Given the lack of exit radiation dose, proton therapy (PT) has been used for primary CNS tumors to minimize dose to adjacent, radiosensitive brain structures. For example, exposure of the hippocampus to even a low radiation dose has been associated with neurocognitive impairment.1,2 While PT efficacy and toxicity for CNS tumors is being studied, data on the patterns of care for PT use is limited and based on only pediatric patients.3–5 We used a multicenter registry to identify which CNS patients receive PT and whether differences exist between adult and pediatric CNS PT patients.
Between 2009 and 2017, primary intracranial CNS tumors diagnosed in 1295 patients were treated with PT from 8 community- and academic-based centers on an institutional review board–approved, prospective Proton Collaborative Group (PCG) registry study (NCT01255748). These included the ProCure Proton Therapy Center (PTC)–Oklahoma City, Northwestern Medicine Chicago Proton Center, ProCure PTC–New Jersey, Seattle Cancer Care Alliance PTC, Scripps PTC, Willis-Knighton PTC, Maryland PTC, and the Mayo Clinic Proton Beam Therapy Program (Phoenix, Arizona). Pathology was locally reviewed and categorized according to the 2007 World Health Organization (WHO) classification.6
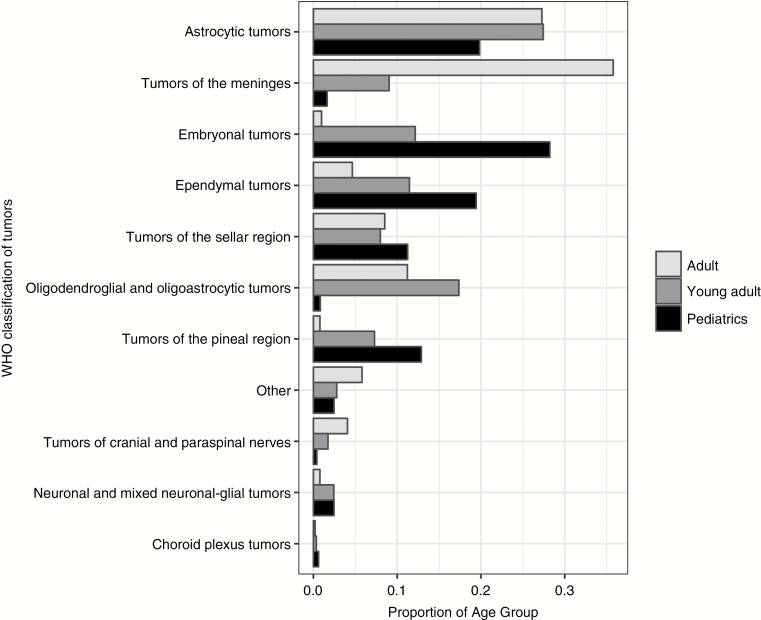
Overall, 37.8% of the patients were pediatric (age ≤18 y), 22.2% were young adult (19–40 y), and 39.9% were adult (>40 y). Males were 52.5%; 78.8% were white (6.3% African American, 2.9% Asian, 12.0% other/unknown), and 67.3% were treated at initial diagnosis. The most commonly treated classification of tumors was astrocytic tumors (24.5%), which included glioblastoma multiforme (GBM; n = 110; 34.7% of astrocytic tumors), followed by pilocytic astrocytoma (n = 74; 23.3%) and WHO grade II astrocytoma (n = 60; 18.9%). The majority of GBM patients were adults (n = 85), 38 had prior radiation, and 10 received >60 Gy. Two centers treated 61% of GBM patients. The frequency of WHO tumor classification varied over age groups (Fig. 1). A minority of patients had prior radiation (14.2%) or were treated for palliation (1.5%). Craniospinal irradiation was used in 195 patients (15.1%), mainly for pediatric and young adult patients.
Fig. 1.
Grouped bar chart of WHO classification of tumors by pediatric, young adult, and adult age category.
To our knowledge, this is the first patterns-of-care study of PT for adult CNS patients. Prior large database3 or survey-based methods4 have evaluated pediatric patients only. Our results confirm that embryonal tumors are the most common to be treated with PT among pediatric CNS patients. In contrast, adults were mainly treated for meningeal and astrocytic tumors. These differences between pediatric and adult CNS patients reflect the overall incidence of these tumors in the respective age populations.7
Nonetheless, we found that the majority of adult patients treated with PT are young with favorable tumors. This likely reflects the selection of patients that may derive the greatest benefit from reduced dose to the brain and body. However, GBM was the most common astrocytic tumor treated with PT and comprised 8.5% of our cohort. In the Central Brain Tumor Registry of the United States (CBTRUS), GBM comprises 14.9% of primary brain tumors.7 Many GBM patients in our study were previously irradiated or likely treated on a clinical trial given that they were treated above standard doses (>60 Gy). Unfortunately, the PCG registry does not capture molecular marker information and/or contemporary CNS patients treated with photon therapy, which would facilitate a comparison of GBM patients treated with protons versus photons. In addition, as PT institutions joined the PCG registry over the study period, we are unable to measure temporal use of PT.
Patients with primary CNS tumors treated with PT are young with relatively favorable tumor types, with few patients treated for aggressive brain tumors such as GBM. This reflects a subset of patients who may derive the greatest benefit from reduction in brain dose. Additional studies are needed to identify which potential factors—physicians, patients, and/or insurance coverage—are the major drivers behind this patient selection.
Funding
No funding was received for this work.
Conflict of interest statement. L.M.H.: research funding from Abbvie; G.L.: equity holder Procure Proton Therapy Center; S.B.: speaker honoraria from Varian Medical Systems.
References
- 1. Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83(4):e487–e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zureick Andrew H, Evans Casey L, Niemierko A, et al. . Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer. 2018;124(10):2238–2245. [DOI] [PubMed] [Google Scholar]
- 3. Odei B, Frandsen JE, Boothe D, Ermoian RP, Poppe MM. Patterns of care in proton radiation therapy for pediatric central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2017;97(1):60–63. [DOI] [PubMed] [Google Scholar]
- 4. Chang AL, Yock TI, Mahajan A, et al. . Pediatric proton therapy: patterns of care across the United States. Int J Particle Ther. 2014;1(2):357–367. [Google Scholar]
- 5. Shen CJ, Hu C, Ladra MM, Narang AK, Pollack CE, Terezakis SA. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer. 2017;123(20):4048–4056. [DOI] [PubMed] [Google Scholar]
- 6. Louis DN, Ohgaki H, Wiestler OD, et al. . The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ostrom QT, Gittleman H, Xu J, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]