Abstract
Osteocytes support dynamic, cell-intrinsic resorption and deposition of bone matrix through a process called perilacunar/canalicular remodeling (PLR). In long bones, PLR depends on MMP13 and is tightly regulated by PTH, sclerostin, TGFβ, and glucocorticoids. However, PLR is regulated differently in the cochlea, suggesting a mechanism that is anatomically distinct. Unlike long bones, the mandible derives from neural crest and exhibits unique susceptibility to medication and radiation induced osteonecrosis. Therefore, we sought to determine if PLR in the mandible is suppressed by glucocorticoids, as it is in long bone. Hemimandibles were collected from mice subcutaneously implanted with prednisolone or vehicle containing pellets for 7, 21, or 55 days (n = 8/group) for radiographic and histological analyses. Within 21 days, micro-computed tomography revealed a glucocorticoid-dependent reduction in bone volume/total volume and trabecular thickness and a significant decrease in bone mineral density after 55 days. Within 7 days, glucocorticoids strongly and persistently repressed osteocytic expression of the key PLR enzyme MMP13 in both trabecular and cortical bone of the mandible. Cathepsin K expression was significantly reduced only after 55 days of glucocorticoid treatment, at which point histological analysis revealed a glucocorticoid-dependent reduction in the lacunocanalicular surface area. In addition to reducing bone mass and suppressing PLR, glucocorticoids also reduced the stiffness of mandibular bone in flexural tests. Thus, osteocyte PLR in the neural crest-derived mandible is susceptible to glucocorticoids, just as it is in the mesodermally-derived femur, highlighting the need to further study PLR as a target of drugs, and radiation in mandibular osteonecrosis.
Keywords: Osteocyte, Perilacunar/canalicular remodeling, Mandible, Glucocorticoids
1. Introduction
Despite the common features of bone throughout the skeleton, bones retain several features that are anatomically distinct, including embryonic derivation, geometry, microarchitecture, and material properties. For example, the femur is mesodermally-derived and has robust cortical bone, whereas the mandible is derived of neural crest and is rich in trabecular bone. While such differences allow bones to meet their unique mechanical demands, different skeletal sites exhibit susceptibility to different clinical problems. Though considerable effort has focused on the restoration of bone mass in the femur or vertebra, many questions remain about the best therapies to treat craniofacial manifestations of skeletal disease such as osteonecrosis of the jaw following radiation therapy, a major unmet clinical need (Cha et al., 2017). Therefore, we seek to examine the role of osteocyte-mediated perilacunar/canalicular remodeling in the mandible.
Bone embedded osteocytes orchestrate bone remodeling by osteoblasts and osteoclasts, and also execute the remodeling of bone matrix directly through a process called perilacunar/canalicular remodeling (PLR). In PLR, osteocytes secrete acid and enzymes, including MMP-2, MMP-13, MMP-14, and cathepsin K (Tang et al., 2012; Inoue et al., 2006; Holmbeck et al., 2005), to resorb bone matrix in the perilacunar and pericanalicular microenvironments. PLR is activated during metabolic stress to increase circulatory levels of calcium and phosphate (JAMA, 1968; Bélanger et al., 1968; Marie and Glorieux, 1983; Zallone and Mueller, 1969; McGee-Lawrence et al., 2011), and is a fundamental process in bone homeostasis. Suppression of PLR, through ablation of PLR enzymes or in skeletal disease, causes severe degeneration of the lacunocanalicular network and compromises bone quality (Tang et al., 2012; Inoue et al., 2006; Holmbeck et al., 2005; Alliston, 2014; Dole et al., 2017; Fowler et al., 2017; Kogawa et al., 2013). Through mechanisms that remain unclear, PLR helps to maintain collagen organization and calibrates the mineralization of bone extracellular matrix. Consequently, disruption of the PLR leads to increased bone fragility (Tang et al., 2012; Dole et al., 2017).
PLR is tightly regulated by several factors, including parathyroid hormone, sclerostin, glucocorticoids, vitamin D, and TGFβ (Dole et al., 2017; Fowler et al., 2017; Kogawa et al., 2013; Qing et al., 2012; Rolvien et al., 2017). In addition, PLR is spatially regulated, such that it varies with anatomic location (Inoue et al., 2006). For example, Jauregui, et al., demonstrated that cochlear bone uniquely maintains bone quality and hearing independently of MMP-13-mediated PLR, which is critical for femoral bone quality (Jáuregui et al., 2016). Because of the mandible's unique derivation from the neural crest cells of the branchial arches rather than mesodermal origin, the question is raised as to whether PLR is differentially regulated in the mandible compared to mesodermally-derived long bones.
Among the many effects of glucocorticoids on bone, they are known to suppress PLR in trabecular and cortical bone of the femur, which likely plays a causal role in glucocorticoid-induced osteonecrosis of the femoral head (Fowler et al., 2017). In the mandible, glucocorticoids alter the biomechanical behavior of the bone as well as decrease overall cortical bone mass and strength (Bozzini et al., 2015; Kozai et al., 2009). However, the extent to which PLR in the mandible is sensitive to glucocorticoids has yet to be studied. Osteonecrosis of mandibular bone occurs in patients with autoimmune diseases, such as vasculitis, who are being treated with high dose steroids (Cowan et al., 1995). Although the extent to which glucocorticoids are responsible for this pathology is unclear, an understanding of the role of PLR in the mandible could improve strategies to prevent and treat osteonecrosis, particularly in this high risk population (Strojan et al., 2017; Chronopoulos et al., 2018). This is important because there are relatively limited options for surgical reconstruction of the necrotic mandible, which most commonly results as a late complication of external beam radiation (Strojan et al., 2017; Chronopoulos et al., 2018). Therefore, in this study, we investigated the extent to which glucocorticoids regulate PLR in the mandible, with the goal of advancing our understanding of osteocyte-mediated bone remodeling in mandibular disease.
2. Materials & methods
2.1. Murine studies
All animal procedures described herein were performed according to national ethical guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at University of California San Francisco. In an established model of glucocorticoid-excess, two-month-old male FVB mice were subcutaneously implanted with slow-release pellets containing placebo or prednisolone (2.8 mg/kg/day) (Innovative Research of America) and sacrificed at 7, 21, 35 and 55 days (n = 8, 8, 5, 6 per group, respectively) (Lane et al., 2006). Mandibles and femora were dissected for the following analyses.
2.2. Murine bone micro-computed tomography (μCT)
Hemimandible specimens were harvested and fixed overnight in 10% neutral buffered formalin and transferred to 70% ethanol for radiographic scanning. Radiologic analysis was performed using a desktop cone-beam micro-computed tomography scanner (μCT 40, Scanco Medical, Bruttisellen, Switzerland) and μCT valuation Software v6.0 (Scanco Medical). Specimens were scanned in 70% ethanol, at an energy of 109 kVp with a voxel size of 10 μm. Scans were reconstructed and three-dimensional digitized images were generated for each specimen (Fig. 1A).
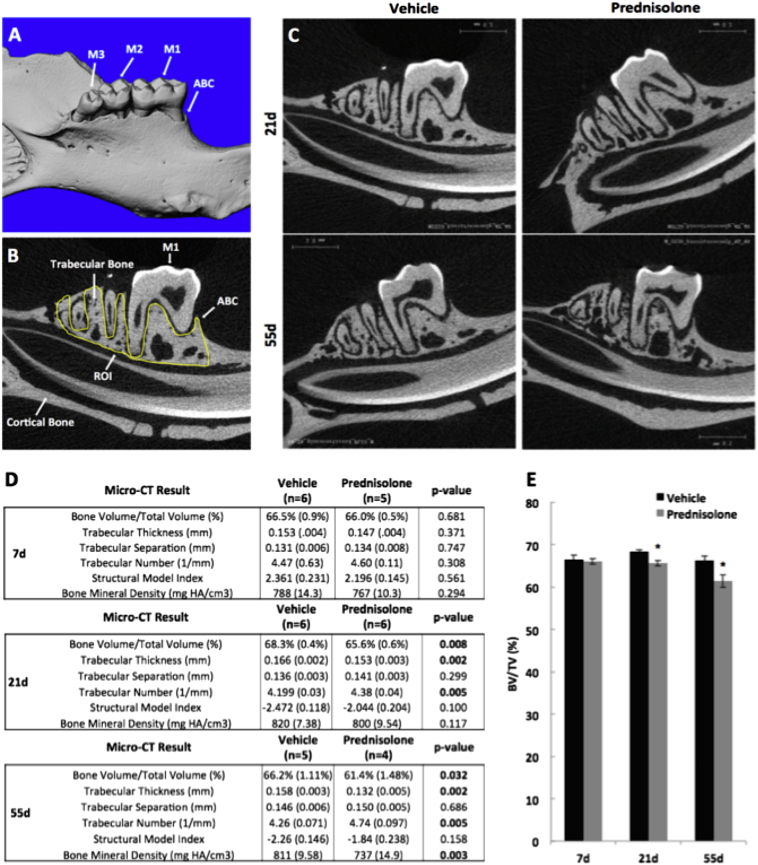
Fig. 1.
Glucocorticoid treatment decreases mandibular bone volume.
Micro-computed tomography (μCT) of hemi-mandibles from 8-week old male FVB mice treated with placebo or prednisolone for 21 or 55 days revealed significant differences in mandibular trabecular and cortical bone. (A) 3-Dimensional images were reconstructed showing the lingual surface of the mandible and the 1st, 2nd and 3rd molars (M1–3, respectively) and the alveolar bone crest (ABC). (B) Using 2-dimensional μCT images, we identified the region of interest (ROI, yellow line), which extends from the mesial aspect of the 1st molar (M1) to the distal aspect of M3. The height of the ROI is defined from the most distal aspect of any root to the alveolar bone crest (ABC). (D) Bone parameters measured by μCT at day 7, 21, and 55 include trabecular bone volume fraction/ total volume (BV/TV), trabecular thickness (Tb. Th), trabecular separation (Tb. Sp), trabecular number (Tb. N), structural model index (SMI), and bone mineral density. (E) Most notably BV/TV was significantly decreased between vehicle- and prednisolone-treated mice at day 21 and day 55. Error bars represent mean ± SEM; *P-value ≤ 0.05, n ≥ 4 compared to Placebo-treatment from Student's t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Volumetric measurements were carried out following the selection of a standardized region of interest (ROI), which was comprised of the alveolar bone surrounding the roots of molars M1, M2 and M3 (Fig. 1B). The length of the ROI extended from the most mesial aspect of the M1 root to the most distal aspect of the M3 root. The width of the ROI extended from the most buccal aspect of any root of the molars to the most lingual aspect of any root. The height of the ROI extended from the inferior most aspect of any root to the alveolar bone crest (ABC) (Chen et al., 2015). A single blinded investigator drew the contour of the desired alveolar bone region so as to maximize the quantification of bone and minimize the inclusion of roots. The abovementioned landmarks defined the borders of the volumetric ROI that was analyzed in 2-D parasagittal images. Bone volume per total volume (BV/TV), trabecular thickness (TT), trabecular separation, trabecular number, Structural Model Index, and bone mineral density were then calculated from each specimen as described (Bouxsein et al., 2010).
2.3. Histology
For paraffin sectioning, dissected murine mandibles were fixed in 10% neutral buffered formalin and incubated in 10% di- and tetra-sodium EDTA for 20–25 days until fully decalcified, followed by serial ethanol dehydration and paraffin embedding. Paraffin sections (7 μm thick) in the sagittal plane of hemimandibles were generated using a microtome (Leica) for polarized light microscopy, Ploton silver stain and tartrate-resistant acid phosphatase (TRAP) staining (Dole et al., 2017; Fowler et al., 2017). In order to standardize evaluation, the trabecular bone was routinely evaluated in the area between molars M1 and M2, whereas cortical bone analysis was performed on the inferior border of the mandible.
Polarized light microscopy was performed as previously described (Jáuregui et al., 2016). Briefly, paraffin-embedded sections were stained in a saturated aqueous solution of picric acid and 0.1% Direct Red-80 (aka: Picrosirius Red) (Sigma-Aldrich). Slides were imaged using polarized filters, which were rotated to achieve the maximum birefringence. Images taken in these conditions were analyzed to quantify collagen fiber orientation.
Ploton silver stain was used to visualize the lacunocanalicular network (Kogawa et al., 2013). Briefly, paraffin-embedded sections were deparaffinized and rehydrated, then incubated in a solution of two-parts 50% silver nitrate and one-part 1% formic acid with 2% gelatin (Fisher Scientific) for 55 min. Stained slides were then washed in 5% sodium thiosulfate (Baker Chemicals) for 10 min and subsequently dehydrated, cleared, and mounted. Image J was used to threshold gray-scale images for quantitative analysis of lacunocanalicular area, which was normalized to total bone area analyzed for each image.
For immunohistochemistry, slides were deparaffinized and hydrated prior to incubation in Innovex Unitrieve low temperature retrieval solution in a 60 °C water bath for 30 min. Endogenous peroxidase activity was quenched using 3% H2O2 for 10 min at room temperature. For all following steps, Innovex Universal Animal IHC kit was utilized. Background buster was applied for 60 min at room temperature. Slides were incubated with rabbit primary antibodies against MMP13 (Abcam #39012, diluted 1:50) and cathepsin K (Abcam #19027, diluted 1:75) in a humid chamber at 37 °C for 24 h. Subsequent incubations with anti-rabbit secondary antibody conjugated to strep avidin, and biotinylated horseradish peroxidase were both performed at room temperature for 10 min each. Fresh DAB solution was applied and incubated at room temperature for 5 min. Slides were mounted with Innovex Advantage Mounting medium. Negative controls were performed by substituting Innovex rabbit negative control sera in place of primary antibody. Quantification was performed with the help of Image J cell counter to determine the average percentage of MMP-13-positive or cathepsin K-positive osteocytes, relative to the total number of osteocytes in the visual field.
For TRAP staining, slides were deparaffinized and hydrated prior to incubation. TRAP solution was prepared according to the package insert (Sigma-Aldrich 386A). Fast Red Violet 7 mg/mL in PBS was used in lieu of Fast Garnet GBC Base Solution. Slides were incubated at 37 °C for 60 min and then counter-stained with nuclear fast green for 10 s. Slides were then air-dried and microscopy and photography were immediately performed and quantified by a single observer at low magnification.
For all histology, images were acquired using a Nikon Eclipse E800 bright-field microscope, unless otherwise noted. For all murine histological analysis, data were collected from n ≥ 3 hemimandibles for each group in an unblinded fashion. Each quantitative average represents 3 high-powered fields from each specimen.
2.4. Flexural testing
Flexural testing was performed on hemimandibles and femurs from animals treated with placebo or prednisolone for 35 days. Bones were gently cleaned of soft tissue and stored in HBSS at 4 °C, then brought to room temperature before testing. All bones were tested in three-point bending under displacement control at 10 μm/s. Hemimandibles were positioned on a 4-mm span with buccal side in compression and the central loading point aligned at the midpoint of the molars. Femurs were positioned on a 6-mm span with the anterior side in compression and the central loading point aligned at the mid-diaphysis. Stiffness was calculated from the slope of the load-displacement curve, and ultimate load was defined as the maximum bending load sustained. Samples that fractured at the condylar process or angular process prior to failure were excluded from analysis. Data from both hemimandibles per animal was averaged so that each data point represents one biological replicate (n = 3 for placebo, n = 4 for prednisolone).
2.5. Statistics
Data were analyzed by one-way ANOVA to determine significant differences between means of multiple groups. Tukey's post-hoc analysis procedure was applied as indicated to determine which groups were significantly different from one another. For experiments involving the comparison of only two groups, Student's t-test was used. P-values < 0.05 were considered significant and are reported as such.
3. Results
3.1. Glucocorticoids decrease mandibular bone volume
To evaluate the effect of glucocorticoids on mandibular osteocytes, we used a well-established prednisolone model that causes bone loss in the appendicular skeleton (Fowler et al., 2017). Similar to observations in long bones, micro-computed tomography analysis of mouse mandibles confirmed that glucocorticoids caused trabecular bone loss in the regions surrounding the mandibular molars (Fig. 1C). Most notably, prednisolone treatment caused a decrease in BV/TV relative to vehicle-treated mice at all time points, with a significant 3.9% decrease at day 21, and a 7.3% decrease at day 55 (Fig. 1D, E). The reduction in trabecular thickness was apparent following 21 days, but not 7 days, of prednisolone treatment. After 55 days of prednisolone treatment, trabecular bone loss was more substantial, with a significant decrease in bone mineral density. Increased trabecular number was also observed within 21 days of prednisolone treatment, and this effect persisted to 55 days.
3.2. Glucocorticoids repress expression of enzymes critical to PLR
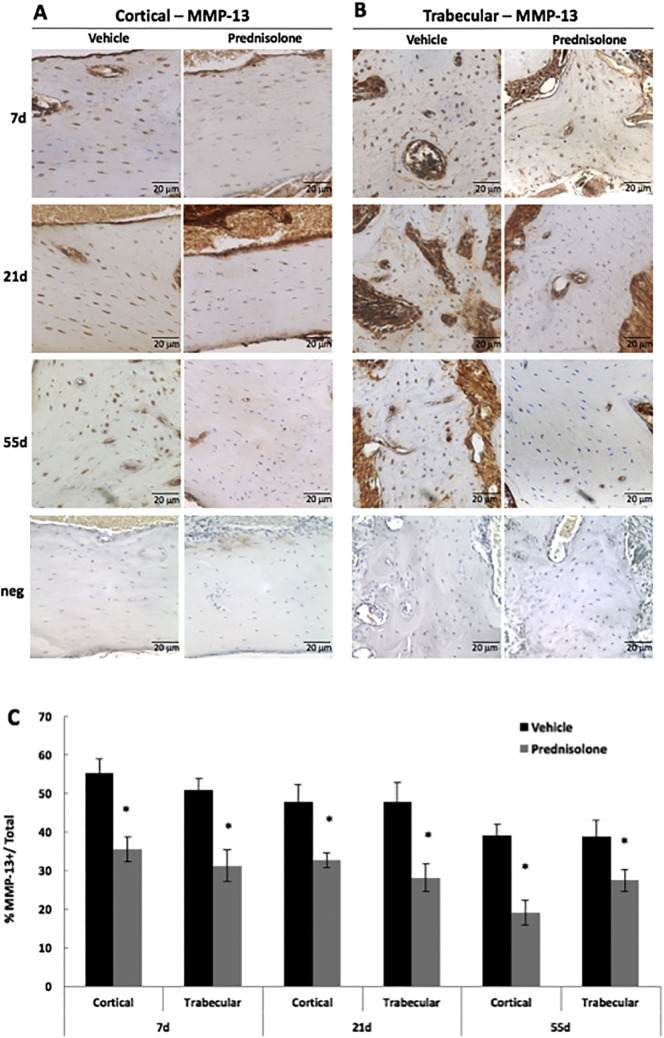
In mesodermally-derived bones, glucocorticoids repress osteocyte expression of genes and proteins required for perilacunar/canalicular remodeling, including MMP2, MMP13, MMP14, and cathepsin K (Fowler et al., 2017). To determine if this regulation was also apparent in neural crest-derived mandibular bone, we evaluated the effect of glucocorticoids on MMP13 and cathepsin K expression in mandibular osteocytes. Even within 7 days of prednisolone treatment, immunohistochemistry revealed qualitatively lower levels of MMP-13 protein expression in both trabecular and cortical bone (Fig. 2A, B). At this time, the percentage of MMP-13 positive osteocytes was significantly reduced by 36% in cortical bone, and 39% in trabecular bone (Fig. 2C). This repressive effect of glucocorticoids on MMP13 levels in both cortical and trabecular bone was persistent. For example, the percentage of MMP-13-positive osteocytes in mandibular cortical bone was significantly reduced by 51% after 55 days of prednisolone treatment.
Fig. 2.
Glucocorticoids suppress MMP-13 expression by mandibular osteocytes.
Immunohistochemistry (IHC) showed qualitatively diminished MMP-13 protein expression in osteocytes of prednisolone-treated mandibular cortical (A) and trabecular bone (B), relative to placebo-treated controls, after 7 days, 21 days, and 55 days. Neg denotes negative controls in which primary antibody was excluded from the IHC protocol. (C) The percent of MMP-13-postively stained osteocytes were normalized to the total number of osteocytes, revealing quantitatively fewer MMP-13-positive osteocytes in both trabecular and cortical bone after 7, 21, and 55 days of prednisolone-treatment. Scale bar = 20 μm (n ≥ 5), error bars indicate mean ± SEM, *P-value ≤ 0.05 compared to vehicle from Student's t-test.
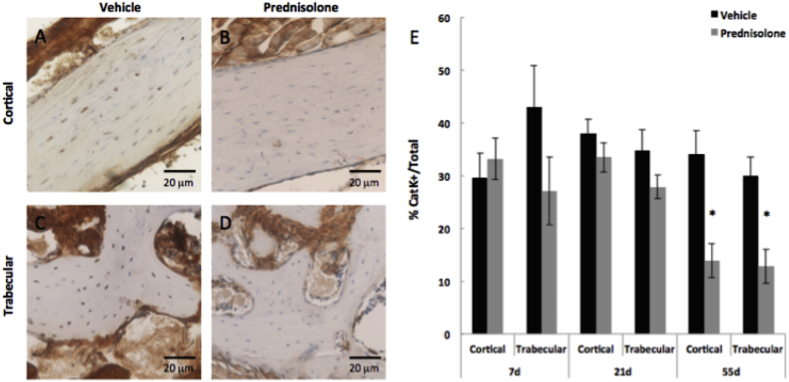
By contrast, osteocyte expression of cathepsin K, was more resistant to the effects of glucocorticoid treatment. A significant reduction in the percentage of cathepsin K-positive osteocytes in trabecular (59%) and cortical bone (57%) was apparent only after 55 days of glucocorticoid treatment (Fig. 3A–E). Significant differences in cathepsin K expression were not detected at either 7 or 21 days. The expression of MMP-13 and cathepsin K was compared between mandibular bone and humeri from untreated animals, with no significant differences detected between bones (N = 4, P > 0.5), suggesting agreement of protein and mRNA expression with previous work (Fowler et al., 2017).
Fig. 3.
Cathepsin K expression by mandibular osteocytes is suppressed by glucocorticoids after 55 days.
Immunohistochemistry (IHC) for cathepsin K (Cat K) in vehicle- and prednisolone-treated cortical (A, B) and trabecular (C, D) bone after 55 days. Diminished positively stained osteocytes for Cat K were observed in both cortical and trabecular bone across all time points. (E) However, significant differences in the percentage of Cat K-positive osteocytes were only detectable at 55 days of treatment. Osteocytes positive for Cat K were quantified and normalized to total number of osteocytes in cortical or trabecular bone. Negative controls are shown in Fig. 2. Scale bar = 20 μm (n ≥ 5). Error bars indicate mean ± SEM, *P-value ≤ 0.05 compared to vehicle from Student's t-test.
3.3. Glucocorticoids cause degeneration of the lacunocanalicular network
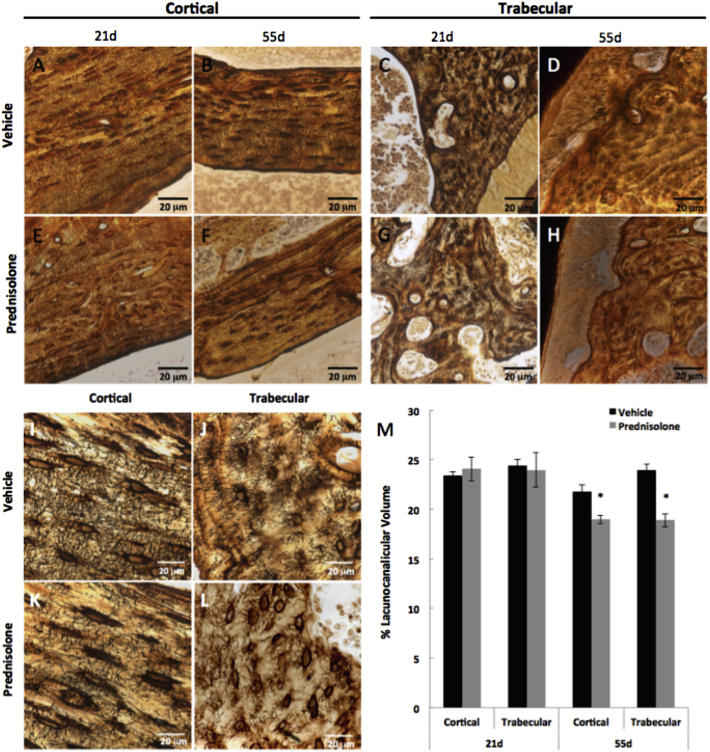
The lacunocanalicular network facilitates osteocyte mediated cell-to-cell communication, enables transport of vital solutes and nutrients, and allows connection of the osteocytes to the bone's vasculature (Bonewald, 2011; Bellido, 2014; Dallas et al., 2013). This network is actively maintained by PLR, and reduced expression of PLR enzymes results in its degeneration (Tang et al., 2012; Inoue et al., 2006; Holmbeck et al., 2005; Qing et al., 2012). We find that prednisolone treatment causes a significant disorganization of mandibular canalicular networks within 55 but not 21 days (Fig. 4A–H). Following 55 days of prednisolone, osteocytes in both cortical and trabecular bone possess fewer and shorter canaliculi (Fig. 4I–L). These qualitative observations correspond to a quantitative reduction in the osteocyte lacunocanalicular area of mandibular cortical (13%) and trabecular (22%) bone (Fig. 4M). Therefore, glucocorticoid-dependent repression of PLR enzyme expression is accompanied by a deterioration of the lacunocanalicular network.
Fig. 4.
Glucocorticoids disrupt the lacunocanalicular network of mandibular bone. Representative silver nitrate-stained histological sections of vehicle- and prednisolone-treated cortical bone after 21 days (A, E) and 55 days (B, F) and trabecular compartments after 21 days (C, G) and 55 days (D, H) respectively. High magnification images (100×) of 55 day vehicle- and prednisolone-treated bones portray the differences in lacunocanalicular network distribution and organization between cortical (I, K) and trabecular (J, L) compartments (scale bar = 20 μm). (M) Lacunocanalicular volume of osteocytes in cortical and trabecular bone is significantly decreased due to prednisolone treatment after 55 days. Scale bar = 20 μm (n ≥ 5). Error bars indicate mean ± SEM, *P-value ≤ 0.05 compared to vehicle from Student's t-test.
3.4. Glucocorticoids cause fragility in femurs and mandibles
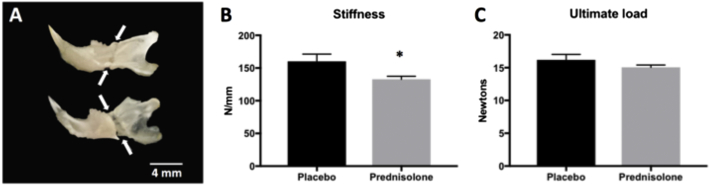
PLR is essential for maintaining bone quality (Tang et al., 2012; Dole et al., 2017). Given that we observe a glucocorticoid-mediated repression of PLR in mandibular bone, we examined the effect of glucocorticoids on bone mechanical properties using flexural tests. As expected, treatment with prednisolone for 35 days was sufficient to cause a 13.8% decrease in ultimate load in the femur, compared to placebo-treated controls (P = 0.007, data not shown). Hemimandibles from placebo and prednisolone-treated mice were additionally subjected to three-point bending. Data were included when hemimandibles from a mouse fractured immediately posterior to the molars without fracturing at the condylar process or angular process (Fig. 5A). Ultimate load was unaffected by prednisolone treatment for 35 days, but stiffness was reduced 17.2% in the glucocorticoid-treated mandibles relative to the vehicle-treated controls (P < 0.05) (Fig. 5B–C).
Fig. 5.
Glucocorticoid treatment causes severe fragility in mouse mandibles.
HBSS-soaked hemi-mandibles were tested in three-point bending by positioning the buccal side in compression and the central loading point aligned at the midpoint of the molars. Hemi-mandibles from both groups consistently broke immediately posterior to the molars as shown by arrows on the buccal (top) and lingual (bottom) sides (A). In the glucocorticoid-treated mandibles, stiffness (B) was reduced by 17.2% relative to the placebo-treated controls after 35 days of treatment. Ultimate load (C) was not significantly affected by the prednisolone treatment after 35 days (N = 3 animals for placebo treatment, N = 4 animals for prednisolone treatment). Error bars indicate mean ± SEM; *P-value ≤ 0.05 compared to vehicle from Student's t-test.
4. Discussion
This study reveals that perilacunar/canalicular remodeling in the mouse mandible, the process by which osteocytes maintain bone extracellular matrix and lacunocanalicular organization, is vulnerable to glucocorticoids. As with our previous study on the impact of glucocorticoids on perilacunar remodeling in the long bones (Fowler et al., 2017), we found that glucocorticoids rapidly and robustly reduce expression of enzymes critical for PLR, and further cause an alteration in the physical structure of the lacunocanalicular network. This glucocorticoid-induced suppression of PLR and thinning of trabecular bone compromises the biological and mechanical integrity of mandibular bone. These findings add to the growing body of evidence that perilacunar/canalicular remodeling is a dynamic process that, if deregulated, can contribute to disease.
Perhaps the most striking finding was that the lacunocanalicular network of glucocorticoid-treated mandibles was disorganized, with a significant reduction in lacunocanalicular area. Given the essential role of the lacunocanalicular network in cell-cell signaling, vascular connectivity, and mechanosensation, deterioration of this network could have profound biological consequences (Bonewald, 2011; Bellido, 2014; Dallas et al., 2013). Degeneration of the lacunocanalicular network is a hallmark in suppressed PLR that is observed in mice deficient in key PLR enzymes and in human disease, such as glucocorticoid-induced osteonecrosis of the femoral head (Tang et al., 2012; Inoue et al., 2006; Holmbeck et al., 2005; Dole et al., 2017; Fowler et al., 2017; Kogawa et al., 2013). PLR enzyme expression is suppressed by glucocorticoids in mesodermally-derived long bone (Hillegass et al., 2007; Vincenti and Brinckerhoff, 2002). Interestingly, in the mandible, we found a divergence in the effect of glucocorticoids on cathepsin K and MMP-13 expression, with a more robust suppression of MMP-13 expression evident after only 7 days of treatment. By contrast, there was no significant difference in cathepsin K expression between groups until 55 days of glucocorticoid treatment. The reason for this difference in cathepsin K regulation by glucocorticoids remains unclear. We speculate it may relate to the neural crest origin or other distinct features of mandibular bone. While we have shown that glucocorticoids cause a robust anatomic and molecular effect on mandibular bone, an important area of future study will be to assess the reversibility of the effect of glucocorticoids.
Perilacunar/canalicular remodeling also plays an instrumental role in maintaining bone quality, such that disruption of PLR can impact both the organic and mineral phases of the bone matrix (Tang et al., 2012; Alliston, 2014; Dole et al., 2017). We previously observed that PLR suppression with glucocorticoid treatment is accompanied by collagen disorganization in femoral cortical and trabecular bone (Fowler et al., 2017). The mandible has a higher proportion of trabecular bone, and the collagen organization in both the cortical and trabecular regions of the mouse mandible is quite heterogeneous, even in the vehicle-treated mice. This heterogeneity may help mandibular bone accommodate the diverse forces encountered during mastication (Panagiotopoulou et al., 2017). Nonetheless, this heterogeneous collagen organization limited our ability to detect significant differences in mandibular bone collagen organization in response to prednisolone treatment (data not shown).
Glucocorticoids are well-known to impact both bone mass and bone quality (Lane et al., 2006; O'Brien et al., 2004; Weinstein, 2011; Kim et al., 2007). The murine mandible has extensive trabecular bone and a relatively thin cortex that is poorly visualized on microCT. Thus, the most pronounced effect of glucocorticoids was the loss of trabecular bone surrounding the mandibular molars. Accordingly, flexural testing of hemimandibles revealed a loss of stiffness with prednisolone treatment, consistent with a prior report (Balooch et al., 2007). In part due to the challenges in reliably quantifying mandibular cortical thickness, we were unable to discriminate the extent to which the reduction in flexural properties resulted from loss of bone mass or bone quality. Nonetheless, given the role of PLR in bone quality, it is likely that glucocorticoid suppression of PLR affects mandibular bone quality to some extent. Because of the intimate relationship between the teeth and mandibular bone, alterations to the bone quality may compromise dentition.
This study of glucocorticoid effects on mandibular bone highlights the potential clinical consequences of PLR suppression on the biological and mechanical health of the mandible. PLR suppression could contribute to an increased risk of pathologic mandibular fracture, and compromise the vascular milieu supporting the dentition. Another interesting clinical correlate identified recently is the damage to the lacunocanalicular network induced by high dose radiation in mouse long bones (Chandra et al., 2017). This particular phenotype shares some features with the lacunocanalicular degeneration precipitated by glucocorticoid treatment. Additional studies are needed to examine the possibility that PLR suppression participates in osteoradionecrosis of the jaw, as it does in glucocorticoid-induced osteonecrosis of the femoral head, especially since treatment with anti-sclerostin antibody (Scl-Ab) mitigates the effects of radiation on osteocytes and other bone cells (Chandra et al., 2017). Understanding these mechanisms is of particular importance as new therapeutic targets are actively being sought for the treatment and prevention of mandibular osteoradionecrosis.
Although glucocorticoid treated mandibular pathology is not a clinical entity that has been described, our data suggests glucocorticoid-suppression of PLR may contribute to the progression of mandibular osteonecrosis. If steroid medications are administered concurrently with other known toxins, such as external beam radiation therapy, there may be an additive injury. This study adds to the growing body of literature supporting the role of osteocyte-mediated perilacunar/canalicular remodeling in skeletal disease and as a potential therapeutic target, including in mandibular osteonecrosis.
Transparency document
Transparency document.
Acknowledgements
This research was supported by NIH-NIDCR R01 DE019284 (TA), DOD PRORP OR130191 (TA), NIH-NCI F32 CA203402-01A1 (TWF), NIH T32 GM008155 (CMM), NSF Graduate Research Fellowship 1650113 (CMM), and the Read Research Foundation. This research used core facilities at UCSF that are supported by NIH-NIAMS P30 AR066262-01 (TA). The authors gratefully acknowledge E. Liebenberg for expert histological assistance.
Footnotes
The Transparency document associated with this article can be found, in the online version.
References
- Alliston T. Biological regulation of bone quality. Curr. Osteoporos. Rep. 2014;12(3):366–375. doi: 10.1007/s11914-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balooch G., Yao W., Ager J.W., Balooch M., Nalla R.K., Porter A.E., Ritchie R.O., Lane N.E. The aminobisphosphonate risedronate preserves localized mineral and material properties of bone in the presence of glucocorticoids. Arthritis Rheum. 2007;56(11):3726–3737. doi: 10.1002/art.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger L.F., Jarry L., Uhthoff H.K. Osteocytic osteolysis in Paget's disease. Rev. Can. Biol. 1968;27(1):37–44. [PubMed] [Google Scholar]
- Bellido T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014;94(1):25–34. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald L.F. The amazing osteocyte. J. Bone Miner. Res. 2011;26(2):229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Bozzini C., Champin G., Alippi R.M., Bozzini C.E. Effect of dexamethasone on mandibular bone biomechanics in rats during the growth phase as assessed by bending test and peripheral quantitative computerized tomography. Acta Odontol. Latinoam. 2015;28(1):83–88. doi: 10.1590/S1852-48342015000100012. [DOI] [PubMed] [Google Scholar]
- Cha Y.H., Hong N., Rhee Y., Cha I.H. Teriparatide therapy for severe, refractory osteoradionecrosis of the jaw. Osteoporos. Int. 2017;29:987–992. doi: 10.1007/s00198-017-4343-2. [DOI] [PubMed] [Google Scholar]
- Chandra A., Lin T., Young T., Tong W., Ma X., Tseng W.J., Kramer I., Kneissel M., Levine M.A., Zhang Y., Cengel K., Liu X.S., Qin L. Suppression of sclerostin alleviates radiation-induced bone loss by protecting bone-forming cells and their progenitors through distinct mechanisms. J. Bone Miner. Res. 2017;32(2):360–372. doi: 10.1002/jbmr.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Xu X., Liu M., Zhang W., Ke H.Z., Qin A., Tang T., Lu E. Sclerostin antibody treatment causes greater alveolar crest height and bone mass in an ovariectomized rat model of localized periodontitis. Bone. 2015;76:141–148. doi: 10.1016/j.bone.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Chronopoulos A., Zarra T., Ehrenfeld M., Otto S. Osteoradionecrosis of the jaws: definition, epidemiology, staging and clinical and radiological findings. A concise review. Int. Dent. J. 2018;68(1):22–30. doi: 10.1111/idj.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan J., Moenning J.E., Bussard D.A. Glucocorticoid therapy for myasthenia gravis resulting in resorption of the mandibular condyles. J. Oral Maxillofac. Surg. 1995;53(9):1091–1096. doi: 10.1016/0278-2391(95)90130-2. [DOI] [PubMed] [Google Scholar]
- Dallas S.L., Prideaux M., Bonewald L.F. The osteocyte: an endocrine cell … and more. Endocr. Rev. 2013;34(5):658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole N.S., Mazur C.M., Acevedo C., Lopez J.P., Monteiro D.A., Fowler T.W., Gludovatz B., Walsh F., Regan J.N., Messina S., Evans D.S., Lang T.F., Zhang B., Ritchie R.O., Mohammad K.S., Alliston T. Osteocyte-intrinsic TGF-β signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;21(9):2585–2596. doi: 10.1016/j.celrep.2017.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T.W., Acevedo C., Mazur C.M., Hall-Glenn F., Fields A.J., Bale H.A., Ritchie R.O., Lotz J.C., Vail T.P., Alliston T. Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Sci. Rep. 2017;7:44618. doi: 10.1038/srep44618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich von Recklinghausen (1833–1910). German pathologistJAMA. 1968;205(9):640–641. [PubMed] [Google Scholar]
- Hillegass J.M., Villano C.M., Cooper K.R., White L.A. Matrix metalloproteinase-13 is required for zebra fish (Danio rerio) development and is a target for glucocorticoids. Toxicol. Sci. 2007;100(1):168–179. doi: 10.1093/toxsci/kfm192. [DOI] [PubMed] [Google Scholar]
- Holmbeck K., Bianco P., Pidoux I., Inoue S., Billinghurst R.C., Wu W., Chrysovergis K., Yamada S., Birkedal-Hansen H., Poole A.R. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J. Cell Sci. 2005;118(Pt 1):147–156. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- Inoue K., Mikuni-Takagaki Y., Oikawa K., Itoh T., Inada M., Noguchi T., Park J.S., Onodera T., Krane S.M., Noda M., Itohara S. A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J. Biol. Chem. 2006;281(44):33814–33824. doi: 10.1074/jbc.M607290200. [DOI] [PubMed] [Google Scholar]
- Jáuregui E.J., Akil O., Acevedo C., Hall-Glenn F., Tsai B.S., Bale H.A., Liebenberg E., Humphrey M.B., Ritchie R.O., Lustig L.R., Alliston T. Parallel mechanisms suppress cochlear bone remodeling to protect hearing. Bone. 2016;89:7–15. doi: 10.1016/j.bone.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Zhao H., Kitaura H., Bhattacharyya S., Brewer J.A., Muglia L.J., Patrick Ross F., Teitelbaum S.L. Glucocorticoids and the osteoclast. Ann. N. Y. Acad. Sci. 2007;1116:335–339. doi: 10.1196/annals.1402.057. [DOI] [PubMed] [Google Scholar]
- Kogawa M., Wijenayaka A.R., Ormsby R.T., Thomas G.P., Anderson P.H., Bonewald L.F., Findlay D.M., Atkins G.J. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J. Bone Miner. Res. 2013;28(12):2436–2448. doi: 10.1002/jbmr.2003. [DOI] [PubMed] [Google Scholar]
- Kozai Y., Kawamata R., Sakurai T., Kanno M., Kashima I. Influence of prednisolone-induced osteoporosis on bone mass and bone quality of the mandible in rats. Dentomaxillofac. Radiol. 2009;38(1):34–41. doi: 10.1259/dmfr/28859075. [DOI] [PubMed] [Google Scholar]
- Lane N.E., Yao W., Balooch M., Nalla R.K., Balooch G., Habelitz S., Kinney J.H., Bonewald L.F. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J. Bone Miner. Res. 2006;21(3):466–476. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie P.J., Glorieux F.H. Relation between hypomineralized periosteocytic lesions and bone mineralization in vitamin D-resistant rickets. Calcif. Tissue Int. 1983;35(4–5):443–448. doi: 10.1007/BF02405074. [DOI] [PubMed] [Google Scholar]
- McGee-Lawrence M.E., Stoll D.M., Mantila E.R., Fahrner B.K., Carey H.V., Donahue S.W. Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) show microstructural bone loss during hibernation but preserve bone macrostructural geometry and strength. J. Exp. Biol. 2011;214(Pt 8):1240–1247. doi: 10.1242/jeb053520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C.A., Jia D., Plotkin L.I., Bellido T., Powers C.C., Stewart S.A., Manolagas S.C., Weinstein R.S. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145(4):1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- Panagiotopoulou O., Iriarte-Diaz J., Wilshin S., Dechow P.C., Taylor A.B., Mehari Abraha H., Aljunid S.F., Ross C.F. In vivo bone strain and finite element modeling of a rhesus macaque mandible during mastication. Zoology (Jena) 2017;124:13–29. doi: 10.1016/j.zool.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing H., Ardeshirpour L., Pajevic P.D., Dusevich V., Jähn K., Kato S., Wysolmerski J., Bonewald L.F. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 2012;27(5):1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolvien T., Krause M., Jeschke A., Yorgan T., Püschel K., Schinke T., Busse B., Demay M.B., Amling M. Vitamin D regulates osteocyte survival and perilacunar remodeling in human and murine bone. Bone. 2017;103:78–87. doi: 10.1016/j.bone.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Strojan P., Hutcheson K.A., Eisbruch A., Beitler J.J., Langendijk J.A., Lee A.W.M., Corry J., Mendenhall W.M., Smee R., Rinaldo A., Ferlito A. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017;59:79–92. doi: 10.1016/j.ctrv.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.Y., Herber R.P., Ho S.P., Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J. Bone Miner. Res. 2012;27(9):1936–1950. doi: 10.1002/jbmr.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti M.P., Brinckerhoff C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4(3):157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R.S. Clinical practice. Glucocorticoid-induced bone disease. N. Engl. J. Med. 2011;365(1):62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- Zallone A.Z., Mueller W.J. Medullary bone of laying hens during calcium depletion and repletion. Calcif. Tissue Res. 1969;4(2):136–146. doi: 10.1007/BF02279115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.