Abstract
Background
Diagnosis of diffuse intrinsic pontine glioma (DIPG) has relied on imaging studies, since the appearance is pathognomonic, and surgical risk was felt to be high and unlikely to affect therapy. The DIPG Biology and Treatment Study (DIPG-BATS) reported here incorporated a surgical biopsy at presentation and stratified subjects to receive FDA-approved agents chosen on the basis of specific biologic targets.
Methods
Subjects were eligible for the trial if the clinical features and imaging appearance of a newly diagnosed tumor were consistent with a DIPG. Surgical biopsies were performed after enrollment and prior to definitive treatment. All subjects were treated with conventional external beam radiotherapy with bevacizumab, and then stratified to receive bevacizumab with erlotinib or temozolomide, both agents, or neither agent, based on O6-methylguanine-DNA methyltransferase status and epidermal growth factor receptor expression. Whole-genome sequencing and RNA sequencing were performed but not used for treatment assignment.
Results
Fifty-three patients were enrolled at 23 institutions, and 50 underwent biopsy. The median age was 6.4 years, with 24 male and 29 female subjects. Surgical biopsies were performed with a specified technique and no deaths were attributed to the procedure. Two subjects experienced grade 3 toxicities during the procedure (apnea, n = 1; hypertension, n = 1). One subject experienced a neurologic deficit (left hemiparesis) that did not fully recover. Of the 50 tumors biopsied, 46 provided sufficient tissue to perform the study assays (92%, two-stage exact binomial 90% CI: 83%–97%).
Conclusions
Surgical biopsy of DIPGs is technically feasible, associated with acceptable risks, and can provide biologic data that can inform treatment decisions.
Keywords: bevacizumab, DIPG, erlotinib, stereotactic biopsy, temozolomide
Importance of the study
This is the first prospective national clinical trial to examine the feasibility and safety of incorporating surgical biopsy into potential treatment strategies for children with DIPG. Our results, which focus on the surgical procedure and tissue acquisition, demonstrate that although there are risks associated with surgical biopsy, these are acceptable, and comparable to biopsies performed for tumors in other brain locations. This tumor type has an extraordinarily poor prognosis, and incorporation of biologic information into the development of future clinical trials is essential to improvements in outcome.
Diffuse intrinsic pontine glioma (DIPG) is the most common primary neoplasm arising in the brainstem of children, and has an extraordinarily poor outcome, with most children dying within 2 years after the initial diagnosis.1 Although the current World Health Organization (WHO) Classification of Tumors of the Central Nervous System now designates a proportion of DIPGs in the larger category of midline tumors based on the presence of the H3K27M mutation, DIPGs are still diagnosed based on clinical and radiologic criteria. These tumors occur in a relatively narrow age range, most between 5 and 15 years, and their location in an eloquent brain region and infiltrative pattern of growth preclude surgical resection. Based on histologic features derived from autopsies and observed clinical outcomes, it was assumed that the biology of DIPGs was similar to glioblastoma (WHO grade IV astrocytoma) in adults. These assumptions led to testing of many experimental agents initially used in adult clinical trials, which did not result in any meaningful improvement in survival.2
In the last 30 years, the characteristic imaging features of a pontine mass in the context of classical clinical findings of cranial neuropathies and pyramidal symptoms have defined the diagnosis of DIPG and have been used as a surrogate for histologic confirmation. Although many other regions of the brain were considered safe in terms of surgical biopsy, the conventional dogma was that surgical biopsy of brainstem tumors would be associated with unacceptable morbidity. Most children were treated without tissue being obtained from the primary tumor at presentation. For these reasons, the molecular pathogenesis of these tumors remained obscure. More recently, detailed analyses of tissue obtained from a variety of sources, predominantly at autopsy, have shown that primary gliomas in children are biologically distinct compared with tumors occurring in adults.3–6 In particular, most DIPGs carry a specific mutation in one of the histone genes (H3.3 or H3.1), which leads to widespread changes in gene expression that are believed to contribute to oncogenesis.7–9 Of note, these specific histone mutations do not typically occur in adult glioblastoma. These data are consistent with our impression that novel therapeutic strategies will need to be developed in order to successfully improve outcomes in children with DIPG.10,11
Treatment strategies individualized to tumor biology (personalized medicine) rely upon an analysis of tissue obtained at diagnosis. Obtaining tissue has been avoided in patients with DIPG but would be required for any directed therapy based on genetic or epigenetic information. Despite historical biases, modern surgical results suggest that biopsy of brainstem tumors can be accomplished safely with acceptable morbidity.12,13 Most series report transient cranial neuropathies, although more serious complications can occur. While the possible surgical morbidity cannot be ignored or minimized, there is a path forward in terms of the molecular and biologic characterization of DIPGs that can be used to further refine therapeutic decisions.14
The basis of the DIPG Biology and Treatment Study (DIPG-BATS), an investigator-initiated investigational new drug study (principal investigator [PI], M.W.K.), was to determine if a national, multi-institutional trial of upfront biopsy of patients with DIPGs could be safely performed using strict preoperative neurosurgical planning, and whether the specimens obtained were sufficient to fully characterize the molecular nature of each individual patient’s tumor. Finally, if the 2 initial criteria were met, the trial prospectively utilized FDA-approved agents chosen on the basis of specific biologic targets to assess activity of personalized therapy in this poor-prognosis disease. Epidermal growth factor receptor (EGFR) signaling has been identified as a relatively common abnormality in high-grade gliomas, including DIPG.15 Similarly, at the time of protocol development, temozolomide was being broadly used in adult and pediatric brain tumors with benefit to those patients with O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation. Finally, since DIPG was assumed to be similar to malignant gliomas in adults, which are characterized by vascular proliferation and increased vascular endothelial growth factor (VEGF) secretion, bevacizumab was added. Therefore, the plan was to treat all subjects with standard, fractionated external beam radiotherapy concurrently with the VEGF inhibitor bevacizumab and then to stratify subjects to receive the EGFR inhibitor erlotinib or temozolomide, both agents, or neither agent, based on the analysis of each subject’s tumor.
In this report, we describe the initial feasibility and surgical safety results from the DIPG-BATS, a prospective, multicenter therapeutic clinical trial, which demonstrates that surgical biopsy of patients with DIPG can be performed safely by pediatric neurosurgeons in tertiary medical centers, with acceptable morbidity in a multi-institutional clinical trial setting. The feasibility of this approach provides a rationale for including biologic information in the design and conduct of future trials for this disease.
Materials and Methods
Overall Study Design
The objective of the study was to assess the overall survival of children and young adults with DIPGs through treatment with a molecularly based strategy, compared with historical controls (COG ACNS0126). Secondary objectives were to determine the safety and potential morbidity associated with biopsy of classic DIPGs based on imaging and clinical history as well as ability to perform biologic analyses on the biopsy material obtained to guide therapy. Study procedures were conducted at 23 sites in the United States. All study procedures were reviewed and approved by the institutional review board at each site.
In order to achieve consistency, a standardized surgical protocol was reviewed with the pediatric neurosurgeons performing the procedure at each participating site using a set of training slides developed by the lead surgical PI of the study (N.G.) prior to study initiation. Specific topics reviewed included inclusion criteria, tumor features associated with higher surgical risk, surgical adjuncts, surgical technique, tissue handling, and postoperative management.
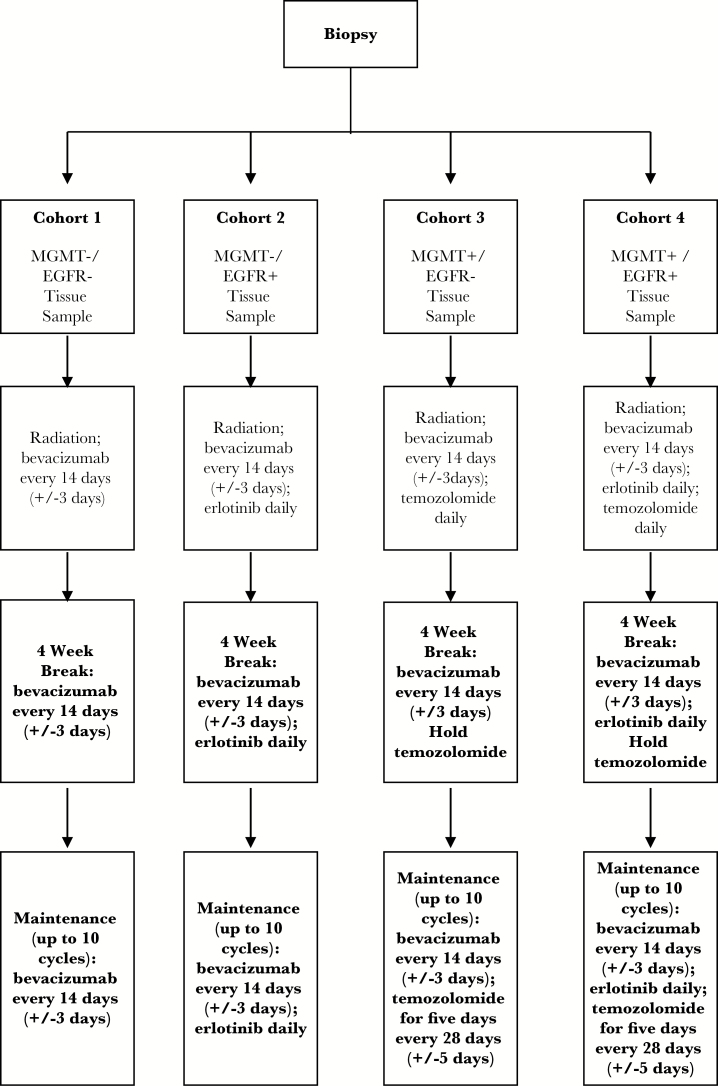
Potential subjects were evaluated at the time of presentation and those with typical DIPGs as assessed by standard clinical exam and MR imaging were considered for enrollment. Study procedures including surgical risk were reviewed with the patient’s parents/guardians and informed consent was obtained prior to enrollment. Assent was obtained in patients old enough to participate in the treatment discussion, according to local institutional guidelines. Assessment of surgical toxicity was performed through day 14 after biopsy. Protocol treatment lasted approximately 52 weeks from the start of radiation therapy in the absence of significant toxicity or progression of disease. Treatment was administered based on the following 4 cohorts:
Cohort 1: Bevacizumab plus irradiation (documentation of neither MGMT promoter methylation nor EGFR overexpression)
Cohort 2: Bevacizumab plus irradiation plus erlotinib (documented EGFR overexpression)
Cohort 3: Bevacizumab plus irradiation plus temozolomide (documented MGMT promoter methylation)
Cohort 4: Bevacizumab plus irradiation plus erlotinib plus temozolomide (documented MGMT promoter methylation and EGFR overexpression)
Treatment doses were as follows: radiation therapy was given in 180 cGy fractions to a total dose of 59.4 + 1.8 Gy/−5.4 Gy. Bevacizumab was administered at 10 mg/kg i.v. every 14 ± 3 days. Erlotinib was administered orally at 85 mg/m2 daily. Temozolomide was administered orally at 90 mg/m2 for 42 days during and after the completion of radiation therapy and then administered at 200 mg/m2 per day for 5 days every 28-day cycle (Figure 1).
Fig. 1.
Study design.
Subjects received radiation therapy beginning 7–21 days after biopsy. Patients in Cohorts 2–4 received concurrent daily erlotinib, temozolomide, or both followed by an approximate 4-week interim period during which bevacizumab and erlotinib was continued (if enrolled on Cohort 2 or 4). Temozolomide was held during this interim period after the 42nd daily dose was provided. Subjects began the maintenance phase of therapy, approximately 10 cycles, based upon cohort assignment. A cycle consisted of 28 ± 3 days. Bevacizumab was given every 2 weeks for all subjects; for subjects in Cohort 2 or 4, erlotinib was given daily; and for subjects in Cohort 3 or 4, temozolomide was given using a 5-day schedule every 28 days. Disease status was assessed every 2 cycles using MR imaging. Subjects without disease progression could continue therapy beyond study completion, but all protocol-specific evaluations (other than survival) concluded after one year. All subjects were followed until death.
Statistical Design
A previous report from a French group described a cohort of 60 children with DIPG who underwent biopsy without any mortality.13 The upper 95% confidence bound for the rate of lethal consequences of surgery, based on their data, would be 4.88%. Therefore, we proposed that if 3 or more of the first 25 subjects enrolled died as a direct result of surgical biopsy, the study would be closed. If the true rate of lethal complications of surgery is 5%, the probability of observing 3 or more deaths in the first 25 subjects enrolled is 0.13.
Subjects enrolled in the study were required to start radiotherapy no sooner than 1 week (and then revised to no sooner than 3 days) but no more than 3 weeks, after biopsy. If the complications arising from surgical biopsy were such that 4 or more subjects of the first 25 patients were unable to start therapy until 3 or more weeks after the date of biopsy, the protocol would be halted. We considered a 20% rate of delay unacceptable. The probability of observing 4 or more subjects with delay of the first 25 if the true rate of delay is 20% would be 0.77.
The feasibility of the proposed molecular approach to therapy was dependent on the ability to assess EGFR expression and MGMT methylation from the biopsy sample, and to return the results to the treating institution within 3 weeks so that the subject could initiate therapy in a timely fashion concurrent with radiation. For statistical design purposes we assumed that the tissue obtained would be nondiagnostic for DIPG in less than 10% of subjects and that an additional 10% of samples would not yield interpretable results for EGFR expression and/or MGMT methylation status. Subjects in whom neither molecular marker was identified were to receive radiotherapy plus bevacizumab (Cohort 1); subjects for whom we were unable to obtain MGMT and EGFR immunohistochemistry results from the biopsy specimen were also treated with radiotherapy plus bevacizumab (Cohort 1). If more than 20% of subjects provided a diagnosis that was not consistent with DIPG or did not provide a complete molecular result, we considered the molecular strategy infeasible. If we failed to confirm a diagnosis or to obtain a molecular result in 8 or more of the first 25 subjects, we considered the attempt to treat with a molecular strategy a failure. If the true rate of inability to provide a molecular result was 20%, the probability that 8 or more of 25 subjects lacked molecular findings was 0.11.
This protocol was designed to allow accrual up to 100 patients with modification of this number based on the distribution of patients between the 4 cohorts. Because there was little information on the distribution of these molecular cohorts across the DIPG population, we had set up stopping rules as follows: if, in the first 25 patients with informative biopsies, no patient was assigned to the targeted treatment group (Cohort 2, 3, or 4), the study would close on the assumption that the proportion of patients with the molecular features we were screening for was so low that it would not be feasible to capture enough patients to make robust inference about the impact of molecularly targeted therapy. If, in the first 25 patients, at least 22 patients were assigned to a single cohort, accrual would stop, as it would be unlikely that the other cohorts would accrue enough patients for robust estimation. If the above conditions were not met, we would continue to accrue another 25 patients with informative biopsies. If, in these 50 patients, we observed 3 or more cohorts with at least 10 patients each, or 2 cohorts with at least 15 patients each, we would add another 50 patients with informative biopsies. If the above rule was not met, accrual would stop at 50 patients with treatment assigned based on molecular findings (which was the eventual sample size achieved). For cohorts with 10 patients or less, statistical analysis within the cohort is descriptive. All patients were followed for progression-free survival and overall survival to evaluate the clinical benefit of adding molecularly targeted agents to bevacizumab and radiation therapy.
Subject Demographics and Subject Inclusion
All subjects underwent MR imaging to confirm the diagnosis of classic DIPG. Central imaging review prior to inclusion was not performed, but if atypical features were present, imaging studies were reviewed by the study chairs with the site investigators prior to subject enrollment.
Operative Procedure
Subjects were included in the study if clinical features were of short duration (<3 mo) and consisted of cranial nerve deficits, long tract signs such as hyperreflexia, clonus, increased tone, and/or ataxia, in addition to a classic MRI appearance of a DIPG. These included but were not restricted to the presence of a diffuse, expansile mass centered in the pons and involving more than 50% of the area; envelopment of the basilar artery; lesions were to be dark on T1-weighted sequences and bright on T2-weighted fluid attenuated inversion recovery sequences, and appear to preserve the pontomedullary boundary, and no other primary CNS lesions or dissemination could be present. Tumors could have a cystic or necrotic component but if this was a dominant feature, subjects were excluded. Areas of contrast enhancement, especially around necrotic areas, were permitted. Diffuse enhancement of the pons was considered atypical and these subjects were not eligible.
All biopsies were performed using standard stereotactic techniques. Open craniotomy was not recommended because of the higher associated morbidity, the increased likelihood of cranial nerve or long tract injury, and slower patient recovery. The biopsy target was selected at the neurosurgeon’s discretion to minimize operative risk, and images of the biopsy location from the navigation software were obtained to allow correlation of the biopsy location and tissue analysis.
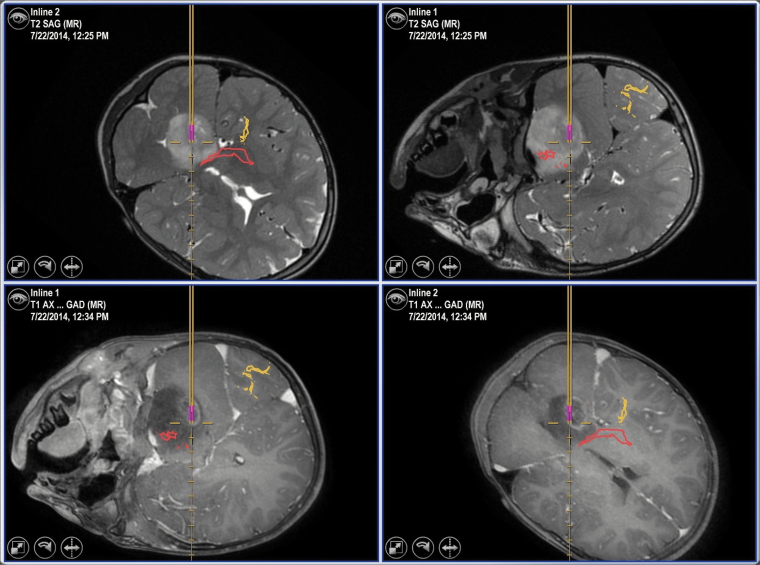
High resolution preoperative MR images were used with frameless stereotactic systems (either Brainlab or Medtronic Stealth platforms) to provide 3D registration after patient positioning. A suboccipital transcerebellar approach was favored, with placement of a small incision (~2 cm) and a burr hole (10–15 mm in diameter) posterior to the ear and below the transverse sinus. The trajectory used passed through the cerebellar white matter, the middle cerebellar peduncle, and then into the main portion of tumor located in the pontine tegmentum (Figure 2). Major descending motor tracts in the ventral pons were avoided, although the choice of mapping tools was left to the neurosurgeon’s discretion. The recommended surgical protocol described above was reviewed by the study surgical chair with each of the neurosurgeons at the participating institutions. The initial reviews were done during in-person meetings convened at professional conferences prior to study initiation. Neurosurgeons from institutions that joined at later times reviewed the surgical protocol with the study surgical chair during teleconferences. A standard set of slides were used to review the surgical protocol with the study neurosurgeons.
Fig. 2.
Representative MR images (T2-weighted images are in the top row, with postcontrast T1-weighted images in the bottom row) obtained from an intraoperative neuronavigation station demonstrating a surgical biopsy of a typical DIPG. In this case, the corticospinal tract (red) and visual pathways (yellow) obtained from DTI sequences are superimposed on the images.
A standard side-biting biopsy needle was used to obtain an initial specimen (~3 mm in length and 1 mm in diameter) for intraoperative pathologic confirmation of a glial neoplasm. Initially up to 3 additional samples were allowed to be obtained for a total of 4 core biopsies; this total number was increased to 6 specimens through a subsequent protocol amendment for the purpose of 2 additional cores to be used to test feasibility of patient cell line and xenograft generation. An upper limit of needle passes was not specified in the event that adequate tissue was not obtained.
Pathology and Immunohistochemical Analyses
Handling of tissue specimens followed a standard operating procedure with priority for usage of biopsy cores. One or 2 core biopsies were reviewed for formalin-fixed paraffin embedded evaluation and standard clinical assessment at each site. All cores were then submitted to the Dana-Farber Cancer Institute for central pathology review for confirmation of the diagnosis as an eligibility requirement (diagnosis compatible with DIPG was required), and adequacy assessment. Immunohistochemical staining for EGFR total protein expression and molecular determination of MGMT promoter methylation status (bisulfite methylation specific PCR) were performed. All evaluations were performed in real time and results were generated from all testing prior to 21 days from day of diagnosis. Per study design, if MGMT and/or EGFR results were not determinable, subjects were treated under the same regimen as Cohort 1.
Results
Eligibility and Subject Inclusion
Fifty-three subjects were enrolled between September 2011 and September 2015. An additional 33 patients were screened for inclusion but were not enrolled for a number of reasons (eg, parents declined a biopsy, low performance scores). Eligibility of subjects for the study to confirm the diagnosis based on imaging and clinical parameters for this national multi-institutional trial was achieved with a mean turnaround time of 8.8 days (SD = 3.99). Subject demographics are described in Table 1. The median age was 6.4 years (range, 3.3–17.5), and the majority was female (M:F = 1:1.2). A total of 50 subjects underwent stereotactic biopsy. Of the 3 nonbiopsied subjects, one had no biopsy performed because of insurance and billing matters; one had evidence of a hemorrhage >0.5 cm on the surgical planning scan; one had low blood pressure (grade 4 hypotension) and heart rate destabilization (grade 4 sinus tachycardia) at the time of induction for general anesthesia. For this last subject, although a skin incision was made, the surgeon elected to stop the procedure prior to performing the biopsy due to the cardiac instability. Of the 50 subjects who underwent biopsy, 48 were taking steroids at baseline or within 14 days after surgery. All 50 subjects received steroids within a month after the date of surgery. All subjects received dexamethasone, except one who received hydrocortisone. The median time from diagnosis to surgery was 6 days (range, 0–34). The median interval between surgery and initiation of radiation treatment was 10 days (range, 4–22).
Table 1.
Demographics for subjects undergoing biopsy (n = 50)
| N | % | |
|---|---|---|
| Age at Registration | ||
| Median | 6.3 (3.3–17.5) | |
| 3–4 | 14 | 28 |
| 5–9 | 27 | 54 |
| 10–14 | 5 | 10 |
| 15–18 | 4 | 8 |
| Sex | ||
| Male | 23 | 46 |
| Female | 27 | 54 |
| Cohort 1 | 30 | 60 |
| Cohort 2 | 14 | 28 |
| Cohort 3 | 3 | 6 |
| Cohort 4 | 3 | 6 |
Perioperative Morbidity
All biopsies were performed through a transcerebellar approach with the goal of avoiding major descending motor pathways. In 3 subjects, there were transient bradycardic episodes during the procedure but these resolved spontaneously and did not recur during the postoperative period. In one subject blood was noted from the biopsy needle after performing the biopsy, and further biopsies were not obtained. A postoperative CT scan did not demonstrate any significant intracranial hemorrhage. There were no intraoperative events resulting in significant hemodynamic instability. Three subjects had ventriculoperitoneal (VP) shunts inserted at the time of biopsy, while another 2 had endoscopic third ventriculostomies performed at the time of biopsy.
There were no deaths directly attributed to the biopsy procedure. One subject died 2 weeks following biopsy, with the cause of mortality attributed to disease progression. In this subject there was development of hydrocephalus subsequent to the biopsy which prompted placement of a VP shunt. Despite this intervention, the patient did not improve and experienced a further decline associated with diffuse brainstem swelling and punctate hemorrhage. Two subjects experienced grade 3 (Common Terminology Criteria for Adverse Events v4.0) toxicities during the procedure (one subject with apnea and one with hypertension, which were transient and did not persist). One subject experienced a neurologic deficit (left hemiparesis) that did not fully recover. One subject experienced a delay in the start of radiation therapy: 22 days following biopsy, rather than 21. Additional complications and adverse events during the first 2 weeks after the procedure are listed in Tables 2 and 3. A complete list of adverse events occurring within 14 days following biopsy are described in Supplementary Tables 1 and 2.
Table 2.
Total grade 3 and 4 toxicities observed in the postprocedural time frame (14 d)
| N | |
|---|---|
| Grade 3 | |
| Alkalosis | 1 |
| Apnea | 2 |
| Depressed consciousness | 3 |
| Dyspnea | 1 |
| Hematoma | 1 |
| Hydrocephalus | 3 |
| Hypotension | 1 |
| Intracranial hemorrhage | 1 |
| Reduced white cell count or platelets | 4 |
| Somnolence | 1 |
| Grade 4 | |
| Intracranial hemorrhage | 1 |
| Respiratory failure | 1 |
Table 3.
Grade 3 and 4 toxicities in the postprocedural time frame (14 d) attributed to the surgical procedure
| Subject | Adverse Event | Outcome |
|---|---|---|
| 16 | Hypertension and hyponatremia | Resolved |
| 17 | Dysarthria | Did not resolve but also thought to be possibly related to radiation |
| 30 | Ataxia | Resolved |
| 35 | Hematoma, depressed level of consciousness, dysphagia | Resolved |
| 53 | Dysphagia, dysarthria | Resolved |
Tissue Specimens and Pathologic Confirmation
Tissue specimens from 50 patients were sent to the Dana-Farber Cancer Institute for central pathology review and molecular analysis prior to stratification. Review of cases and assignment of subjects to treatment cohort based on immunohistochemical and MGMT PCR results occurred within a median of 15 days (range, 8–26) after the biopsy procedure. Among the cohort who had tissue obtained, in 2 subjects there was insufficient tissue to determine a pathologic diagnosis. In one subject, the frozen section diagnosis was not obtained prior to obtaining additional sample, which was a protocol deviation. In a second subject, only a small number of infiltrating tumor cells were noted within the sample, which did not permit a molecular analysis due to low tumor content. These 2 subjects were assigned to Cohort 1. In 2 additional subjects, there was insufficient tissue to obtain an MGMT result and the subjects were placed into Cohorts 1 and 2, respectively, on the basis of EGFR expression. In the remaining subjects, sufficient tissue was obtained to determine both EGFR expression and MGMT status. Overall, of the 50 subjects biopsied, 46 provided sufficient tissue to perform all of the assays (92%, two-stage exact binomial 90% confidence interval: 83%–97%).
Discussion
Until recently, selection of new therapeutic agents for early phase clinical trials for DIPG was empiric and results were uniformly unsuccessful. Only in the last 5 years has the distinct biology of DIPGs been identified.6,16 These findings suggest that effective therapies may require more tumor-specific approaches tailored for biologic targets.11 The design of DIPG-BATS relied on the limited genetic information available regarding these tumors at the time of study development, and the biologic assays that could be performed in a timely manner, namely presence of MGMT promoter methylation and EGFR overexpression. In addition, only combinations for which human data were available could be used in this patient population. Based on these constraints, radiation, bevacizumab, temozolomide, and erlotinib were permitted from a regulatory point of view.
An important goal of the study was to demonstrate that surgical biopsy of DIPGs can be performed with acceptable morbidity, comparable to other neurosurgical procedures in a multi-institutional setting. In this study, there were transient hemodynamic changes noted in 3 of 50 (6%) subjects during the surgical procedure that were not associated with postoperative complications. No mortality was attributed to the surgical procedure. Only one subject (2%) experienced a persistent neurologic deficit (hemiparesis).
Based on the results of this study and other reports,13 stereotactic biopsy is feasible using well-planned surgical procedures to obtain tissue from patients with DIPG with acceptable morbidity. The complications observed were expected and the overall frequency of complications was low. Puget et al reported their experience from a large single-institution case series in which 130 patients with DIPG underwent a stereotactic biopsy.17 Their complication rate was 3.9%, which is in the range of what was noted in this report. In order to achieve consistency across the different centers, surgical procedures performed for this study were done at centers with experience in pediatric neurosurgery and access to current technology. For example, modern neuronavigation tools allow accurate 3D localization of deep structures in the brain and identification of major vessels prior to surgery, which greatly minimizes surgical complications. In this study, we recognized the potential for complications, and specific steps were taken to minimize adverse events. All biopsies were performed through a transcerebellar route, which minimized the extent of brain tissue traversed before reaching the actual tumor. In addition, biopsies were usually obtained from the dorsal half of the tumor, thereby avoiding perforating arteries from the ventral surface of the brainstem and descending corticospinal tracts. Although not mandated in this study, newer neuroimaging tools such as diffusion tensor imaging–based fiber tractography or MR angiography could be theoretically used as surgical adjuncts in subsequent trials to further reduce risk. We recognized that transient cranial neuropathies could impact a patient’s functional status substantially by interfering with activities such as the ability to ambulate or swallow. Patients with a poor functional status (Karnofsky or Lansky ≤40) were excluded from the study. The number of samples obtained during the biopsy was increased from the initial number of 4 to 6, without a resultant increase in adverse events.
Although the size of the specimens obtained from stereotactic biopsy was small, we found that histopathology and immunohistochemical and MGMT testing can be accomplished without difficulty. In only 4 subjects was there insufficient tissue for a complete immunohistochemical analysis. Beyond the primary endpoints, in our ongoing exploratory studies, we have shown that the specimens were sufficient to obtain high-quality nucleic acid samples in 44 of 49 samples. This allows detailed expression and genomic studies, including performance of whole genomic sequencing, RNA sequencing, methylation arrays, single cell sequencing, and patient-derived cell line/xenograft generation (unpublished observations). This is an unprecedented level of analysis which should enable substantial advances for the treatment of patients with DIPG. In fact, an early pilot analysis of the tissue obtained from an initial subset of subjects contributed to the identification of novel mutations in the activin A receptor type 1 gene in a group of midline high-grade tumors.4 Clearly, this work shows that future studies with adequate physician training will be able to rely on incorporation of more detailed genetic information to guide treatment choices. As demonstration of this and partly based on the work described in this manuscript, recently opened trials using advanced sequencing techniques to identify molecular alterations within specific pathways have informed the design of recently opened trials using therapy to be directed against those targets—an example of personalized medicine (ClinicalTrials.gov NCT02274987 and NCT02233049).
Funding
This work was supported by the Zach Carson Fund, Ellie Kavalieros DIPG Fund, Ryan Harvey Fund, Mikey Czech DIPG Foundation, Caroline Cronk Fund, Prayers from Maria, Guglietti DIPG Fund, Markoff Art in Giving Foundation, Brock Fleming Fund, Stop and Shop Pediatric Brain Tumor Program, the DIPG Collaborative (which includes the Hope for Caroline Foundation, Reflections of Grace Foundation, Cure Starts Now Foundation, Soar with Grace Foundation, Abbie’s Army Charity Trust, Julian Boivin Courage for Cures Foundation, Smiles for Sophie Forever, Caroline’s Miracle Foundation, Love, Chloe Foundation, Benny’s World Foundation, Pray Hope Believe Foundation, Jeffrey Thomas Hayden Foundation), and 5P30 CA006516.
Supplementary Material
Acknowledgments
We would like to thank and acknowledge the many children and families affected by pediatric brain tumors for their generous contributions to this research, and the funding groups noted above.
Conflict of interest statement. N.G. receives research funding from Pfizer. P.B. receives research funding from Novartis Institutes for Biomedical Research. M.W.K. has advisory board roles with Novartis, Boehringer, Lilly, Sanofi, Incyte, Sigma-Tau, Merck, BMS, Bayer, and Takeda Pharmaceutical. All other authors have no declared potential conflicts of interest.
References
- 1. Kebudi R, Cakir FB. Management of diffuse pontine gliomas in children: recent developments. Paediatr Drugs. 2013;15(5):351–362.23719782 [Google Scholar]
- 2. Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38(1):27–35. [DOI] [PubMed] [Google Scholar]
- 3. Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46(5):451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet. 2014;46(5):462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor KR, Mackay A, Truffaux N, et al. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet. 2014;46(5):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46(5):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan KM, Fang D, Gan H, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paugh BS, Broniscer A, Qu C, et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol. 2011;29(30):3999–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21(6):555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashizume R, Andor N, Ihara Y, et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med. 2014;20(12):1394–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cage TA, Samagh SP, Mueller S, et al. Feasibility, safety, and indications for surgical biopsy of intrinsic brainstem tumors in children. Childs Nerv Syst. 2013;29(8):1313–1319. [DOI] [PubMed] [Google Scholar]
- 13. Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107 (Suppl 1):1–4. [DOI] [PubMed] [Google Scholar]
- 14. Robison NJ, Kieran MW. Diffuse intrinsic pontine glioma: a reassessment. J Neurooncol. 2014;119(1):7–15. [DOI] [PubMed] [Google Scholar]
- 15. Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9(10):3620–3624. [PubMed] [Google Scholar]
- 16. Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28(18):3061–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puget S, Beccaria K, Blauwblomme T, et al. Biopsy in a series of 130 pediatric diffuse intrinsic pontine gliomas. Childs Nerv Syst. 2015;31(10):1773–1780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.