Abstract
Objective
To evaluate sufentanil sublingual tablet 30 mcg (SST 30 mcg) for postoperative pain in an older patient population with comorbidities.
Design
Multicenter, open-label, single-arm study.
Setting
Nine hospitals across the United States.
Subjects
Adults aged ≥40 years who had undergone a surgical procedure.
Methods
Patients with a postoperative pain intensity score ≥4 on an 11-point numeric rating scale (NRS) were allowed to enter the study and receive SST 30 mcg as requested for pain (minimum 60-minute redosing interval) over the 12-hour study period. Efficacy was assessed by patient reports of pain intensity on the NRS and a five-point pain relief scale. Safety was monitored throughout the study; plasma sufentanil concentrations were also measured. The primary efficacy endpoint was the time-weighted summed pain intensity difference (SPID) to baseline over 12 hours (SPID12).
Results
Of the 140 patients enrolled, 69% were American Society of Anesthesiologists Physical Class II or III, 44% had a body mass index (BMI) ≥30 mg/kg2, and 29% had hepatic and/or renal impairment. Average age was 54.7 years (SD = 9.9 years), and average baseline pain intensity was 6.2 (SD = 1.9). The most common surgeries were abdominal (59%) and orthopedic (20%). The mean SPID12 was 36.0 (standard error of the mean = 2.2); mean scores were similar, regardless of age, sex, race, and BMI. From baseline, mean pain intensity decreased significantly starting 30 minutes postdose, and mean pain relief increased significantly starting 15 minutes postdose, remaining relatively stable through 12 hours (P < 0.001 at each time point). Four (3%) patients discontinued due to inadequate analgesia, and 45 (32%) patients had one or more adverse events that were considered possibly or probably related to the study drug. Mean plasma sufentanil concentrations were generally similar regardless of age, sex, BMI, or organ impairment status.
Conclusions
SST 30 mcg was effective and well tolerated for the management of moderate-to-severe acute postoperative pain.
Keywords: Sufentanil, Sublingual, Postoperative Pain, Opioid Analgesic, Pain Assessment, Open-Label Trial
Introduction
Despite improvements in medical techniques and the development of novel drugs to treat acute pain, postoperative pain remains clinically undermanaged [1,2]. Intravenous (IV) morphine remains a common treatment for postoperative moderate-to-severe pain control [3]. IV morphine has been consistently shown to reduce pain following a surgical procedure [4,5] and has similar efficacy compared with other opioids in providing postoperative analgesia [6–8]. However, IV morphine and other opioids with active metabolites, including hydromorphone, have the potential issue of increased side effects (respiratory depression, sedation, and delirium), due to the variability in metabolite clearance, that could potentially limit their use in certain populations including the elderly and those with renal or hepatic impairment [9–13]. Not surprisingly, data from a retrospective study of 319 898 surgeries from a national hospital database showed that patients who experienced adverse events (AEs) related to opioids had longer hospitalizations, incurred greater costs, and were more likely to be readmitted after discharge compared with patients who did not have any AEs [14].
Sufentanil is a synthetic opioid analgesic that, unlike morphine and hydromorphone, has no active (ie, clinically relevant) metabolites, and dosing does not need to be adjusted with regard to age [15], liver, or kidney function [16,17]. Compared with other opioids, earlier clinical studies suggest that sufentanil produces fewer respiratory depressive effects relative to its analgesic effects [18–21]; however, these studies only included a small number of patients. Sufentanil has recently been investigated as a 15-mcg sublingual tablet in a multidose cartridge for patient-controlled analgesia (PCA) in phase III trials for the treatment of postoperative pain [22–25], with fewer patients experiencing oxygen desaturation events than patients administering IV morphine PCA [23].
The sufentanil sublingual tablet 30 mcg (SST 30 mcg; DSUVIA, AcelRx, Redwood City, CA, USA), administered by a healthcare practitioner via a single-dose applicator, is in development for more short-term analgesia settings, such as the emergency department, ambulatory surgical settings, and for battlefield use. SST 30 mcg has the above-mentioned advantages of sufentanil while providing noninvasive pain management for acute pain. In a phase III study of patients treated with SST 30 mcg or placebo following outpatient abdominal surgery, SST 30 mcg was well tolerated and showed superior pain control compared with placebo, with reductions in pain intensity as early as 15 minutes after the start of drug dosing [22]. An earlier phase II placebo-controlled study demonstrated favorable efficacy and tolerability in patients following bunionectomy [26]. However, these studies did not report on the safety of at-risk populations, such as older patients or patients with hepatic or renal dysfunction.
The purpose of this study was to evaluate the safety and efficacy of SST 30 mcg for the management of postoperative pain in patients age ≥40 years, with an emphasis on enrolling patients with comorbidities (e.g., organ impairment, body mass index [BMI] ≥ 30 mg/kg2, hyperlipidemia, hypertension, diabetes).
Methods
This was a multicenter, single-arm, open-label trial conducted at nine investigational sites across the United States. The protocol was approved by the institutional review board for each study site, and written informed consent was obtained from all patients. This study was conducted under the International Conference on Harmonisation, Harmonised Tripartite Guideline for Good Clinical Practice (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; http://www.ich.org/home.html) and the Guidelines of the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013; http://www.wma.net/en/30publications/10policies/b3/). The study was registered with clinicaltrials.gov (NCT02662556) in January 2016.
Primary Inclusion and Exclusion Criteria
All patients were approached for study participation and consented and screened up to 30 days prior to surgery. Male and nonpregnant female patients age ≥40 years who were scheduled to undergo a surgical procedure with general anesthesia or spinal anesthesia that did not include intrathecal opioids during the operation, and who were classified as American Society of Anesthesiologists (ASA) physical class І–ІІІ [27], were eligible for inclusion.
Patients were excluded if they were opioid tolerant (>15 mg oral morphine sulfate-equivalent daily) or were dependent on supplemental oxygen at home.
Study Treatment and Rescue Medication
Before the study drug was administered, the patient must have reported a pain intensity score ≥4 on an 11-point numeric rating scale (NRS; 0 = no pain; 10 = worst possible pain) just prior to administration of the first dose of SST 30 mcg. Eligible patients received their SST 30 mcg dose sublingually by a healthcare practitioner, and following administration patients were evaluated over a 12-hour study period. The minimum re-dosing interval for SST 30 mcg was 60 minutes (up to the end of the 12-hour study period). Patients could also receive—upon request—rescue medication (1 mg IV morphine) if analgesia with the study medication was insufficient, but no sooner than 10 minutes following SST 30 mcg dosing.
Efficacy and Safety Assessments Included in the Analysis
Efficacy was assessed by patient reports of pain intensity on the NRS and a five-point categorical pain relief verbal rating scale (0 = no relief; 1 = a little relief; 2 = moderate relief; 3 = a lot of relief; 4 = complete relief) [28]. Pain intensity was initially recorded at baseline (immediately prior to dosing). Pain intensity and pain relief scores were evaluated at 15-minute intervals for the first 60 minutes, followed by every hour (from 2–12 hours) after administration of the first dose of SST 30 mcg.
The primary efficacy endpoint was the time-weighted summed pain intensity difference (SPID) to baseline over the 12-hour study period (SPID12). Secondary efficacy endpoints included total pain relief throughout the study and individual pain intensity and pain relief scores over each evaluation time point. Patient global assessments (PGAs) [29,30] and healthcare professional global assessments (HPGA) [31] were used to evaluate the overall satisfaction (indicated as “poor,” “fair,” “good,” or “excellent”), with SST 30 mcg as a pain control method. Other secondary efficacy endpoints included the percentage of patients requiring rescue medication, the total number of doses of study and rescue medication, and the proportion of patients terminated from the study due to inadequate analgesia.
Safety was monitored via periodic measurement of vital signs (i.e., respiratory rate, radial pulse rate, and blood pressure) and continuous monitoring of oxygen saturation, as well as assessment of AEs and the use of any type of concomitant medications (including those used to treat AEs). AEs—per the Medical Dictionary for Regulatory Activities v. 11.0—were defined as any illness, sign, laboratory value, or symptom that appeared or worsened during the study, regardless of causal relationship to SST 30 mg. AE severity (mild, moderate, or severe) and relationship to SST 30 mcg was assessed by the investigators. AE relatedness, described as “possibly or probably related” was defined as having drug administration and AE occurrence being reasonably related in time. Relatedness was further defined as the AE being explained equally well by causes other than SST 30 mcg (possibly related) or more likely explained by exposure to SST 30 mcg than by other mechanisms (probably related).
Respiratory rate and other vital signs could be checked at additional times on an ad hoc basis per clinical judgment. Oxygen saturation was measured continuously by pulse oximetry, but only recorded at time points when respiratory rates were measured and recorded. Patients with oxygen saturation values that could not be maintained above 95% or with respiratory rates below 8 breaths per minute were discontinued from the study. Plasma samples for determination of sufentanil concentrations were drawn 1, 4, 8, and 12 hours following the first dose of SST 30 mcg, or at the time of patient withdrawal from the study.
Classification of organ impairment was based on prestudy laboratory values. Hepatic impairment was determined by aspartate transaminase and alanine transaminase levels (normal: ≤upper limit of normal [ULN]; mild: >1–3 × ULN; moderate: >3–5 × ULN; severe: >5–20 × ULN) and total bilirubin levels (normal: ≤ULN; mild: >1–1.5 × ULN; moderate: >1.5–3 × ULN; severe: >3–10 × ULN), and classification of renal impairment was determined by creatinine levels and estimated glomerular filtration rate (eGFR; normal: ≤ULN of the creatinine test; mild: abnormally high creatinine and eGFR ≤ 60 mL/min/1.73 m2; moderate: abnormally high creatinine and eGFR 30 to < 60 mL/min/1.73 m2; severe: abnormally high creatinine and eGFR <30 mL/min/1.73 m2).
Statistical Analysis
A sample size of approximately 150 patients was planned for this analysis. All enrolled patients were those who were enrolled and received treatment. For patients who used any rescue medication during the study period, the last observed pain intensity score prior to using each dose of rescue medication was carried throughout a follow-up one-hour time interval. Any pain intensity data collected one hour or less after the start of any rescue medication was excluded from the derivation of the efficacy endpoint (SPID).
All enrolled patients were included in the descriptive summaries of efficacy and safety data. The pain assessment data, pain intensity and pain relief, were considered as continuous measurements. A two-sided paired t test was used for the test of pain intensity difference (i.e., change in pain intensity from baseline) and pain relief at follow-up time points within the group. All tests were performed at a significance level of α = 0.05.
Results
Baseline Demographics and Patient Disposition
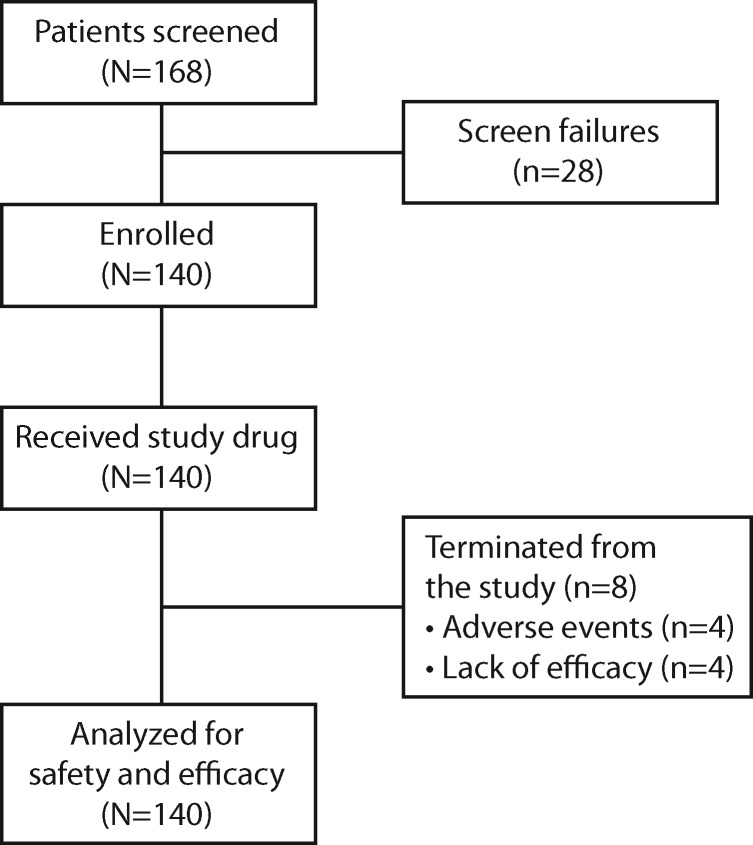
The first patient enrolled March 4, 2016, and the final patient completed the study June 22, 2016. A total of 140 patients were enrolled and received study drug. All were included in the ITT population and efficacy and safety analyses (Figure 1). One-hundred and thirty-two (94%) patients completed the 12-hour study period, and eight (6%) patients prematurely terminated the study. Reasons for early termination were AEs (four patients, 3%) and lack of efficacy (four patients, 3%). Overall, 40 (29%) patients had prestudy renal and/or hepatic impairment (renal: N = 10; hepatic: N = 37; renal + hepatic: N = 7), and the average baseline pain intensity was 6.2 (on a scale from 0–10) (Table 1). Before the end of the 12-hour study period, four (3%) patients discontinued the study due to inadequate analgesia.
Figure 1.
Patient disposition.
Table 1.
Patient demographics and baseline characteristics (ITT population)
| Characteristic | N = 140 |
|---|---|
| Age, No. (%), y | |
| <65 | 116 (83) |
| ≥65 | 24 (17) |
| Age, mean (SD) | 54.7 (9.9) |
| Sex, No. (%) | |
| Female | 75 (54) |
| Male | 65 (46) |
| Race, No. (%) | |
| White | 117 (84) |
| Black or African American | 20 (14) |
| Asian | 2 (1) |
| American Indian or Alaska Native | 1 (1) |
| Ethnicity, No. (%) | |
| Hispanic or Latino | 22 (16) |
| BMI, No. (%), kg/m2 | |
| <30 | 78 (56) |
| ≥30 | 62 (44) |
| BMI, mean (SD), kg/m2 | 30.0 (6.8) |
| ASA classification,* No. (%) | |
| I | 44 (31) |
| II | 73 (52) |
| III | 23 (16) |
| Type of surgery, No. (%) | |
| Laparoscopic abdominal | 58 (41) |
| Open abdominal | 25 (18) |
| Breast augmentation/reconstruction | 23 (16) |
| Knee replacement | 12 (9) |
| Bunionectomy | 10 (7) |
| Hip replacement | 6 (4) |
| Other† | 6 (4) |
| Hepatic function, No. (%) | |
| Normal | 103 (74) |
| Impaired, by severity | |
| Mild | 34 (24) |
| Moderate | 2 (1) |
| Severe | 1 (1) |
| Renal function, No. (%) | |
| Normal | 130 (93) |
| Impaired, by severity | |
| Mild | 5 (4) |
| Moderate | 5 (4) |
| Severe | 0 (0) |
| Hypertension | 47 (34) |
| Diabetes | 22 (16) |
| Hyperlipidemia | 21 (15) |
BMI = body mass index; ITT = intent-to-treat.
American Society of Anesthesiologists (ASA) physical status classification (https://www.asahq.org/quality-and-practice-management/standards-and-guidelines/): 1 = normal healthy; 2 = mild systemic disease, no functional limitation (e.g., smoker with well-controlled hypertension); 3 = severe systemic disease, definite functional impairment (e.g., diabetes and angina with relatively stable disease, but requiring therapy).
Other surgeries (N = 1 each) included bronchoscopy (uniportal right vats completion, right upper lobectomy), osteotomy, parathyroidectomy, right anterior mini-thoracotomy with excision of pericardial phrenic nodules, right thoracotomy (right upper lobe wedge resection, right lower lobe wedge resection), and spinal laminectomy.
Study Drug Doses
Over the 12-hour study period, the mean total number of study drug doses used for all patients was 3.3 (SD = 1.8) and was similar, regardless of hepatic or renal function (Table 2). The mean interdosing interval for all patients was 193.2 (SD = 106.6) minutes.
Table 2.
Total SST 30 mcg doses used over 12 hours
| Patient Subgroup | Number of Patients | Total Number of Doses |
|
|---|---|---|---|
| Mean (SD) | Median (min, max) | ||
| All patients | 140 | 3.3 (1.8) | 3.0 (1, 8) |
| Hepatic function | |||
| Normal | 103 | 3.3 (1.9) | 3.0 (1, 8) |
| Impaired, by severity | |||
| Mild | 34 | 3.6 (1.9) | 3.0 (1, 8) |
| Moderate | 2 | 3.0 (0.0) | 3.0 (3, 3) |
| Severe | 1 | 3.0 (NA) | 3.0 (3, 3) |
| Renal function | |||
| Normal | 130 | 3.3 (1.8) | 3.0 (1, 8) |
| Impaired, by severity | |||
| Mild | 5 | 4.2 (2.3) | 4.0 (2, 8) |
| Moderate | 5 | 3.4 (1.1) | 3.0 (2, 5) |
max = maximum; min = minimum; NA = not applicable; SST 30 mcg = sufentanil sublingual tablet 30 mcg.
Rescue Medication
Overall, 20 (14%) patients required rescue medication due to inadequate analgesia. The median (range) time to take the first dose of rescue medication due to inadequate analgesia for those patients was 36.5 (12–650) minutes. Thirteen of the 20 patients required a single dose of rescue medication, three patients required two rescue doses, and four patients required three or more rescue doses.
Efficacy
SPID12 scores (primary endpoint), a cumulative measure of pain intensity over the duration of the study, were assessed to analyze pain control. The mean SPID12 for all patients was 36.0 (standard error of the mean = 2.2; 95% confidence interval [CI] = 31.7–40.4). When analyzed by patient subgroup for age, sex, race, and BMI, the mean SPID12 scores were similar (overlapping CIs) for patients age ≥65 years vs < 65 years, males vs females, Caucasian patients vs non-Caucasian patients, and patients with a BMI ≥ 30 kg/m2 vs < 30 kg/m2 (Table 3).
Table 3.
SPID at 12 hours (ITT population)
| Baseline Pain Intensity |
SPID12 |
||||
|---|---|---|---|---|---|
| Patients, No. | Mean (SEM) | Patients, No. | Mean (SEM) | 95% CI | |
| Age, y | |||||
| <65 | 115 | 6.3 (0.2) | 109 | 37.2 (2.5) | 32.2–42.2 |
| ≥65 | 22 | 5.8 (0.4) | 18 | 29.1 (3.54) | 21.7–36.6 |
| Sex | |||||
| Male | 63 | 6.2 (0.3) | 63 | 35.3 (3.2) | 28.9–41.8 |
| Female | 74 | 6.2 (0.2) | 64 | 36.7 (3.1) | 30.6–42.9 |
| Race | |||||
| Caucasian | 93 | 6.0 (0.2) | 86 | 32.4 (2.5) | 27.5–37.3 |
| Non-Caucasian | 44 | 7.0 (0.3) | 41 | 43.7 (4.3) | 34.9–52.5 |
| BMI, kg/m2 | |||||
| <30 | 76 | 6.3 (0.2) | 71 | 38.1 (3.0) | 32.0–44.1 |
| ≥30 | 61 | 6.1 (0.2) | 56 | 33.5 (3.2) | 27.0–39.9 |
| Total | 137 | 6.2 (0.2) | 127 | 36.0 (2.2) | 31.7–40.4 |
BMI = body mass index; CI = confidence interval; ITT = intent-to-treat; SEM = standard error of the mean; SPID12 = time-weighted summed pain intensity difference at 12 hours.
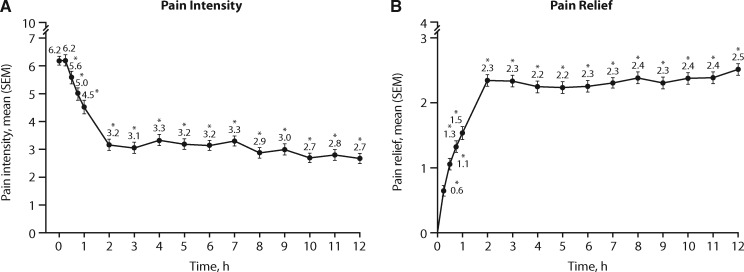
Mean pain intensity significantly decreased from baseline starting at 30 minutes post–treatment initiation through the first two hours (from mean = 6.2, SEM = 0.16, to mean = 3.2, SEM = 0.20, on a scale from 0–10) and remained relatively stable for the duration of the 12-hour study period (P < 0.001 at each time point) (Figure 2A). Mean pain relief also significantly improved from baseline, starting as early as 15 minutes postdose, from a mean of 0.6 (SEM = 0.08; on a scale of 0–4) at 15 minutes to a mean of 2.3 (SEM = 0.09) at two hours, and then remained relatively stable (from 2.3–2.5) for the remainder of the 12-hour study period (P < 0.001 at each time point) (Figure 2B).
Figure 2.
Mean pain intensity and pain relief over 12 hours of treatment (ITT population). *Difference from baseline (P < 0.001). ITT = intent-to-treat; PI = pain intensity; PR = pain relief; SEM = standard error of the mean.
Patient and HealthCare Professional Global Assessments of Methods of Pain Control
In the global assessments of method of pain control, 53%, 35%, 7%, and 5% of 135 patients (PGA) and 50%, 40%, 8%, and 2% of 123 healthcare professionals (HPGA), responded “excellent,” “good,” “fair,” or “poor,” respectively, at the 12-hour time point.
Safety
Adverse Events
Over the 12-hour study period, the majority (63%, 88/140) of patients did not incur an AE. Forty-five (32%) patients had one or more AEs considered possibly or probably related to study drug. The most frequently reported AEs overall were nausea (27%), headache (6%), and dizziness (4%) (Table 4). Oxygen saturation levels were decreased in three (2.1%) patients, and respiratory rate was decreased in one (0.7%) patient. Psychiatric disorders (i.e., confusional state, euphoria, and nervousness) and memory impairment considered related to study drug were reported infrequently (0.7% for each AE). One patient had a serious AE of acute stroke that was not considered related to treatment as it occurred more than 12 hours after discontinuation. Four patients had AEs that led to discontinuation of SST 30 mcg; these were mild oxygen saturation decrease (two patients < 95%), mild pruritus (one patient), and mild dizziness (one patient).
Table 4.
Summary of AEs and related AEs
| Patients | Hepatic Function, No. (%) |
Renal Function,* No. (%) |
Total AEs, No. (%)(N = 140) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal (n = 103) | Impaired |
Normal (n = 130) | Impaired |
|||||||
| Mild (n = 34) | Moderate (n = 2) | Severe (n = 1) | All (N = 37) | Mild (n = 5) | Moderate (n = 5) | All (N = 10) | ||||
| Number of AEs | ||||||||||
| None | 65 (63) | 20 (59) | 2 (100) | 1 (100) | 23 (62) | 81 (62) | 4 (80) | 3 (60) | 7 (70) | 88 (63) |
| ≥1 | 38 (37) | 14 (41) | 0 | 0 | 14 (38) | 49 (38) | 1 (20) | 2 (40) | 3 (30) | 52 (37) |
| All AEs (≥2% of all patients) | ||||||||||
| Nausea | 25 (24) | 13 (38) | 0 | 0 | 13 (35) | 35 (27) | 1 (20) | 2 (40) | 3 (30) | 38 (27) |
| Headache | 5 (5) | 3 (9) | 0 | 0 | 3 (8) | 6 (5) | 0 | 2 (40) | 2 (20) | 8 (6) |
| Dizziness | 4 (4) | 2 (6) | 0 | 0 | 2 (5) | 5 (4) | 0 | 1 (20) | 1 (10) | 6 (4) |
| Pruritus | 4 (4) | 0 | 0 | 0 | 0 | 4 (3) | 0 | 0 | 0 | 4 (3) |
| Hypotension | 2 (2) | 1 (3) | 0 | 0 | 1 (3) | 3 (2) | 0 | 0 | 0 | 3 (2) |
| Oxygen saturation decreased | 2 (2) | 1 (3) | 0 | 0 | 1 (3) | 2 (2) | 1 (20) | 0 | 1 (10) | 3 (2) |
| AEs related to SST 30 mcg (≥2% of all patients) | ||||||||||
| Nausea | 25 (24) | 13 (38) | 0 | 0 | 13 (35) | 35 (27) | 1 (20) | 2 (40) | 3 (30) | 38 (27) |
| Dizziness | 4 (4) | 2 (6) | 0 | 0 | 2 (5) | 5 (4) | 0 | 1 (20) | 1(10) | 6 (4) |
| Headache | 1 (1) | 3 (9) | 0 | 0 | 2 (4) | 0 | 2 (40) | 4 (3) | ||
| Pruritus | 4 (4) | 0 | 0 | 0 | 0 | 4 (3) | 0 | 0 | 0 | 4 (3) |
| Oxygen saturation decreased | 2 (2) | 1 (3) | 0 | 0 | 1 (3) | 2 (2) | 1 (20) | 0 | 1 (20) | 3 (2) |
AE = adverse event; SST 30 mcg = sufentanil sublingual tablet 30 mcg.
Data in patients with renal impairment should be interpreted with caution due to low number of patients (N = 10) in this subgroup.
Overall, 37% (38/103) of patients with normal hepatic function and 38% (14/37) of patients with impaired hepatic function had one or more AEs (see Table 4). Similarly, 38% (49/130) of patients with normal renal function and 30% (3/10) of patients with impaired renal function had one or more AEs. The number and types of AEs were similar for patients with normal or impaired renal or hepatic function. Data in patients with renal impairment should be interpreted with caution due to the small number of patients (N = 10) in this subgroup.
There were no clinically meaningful changes in mean vital signs, and no patient required naloxone during the 12-hour study period.
Plasma Sufentanil Concentration
The mean plasma sufentanil concentrations for all patients were 37.2 (SD = 22.5), 44.1 (SD = 35.1), 35.7 (SD = 27.7), and 30.4 (SD = 28.5) pg/mL at 1, 4, 8, and 12 hours, respectively, and they were generally similar at the beginning and end of the study, regardless of patient age (≥65 vs < 65 years), sex, BMI (<30 kg/m2 vs ≥ 30 kg/m2), or organ impairment (Table 5). At all evaluation time points (data not shown), plasma sufentanil concentrations were similar for patients with normal hepatic and/or renal function (N = 103) and those with mild (N = 34) or moderate (N = 2) hepatic impairment or mild (N = 5) or moderate (N = 5) renal impairment.
Table 5.
Sufentanil plasma concentrations, by subgroup
| 1 h |
12 h |
|||
|---|---|---|---|---|
| Patients, No. | Mean (SD) pg/mL | Patients, No. | Mean (SD) pg/mL | |
| Age, y | ||||
| <65 | 110 | 35.1 (20.5) | 110 | 27.1 (24.6) |
| ≥65 | 24 | 47.1 (28.4) | 20 | 48.3 (40.6) |
| Sex | ||||
| Male | 62 | 37.0 (19.4) | 65 | 29.8 (26.5) |
| Female | 72 | 37.4 (25.0) | 65 | 30.9 (30.5) |
| BMI, kg/m2 | ||||
| <30 | 75 | 36.4 (23.3) | 73 | 29.8 (30.1) |
| ≥30 | 59 | 38.3 (21.6) | 57 | 31.1 (26.5) |
| Hepatic function* | ||||
| Normal | 98 | 37.7 (22.9) | 96 | 29.4 (28.4) |
| Impaired, by severity | ||||
| Mild | 33 | 35.9 (22.3) | 32 | 33.3 (30.5) |
| Moderate | 2 | 33.6 (15.5) | 2 | 26.4 (4.4) |
| Severe | 1 | 42.4 (NA) | 0 | NA |
| Renal function† | ||||
| Normal | 124 | 37.0 (22.6) | 120 | 29.4 (28.5) |
| Impaired, by severity | ||||
| Mild | 5 | 42.1 (11.3) | 5 | 46.9 (36.2) |
| Moderate | 5 | 36.5 (30.1) | 5 | 35.9 (16.1) |
| Total | 134 | 37.2 (22.5) | 130 | 30.4 (28.5) |
BMI = body mass index.
The mean number of study drug doses used was similar for patients with normal hepatic function (3.3, SD = 1.9) and those with mild (3.6, SD = 1.9), moderate (3.0, SD = 0.0), or severe (3.0, SD = not applicable) hepatic impairment.
The mean number of study drug doses used was similar for patients with normal renal function (3.3, SD = 1.8) and those with mild (4.2, SD = 2.3) or moderate (3.4, SD = 1.1) renal impairment.
Discussion
SST 30 mcg was effective for the management of moderate-to-severe postoperative pain in patients who had undergone a wide variety of surgery types. Most commonly, these included abdominal (e.g., laparoscopic or open-abdominal) and orthopedic (e.g., knee or hip replacement, bunionectomy) procedures. Statistical improvements in pain control were observed within 15 to 30 minutes, with increases observed up until two hours, and maintained through the remainder of the 12-hour study period. Clinically relevant changes in pain intensity in the acute postoperative setting have been shown to be a decrease of 1.3 on the 11-point NRS or a 20% decrease from baseline pain intensity [32]. This occurred at approximately the 30-minute time point in this study. Clinically relevant acute postoperative pain relief scores have not been adequately validated in trials.
Mean SPID12 scores demonstrated that there were no clinically meaningful differences in pain control due to age, sex, race, or BMI. When considering the global assessment of SST 30 mcg as a method of pain control, more than 85% of patients and 90% of healthcare providers responded favorably (i.e., good or excellent).
The first plasma sample was drawn 60 minutes after the first SST 30 mcg dose had been administered, thereby reflecting plasma concentrations achieved with a single dose. The mean sufentanil plasma concentrations at the three other time points were similar to that of the first dose, suggesting that analgesic sufentanil concentrations are achieved with the first dose and, on average, patients are redosing to maintain these plasma drug concentrations. Sufentanil plasma concentrations were similar between subgroups (age, sex, and BMI) and between patients with and without hepatic or renal impairment, as compared between the one-hour and 12-hour time points. A previous phase III study by Melson and colleagues compared patient-administered sublingual sufentanil 15 mcg with patient-controlled IV morphine and found no difference in sufentanil concentrations in patients with or without renal or hepatic impairment at 24 or 48 hours [23]. In contrast, patients with renal impairment had significantly increased concentrations of active metabolites of morphine (morphine-3-glucuronide and morphine-6-glucuronide) compared with patients with normal renal functioning at 24 (P < 0.01) and 48 (P < 0.01) hours.
Active metabolites of morphine, as well as hydromorphone [33], can contribute to opioid-related AEs, such as nausea, sleep disturbance, neuroexcitation, cognitive impairment, dysphoria, and agitation [34–44]. For example, nausea has been reported in up to 68% and 64% [34,44] of patients and sleep disturbance in up to 82% and 73% of patients [44,45] treated with morphine and hydromorphone, respectively, for postoperative acute pain [44]. Delayed penetration of active glucuronide metabolites into the CNS (equilibration half-life between the plasma and CNS [t1/2ke0] is more than six hours for M6G, more than twice as long as the t1/2ke0 of its parent compound) [46,47], as well as the relatively slow equilibration for morphine (t1/2ke0 = 168 minutes) [47] and hydromorphone (t1/2ke0 = 46 minutes) [48], creates a high likelihood of “dose stacking” and delayed AEs. In comparison, drug equilibration for the lipophilic opioids fentanyl and sufentanil is relatively rapid, with a median t1/2ke0 of 6.6 and 6.2 minutes [49], respectively, allowing for faster onset of analgesia and less risk of dose-stacking, and eliminating concern for delayed effects of active metabolites.
The types of AEs reported in this study were generally as expected in this type of patient population in a postoperative setting. Overall, approximately one-third of patients experienced at least one AE, with nausea being the most common. Whereas IV morphine and other similar opioids require dose adjustments and careful monitoring of patients with hepatic or renal impairment due to decreased drug clearance [9,12], no dose adjustment was necessary within our small sample size, and AEs were similar between patients with normal or impaired hepatic function or between those with normal or impaired renal function.
This was an open-label, single-arm study, and therefore results must be interpreted with caution. In a previous placebo-controlled study of younger patients recovering from abdominal surgery, SST 30 mcg was well tolerated [22], with similar safety results between the active and placebo treatment groups, as well as similar rates of AEs, as observed in the current study. Results of the renal impairment subgroup in the current study should be interpreted with caution due to the low number of patients (N = 10) with renal impairment in this study.
The results of this study demonstrate that the sufentanil sublingual tablet 30 mcg was effective, with onset of analgesia within 15 to 30 minutes postdose, and that it was well tolerated for the management of moderate to severe acute pain in postoperative patients, the majority of whom had comorbidities prior to surgery.
Author Contributions
All authors analyzed and interpreted the data, drafted or revised the manuscript for important intellectual content, and provided approval of the final version. In addition, PPP and KPD were involved in data acquisition, and PPP was involved in study concept and design.
Acknowledgments
The authors would like to thank Melissa Cohen (research assistant to JLH) at the University of Minnesota, Minneapolis, Minnesota, for aiding in patient recruitment and data collection. The authors would also like to thank Pam Lindley (research nurse to HM) and the research team at Research Concepts GP, LLC, Bellaire, Texas, for their assistance in executing the study. Editorial assistance was provided by Penny Baron and Robert Steger (BlueMomentum, a division of Ashfield Healthcare Communications, a UDG Healthcare plc company), Burlingame, California, and funded by AcelRx Pharmaceuticals.
Funding sources: This study was funded by AcelRx Pharmaceuticals and by the Clinical and Rehabilitative Medicine Research Program (CRMRP) of the US Army Medical Research and Materiel Command (USAMRMC) under contract No. W81XWH-15-C-0046. The CRMRP was established in 2008 to foster research and technology advances for the regeneration, restoration, and rehabilitation of traumatic injuries. In accordance with USAMRMC guidelines, in the conduct of clinical research, AcelRx has adhered to the policies regarding the protection of human subjects as prescribed by Code of Federal Regulations (CFR) Title 45, Volume 1, Part 46; Title 32, Chapter 1, Part 219; and Title 21, Chapter 1, Part 50 (Protection of Human Subjects).
Disclosures and conflicts of interest: JH, DL, HM, and MJ were investigators of this study; their institutions received funding for their participation in the trial. JH is also a consultant, speaker, and has received research funding from Pacira Pharmaceuticals. He also owns stock options in Insitu Biologics. HM is also a consultant to AcelRx. KPD is an employee and has stock ownership of AcelRx Pharmaceuticals. PPP is an employee and has stock ownership of AcelRx Pharmaceuticals.
References
- 1. Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL.. Incidence, patient satisfaction, and perceptions of post-surgical pain: Results from a US national survey. Curr Med Res Opin 2014;30:149–60. [DOI] [PubMed] [Google Scholar]
- 2. Correll DJ, Vlassakov KV, Kissin I.. No evidence of real progress in treatment of acute pain, 1993–2012: Scientometric analysis. J Pain Res 2014;7:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garimella V, Cellini C.. Postoperative pain control. Clin Colon Rectal Surg 2013;26:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aubrun F, Monsel S, Langeron O, Coriat P, Riou B.. Postoperative titration of intravenous morphine. Eur J Anaesthesiol 2001;18:159–65. [DOI] [PubMed] [Google Scholar]
- 5. Tveita T, Thoner J, Klepstad P, et al. A controlled comparison between single doses of intravenous and intramuscular morphine with respect to analgesic effects and patient safety. Acta Anaesthesiol Scand 2008;52:920–5. [DOI] [PubMed] [Google Scholar]
- 6. Silvasti M, Rosenberg P, Seppälä T, Svartling N, Pitkänen M.. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient‐controlled analgesia. Acta Anaesthesiol Scand 1998;42:576–80. [DOI] [PubMed] [Google Scholar]
- 7. Hippard HK, Govindan K, Friedman EM, et al. Postoperative analgesic and behavioral effects of intranasal fentanyl, intravenous morphine, and intramuscular morphine in pediatric patients undergoing bilateral myringotomy and placement of ventilating tubes. Anesth Analg 2012;115:356–63. [DOI] [PubMed] [Google Scholar]
- 8. Zeng Z, Lu J, Shu C, et al. A comparison of nalbuphine with morphine for analgesic effects and safety: Meta-analysis of randomized controlled trials. Sci Rep 2015;5:10927.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith HS. Opioid metabolism. Mayo Clin Proc 2009;84:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Honneur G, Gilton A, Sandouk P, Scherrmann JM, Duvaldestin P.. Plasma and cerebrospinal fluid concentrations of morphine and morphine glucuronides after oral morphine. The influence of renal failure. Anesthesiology 1994;81:87–93. [DOI] [PubMed] [Google Scholar]
- 11. Wolff J, Bigler D, Christensen CB, et al. Influence of renal function on the elimination of morphine and morphine glucuronides. Eur J Clin Pharmacol 1988;34:353–7. [DOI] [PubMed] [Google Scholar]
- 12. Morphine Sulfate Injection [package insert]. Lake Forest, IL: Hospira, Inc.; 2011. [Google Scholar]
- 13. Hagen N, Foley K, Cerbone D, Portenoy R, Inturrisi C.. Chronic nausea and morphine-6-glucuronide. J Pain Symptom Manage 1991;6:125–8. [DOI] [PubMed] [Google Scholar]
- 14. Oderda GM, Gan TJ, Johnson BH, Robinson SB.. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Pharmacother 2013;27:62–70. [DOI] [PubMed] [Google Scholar]
- 15. Matteo RS, Schwartz AE, Ornstein E, Young WL, Chang W.. Pharmacokinetics of sufentanil in the elderly surgical patient. Can J Anaesth 1990;37:852–6. [DOI] [PubMed] [Google Scholar]
- 16. Fyman PN, Reynolds JR, Moser DF, et al. Pharmacokinetics of sufentanil in patients undergoing renal transplantation. Can J Anaesth 1988;35:312–5. [DOI] [PubMed] [Google Scholar]
- 17. Chauvin M, Ferrier C, Haberer JP, et al. Sufentanil pharmacokinetics in patients with cirrhosis. Anesth Analg 1989;68:1–4. [PubMed] [Google Scholar]
- 18. Clark N, Meuleman T, Liu W, et al. Comparison of sufentanil-N2O and fentanyl-N2O in patients without cardiac disease undergoing general surgery. Anesthesiology 1987;66:130–5. [DOI] [PubMed] [Google Scholar]
- 19. Ved SA, Dubois M, Carron H, Lea D.. Sufentanil and alfentanil pattern of consumption during patient-controlled analgesia: A comparison with morphine. Clin J Pain 1989;5(suppl 1):S63–70. [DOI] [PubMed] [Google Scholar]
- 20. Bailey PL, Streisand JB, East KA, et al. Differences in magnitude and duration of opioid induced respiratory depression and analgesia with fentanyl and sufentanil. Anesth Analg 1990;70:8-15.. [DOI] [PubMed] [Google Scholar]
- 21. Conti G, Arcangeli A, Antonelli M, et al. Sedation with sufentanil in patients receiving pressure support ventilation has no effects on respiration: A pilot study. Can J Anesth 2004;51:494–9. [DOI] [PubMed] [Google Scholar]
- 22. Minkowitz HS, Leiman D, Melson T, et al. Sufentanil sublingual tablet 30 mcg for the management of pain following abdominal surgery: A randomized, placebo‐controlled, phase‐3 study. Pain Pract. 2017;17:848–58. [DOI] [PubMed] [Google Scholar]
- 23. Melson TI, Boyer DL, Minkowitz HS, et al. Sufentanil sublingual tablet system vs. intravenous patient‐controlled analgesia with morphine for postoperative pain control: A randomized, active‐comparator trial. Pain Pract 2014;14:679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jove M, Griffin DW, Minkowitz HS, et al. Sufentanil sublingual tablet system for the management of postoperative pain after knee or hip arthroplasty: A randomized, placebo-controlled study. Anesthesiology 2015;123:434–43. [DOI] [PubMed] [Google Scholar]
- 25. Ringold FG, Minkowitz HS, Gan TJ, et al. Sufentanil sublingual tablet system for the management of postoperative pain following open abdominal surgery: A randomized, placebo-controlled study. Reg Anesth Pain Med 2015;40:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singla NK, Muse DD, Evashenk MA, Palmer PP.. A dose-finding study of sufentanil sublingual microtablets for the management of postoperative bunionectomy pain. J Trauma Acute Care Surg 2014;77:S198–203. [DOI] [PubMed] [Google Scholar]
- 27. American Society of Anesthesiologists. ASA physician status classification system. 2014. Available at: https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system (accessed October 2017).
- 28. Jensen MP, Karoly P, Braver S.. The measurement of clinical pain intensity: A comparison of six methods. Pain 1986;27:117–26. [DOI] [PubMed] [Google Scholar]
- 29. Rothman M, Vallow S, Damaraju CV, Hewitt DJ.. Using the patient global assessment of the method of pain control to assess new analgesic modalities in clinical trials. Curr Med Res Opin 2009;25:1433–43. [DOI] [PubMed] [Google Scholar]
- 30. Kamper SJ, Maher CG, Mackay G.. Global rating of change scales: A review of strengths and weaknesses and considerations for design. J Man Manip Ther 2009;17:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 1993;36:729–40. [DOI] [PubMed] [Google Scholar]
- 32. Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB.. What decline in pain intensity is meaningful to patients with acute pain? Pain 2003;105: 151–7. [DOI] [PubMed] [Google Scholar]
- 33. Smith HS. The metabolism of opioid agents and the clinical impact of their active metabolites. Clin J Pain 2011;27:824–38. [DOI] [PubMed] [Google Scholar]
- 34. Hong D, Flood P, Diaz G.. The side effects of morphine and hydromorphone patient-controlled analgesia. Anesth Analg 2008;107:1384–9. [DOI] [PubMed] [Google Scholar]
- 35. Murray A, Hagen NA.. Hydromorphone. J Pain Symptom Manage 2005;29(5 suppl):S57–66. [DOI] [PubMed] [Google Scholar]
- 36. Sear JW, Hand CW, Moore RA, McQuay HJ.. Studies on morphine disposition: Influence of renal failure on the kinetics of morphine and its metabolites. Br J Anaesth 1989;62:28–32. [DOI] [PubMed] [Google Scholar]
- 37. Smith MT. Neuroexcitatory effects of morphine and hydromorphone: Evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol 2000;27:524–8. [DOI] [PubMed] [Google Scholar]
- 38. Wright AW, Mather LE, Smith MT.. Hydromorphone-3-glucuronide: A more potent neuro-excitant than its structural analogue, morphine-3-glucuronide. Life Sci 2001;69:409–20. [DOI] [PubMed] [Google Scholar]
- 39. Ratka A, Wittwer E, Baker L, Kern S.. Pharmacokinetics of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in healthy older men and women. Am J Pain Manag 2004;14:45–55. [Google Scholar]
- 40. Dolin SJ, Cashman JN.. Tolerability of acute postoperative pain management: Nausea, vomiting, sedation, pruritis, and urinary retention. Evidence from published data. Br J Anaesth 2005;95: 584–91. [DOI] [PubMed] [Google Scholar]
- 41. Ashby M, Fleming B, Wood M, Somogyi A.. Plasma morphine and glucuronide (M3G and M6G) concentrations in hospice inpatients. J Pain Symptom Manage 1997;14:157–67. [DOI] [PubMed] [Google Scholar]
- 42. Paramanandam G, Prommer E, Schwenke DC.. Adverse effects in hospice patients with chronic kidney disease receiving hydromorphone. J Palliat Med 2011;14:1029–33. [DOI] [PubMed] [Google Scholar]
- 43. Klimas R, Mikus G.. Morphine-6-glucuronide is responsible for the analgesic effect after morphine administration: A quantitative review of morphine, morphine-6-glucuronide, and morphine-3-glucuronide. Br J Anaesth 2014;113:935–44. [DOI] [PubMed] [Google Scholar]
- 44. Rapp SE, Egan KJ, Ross BK, et al. A multidimensional comparison of morphine and hydromorphone patient-controlled analgesia. Anesth Analg 1996;82: 1043–8. [DOI] [PubMed] [Google Scholar]
- 45. Mahler DL, Forrest WH Jr.. Relative analgesic potencies of morphine and hydromorphone in postoperative pain. Anesthesiology 1975;42:602–7. [DOI] [PubMed] [Google Scholar]
- 46. Lötsch J, Skarke C, Schmidt H, Grösch S, Geisslinger G.. The transfer half-life of morphine-6-glucuronide from plasma to effect site assessed by pupil size measurement in healthy volunteers. Anesthesiology 2001;95:1329–38. [DOI] [PubMed] [Google Scholar]
- 47. Lötsch J. Pharmacokinetic-pharmacodynamic modeling of opioids. J Pain Symptom Manage 2005;29(5 suppl):S90–S103. [DOI] [PubMed] [Google Scholar]
- 48. Shafer SL, Flood PD.. The pharmacology of opioids. In: Silverstein JH, Rooke A, Reves JG, McLeskey CH, eds. Geriatric Anesthesiology. 2nd edition.New York: Springer Science & Business Media; 2007:209–28. [Google Scholar]
- 49. Scott JC, Cooke JE, Stanski DR.. Electroencephalographic quantitation of opioid effect: Comparative pharmacodynamics of fentanyl and sufentanil. Anesthesiology 1991;74:34–42. [DOI] [PubMed] [Google Scholar]