Abstract
The development of brain metastasis (BM) of breast cancer is usually a late event with deleterious effect on the prognosis. Treatment options for intracerebral seeding of breast cancer are limited and, so far, nonspecific. Molecular detailing of subsequent events of penetration, seeding, and outgrowth in brain is highly relevant for developing therapeutic strategies to treat, or prevent, BM.
We scrutinize recent literature for molecules and pathways that are operative in the formation of breast cancer BM. We also summarize current data on therapeutic efforts to specifically address BM of breast cancer. Data on molecular pathways underlying the formation of BM of breast cancer are sketchy and to some extent inconsistent. The molecular makeup of BM differs from that of the primary tumors, as well as from metastases at other sites. Current efforts to treat breast cancer BM are limited, and drugs used have proven effects on the primary tumors but lack specificity for the intracerebral tumors.
More basic research is necessary to better characterize BM of breast cancer. Apart from the identification of drug targets defined by the intracerebral tumors, also targets in the molecular pathways involved in passing the blood–brain barrier and intracerebral tumor cell growth should be revealed.
Keywords: brain metastasis, breast cancer, blood-brain barrier, chemotherapy, molecular mechanisms
Breast cancer is a histologically and genetically heterogeneous disease, categorized by the expression of estrogen receptor (ER) and progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2/neu). There are 6 basic molecular subtypes of breast cancer. Based on the ER, PR, and HER2/neu status, only 4 categories are clinically distinguished.1 Each subtype exhibits a particular natural history, metastatic potential, and outcome. Breast cancer tends to metastasize to bone, lung, liver, and brain. Seeding to brain is usually a late event. Tumor cell growth in the brain microenvironment is the result of genetic predisposition and cellular adaptation mechanisms, and is largely dependent on cross-talk between tumor cells and brain-resident cells. Brain metastasis (BM) develops more frequently in triple-negative (TN) (25%–27%) and HER2+ breast cancers (11%–20%), while the incidence of BM in luminal A and B is much lower (8%–15% and 11%, respectively).2 TN breast cancer BM tends to develop earlier in the course of disease and is almost invariably associated with extracranial metastases.3 BM in HER2+ tumors occurs in up to 50% of patients receiving HER2+ targeted therapies (in particular trastuzumab). The TN subtype displays the worse (3–4 mo) and luminal B the best (19–20 mo) median survival in the presence of BM.4 This review will focus on the presently known molecular mechanisms that grant breast circulating tumor cells (CTCs) extravasation into the brain parenchyma. Discovering specific gene mutations or expressional profiles in tumor cells prone to disseminate to brain will open avenues to intervene with the rise of intracerebral dissemination and offer a great step forward in the treatment of breast cancer.
Genetic Predictors of Brain Metastasis
Over the last decades improvements of microarray technology and the capacity to carry out massive parallel gene expression analyses have provided awareness of the complexity of breast cancer. Data on the breast cancer subclones and molecular pathways involved in metastasis, in particular to brain, are sketchy and not univocal. Using next-generation sequencing data of 4 DNA samples collected from one breast cancer patient, it was suggested that the development of metastases may be triggered by a minority of cells within the primary tumor,5 and the results of various studies point to large similarities between the parent tumors and their metastatic offspring. In a study where 15 primary breast cancer samples were compared with their intracerebral metastases by next-generation sequencing, no significant differences in mutation profiles were detected, while actionable gene alterations in the breast cancer BM (such as TP53, PIK3CA, KIT, MLH-1, and RB1) had been preserved.6 Similarly, in a study of 12 primary breast cancers and their BM in which 19 oncogenes were scrutinized, all somatic mutations were found in both primary and metastatic specimens except for one epidermal growth factor receptor (EGFR) mutation identified only in the primary tumor.7 However, whole-exome sequencing of 86 matched primary tumors and BMs from patients with breast, lung, and renal cancer revealed that 53% of the BMs harbored at least one clinically actionable alteration that was not detected in the matched primary tumor, while the BMs had more unique mutations than the primary and lung metastases combined.8 Further assessment of different intracranial metastatic sites in the same patient showed that almost all of the potentially clinically informative driver alterations were shared, suggesting that BM is homogeneous within an individual.8 Clearly, differences in findings between studies depend on the extent of screenings applied.
Interestingly, between 16% and 22% of Erb-B2 receptor tyrosine kinase 2 (ERBB2)/HER2-negative breast cancers have been reported to acquire ERBB2/HER2 amplifications and/or mutations in the BM. The discordance between primary tumors and their BM could be even greater regarding the ER status. Similarly, EGFR expression was increased in the brain-seeking, but not the bone-seeking, subline of an MDA-MB-231 model system.9 The induction of HER2 in this cell line resulted in a 3-fold increase of brain macrometastasis in a xenograft model.9 A significant gain of EGFR copy number status was found in breast cancer BM compared with the primary breast cases. EGFR amplification is also more frequently found in brain metastatic adenocarcinomas from the lung compared with corresponding primary tumors. Furthermore, EGFR amplified primary lung tumors are significantly correlated with shorter time to BM development.10 The identification of exclusive mutations in tumor cells at different sites is suggestive of a metastatic cascade-dependent evolution influenced by, or dependent on, the cross-talk between CTCs and their variable microenvironments. Some of these mutations may be necessary for the establishment of the initial metastatic seeding in the brain, but not for its continued growth or maintenance. While phosphatase and tensin homolog (PTEN) mutations are rarely found in primary breast11 or lung12 cancers, 21% of the brain metastatic breast tumors were reported to carry PTEN mutations and 31% were determined with loss of PTEN protein expression.11 Results of comparative genetic hybridization of arrays of breast tumors and BM emphasized the relevance of PTEN loss and the role of the EGFR gene in BM formation.13 In a study of 119 primary TN breast tumors it was shown that analysis of gene transcripts in the primary tumors is insufficient to predict the development of BM.14 It is clear that BM of breast cancer does differ from the primary tumors in terms of mutations and expression. Importantly, some data indicate that therapeutic actionable mutations can be present in BM while not in the primary tumors. This difference has consequences for choosing the right therapy for BM, and therefore intensified research aiming at the characterization of primary breast tumors and their BM is important for the development of targeted therapies.
Although Wnt/β-catenin signaling activation is associated with breast cancer BM, the significance of this pathway to predict BM remains unclear. Two potential Wnt ligands, Wnt5A and Wnt5B and their respective receptors, ROR1 and ROR2, were identified as signal mediators and potential therapeutic targets,15 and it has been suggested that Wnt signaling in breast tumor progression occurs independently of β-catenin. Microglia reportedly promotes colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Moreover, the Wnt ligand Wnt5A was found to be constitutively expressed in microglia and is a critical mediator of CXCR4-induced T-cell migration.16
Influence of the Immune System
In a recent study in which gene expressions of breast cancer from women with metastasized disease with and without cerebral metastases were compared, T-cell response to the primary tumors appeared crucial for the rise of BM.17 The T cells appeared to change the tumor cell proteome, and the expression of some of these crucial proteins was confirmed in those primary tumors that gave rise to BM. The findings could be confirmed in a blood–brain barrier (BBB) cell culture model in which breast cancer cell lines were incubated with T cells. Moreover, injecting breast cancer cells that were co-cultured with T cells in mice gave a brain phenotype as well. The results need further explorations in order to scrutinize current anticancer T-cell therapies and to find targets for therapeutic interference with the T-cell response and the resulting upregulated proteins. The influence of the immune system on the formation of BM needs further scrutiny in order to find ways to prevent the formation of BM in breast cancer patients.
Stages of Metastasizing to Brain
Passage Through the BBB
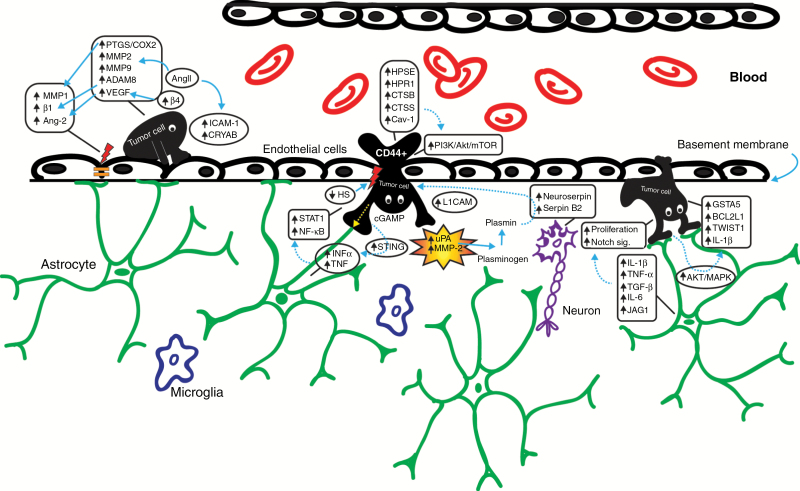
Tumor cells (TCs) mimic mechanisms used by immune cells, but the adhesion molecules and ligands they use for extravasation are different. It is unclear whether attachment characteristics of leukocytes are a precondition for transendothelial migration (TEM).18 Migration into the brain occurs foremost by opening tight junctions (TJs).19 In an in vitro experiment, melanoma cells disrupted the intercellular junctions and adherens junctions of the endothelial cells,19 and their sizes are more important for the initial arrest than the capacity to adhere to vascular walls.20 It is not yet clear how and whether CTCs affect the function of the BBB. So far, apoptosis or hypoxia has not been recorded in the endothelial cells at the site of extravasation, suggesting that extravasation events do not directly correlate with damage of the vessel walls. To enter the brain parenchyma, cancer cells must pass through microcapillary walls that constitute the BBB.21 The TJs and the absence of extensive pinocytosis and fenestrae account for protecting the brain from cellular and molecular intruders. The extravasation strategy of TCs comprises arrest and adherence to the endothelium (docking), establishment of intercellular contacts (locking), TEM, adhesion to the subendothelial matrix (foothold), and subsequent modification of the surrounding host tissue microenvironment (colonization) (Fig. 1). Reciprocal interaction with stromal cells grants the successful formation of macrometastasis.22 Initial investigations showed that the production of cytokines resulting from inflammation increases the ability of CTCs to adhere to the vascular endothelium, while the endothelium itself was thought to be a mere passive participant. However, later studies revealed that the attachment of TCs to brain endothelial cells is mediated by bilateral cell surface receptors and adhesion molecules, such as integrins, selectins, and chemokines. Therefore, endothelial cells actively influence TC extravasation and proliferation20 (Fig. 1; Table 1). Only a minority of TCs, known as tumorigenic cells or cancer-initiating cells, trigger tumor proliferation and self-renewal.23 Only a small proportion of breast cancer cells display a CD44high/CD24low antigenic phenotype, contrasting with the predominant CD44low/CD24high phenotype.24 Breast cancer cells with a CD44+/CD24− phenotype display highly invasive properties, and levels of pro-invasive genes, like interleukin (IL)-1α, IL-6, IL-8, and urokinase plasminogen activator (uPA) are elevated.25 Stromal uPA and matrix metalloproteinase 2 (MMP-2) are produced as nonactive precursors and become responsive on the surface of the TCs, allowing the neoplastic cells to break through the basement membrane.26 uPA converts plasminogen into plasmin. Suppression of plasmin is caused by overexpression of neuroserpin and serpin B2, which in turn enable the infiltration of metastatic breast cancer cells through the BBB. This mechanism is mediated by L1 cell adhesion molecule (L1CAM), which will empower the metastatic process.27 Through stimulation of the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) pathway, uPA activates MMPs, endopeptidases that can cleave any component of the extracellular matrix (ECM).28
Fig. 1.
Schematic representation of the stages of the formation of cerebral metastasis. From left to right, the processes of vascular adhesion, transgression through the BBB, extravasation, interaction with the brain microenvironment of the tumor cells and involved molecules are presented. MMP, matrix metalloproteinase; β1, beta 1 integrin subunit; PTGS2/COX-2, prostaglandin-endoperoxide synthase 2/cyclooxygenase-2; ADAM8, a disintegrin and metalloproteinase domain-containing protein 8; β4, beta 4 integrin subunit; CRYAB, αβ-crystallin; INFα, interferon alpha; STING, stimulator of interferon genes; L1CAM, L1 cell adhesion molecule; JAG1, jagged 1; GSTA5, glutathione S-transferase alpha 5; BCL2L1, BCL2 like 1; TWIST1, twist family BHLH transcription factor 1.
Table 1.
Molecular mechanisms and potential therapeutic targets in breast cancer brain metastasis
| Mechanism | Potential Therapeutic Target | Location | Regulated by | Regulates | Action | Reference |
|---|---|---|---|---|---|---|
| Passage through BBB | Wnt5A/ROR1; Wnt5B/ ROR2 | NA | Wnt signaling | NA | ↑ invasion | 15 |
| αvβɜ integrin | NA | NA | ↑ tumor-platelet interaction | ↑ arrest in vasculature | 29 | |
| αvβɜ integrin | NA | NA | ↑ VEGF | ↑ intracerebral growth | 30 | |
| β4 integrin signaling | NA | NA | ↑ VEGF | ↑ disruption of inter- endothelial junctions | 31 | |
| β1 integrin | NA | NA | NA | ↑ adhesion | 32 | |
| P-selectin | endothelial cells and activated platelets | NA | communication/ protection of arrested TC | ↑ metastatic ability | 34 | |
| CXCL12/CXCR4 signaling | metastatic site/ TC | NA | ↑ PI3K/Akt | ↑ adhesion, ↑ TEM | 36 | |
| NF-κB signaling | NA | CXCR4 | organ-specific metastasis of breast cancer | ↑ migration, ↑ tumor growth | 38 | |
| Intracerebral progression | CD44+/ CD24− phenotype | breast cancer cells | NA | ↑ IL-1α, ↑ IL-6, ↑ IL-8, ↑ uPA | ↑ invasion | 25 |
| uPA | breast cancer cells | NF-κB pathway | Plasmin production, MMPs | ↑ ECM cleavage | 28 | |
| ↓ Plasmin | NA | ↑ neuroserpin, ↑ serpin B2, ↑ L1CAM | NA | ↑ TEM | 27 | |
| Interaction with astrocytes | IL-1β, TNF-α, TGF-β, IL-6 | Astrocytes | NA | NA | ↑ proliferation (breast and lung cancer cells) | 41 |
| cyclic GAMP | Astrocytes | TCs | ↑ INFα, ↑ TNF ->↑STAT1 and NF-κB pathways | ↑ tumor growth, ↑ chemo-resistance | 42 | |
| Akt/MAPK pathways | TCs | astrocytes-TC interaction | ↑ GSTA5, ↑ BCL2L1, ↑ TWIST1 | ↑ resistance, ↑ survival | 43 | |
| IL-1β | (secreted by) BM breast cancer | NA | ↑ JAG1 | ↑ Notch signaling | 44 | |
| Interaction with ECM | MMP-14 (or MT1-MMP) | target site | COX-2 (or PTGS2) | MMP-2 | ↑ TEM | 47 |
| MMP1 | (secreted by) breast cancer cells | NA | degrades tight junctions of BBB | ↑ TEM | 48 | |
| HPSE | BM breast cancer, endothelial cells and glial cells | EGFR/Her2 signaling | NA | ↑ TEM | 53 | |
| HPR1 | NA | ↓ HS | NA | ↑ metastatic ability | 55 | |
| CTSB | breast cancer cells | NA | ↑ angiogenesis, ↑ MMPs | ↑ ECM cleavage, ↑ invasion, ↑ tumor growth | 57 | |
| CTSS | NA | NA | proteolytic processing of JAM-B | ↑ TEM | 59 | |
| Cav-1 | NA | NA | ↓ MMP-9 and MMP-2, ↓ STAT3 / (LSS exposure):↑ PI3K/Akt/mTOR signaling | ↓ tumor growth, ↓ invasion / (LSS exposure):↑ motility, ↑ invadopodia formation, ↑ invasion | 60,61 | |
| MEK5 | NA | STAT3 | ↑ EMT | ↑ invasion | 64 | |
| exo-AnxA2 | breast cancer cells | NA | ↑ p38MAPK, ↑NF-κB, ↑ stat3 pathways; (secretion of):↑ IL-6, ↑ TNF-α | ↑ angiogenesis, ↑ proliferation | 65 | |
| TUBB3 | breast cancer BM cell line | NA | NA | ↑ metastatic ability | 66 | |
| AngII | vasoactive peptide | NA | ↑ MMP-2, ↑ MMP-9 in breast cancer cells; ↑ICAM-1 | ↑ adhesion, ↑ TEM, ↑ motility | 67 | |
| CRYAB | (independent predictor of BM) | NA | NA | ↑ adhesion, ↑ TEM | 68,69 | |
| Ang-2 | activated brain endothelial cells | VEGF secreted by TN breast cancer cells | impairment of TJ structures | ↑ TEM | 70 |
Akt, protein kinase B; TEM, transendothelial migration; L1CAM, L1 cell adhesion molecule; TNF, tumor necrosis factor; TGF, tumor growth factor; cGAMP, cyclic guanosine monophosphate–adenosine monophosphate; INF, interferon; GSTA5, glutathione S-transferase alpha 5; BCL2L1, BCL2 like 1; TWIST1, twist family BHLH transcription factor 1; JAG1, jagged 1; PTGS2, prostaglandin-endoperoxide synthase 2; JAM-B, junctional adhesion molecule B; Cav-1, caveolin-1; LSS, low shear stress; MEK5, mitogen extracellular-signal-regulated kinase 5; exo-AnxA2, exosomal-annexin A2; ICAM-1, intercellular adhesion molecule 1; CRYAB, αβ-crystallin; TN, triple negative; TJ, tight junction; NA, not available
In a mouse model of breast cancer metastasis, upregulation of αvβɜ integrin in TCs appeared to interact with platelets, resulting in thrombus formation that facilitates the arrest of TCs within the vasculature.29 Activation of αvβɜ integrins also assists in the intracerebral growth of TCs through continued upregulation of vascular endothelial growth factor (VEGF).30 Enhanced β4 integrin signaling induces HER2-dependent expression of VEGF, which is responsible for the disruption of the interendothelial junctions.31 VEGF is highly expressed in breast cancer cells and is known as a vascular permeability factor and major regulator of angiogenesis during tumor development. The interaction between TCs and blood vessels relies on β1 integrin-mediated adhesion, and it has been suggested that antagonisms of the β1 integrin subunit expressed in the vascular basal membrane would reduce the formation of CNS metastases.32 Another role in the interaction between endothelial cells and TCs is played by the selectins. E-selectin is expressed on activated endothelial cells and P-selectin is found on both endothelial cells and activated platelets.33 P-selectin may directly assist the metastatic process through communication with arrested TCs, masking and protecting TCs from the immune response.34 Other soluble factors affecting the formation of metastases are chemokines and their specific receptors. The chemokine stromal cell–derived factor 1α (SDF-1α or CXCL12) and its receptor CXCR4 play a role in such multiple biological functions as homing, motility, and progression of metastases.35 CXCL12/CXCR4 signaling is involved in the migration of breast cancer cells through the BBB. SDF-1α induces blood vessel instability and is significantly more highly expressed in breast cancer cells compared with normal breast tissue.36 CXCL12 appeared to be overexpressed in BM from breast and lung cancer.35 CXCR4 is among the chemokine receptors that are most commonly expressed in cancer. SDF-1α expression is frequently observed at common metastatic sites of breast cancer.37 The stimulation of SDF-1α increases adhesion and activates TEM by activating the phosphatidylinositol-3 kinase pathway (PI3K/Akt).36 NF-κB signaling, mediated by CXCR4, plays a role in organ-specific metastasis of breast cancer, migration, and tumor growth.38 These data taken together suggest that the expression of chemokine receptors in primary breast cancers correlates with the appearance of BM. Whether any of these receptors will become useful as a biomarker predictive of the appearance of BM is an unanswered question.
Intracerebral Progression
Astrocytes are confronted by breast cancer cells that have breached the endothelial side of the BBB. Astrocytes have a pivotal role in the maintenance of the BBB, contributing to cerebral and extracellular homeostasis.39 Direct contact between astrocytes and TCs induces calcium sequestration.40 The production of IL-1β, tumor necrosis factor alpha (TNF-α), tumor growth factor beta (TGF-β), and IL-6 by astrocytes was shown to induce proliferation of breast and lung cancer cells.41 TCs use astrocyte gap junctions to transfer cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) to astrocytes, boosting the production of inflammatory cytokines such as interferon alpha (INFα) and TNF (Fig. 1). In turn, these factors activate the signal transducer and activator of transcription 1 (STAT1) and NF-κB pathways in brain metastatic cells, promoting tumor growth and chemoresistance.42 Direct contact between astrocytes and TCs induces activation of the Akt/mitogen-activated protein kinase (MAPK) pathways, leading to the upregulation of anti-apoptotic genes (GSTA5, BCL2L1, and TWIST1) in TCs that mediate resistance against cytotoxic drugs.43 In addition, IL-1β, secreted by the breast cancer cells, specifically upregulates the Notch ligand JAG1 on primary rat astrocytes, which in turn promote Notch signaling in CTCs.44
Matrix metalloproteinases are produced by TCs or their environment and play a role in growth, angiogenesis, and migration in multiple stages of tumor progression. MMPs are characterized according to the specificity of the degrading substrate; MMP-2 and MMP-9 degrade type IV collagen, which is the main component of the vascular basement membrane.45 In a rat model, MMP-2, MMP-9, and MMP-3 have been proposed to play a role in the establishment of breast cancer BM.46 MMP-2 is initially secreted in a proactive form and is activated through interaction with MMP-14 (or MT1-MMP) on the cell surface at the target site, which expression is increased by cyclooxygenase-2 (COX-2, also known as PTGS2).47 MMP-1, secreted by 231BrM cells, degrades occludin and claudin-5, which are major components of the TJs of the BBB. MMP-1 expression strongly correlates with COX-2 expression, indicating its active role in BM of breast cancer patients.48 COX-2, the EGFR ligand HBEGF, and the α2,6-sialyltransferase ST6GALNAC5 genes were identified as mediators of breast cancer cell passage through the BBB. COX-2 changes the permeability of the BBB,49 and its overexpression is observed in about 40% of breast tumors. While COX-2 and HBEGF expression in primary tumors enhances tumor cell extravasation into both brain and lungs, ST6GALNAC5 was found as a specific mediator of BM of breast cancer.50 In contrast, upregulation of ST6GALNAC5 in brain-seeking breast cancer cells (MDA-MB-231 BrM2) decreased their adhesion to the endothelial component of a well-characterized human BBB in vitro model.51 Various other genes involved in transcription, translation, and metabolism were associated with BM.52 Heparanase (HPSE) is a pro-tumorigenic, pro-angiogenic, and pro-metastatic endoglycosidase overexpressed in brain metastatic breast cancer.53 This downstream target of EGFR/HER2 signaling is also produced by brain endothelial and glial cells, promoting tumor cell invasion.54 The expression of heparanase-1 (HPR1) reportedly is inversely correlated with deposition of heparin sulfate (HS), a component of proteoglycans and constituent of the ECM and basement membrane, correlated with the metastatic potential of breast cancer.55 The coordinated action of MMP-9 and HPSE contributes to breast cancer cell carcinogenesis and progression.56 Angiogenesis and MMP activity are enhanced by cathepsin B (CTSB).57 This cysteine protease is often overexpressed in breast cancer, increasing matrix degradation, invasiveness, and tumor growth.58 Cathepsin S (CTSS) was identified as a breast cancer BM promoter, crucial for metastatic seeding and outgrowth; CTSS mediates BBB transmigration of breast CTCs by proteolytic processing of junctional adhesion molecule B (JAM-B).59 Various studies have shown that caveolin-1 (Cav-1) acts as a tumor suppressor protein in human breast cancer. Cav-1 also inhibits discharge and expression of MMP-9 and MMP-2.60 Upregulation of Cav-1 in vitro and in nude mice was shown to mimic effects of STAT3 activation, suppressing tumor growth and attenuating the invasiveness of breast cancer.61 Conversely, in another recent in vivo study, Cav-1 was shown to be mechanosensitive to low shear stress exposure, and its activation-induced PI3K/Akt/mammalian target of rapamycin (mTOR) signaling promoted motility, invadopodia formation, and lung metastasis of breast carcinoma MDA-MB-231 cells.62 STAT3 controls constitutive and inducible VEGF receptor 2 expression in tumor-associated brain endothelial cells, and its inhibition suppresses BM of breast cancer cells.63 STAT3 intercedes with the upregulation of mitogen extracellular-signal-regulated kinase 5 (MEK5), which promotes breast cancer cell invasion through epithelial to mesenchymal transition (EMT). Ectopic expression of MEK5 could provide non-invasive breast cancer cells with invasive capacity.64 Recent work on expressed proteins in tumor-derived exosomes demonstrated an important role of exosomal-annexin A2 (exo-AnxA2) in breast cancer pathogenesis. Moreover, exo-AnxA2, overexpressed in malignant breast cells, was shown to mediate activation of the p38MAPK, NF-κB, and STAT3 pathways as well as the secretion of IL-6 and TNF-α, increasing angiogenesis and breast cancer proliferation. Furthermore, in vivo analysis revealed that priming with AnxA2-depleted exosomes reduced BM formation ~4-fold.65 Altogether, these findings highlight the critical role of the tumor microenvironment in breast cancer progression and metastatic behavior.
Several other genes were reported to be upregulated in breast cancer BM, being potential targets of treatment. Hexokinase II and βIII-tubulin (TUBB3) expression is significantly associated with distant metastases. Knockdown of TUBB3 in a breast cancer BM cell line compromised its metastatic ability in vivo, improving survival in a BM model.66 Angiotensin II (AngII), a potent vasoactive peptide, was shown to contribute to increased tumor-endothelial cell adhesion, transendothelial migration, and motility, accelerating metastatic progression in an experimental mouse model. Besides upregulating MMP-2 and MMP-9 gene expression in breast cancer cells, AngII was also reported to upregulate the expression of intercellular adhesion molecule 1 (ICAM-1).67 In the same line, αβ-crystallin (CRYAB) promoted adhesion of TN breast cancer cells to human brain microvascular endothelial cells, enhanced penetration through the BBB in vivo, and was indicated as an independent predictor for the development of BM.68,69 Using in vivo mouse models for breast cancer BM, angiopoietin-2 (Ang-2) was observed to be elevated in activated brain microvascular endothelial cells, due to the presence of VEGF secreted by TN breast cancer cells and by their “brain-seeking” variant. Secreted Ang-2 impaired TJ structures and increased BBB permeability, resulting in TN breast cancer colonization of the brain.70
Clinical Perspectives
So far, only a few clinical trials have allowed for the inclusion of patients with breast cancer and CNS metastases. There is a trend of initiating trials that explore the efficacy of new targeted therapies with specific focus on BM71 (Table 2). None of the agents used to treat BM in a clinical setting are directed against any of the molecules that are operative in crossing the BBB or the intracerebral propagation of TCs. Conversely, the available studies have investigated BM from breast cancer compounds with proven efficacy in the systemic disease. Their activity has been evaluated in terms of either intracranial response and/or progression-free survival (PFS) in patients with established BM, or prevention of the development of BM in patients without or with stable BM following local treatments (chemoprevention). So far, no targeted agents with the specific indication of BM are registered in the US or Europe.72 Most investigations concern the HER2+ subtype of breast cancer. Monoclonal antibodies with action against HER2, such as trastuzumab, pertuzumab, and trastuzumab emtansine (TDM1), are considered to be too large to cross an intact BBB for an effective chemoprevention. However, the BBB may be damaged in case of macrometastases, or following radiotherapy, resulting in increased CSF levels of trastuzumab.73,74 HER2+ metastatic brain lesions can be visualized by 64Cu-DOTA-trastuzumab PET.75 Continuing treatment with trastuzumab beyond the development of BM may result in a survival benefit,76 and there is now renewed interest in studying high doses of trastuzumab as a treatment of HER2+ BM.77 Following treatment with TDM1, response rates in the brain are reported to be similar to those observed in extracranial sites.78
Table 2.
Overview of actionable targets and clinical studies on targeted therapies in established brain metastasis
| Target | Targeted Agent | Pretreatment with Radiotherapy |
Response Rate | Progression-free Survival (mo) | Overall Survival | Type of Trial | Reference or Clinicaltrials.Gov |
|---|---|---|---|---|---|---|---|
| HER2, EGFR | Lapatinib | Yes | 6% | 2.4 | 6.4 | Phase II | 81 |
| Lapatinib + Capecitabine |
No | 66% | 5.5 | ˃70% (1 y) |
Phase II | 83 | |
| Her2 | Neratinib | Yes | 8% | 1.9 | 8.7 | Phase II | 94 |
| Neratinib + Capecitabine |
Yes | 49% | NA | 63% (1 y) |
Phase II | 95(preliminary results) | |
| Tucatinib (ONT- 380) + (TDM1) | Yes | 33% | 6.5 | NA | Phase I | 97 | |
| PARP | Iniparib c | Yes | 27% | 2.14 | NA | Phase II | 102 |
| HER2 | Pertuzumab + High-dose Trastuzumab (intravenous) |
Yes | NA | NA | NA | Phase II | NCT02536339 |
| Pertuzumab + Trastuzumab (intrathecal) |
No | NA | NA | NA | Phase I | NCT02598427 | |
| Tucatinib (ONT- 380) + Trastuzumab |
Yes | NA | NA | NA | Phase I | NCT019221335 | |
| CDK4/6 | Abemaciclib | Yes | NA | NA | NA | Phase II | NCT02774681 |
| Palbociclib | No | NA | NA | NA | Phase II | NCT02308020 | |
| P13K/Akt | Everolimus | Yes | NA | NA | NA | Phase II |
NCT01305941 a NCT01783756 b |
| PARP | Veliparib | Yes (in association) |
NA | NA | NA | Phase II | NCT00649207 |
TDM1, trastuzumab emtansine; CDK4/6, cyclin-dependent kinase 4 and 6; Akt, protein kinase B; NA, not available.
aIn association with trastuzumab and vinorelbine. bIn association with lapatinib and capecitabine. cIn association with irinotecan.
HER2 receptors enhance EGFR signaling,79 and therefore simultaneous inhibition of HER2 and EGFR may be superior to HER2 inhibition alone.80 Lapatinib, a small-molecule tyrosine kinase inhibitor with activity against HER2 and EGFR, is approved in combination with capecitabine for the treatment of metastatic breast cancer. The activity of lapatinib used as a single agent in pretreated BM is modest (response rate of 6%)81 but increases to 66% by adding capecitabine.82–84 The potential chemopreventive activity of lapatinib was suggested by the results of a phase III randomized trial, in which the effects of lapatinib plus capecitabine versus capecitabine alone were compared in patients with advanced breast cancer who had progressed on trastuzumab: fewer patients with CNS involvement at first progression were in the lapatinib-containing arm (2% vs 6%).85 However, prospective validation of this finding was inconclusive in the CEREBEL86 and EMILIA87 trials. Recent preclinical studies have shown that lapatinib is a substrate of ATP-binding cassette (ABC) transporters (in particular ABCB1), potentially limiting the capacity of the drug to penetrate an intact BBB.88 Therapeutic levels of lapatinib may be reached in established BM, but concentrations are by far lower than those reached for extracranial metastases.89 A dual HER2 inhibition, for instance by the combined use of pertuzumab and trastuzumab, could be active in the prevention of BM. A post hoc analysis of the CLEOPATRA trial suggested that the combination of pertuzumab, trastuzumab, and docetaxel could delay the onset of CNS disease compared with docetaxel alone.90
Novel HER2 targeted tyrosine kinase inhibitors that are potentially active in BM include neratinib and tucatinib (ONT-380, ARRY 380). Neratinib is an irreversible inhibitor of HER2, Erb1, and ErbB4 and preclinical data suggest that it may penetrate an intact BBB. In contrast to lapatinib, neratinib reverses ABCB1-mediated chemoresistance, and is unaltered by the presence of ABCB2 transporter.91 Neratinib was reported to target activating HER2 mutations in HER2 gene amplification–negative breast cancer, thereby overcoming resistance to trastuzumab or lapatinib.92,93 Thus far, the efficacy of neratinib on established BM is modest and similar to that observed with lapatinib. A recent phase II study on HER2-positive BM in patients who had progressed on at least one prior CNS-directed therapy has shown an intracranial response rate of 8% and a median PFS of 1.9 months.94 The association of neratinib and capecitabine is being investigated in an ongoing trial with interesting preliminary results in terms of response rate (49%) and 12-month survival (63%).95 Neratinib may be more active in the prevention than in the treatment of BM. A randomized clinical trial in previously untreated metastatic HER2-positive breast cancer showed that symptomatic or progressive CNS recurrences occurred in 8.3% of patients in the neratinib-paclitaxel group versus 17% of patients in the trastuzumab-paclitaxel group.96 The estimated Kaplan–Meier 2-year incidence of CNS recurrences was 16.3% in the neratinib-paclitaxel group versus 31.2% in the trastuzumab-paclitaxel group. ONT-380 selectively targets HER2, has the potential to cross the BBB, and has minimal activity against EGFR, leading to a more favorable toxicity profile. ONT-380 has shown antitumor activity in heavily pretreated HER2+ metastatic breast cancer patients in a phase I study.97 In another phase I study in which ONT-380 was combined with TDM1, a response rate of 33% and an intracranial PFS of 6.5 months among 26 evaluable patients with BM were reached.98 Another phase I study on the association of escalation doses of ONT-380 in combination with trastuzumab is ongoing.99
For the treatment of patients with BM in hormone receptor positive (HR+) breast cancer, CDK4/6 inhibitors (abemaciclib, palbociclib) are of interest. In particular abemaciclib, which has good CNS penetration in preclinical models, can reach therapeutic levels in human BM.100 Other potential therapeutic targets of patients with breast cancer BM include the PI3K/Akt/mTOR pathway and everolimus, an mTOR inhibitor currently being investigated in trials on BM.
Given the frequency of BM in patients with TN breast cancer and the limited efficacy of conventional chemotherapy, there is urgent need of new molecular approaches. Poly(ADP-ribose) polymerase (PARP) inhibitors are now being investigated, and preliminary results from a phase III trial (the OlympiAD study) suggest that olaparib, as compared with single-agent standard chemotherapy, could be an effective option, especially in patients with TN breast cancer and breast cancer mutations.101 Other PARP inhibitors under investigation in breast cancer are veliparib and iniparib102 in ongoing trials allowing the inclusion of patients with BM. Alternative new avenues of treatments being investigated in TN breast cancer are inhibitors of the PI3K/Akt pathway, selective androgen antagonists, and newer antibody–drug conjugates. VEGF plays an important role in angiogenesis of BM. Vascular normalization induced by bevacizumab, a monoclonal antibody targeting VEGF, delivered prior to chemotherapy, could enhance its efficacy, and trials have been launched to confirm these preliminary results.103
There are several challenges for the development of new drugs with better activity in BM from breast cancer. Regarding established BM, there is a need to improve the quality of results of novel clinical trials by employing specific inclusion criteria and more homogeneous and well-defined endpoints adapted for the valuation of targeted agents, such as the recently proposed criteria of Response Assessment in Neuro-Oncology.104,105 In particular, promising new agents should be tested upfront in clinical trials on patients with small and asymptomatic metastatic lesions, reserving radiotherapy (whole-brain radiotherapy, stereotactic radiosurgery) as salvage. This is important, as lesions that emerge after radiotherapy are often resistant to drugs. On the other hand, new compounds with radio-potentiating properties should be investigated in clinical trials in association with radiotherapy with careful monitoring of acute/early adverse effects and late cognitive function deterioration.
Phase 0 trials that investigate the distribution and activity of molecular compounds, given before neurosurgical resection of BM, are needed for monitoring potential intra- and/or interpatient heterogeneity.106 Trials focusing on chemoprevention are important. In addition to compounds that are active in preclinical models and able to penetrate an intact BBB, there is need to identify those patients who are at high risk of developing BM, particularly those at higher risk of first or isolated brain relapse, such as HER2+ patients.3 The development of biomarkers associated with the risk of brain colonization in humans is another unmet need.107
Concluding Remarks
BM is considered one of the major causes of mortality in breast cancer patients. TN breast cancer, basal-like subtype, and HER2-enriched breast cancers are most associated with BM. Patients with active BM are often excluded from clinical trials in part because systemic response and brain response do not correlate. The divergent therapeutic responses might be due to the molecular alterations that are specifically present in brain metastatic lesions while not in the primary tumors. Results so far indicate that tumor tissue in regional lymph nodes or extracranial metastasis does not resemble BM. A more comprehensive characterization of the primary lesion might disclose subclones that more closely feature intracranial disease. The identification of genomic and expressional alterations specific to BM is crucial to the development of BM-specific therapies. In addition, following up on discoveries regarding the molecular pathways of TCs involved in crossing the BBB and entering the brain will yield targets for BM preventive strategies.
Funding
This work was supported by the Dutch Cancer Society, KWF grant # EMCR 2009-4553.
Conflict of interest statement. None.
References
- 1. Goldhirsch A, Winer EP, Coates AS, et al. ; Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. [DOI] [PubMed] [Google Scholar]
- 3. Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zagar TM, Van Swearingen AE, Kaidar-Person O, Ewend MG, Anders CK. Multidisciplinary management of breast cancer brain metastases. Oncology (Williston Park). 2016;30(10):923–933. [PubMed] [Google Scholar]
- 5. Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464(7291):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee JY, Park K, Lim SH, et al. Mutational profiling of brain metastasis from breast cancer: matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget. 2015;6(41):43731–43742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Da Silva L, Simpson PT, Smart CE, et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010;12(4):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67(9):4190–4198. [DOI] [PubMed] [Google Scholar]
- 10. Sun M, Behrens C, Feng L, et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res. 2009;15(15):4829–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hohensee I, Lamszus K, Riethdorf S, et al. Frequent genetic alterations in EGFR- and HER2-driven pathways in breast cancer brain metastases. Am J Pathol. 2013;183(1):83–95. [DOI] [PubMed] [Google Scholar]
- 12. Soria JC, Lee HY, Lee JI, et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8(5):1178–1184. [PubMed] [Google Scholar]
- 13. Wikman H, Lamszus K, Detels N, et al. Relevance of PTEN loss in brain metastasis formation in breast cancer patients. Breast Cancer Res. 2012;14(2):R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duchnowska R, Jarząb M, Żebracka-Gala J, et al. ; Polish Brain Metastasis Consortium Brain metastasis prediction by transcriptomic profiling in triple-negative breast cancer. Clin Breast Cancer. 2017;17(2):e65–e75. [DOI] [PubMed] [Google Scholar]
- 15. Klemm F, Bleckmann A, Siam L, et al. β-catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis. 2011;32(3):434–442. [DOI] [PubMed] [Google Scholar]
- 16. Pukrop T, Dehghani F, Chuang HN, et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia. 2010;58(12):1477–1489. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa DAM, Pedrosa R, Smid M, et al. T lymphocytes facilitate brain metastasis of breast cancer by inducing guanylate-binding protein 1 expression. Acta Neuropathol. 2018. doi:10.1007/s00401-018-1806-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stewart DA, Cooper CR, Sikes RA. Changes in extracellular matrix (ECM) and ECM-associated proteins in the metastatic progression of prostate cancer. Reprod Biol Endocrinol. 2004;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fazakas C, Wilhelm I, Nagyoszi P, et al. Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases. PLoS One. 2011;6(6):e20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kienast Y, von Baumgarten L, Fuhrmann M, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1):116–122. [DOI] [PubMed] [Google Scholar]
- 21. Wilhelm I, Molnár J, Fazakas C, Haskó J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. 2013;14(1):1383–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25(4):305–324. [DOI] [PubMed] [Google Scholar]
- 23. Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. [DOI] [PubMed] [Google Scholar]
- 24. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8(5):R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edwards DR, Murphy G. Cancer. Proteases—invasion and more. Nature. 1998;394(6693):527–528. [DOI] [PubMed] [Google Scholar]
- 27. Valiente M, Obenauf AC, Jin X, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. [DOI] [PubMed] [Google Scholar]
- 29. Felding-Habermann B, O’Toole TE, Smith JW, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98(4):1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of tumor cell integrin alphavbeta3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci U S A. 2009;106(26):10666–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan J, Cai B, Zeng M, Hao Y, Giancotti FG, Fu BM. Integrin β4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF. Ann Biomed Eng. 2011;39(8):2223–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as “soil” in brain metastasis. PLoS One. 2009;4(6):e5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sathiyanadan K, Coisne C, Enzmann G, Deutsch U, Engelhardt B. PSGL-1 and E/P-selectins are essential for T-cell rolling in inflamed CNS microvessels but dispensable for initiation of EAE. Eur J Immunol. 2014;44(8):2287–2294. [DOI] [PubMed] [Google Scholar]
- 34. Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11(11):1473–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salmaggi A, Maderna E, Calatozzolo C, et al. CXCL12, CXCR4 and CXCR7 expression in brain metastases. Cancer Biol Ther. 2009;8(17):1608–1614. [DOI] [PubMed] [Google Scholar]
- 36. Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis. 2010;27(2):97–105. [DOI] [PubMed] [Google Scholar]
- 37. Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2(6):327–338. [PubMed] [Google Scholar]
- 38. Helbig G, Christopherson KW II, Bhat-Nakshatri P, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278(24):21631–21638. [DOI] [PubMed] [Google Scholar]
- 39. Seth P, Koul N. Astrocyte, the star avatar: redefined. J Biosci. 2008;33(3):405–421. [DOI] [PubMed] [Google Scholar]
- 40. Kim SW, Choi HJ, Lee HJ, et al. Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro Oncol. 2014;16(12):1585–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seike T, Fujita K, Yamakawa Y, et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis. 2011;28(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Q, Boire A, Jin X, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim SJ, Kim JS, Park ES, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia. 2011;13(3):286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xing F, Kobayashi A, Okuda H, et al. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol Med. 2013;5(3):384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang M, Teng XD, Guo XX, Li ZG, Han JG, Yao L. Expression of tissue levels of matrix metalloproteinases and their inhibitors in breast cancer. Breast. 2013;22(3):330–334. [DOI] [PubMed] [Google Scholar]
- 46. Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis. 2005;22(3):237–246. [DOI] [PubMed] [Google Scholar]
- 47. Mohammad MA, Zeeneldin AA, Abd Elmageed ZY, et al. Clinical relevance of cyclooxygenase-2 and matrix metalloproteinases (MMP-2 and MT1-MMP) in human breast cancer tissue. Mol Cell Biochem. 2012;366(1–2):269–275. [DOI] [PubMed] [Google Scholar]
- 48. Wu K, Fukuda K, Xing F, et al. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290(15):9842–9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee KY, Kim YJ, Yoo H, Lee SH, Park JB, Kim HJ. Human brain endothelial cell-derived COX-2 facilitates extravasation of breast cancer cells across the blood-brain barrier. Anticancer Res. 2011;31(12):4307–4313. [PubMed] [Google Scholar]
- 50. Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Drolez A, Vandenhaute E, Delannoy CP, et al. ST6GALNAC5 expression decreases the interactions between breast cancer cells and the human blood-brain barrier. Int J Mol Sci. 2016;17(8):doi: 10.3390/ijms17081309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klein A, Olendrowitz C, Schmutzler R, et al. Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett. 2009;276(2):212–220. [DOI] [PubMed] [Google Scholar]
- 53. Zhang L, Sullivan PS, Goodman JC, Gunaratne PH, Marchetti D. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 2011;71(3):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marchetti D, Li J, Shen R. Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res. 2000;60(17):4767–4770. [PubMed] [Google Scholar]
- 55. Maxhimer JB, Quiros RM, Stewart R, et al. Heparanase-1 expression is associated with the metastatic potential of breast cancer. Surgery. 2002;132(2):326–333. [DOI] [PubMed] [Google Scholar]
- 56. Tang D, Piao Y, Zhao S, et al. Expression and correlation of matrix metalloproteinase-9 and heparanase in patients with breast cancer. Med Oncol. 2014;31(7):26. [DOI] [PubMed] [Google Scholar]
- 57. Kostoulas G, Lang A, Nagase H, Baici A. Stimulation of angiogenesis through cathepsin B inactivation of the tissue inhibitors of matrix metalloproteinases. FEBS Lett. 1999;455(3):286–290. [DOI] [PubMed] [Google Scholar]
- 58. Bengsch F, Buck A, Günther SC, et al. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene. 2014;33(36):4474–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sevenich L, Bowman RL, Mason SD, et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol. 2014;16(9):876–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Williams TM, Medina F, Badano I, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279(49):51630–51646. [DOI] [PubMed] [Google Scholar]
- 61. Chiu WT, Lee HT, Huang FJ, et al. Caveolin-1 upregulation mediates suppression of primary breast tumor growth and brain metastases by stat3 inhibition. Cancer Res. 2011;71(14):4932–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang H, Guan L, Li S, et al. Mechanosensitive caveolin-1 activation-induced PI3K/Akt/mTOR signaling pathway promotes breast cancer motility, invadopodia formation and metastasis in vivo. Oncotarget. 2016;7(13):16227–16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee HT, Xue J, Chou PC, et al. Stat3 orchestrates interaction between endothelial and tumor cells and inhibition of Stat3 suppresses brain metastasis of breast cancer cells. Oncotarget. 2015;6(12):10016–10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu F, Zhang H, Song H. Upregulation of MEK5 by Stat3 promotes breast cancer cell invasion and metastasis. Oncol Rep. 2016;37(1):83–90. [DOI] [PubMed] [Google Scholar]
- 65. Maji S, Chaudhary P, Akopova I, et al. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol Cancer Res. 2017;15(1):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kanojia D, Morshed RA, Zhang L, et al. βIII-Tubulin regulates breast cancer metastases to the brain. Mol Cancer Ther. 2015;14(5):1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rodrigues-Ferreira S, Abdelkarim M, Dillenburg-Pilla P, et al. Angiotensin II facilitates breast cancer cell migration and metastasis. PLoS One. 2012;7(4):e35667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Malin D, Strekalova E, Petrovic V, et al. αB-crystallin: a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res. 2014;20(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Voduc KD, Nielsen TO, Perou CM, et al. alphaB-crystallin expression in breast cancer is associated with brain metastasis. NPJ Breast Cancer. 2015;1:doi: 10.1038/npjbcancer.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Avraham HK, Jiang S, Fu Y, Nakshatri H, Ovadia H, Avraham S. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J Pathol. 2014;232(3):369–381. [DOI] [PubMed] [Google Scholar]
- 71. Costa R, Carneiro BA, Wainwright DA, et al. Developmental therapeutics for patients with breast cancer and central nervous system metastasis: current landscape and future perspectives. Ann Oncol. 2017;28(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Soffietti R, Abacioglu U, Baumert B, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. 2017;19(2):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18(11):2349–2351. [DOI] [PubMed] [Google Scholar]
- 74. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18(1):23–28. [DOI] [PubMed] [Google Scholar]
- 75. Kurihara H, Hamada A, Yoshida M, et al. (64)Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bartsch R, Rottenfusser A, Wenzel C, et al. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol. 2007;85(3):311–317. [DOI] [PubMed] [Google Scholar]
- 77. Lin NU, Prowell T, Tan AR, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology-friends of cancer research brain metastases working group. J Clin Oncol. 2017;35(33):3760–3773. [DOI] [PubMed] [Google Scholar]
- 78. Jacot W, Pons E, Frenel JS, et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat. 2016;157(2):307–318. [DOI] [PubMed] [Google Scholar]
- 79. Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274(13):8865–8874. [DOI] [PubMed] [Google Scholar]
- 80. Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–1639. [DOI] [PubMed] [Google Scholar]
- 81. Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. [DOI] [PubMed] [Google Scholar]
- 82. Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22(3):625–630. [DOI] [PubMed] [Google Scholar]
- 83. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 84. Sutherland S, Ashley S, Miles D, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases—the UK experience. Br J Cancer. 2010;102(6):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–543. [DOI] [PubMed] [Google Scholar]
- 86. Pivot X, Manikhas A, Żurawski B, et al. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33(14):1564–1573. [DOI] [PubMed] [Google Scholar]
- 87. Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Polli JW, Humphreys JE, Harmon KA, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36(4):695–701. [DOI] [PubMed] [Google Scholar]
- 89. Taskar KS, Rudraraju V, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29(3):770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Swain SM, Im YH, Im SA, et al. Safety profile of pertuzumab with trastuzumab and docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncologist. 2014;19(7):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhao XQ, Xie JD, Chen XG, et al. Neratinib reverses ATP-binding cassette B1-mediated chemotherapeutic drug resistance in vitro, in vivo, and ex vivo. Mol Pharmacol. 2012;82(1):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Canonici A, Gijsen M, Mullooly M, et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget. 2013;4(10):1592–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Freedman RA, Gelman RS, Wefel JS, et al. Translational Breast Cancer Research Consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2016;34(9):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rachel AF, Rebecca Sue G, Michelle EM, et al. TBCRC 022: Phase II trial of neratinib + capecitabine for patients (Pts) with human epidermal growth factor receptor 2 (HER2+) breast cancer brain metastases (BCBM). J Clin Oncol. 2017;35(15 suppl):1005. [Google Scholar]
- 96. Awada A, Colomer R, Inoue K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2(12):1557–1564. [DOI] [PubMed] [Google Scholar]
- 97. Moulder SL, Borges VF, Baetz T, et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2+-advanced solid tumors, with an expansion cohort in HER2+metastatic breast cancer (MBC). Clin Cancer Res. 2017;23(14):3529–3536. [DOI] [PubMed] [Google Scholar]
- 98. Virginia FB, Cristiano F, Nathalie A, et al. Efficacy results of a phase 1b study of ONT-380, a CNS-penetrant TKI, in combination with T-DM1 in HER2+ metastatic breast cancer (MBC), including patients (pts) with brain metastases. J Clin Oncol. 2016;34(15 suppl):513.26729429 [Google Scholar]
- 99. Otto M-F, William Thomas B, Ian EK, et al. Phase I dose-escalation trial of ONT-380 in combination with trastuzumab in participants with brain metastases from HER2+ breast cancer. J Clin Oncol. 2014;32(15 suppl):TPS660. [Google Scholar]
- 100. Solmaz S, Emilie Le R, Palaniappan K, et al. Assessment of concentrations of abemaciclib and its major active metabolites in plasma, CSF, and brain tumor tissue in patients with brain metastases secondary to hormone receptor positive (HR+) breast cancer. J Clin Oncol. 2016;34(15 suppl):526. [Google Scholar]
- 101. Mark ER, Seock-Ah I, Elżbieta S, et al. OlympiAD: Phase III trial of olaparib monotherapy versus chemotherapy for patients (pts) with HER2-negative metastatic breast cancer (mBC) and a germline BRCA mutation (gBRCAm). J Clin Oncol. 2017;35(18 suppl):LBA4. [Google Scholar]
- 102. Anders C, Deal AM, Abramson V, et al. TBCRC 018: phase II study of iniparib in combination with irinotecan to treat progressive triple negative breast cancer brain metastases. Breast Cancer Res Treat. 2014;146(3):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lu YS, Chen TW, Lin CH, et al. ; Taiwan Breast Cancer Consortium Bevacizumab preconditioning followed by etoposide and cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy. Clin Cancer Res. 2015;21(8):1851–1858. [DOI] [PubMed] [Google Scholar]
- 104. Lin NU, Lee EQ, Aoyama H, et al. ; Response Assessment in Neuro-Oncology (RANO) group Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–e278. [DOI] [PubMed] [Google Scholar]
- 105. Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology brain metastases working group. Lancet Oncol. 2018;19(1):e20–e32. [DOI] [PubMed] [Google Scholar]
- 106. Morikawa A, Peereboom DM, Thorsheim HR, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol. 2015;17(2):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Duchnowska R, Jassem J, Goswami CP, et al. Predicting early brain metastases based on clinicopathological factors and gene expression analysis in advanced HER2-positive breast cancer patients. J Neurooncol. 2015;122(1):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]