis via one-way ANOVA. When F values were significant, Fischer’s least square means post hoc analysis was used to determine statistically significant differences between individual treatment groups. All data are presented as mean ± standard error (SE) and the alpha level was set at 0.05.
3. Results
3.1 Body Weight and Composition
Prior to determining the effects of the different bioactive components in dried plum on bone, it was important to consider the influence of the interventions on body weight, body composition and estrogen-sensitive tissue. As expected, ovariectomy (OVX) resulted in a significant decrease in uterine weight and an increase in body weight over the 5-month postoperative period compared to sham-operated animals (Sham) (Table 2). Uterine weight was not affected by the dietary treatments. Despite match feeding all groups, only dried plum supplementation attenuated the OVX-induced increase in body weight and this response was not achieved with the bioactive components alone or in combination. However, body composition analyses indicated that OVX animals consuming diets supplemented with polyphenols, potassium, and the combination of bioactive components had significantly lower fat mass than those on a control diet but maintained lean mass (Table 2). The reduced fat mass in these groups was similar to that of OVX animals consuming a diet supplemented with dried plum. Vitamin K supplementation alone did not have an effect on fat mass. There was no difference between any groups in lean mass (Table 2).
Table 2.
Body weight, and final body composition and bone densitometry
| Sham | OVX-CON | OVX-DP | OVX-PP | OVX-K+ | OVX-VitK | OVX-Combo | |
|---|---|---|---|---|---|---|---|
| Uterus (g) | 0.75 ± 0.07a | 0.21 ± 0.02b | 0.22 ± 0.04b | 0.16 ± 0.01b | 0.20 ± 0.03b | 0.21 ± 0.01b | 0.20 ± 0.02b |
| Body weight | |||||||
| Baseline (g) | 298.5 ± 6.8 | 298.2 ±5.7 | 297.2± 4.8 | 298.2 ± 5.8 | 298.2 ± 6.6 | 297.7 ± 6.3 | 297.5 ± 7.0 |
| Final (g) | 345.3 ± 13.3c | 402.1 ± 8.2a | 373.4 ± 6.8b | 391.9 ± 5.6ab | 389.5 ± 6.8ab | 401.0 ± 9.9a | 394.8 ± 7.3ab |
| Body composition | |||||||
| Lean mass (g) | 273.9 ± 7.0 | 265.21 ± 5.0 | 280.7 ± 6.6 | 277.9 ± 3.4 | 273.4 ± 4.6 | 267.9 ± 4.7 | 280.7 ± 5.6 |
| Fat mass (g) | 64.2 ± 7.9d | 136.8 ± 6.9a | 89.6 ± 6.6c | 116.3 ± 7.2b | 114.0 ± 6.7b | 128.4 ± 10.2ab | 110.9 ± 8.1bc |
| Percent fat (%) | 18.4 ± 1.8d | 33.9 ± 1.3a | 24.1 ± 1.6c | 29.3 ± 1.4b | 29.3 ± 1.3b | 32.1 ± 1.7ab | 28.1 ± 1.7bc |
| Bone densitometry | |||||||
| Spine | |||||||
| BMA (cm2) | 0.77 ± 0.01a | 0.64 ± 0.02d | 0.75 ± 0.01ab | 0.70 ± 0.01bc | 0.68 ± 0.01cd | 0.69 ± 0.01c | 0.73 ± 0.02ab |
| BMC (g) | 0.18 ± 0.01a | 0.10 ± 0.01d | 0.16 ± 0.01b | 0.13 ± 0.01c | 0.12 ± 0.01cd | 0.12 ± 0.01cd | 0.15 ± 0.01b |
| BMD (g/cm2) | 0.24 ± 0.01a | 0.16 ± 0.00d | 0.21 ± 0.01b | 0.19 ± 0.01c | 0.18 ± 0.00cd | 0.17 ± 0.00d | 0.21 ± 0.01b |
| Femur | |||||||
| BMA (cm2) | 1.96 ± 0.01 | 1.96 ± 0.00 | 2.02 ± 0.03 | 1.96 ± 0.00 | 1.99 ± 0.00 | 1.97 ± 0.03 | 1.96 ± 0.03 |
| BMC (g) | 0.55 ± 0.03a | 0.48 ± 0.01c | 0.54 ± 0.01ab | 0.51 ± 0.01abc | 0.51 ± 0.01bc | 0.48 ± 0.01c | 0.54 ± 0.01ab |
| BMD (g/cm2) | 0.28 ± 0.01a | 0.24 ± 0.00d | 0.27 ± 0.00b | 0.26 ± 0.00bc | 0.25 ± 0.01cd | 0.24 ± 0.00d | 0.27 ± 0.00ab |
Bone densitometry measures include bone mineral area (BMA), bone mineral content (BMC) and bone mineral density (BMD). Data is presented as mean ± SE. Groups that do not share the same letter are statistically different from each other, p < 0.05. Abbreviations: OVX, ovariectomized; Con, control diet; DP, dried plum; PP, polyphenol; K+, potassium; VitK, vitamin K; Combo, combination of PP, K+, and VitK.
3.2 Whole Body, Spine, and Femoral Bone Densitometry
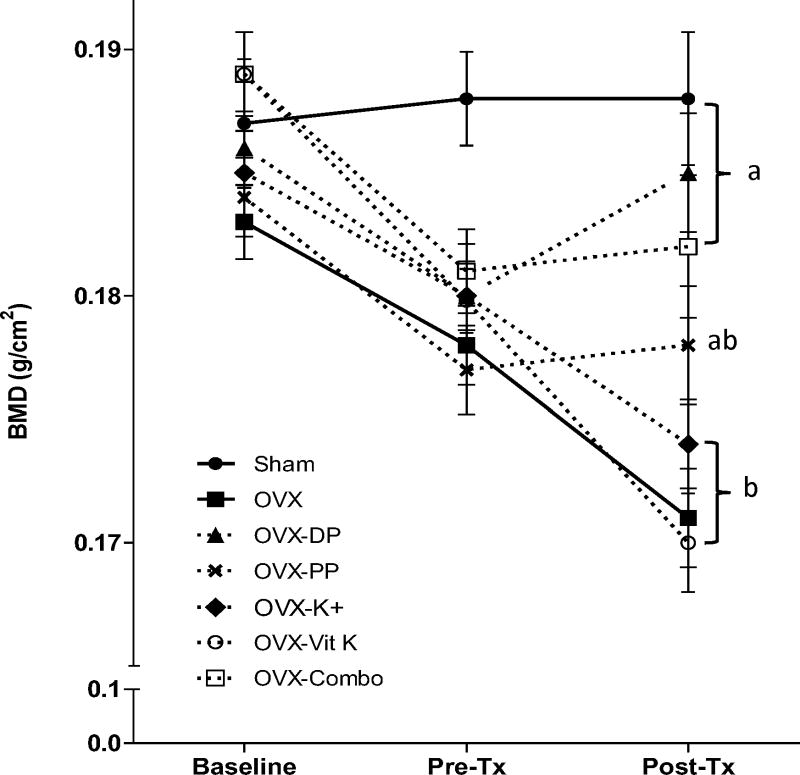
Two months post-OVX and prior to treatment, whole body BMD was reduced by ~3–4% in the OVX groups compared to the Sham group (Figure 1). Even in these aging, osteopenic animals, three months of dietary treatment with the polyphenols and the combination of bioactive components resulted in improved whole body BMD (p < 0.0001) compared to that of OVX animals consuming the control diet (Figure 1). Both of these treatment groups (OVX-PP and OVX-Combo) exhibited a similar response as the dried plum group. Supplementing the diet with potassium or vitamin K alone did not result in an increase in whole body BMD.
Figure 1.
Whole body bone mineral density (BMD) was measured at baseline, 2 months after ovariectomy but prior to beginning dietary treatment (Pre-Tx), and following 3 months of dietary treatment (Post-Tx). Bars represent the mean ± SE. Data points that do not share the same superscript letter are statistically different from each other, p < 0.05. Abbreviations: OVX, ovariectomized; Con, control diet; DP, dried plum; PP, polyphenol; K+, potassium; VitK, vitamin K; Combo, combination of PP, K+, and VitK.
Coinciding with the improvement in whole body BMD, bone density of the spine and femur were also increased in the groups receiving the polyphenol and the combination diet (Table 2). Vitamin K and potassium supplementation alone failed to increase BMD at these sites. The BMD response to the combination of bioactive components in the spine was similar to that of the dried plum group (Table 2). Dietary supplementation with the polyphenols resulted in a spine BMD greater than that of OVX animals consuming a control diet (OVX-CON), but did not reach the level of the dried plum group (Table 2). The increase in spine BMD was a result of the robust 30% and 47% increase in bone mineral content (BMC) in the polyphenol and combination of bioactive components groups, respectively (Table 2). Significant improvements in femoral BMD were also observed in the OVX groups consuming the polyphenol and combination diet compared to OVX-CON (p < 0.0001), and both groups experienced a similar increase to that of dried plum-treated group (Table 2). In fact, OVX animals consuming a diet supplemented with the combination of bioactive components had a femoral BMD that was restored to that of the intact Sham animals. This increase in BMD resulted from a 12% increase in BMC (p = 0.0010), with no change in BMA.
3.3 Trabecular and Cortical Bone Analyses
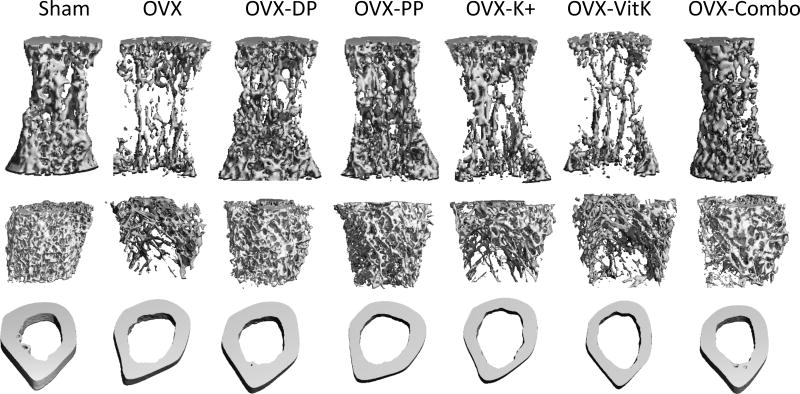
Next, the treatment effects on trabecular and cortical bone microarchitecture were evaluated using microCT (Table 3). Representative three-dimensional images of the lumbar vertebra and the femur following 3 months of dietary treatment are shown in Figure 2. In the vertebral body, BV/TV was reduced by 80% in OVX-CON animals. However, this decrease in vertebral BV/TV was countered (p < 0.0001) in OVX animals consuming diets supplemented with the polyphenol extract and the combination of bioactive components, with an increase in vertebral BV/TV of 155% and 197%, respectively (Table 3). In fact, consumption of the combination of bioactive components resulted in a trabecular bone volume similar to that of animals consuming dried plum. The improvements in BV/TV coincided with a significant increase in TbN and TbTh, and a decrease in TbSp. These changes in trabecular bone morphometric parameters were similar to those observed with the dried plum treatment (Table 3). Potassium supplementation did result in an increase in TbN (p = 0.0232) and a decrease in TbSp (p = 0.0029) compared to OVX animals consuming a control diet, but these improvements did not result in a significant improvement in trabecular bone volume. Vitamin K supplementation alone did not provide any benefit to morphometric parameters of vertebral trabecular bone.
Table 3.
Micro-computed tomography analyses of the lumbar vertebra and the femur distal metaphysis and mid-diaphysis
| Sham | OVX-CON | OVX-DP | OVX-PP | OVX-K+ | OVX-VitK | OVX-Combo | |
|---|---|---|---|---|---|---|---|
| Lumbar vertebra | |||||||
| BV/TV (%) | 31.1 ± 3.4a | 6.2 ± 0.6e | 23.4 ± 2.0b | 15.8 ± 2.0cd | 9.9 ± 1.3de | 7.6 ± 1.4e | 18.4 ± 3.3bc |
| TbN (1/mm2) | 3.21 ± 0.14a | 1.52 ± 0.13e | 2.91 ± 0.16ab | 2.46 ± 0.21bc | 2.06 ± 0.07cd | 1.76 ± 0.16de | 2.63 ± 0.20b |
| TbTh (μm) | 125.3 ± 5.8a | 87.0 ± 0.2d | 111.2 ± 2.3b | 100.6 ± 2.6bc | 92.6 ± 2.5cd | 88.7 ± 3.8d | 104.8 ± 5.3b |
| TbSp (μm) | 316.0 ± 17.5d | 687.1 ± 54.2a | 356.4 ± 23.7d | 431.2 ± 43.7cd | 498.4 ± 16.6bc | 604.0 ± 68.8ab | 391.0 ± 31.0cd |
| ConnDen (1/mm3) | 24.12 ± 1.59a | 3.65 ± 0.79c | 20.36 ± 1.73ab | 15.03 ± 2.47b | 7.578 ± 1.80c | 4.80 ± 1.81c | 15.35 ± 3.55b |
| SMI | 0.58 ± 0.47d | 3.18 ± 0.09a | 1.46 ± 0.15c | 2.34 ± 0.18b | 2.85 ± 0.18ab | 3.13 ± 0.15a | 2.17 ± 0.39bc |
| Femur distal metaphysis | |||||||
| BV/TV (%) | 19.9 ± 1.9a | 5.2 ± 0.4cd | 12.5 ± 1.6b | 8.0 ± 0.4c | 5.6 ± 0.7cd | 4.6 ± 0.6d | 8.0 ± 0.7c |
| TbN (1/mm2) | 4.08 ± 0.15a | 1.66 ± 0.10d | 2.87 ± 0.24b | 2.14 ± 0.12c | 1.67 ± 0.19d | 1.42 ± 0.11d | 2.16 ± 0.09c |
| TbTh (μm) | 69.0 ± 2.7 | 65.7 ± 3.0 | 69.8 ± 2.2 | 65.1 ± 1.4 | 63.6 ± 1.6 | 62.9 ± 2.3 | 64.9 ± 1.2 |
| TbSp (μm) | 245.6 ± 10.1d | 656.7 ± 38.9a | 362.7 ± 26.3cd | 488.1 ± 30.6b | 666.5 ± 84.4a | 737.8 ± 49.8a | 475.9 ± 18.4bc |
| ConnDen (1/mm3) | 84.14 ± 7.00a | 10.86 ± 1.16c | 36.15 ± 7.42b | 22.04 ± 1.61c | 13.56 ± 2.34c | 10.86 ± 1.80c | 21.96 ± 2.56c |
| SMI | 1.36 ± 0.15d | 2.39 ± 0.06a | 1.85 ± 0.11c | 2.08 ± 0.05bc | 2.39 ± 0.07a | 2.41 ± 0.08a | 2.16 ± 0.09ab |
| Femur mid-diaphysis | |||||||
| CortTh (mm) | 0.71 ± 0.01a | 0.58 ± 0.02c | 0.68 ± 0.02a | 0.63 ± 0.01b | 0.56 ± 0.02c | 0.58 ± 0.01c | 0.63 ± 0.01b |
| CortArea (mm2) | 2.07 ± 0.03a | 1.67 ± 0.07d | 1.91 ± 0.03b | 1.82 ± 0.04bc | 1.72 ± 0.04cd | 1.68 ± 0.05d | 1.92 ± 0.04b |
| MedArea (mm2) | 0.25 ± 0.04 | 0.20 ± 0.03 | 0.19 ± 0.01 | 0.20 ± 0.03 | 0.23 ± 0.03 | 0.18 ± 0.003 | 0.24 ± 0.02 |
| Porosity (%) | 3.3 ± 0.6 | 3.2 ± 0.4 | 2.7 ± 0.2 | 3.0 ± 0.4 | 3.6 ± 0.4 | 2.9 ± 0.1 | 3.4 ± 0.2 |
Bone microarchitectural measurements include: trabecular bone volume/total volume (BV/TV); trabecular number (TbN); trabecular thickness (TbTh); trabecular separation (TbSp); connectivity density (ConnDens); structural model index (SMI); cortical thickness (CortTh); cortical area (CortArea); and medullary area (MedArea). Data is presented as mean ± SE. Groups that do not share the same letter are statistically different from each other, p < 0.05. Abbreviations: OVX, ovariectomized; Con, control diet; DP, dried plum; PP, polyphenol; K+, potassium; VitK, vitamin K; Combo, combination of PP, K+, and VitK.
Figure 2.
Bone microarchitecture was assessed in the 4th lumbar vertebra and the femur following 3 months of dietary treatment. Three dimensional micro-computed tomography images are shown. Trabecular bone volume was assessed in the vertebra and the distal femur metaphysis (top two images). Cortical bone was assessed in the femur mid-diaphysis (bottom). Abbreviations: OVX, ovariectomized; Con, control diet; DP, dried plum; PP, polyphenol; K+, potassium; VitK, vitamin K; Combo, combination of PP, K+, and VitK.
In contrast to the trabecular bone response in the vertebra, bone volume of the distal femur metaphysis was increased by dried plum supplementation only (Table 3). Coincident with this increase in BV/TV, positive effects were observed in TbN and TbSp compared to OVX-CON group, but the microarchitecture was not restored to that of Sham at this site. Whereas the combination of polyphenols and bioactive components increased TbN compared to the OVX-CON group, there were no effects on TbTh (Table 3). Vitamin K and potassium supplementation alone did not affect any of these trabecular bone morphometric parameters in the distal femur metaphysis (Table 3).
Non-morphometric analyses of trabecular bone in the vertebra also indicated a positive effect of treatment in OVX animals consuming diets supplemented with the polyphenols and the combination of bioactive components; however, this was not the case in the vitamin K or potassium treatments alone (Table 3). The connectivity density (ConnDen) of the trabecular bone was greater (p < 0.0001) in the groups receiving the polyphenol and combination supplemented diets compared to OVX animals on a control diet, and was similar to that of the dried plum group (Table 3). In addition, consumption of the polyphenols or the combination of bioactive components resulted in a lower the structural model index (SMI), indicating a more plate-like arrangement of trabeculae, compared to OVX animals consuming a control diet (p < 0.0001). In fact, the combination of bioactive components resulted in a SMI similar to that of the dried plum group. Like BV/TV in the distal femur metaphysis, ConnDen was only greater in OVX animals consuming dried plum compared to OVX animals on a control diet (Table 3). Notably, femoral SMI was reduced by supplementation with the polyphenols compared to OVX-CON (p < 0.0001) which suggests the potential for biomechanical benefit in the trabecular arrangement, and this response was similar to that of the dried plum group (Table 3).
Cortical bone analyses of the femur mid-diaphysis of these aged, osteopenic animals indicated enhanced cortical thickness (CortTh) and cortical area (CortArea) due to dietary treatment with polyphenols and the combination of bioactive components (Table 3). CortTh was significantly increased ~8% with dietary supplementation with the polyphenols and the combination of bioactive components compared to OVX-CON, but neither group was able to achieve the benefit on CortTh observed with dried plum supplementation. CortArea was improved (p < 0.0001) in both the polyphenol-supplemented group and the animals consuming the combination of bioactive components compared to those on a control diet (p < .0001). This cortical bone response was similar to that of the dried plum group. Notably, there was no difference in medullary area (MedArea) or porosity between any of the groups.
3.4 Biochemical Assessment of Bone Resorption
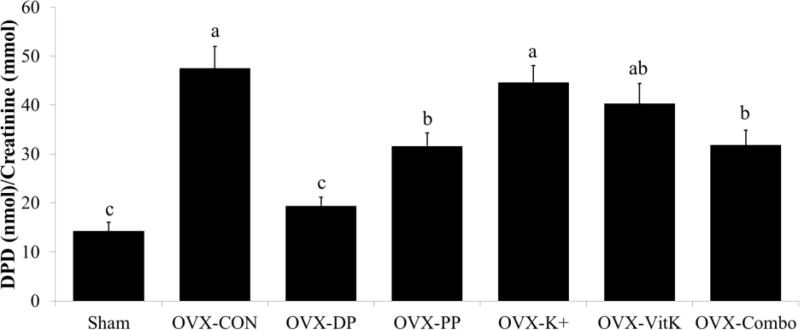
Even five months post-surgery, urinary excretion of deoxypyridinoline (Dpd), an indicator of bone resorption, was elevated ~3 fold by OVX (Figure 3). Dietary supplementation with the polyphenols and the combination of bioactive components attenuated this response. Urinary Dpd was decreased (p < 0.0001) by 33% in both of these groups compared to OVX-CON, but this response did not achieve the magnitude of the response observed with the dried plum treatment. Only the dried plum was able to restore urinary Dpd excretion to that of Sham. Interestingly, vitamin K and potassium supplementation had no effect on urinary Dpd excretion in this study.
Figure 3.
Urinary excretion of deoxypyridinoline (DPD) was measured as an indicator of bone resorption. The animals were placed in metabolic cages and 12-hour urine samples were collected. Bars that do not share the same superscript letter are statistically different from each other, p < 0.05. Abbreviations: OVX, ovariectomized; Con, control diet; DP, dried plum; PP, polyphenol; K+, potassium; VitK, vitamin K; Combo, combination of PP, K+, and VitK.
3.5 Gene Expression Related to Osteogenesis
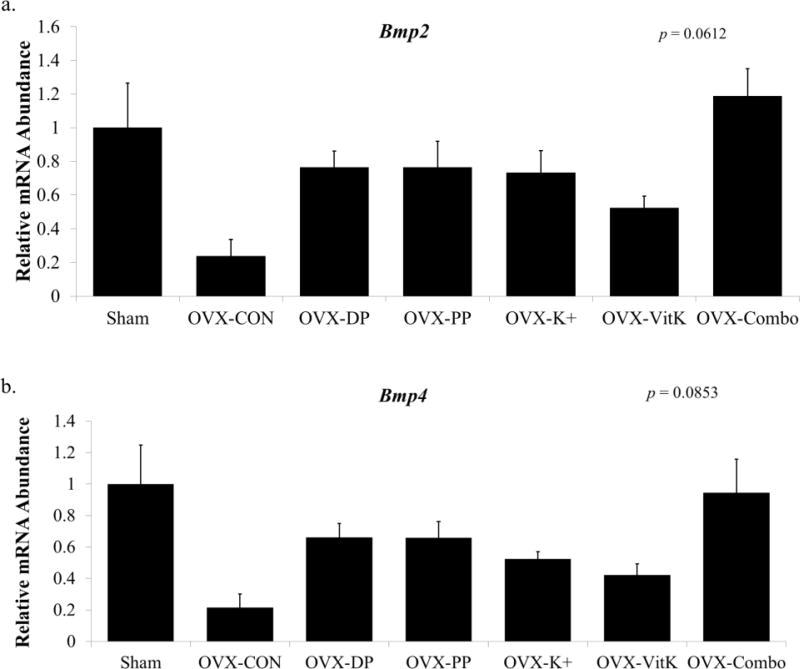
To assess indicators of osteogenesis that we have previously shown to be affected by dried plum (Smith, Bu, et al., 2014), the relative mRNA abundance of Bmp2 and Bmp4 was determined in the femur metaphysis (Figure 4). There was as trend for a treatment effect on expression of both Bmp2 (p = 0.0612) and Bmp4 (p = 0.0853), with OVX resulting in more than a 4-fold decrease in the expression of both genes. Conversely, the combination of polyphenols, vitamin K and potassium resulted in a ~5-fold increase in Bmp2 expression and a ~4.5-fold increase in Bmp4 expression compared to OVX-CON, and expression of both genes in this treatment group was similar to that of the Sham group.
Figure 4.
Alterations in the relative abundance of mRNA for bone morphogenetic proteins, Bmp2 and Bmp4 in the distal femur metaphysis. Relative mRNA expression of (a) Bmp2 and (b) Bmp4 was assessed in the distal femur metaphysis following 3 months of dietary treatment. Bars represent the mean fold change ± SE. Data points that do not share the same superscript letter are statistically different from each other, p < 0.05. Abbreviations: OVX, ovariectomized; Con, control diet; DP, dried plum; PP, polyphenol; K+, potassium; VitK, vitamin K; Combo, combination of PP, K+, and VitK.
4. Discussion
The bone protective properties of dried plums were first reported more than a decade ago when dietary supplementation with dried plum was shown to prevent bone loss in an animal model of estrogen deficiency (Arjmandi et al., 2001). Since that time, the efficacy of dried plum (‘Improved French’) to prevent and even reverse bone loss due to age and gonadal hormone deficiency, as well as its mechanisms of action, have been reported in the literature (Bu et al., 2007; Franklin et al., 2006; Halloran et al., 2010; Hooshmand et al., 2011; Rendina et al., 2013; Rendina et al., 2012; Smith, Bu, et al., 2014; Smith, Graef, et al., 2014). However, the bioactive component(s) responsible for this osteoprotective effects have remained in question. Candidate components with known anti-resorptive or osteogenic effects have been proposed, but this is the first in vivo study to investigate the contribution of the polyphenols, vitamin K and potassium to dried plum’s anabolic effects on bone. Furthermore, this model represents one of the most challenging physiological scenarios to test dried plum and its bioactive components ability to restore bone to date, the aged, osteopenic ovariectomized animal model.
As we have shown, the most pronounced improvements in bone density as well as trabecular and cortical bone parameters were observed when the polyphenols extracted from dried plum were combined with supplemental doses of vitamin K and potassium consistent with the amount found dried plum. Our findings demonstrate that the polyphenol extract is the primary contributor to this response, as a number of bone parameters were restored with polyphenol supplementation alone and that was not the case with the potassium or the vitamin K groups alone. This point was demonstrated at the spine, where supplementing the diet with both the polyphenols and the combination of bioactive components resulted in similar improvements in trabecular bone. In fact, approximately 82% of the increase in vertebral trabecular bone observed with the combination of bioactive components could be attributed to the polyphenols. Interestingly, none of the bioactive components that were tested were able to restore femoral trabecular bone to that of the Sham group. This may have been due to the fact that there was a 74% reduction in trabecular bone in these older osteopenic animals, leaving little trabecular network for new strut connections to form. Yet the most robust bone response observed with the polyphenols was in cortical bone of the femur mid-diaphysis where no further improvements were observed in the thickness of the cortices with the addition of potassium and vitamin K. The polyphenolic extract induced a similar increase in cortical area as that observed with dried plum and combination of polyphenols, vitamin k and potassium. Although the magnitude of the effect of the polyphenols on cortical thickness was not as great as that of cortical area, this effect on cortical bone is in line with our previous report that dried plum increased endocortical bone mineralization rate in long bones, while (Smith, Bu, et al., 2014).
Although the focus of this study was not on the mechanism through which the effects of the extract alters bone, the polyphenols likely contribute to the fruit’s antioxidant and anti-inflammatory properties (Bu et al., 2009; Bu et al., 2008; Rendina et al., 2013; Rendina et al., 2012; Smith, Graef, et al., 2014). An increase in circulating glutathione peroxidase activity, a marker of endogenous antioxidant capacity, has been reported with dried plum supplementation in age-related and gonadal hormone deficiency-related models of osteoporosis (Rendina et al., 2013; Smith, Graef, et al., 2014). Oxidative stress, including an increase in reactive oxygen species and a decrease in endogenous antioxidants, increases with age and with estrogen deficiency and can upregulate osteoclast activity as well and osteoblast apoptosis (Almeida et al., 2007; Muthusami et al., 2005). Reactive oxygen species also upregulate an inflammatory response that results in deterioration of bone integrity (Chung et al., 2009; Roggia et al., 2001). We have previously reported dried plum supplementation suppressed T-cell activation in estrogen-deficient mice (Rendina et al., 2012). Therefore, the polyphenols in dried plum may protect bone by exerting antioxidant and anti-inflammatory responses. Alternatively, it is possible that the polyphenols may be affecting cell signaling directly in pre-osteoclasts and pre-osteoblasts. Recent in vitro work in our laboratory using primary osteoblasts derived from the bone marrow of C57BL/6 mice has suggested that certain fractions of the polyphenols in dried plum upregulate BMP signaling in differentiating osteoblasts, leading to an increase in mineralizing activity (Graef et al., 2017). Additionally, we found that osteoclast differentiation was inhibited when pre-osteoclasts with some fractions of polyphenols from dried plum (Graef et al., 2017). These in vitro findings are consistent with our data in the current study in that both bone resorption as well as regulators of osteogenesis (i.e., Bmp2 and Bmp4) tended to be affected.
Our data indicated the polyphenols were the primary bioactive component responsible for the bone response, but the addition of the potassium and vitamin K to the polyphenol extract did provide an additional benefit on BMD of the spine. Potassium has been found to be important in regulating bone resorption (Macdonald, New, Fraser, Campbell, & Reid, 2005), and this is likely due to its ability to alter the pH of the bone microenvironment (Bushinsky et al., 1997). Conversely, vitamin K promotes the carboxylation of osteocalcin, and therefore plays a role in bone mineralization (Binkley et al., 2000; Knapen et al., 1989). Therefore, it stands to reason that the addition of potassium and vitamin K to the polyphenol extract provides an ideal combination of bioactive components that further enhance the anti-resorptive and pro-anabolic properties of the polyphenolic extract. A recent report by Léoting and colleagues (Leotoing et al., 2016) brought into question the influence of dried plum’s polyphenols effects on bone. They reported that a diet supplemented with a cultivar of dried plum rich in chlorogenic acids was not more effective at preventing OVX-induced bone loss than a diet supplemented with a cultivar of plum low in chlorogenic acids. However, the amount of plum supplemented (15% w/w) was lower than the amount reported to be most effective (25% w/w) at preventing and reversing bone loss. Furthermore, they showed that a combination of synthetic polyphenols found in dried plum (neochlorogenic acid, chlorogenic acid, and caffeic acid) did not have osteoprotective effects. Although the source of the polyphenols is an important distinction to be made, our findings show that polyphenols extracted from dried plum are important bioactive components and, when combined with the dose of vitamin K and potassium found in the dried plum, they can restore many of the bone parameters that have been compromised due to age and estrogen deficiency.
In addition to polyphenols, it should also be noted that the polyphenol extract also contained some carbohydrates. The rationale for not further purifying the extract was based on the fact that we had used this same ethanol extract in our previous in vitro studies as well as concerns related to potential loss of or alterations in polyphenols that can occur with purification. To account for this, all diets were formulated to provide the same amount of total carbohydrates. Typically, non-digestible carbohydrates make up approximately 11–12% of dried plum’s dry matter (Dikeman, Bauer, & Fahey, 2004). Long-chain oligosaccharides have been shown to increase absorption of minerals (e.g., calcium, magnesium, and phosphorus) that are important in bone metabolism (Bryk et al., 2014). The addition of fructo-oligosaccharides to a dried plum supplemented diet has also been shown to result in greater improvements in spine and femoral bone mineral density and a decrease in urinary calcium excretion in an estrogen-deficient animal model (Arjmandi et al., 2010). Consumption of non-digestible oligosaccharides is also known to suppress bone turnover and increase BMD (Zafar, Weaver, Zhao, Martin, & Wastney, 2004). Therefore, the possibility exists that the carbohydrate component of the polyphenol extract also contributed to the skeletal response observed in this study. Experiments are underway in our laboratory to further elucidate the contribution of the extract’s polyphenols compared to the carbohydrates in understanding the bioactive components in dried plum and their effects on bone.
4.1 Conclusions
In summary, this study is the first to demonstrate that a polyphenol extract from dried plum accounts for 60–80% of the anabolic effect of dried plum on bone in vivo; however, it is when the extract is combined with vitamin K and potassium that the response most closely mimics that of the dried fruit. These findings indicate that the bioactive components in dried plum include not only the polyphenols but also potassium and vitamin K. Future studies are warranted to determine if certain types of polyphenols or other components of the polyphenol extract are responsible for the osteoprotective effects and whether other cultivars of the dried plum, aside from the Improved French provide similar benefits.
Highlights.
Dried plum polyphenol (PP) extract benefits trabecular and cortical bone.
Some bone parameters improved when vitamin K and potassium combined with PP.
PP extract accounts for up to 80% of the bone anabolic response.
Dried plum bioactives counter bone loss in aging, estrogen-deficient model.
Acknowledgments
We thank the California Dried Plum Board for providing the dried plum powder used for this project.
Funding: This manuscript is based on work supported by U.S. Department of Agriculture [grant number 2006-35200-17383] and the National Institutes of Health/National Center for Complimentary and Integrative Health [grant number R21AT006580]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or U.S. Department of Agriculture.
Abbreviations
- BMD
bone mineral density
- TRAP-5b
tartrate resistant acid phosphatase-5b
- Dpd
deoxypyridinoline
- Runx2
runt-related transcription factor 2
- BMP
bone morphogenetic protein
- OVX
ovariectomized
- DP
dried plum
- CON
control diet
- PP
polyphenols
- K+
potassium
- VitK
vitamin K
- DXA
dual x-ray absorptiometry
- BMA
bone mineral area
- BMC
bone mineral content
- BV/TV
bone volume per total volume
- TbN
trabecular number
- TbTh
trabecular thickness
- TbSp
trabecular separation
- ConnDens
connectivity density
- SMI
structural model index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article: Caffeic acid (PubChem CID: 689043); 5-O-Caffeoylquinic acid (PubChem CID: 5280633); 4-O-Caffeoylquinic acid (PubChem CID: 12305664); 3-Caffeoylquinic acid (PubChem CID: 71314735); gallic acid (PubChem CID: 370); p-coumaric acid (PubChem CID: 637542); rutin (PubChem CID: 5280805)
References
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjmandi BH, Johnson CD, Campbell SC, Hooshmand S, Chai SC, Akhter MP. Combining fructooligosaccharide and dried plum has the greatest effect on restoring bone mineral density among select functional foods and bioactive compounds. J Med Food. 2010;13(2):312–319. doi: 10.1089/jmf.2009.0068. [DOI] [PubMed] [Google Scholar]
- Arjmandi BH, Khalil DA, Lucas EA, Georgis A, Stoecker BJ, Hardin C, Wild RA. Dried plums improve indices of bone formation in postmenopausal women. J Womens Health Gend Based Med. 2002;11(1):61–68. doi: 10.1089/152460902753473471. [DOI] [PubMed] [Google Scholar]
- Arjmandi BH, Lucas E, Juma S, Soliman A, Stoecker B, Khalil D, Wang C. Dried plums prevent ovariectomy-induced bone loss in rats. JANA. 2001;4(1):50–56. [Google Scholar]
- Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Kang MI. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010;87(3):226–235. doi: 10.1007/s00223-010-9393-9. [DOI] [PubMed] [Google Scholar]
- Binkley NC, Krueger DC, Engelke JA, Foley AL, Suttie JW. Vitamin K supplementation reduces serum concentrations of under-gamma-carboxylated osteocalcin in healthy young and elderly adults. Am J Clin Nutr. 2000;72(6):1523–1528. doi: 10.1093/ajcn/72.6.1523. [DOI] [PubMed] [Google Scholar]
- Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, Kiel DP. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr. 2003;77(2):512–516. doi: 10.1093/ajcn/77.2.512. [DOI] [PubMed] [Google Scholar]
- Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93(4):1217–1223. doi: 10.1210/jc.2007-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, Kiel DP. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr. 2000;71(5):1201–1208. doi: 10.1093/ajcn/71.5.1201. [DOI] [PubMed] [Google Scholar]
- Bryk G, Coronel MZ, Pellegrini G, Mandalunis P, Rio ME, de Portela ML, Zeni SN. Effect of a combination GOS/FOS prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur J Nutr. 2014 doi: 10.1007/s00394-014-0768-y. [DOI] [PubMed] [Google Scholar]
- Bu SY, Hunt TS, Smith BJ. Dried plum polyphenols attenuate the detrimental effects of TNF-alpha on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-I. J Nutr Biochem. 2009;20(1):35–44. doi: 10.1016/j.jnutbio.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Bu SY, Lerner M, Stoecker BJ, Boldrin E, Brackett DJ, Lucas EA, Smith BJ. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif Tissue Int. 2008;82(6):475–488. doi: 10.1007/s00223-008-9139-0. [DOI] [PubMed] [Google Scholar]
- Bu SY, Lucas EA, Franklin M, Marlow D, Brackett DJ, Boldrin EA, Smith BJ. Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteoporos Int. 2007;18(7):931–942. doi: 10.1007/s00198-007-0335-y. [DOI] [PubMed] [Google Scholar]
- Bugel S. Vitamin K and bone health in adult humans. Vitam Horm. 2008;78:393–416. doi: 10.1016/S0083-6729(07)00016-7. [DOI] [PubMed] [Google Scholar]
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- Bushinsky DA, Riordon DR, Chan JS, Krieger NS. Decreased potassium stimulates bone resorption. Am J Physiol. 1997;272(6 Pt 2):F774–780. doi: 10.1152/ajprenal.1997.272.6.F774. [DOI] [PubMed] [Google Scholar]
- Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Pacifici R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci U S A. 2003;100(18):10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyhim F, Stoecker BJ, Brusewitz GH, Devareddy L, Arjmandi BH. Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause. 2005;12(6):755–762. doi: 10.1097/01.gme.0000185486.55758.5b. [DOI] [PubMed] [Google Scholar]
- Dikeman CL, Bauer LL, Fahey GC., Jr Carbohydrate composition of selected plum/prune preparations. J Agric Food Chem. 2004;52(4):853–859. doi: 10.1021/jf034858u. [DOI] [PubMed] [Google Scholar]
- Donovan JL, Meyer AS, Waterhouse AL. Phenolic composition and antioxidant activity of prunes and prune juice (Prunus domestica) J Agric Food Chem. 1998;46(4):1247–1252. [Google Scholar]
- Feskanich D, Weber P, Willett WC, Rockett H, Booth SL, Colditz GA. Vitamin K intake and hip fractures in women: a prospective study. Am J Clin Nutr. 1999;69(1):74–79. doi: 10.1093/ajcn/69.1.74. [DOI] [PubMed] [Google Scholar]
- Franklin M, Bu SY, Lerner MR, Lancaster EA, Bellmer D, Marlow D, Smith BJ. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone. 2006;39(6):1331–1342. doi: 10.1016/j.bone.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Graef JL, Rendina-Ruedy E, Crockett EK, Wu L, Ouyang P, King JB, Cichewicz RH, Lin D, Lucas EA, Smith BJ. Osteoclast Differentiation is Downregulated by Select Polyphenolic Fractions from Dried Plum via Suppression of MAPKs and Nfatc1 in Mouse C57BL/6 Primary Bone Marrow Cells. Current Developments Nutr. doi: 10.3945/cdn.117.000406. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef JL, Rendina-Ruedy E, Crockett EK, Ouyang P, King JB, Cichewicz RH, Lucas EA, Smith BJ. Fractions of dried plum polyphenols enhance osteoblast activity through BMP-2 signaling. J Nutr Biochem. doi: 10.1016/j.jnutbio.2017.09.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory NS, Kumar R, Stein EM, Alexander E, Christos P, Bockman RS, Rodman JS. Potassium Citrate Decreases Bone Resorption in Postmenopausal Women with Osteopenia: A Randomized, Double-Blind Clinical Trial. Endocr Pract. 2015;21(12):1380–1386. doi: 10.4158/EP15738.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran BP, Wronski TJ, VonHerzen DC, Chu V, Xia X, Pingel JE, Smith BJ. Dietary dried plum increases bone mass in adult and aged male mice. J Nutr. 2010;140(10):1781–1787. doi: 10.3945/jn.110.124198. [DOI] [PubMed] [Google Scholar]
- Hooshmand S, Chai SC, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br J Nutr. 2011;106(6):923–930. doi: 10.1017/S000711451100119X. [DOI] [PubMed] [Google Scholar]
- Hooshmand S, Kern M, Metti D, Shamloufard P, Chai SC, Johnson SA, Arjmandi BH. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: a randomized, controlled trial. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3524-8. [DOI] [PubMed] [Google Scholar]
- Hooshmand S, Kumar A, Zhang JY, Johnson SA, Chai SC, Arjmandi BH. Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food Funct. 2015;6(5):1719–1725. doi: 10.1039/c5fo00173k. [DOI] [PubMed] [Google Scholar]
- Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem. 2003;51(22):6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- Knapen MH, Hamulyak K, Vermeer C. The effect of vitamin K supplementation on circulating osteocalcin (bone Gla protein) and urinary calcium excretion. Ann Intern Med. 1989;111(12):1001–1005. doi: 10.7326/0003-4819-111-12-1001. [DOI] [PubMed] [Google Scholar]
- Leotoing L, Wauquier F, Davicco MJ, Lebecque P, Gaudout D, Rey S, Coxam V. The phenolic acids of Agen prunes (dried plums) or Agen prune juice concentrates do not account for the protective action on bone in a rat model of postmenopausal osteoporosis. Nutr Res. 2016;36(2):161–173. doi: 10.1016/j.nutres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Macdonald HM, New SA, Fraser WD, Campbell MK, Reid DM. Low dietary potassium intakes and high dietary estimates of net endogenous acid production are associated with low bone mineral density in premenopausal women and increased markers of bone resorption in postmenopausal women. Am J Clin Nutr. 2005;81(4):923–933. doi: 10.1093/ajcn/81.4.923. [DOI] [PubMed] [Google Scholar]
- Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res. 2011;26(1):50–62. doi: 10.1002/jbmr.171. [DOI] [PubMed] [Google Scholar]
- Marangella M, Di Stefano M, Casalis S, Berutti S, D’Amelio P, Isaia GC. Effects of potassium citrate supplementation on bone metabolism. Calcif Tissue Int. 2004;74(4):330–335. doi: 10.1007/s00223-003-0091-8. [DOI] [PubMed] [Google Scholar]
- Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, Narasimhan S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360(1–2):81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Nakatani N, Kayano S, Kikuzaki H, Sumino K, Katagiri K, Mitani T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.) J Agric Food Chem. 2000;48(11):5512–5516. doi: 10.1021/jf000422s. [DOI] [PubMed] [Google Scholar]
- Nandi A, Dey S, Biswas J, Jaiswal P, Naaz S, Yasmin T, Bishayi B. Differential induction of inflammatory cytokines and reactive oxygen species in murine peritoneal macrophages and resident fresh bone marrow cells by acute staphylococcus aureus infection: contribution of toll-like receptor 2 (TLR2) Inflammation. 2015;38(1):224–244. doi: 10.1007/s10753-014-0026-8. [DOI] [PubMed] [Google Scholar]
- Piga A, Del Caro A, Corda G. From plums to prunes: influence of drying parameters on polyphenols and antioxidant activity. J Agric Food Chem. 2003;51(12):3675–3681. doi: 10.1021/jf021207+. [DOI] [PubMed] [Google Scholar]
- Rendina E, Hembree KD, Davis MR, Marlow D, Clarke SL, Halloran BP, Smith BJ. Dried plum’s unique capacity to reverse bone loss and alter bone metabolism in postmenopausal osteoporosis model. PLoS One. 2013;8(3):e60569. doi: 10.1371/journal.pone.0060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina E, Lim YF, Marlow D, Wang Y, Clarke SL, Kuvibidila S, Smith BJ. Dietary supplementation with dried plum prevents ovariectomy-induced bone loss while modulating the immune response in C57BL/6J mice. J Nutr Biochem. 2012;23(1):60–68. doi: 10.1016/j.jnutbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ, Iii 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19(12):1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23(2):205–214. doi: 10.1359/jbmr.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98(24):13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JA, Perez-Jimenez J, Neveu V, Medina-Remon A, M’Hiri N, Garcia-Lobato P, Scalbert A. Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford) 2013;2013:bat070. doi: 10.1093/database/bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299C:152–178. [Google Scholar]
- Smith BJ, Bu SY, Wang Y, Rendina E, Lim YF, Marlow D, Lucas EA. A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone. 2014;58:151–159. doi: 10.1016/j.bone.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Graef JL, Wronski TJ, Rendina E, Williams AA, Clark KA, Halloran BP. Effects of dried plum supplementation on bone metabolism in adult C57BL/6 male mice. Calcif Tissue Int. 2014;94(4):442–453. doi: 10.1007/s00223-013-9819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA. USDA National Nutrient Database for Standard Reference. 2011 Available from USDA Nutrient Data Lab The USDA National Nutrient Database for Standard Reference Retrieved 12/07/2011, from USDA Nutrient Data Lab Beltsville Human Nutrition Research Center http://ndb.nal.usda.gov/
- Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110(11):1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting SJ, Boyle JL, Thompson A, Mirwald RL, Faulkner RA. Dietary protein, phosphorus and potassium are beneficial to bone mineral density in adult men consuming adequate dietary calcium. J Am Coll Nutr. 2002;21(5):402–409. doi: 10.1080/07315724.2002.10719242. [DOI] [PubMed] [Google Scholar]
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52(12):4026–4037. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- Zafar TA, Weaver CM, Zhao Y, Martin BR, Wastney ME. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J Nutr. 2004;134(2):399–402. doi: 10.1093/jn/134.2.399. [DOI] [PubMed] [Google Scholar]