Abstract
Background
Immunosuppressive protumoral M2 macrophages are important in pathogenesis, progression, and therapy resistance in glioblastoma (GBM) and provide a target for therapy. Recently oncolytic virotherapy in murine models was shown to change these M2 macrophages toward the pro-inflammatory and antitumoral M1 phenotype. Here we study the effects of the oncolytic virotherapy Delta24-RGD in humans, using both in vitro models and patient material.
Methods
Human monocyte-derived macrophages were co-cultured with Delta24-RGD–infected primary glioma stem-like cells (GSCs) and were analyzed for their immunophenotype, cytokine expression, and secretion profiles. Cerebrospinal fluid (CSF) from 18 Delta24-RGD–treated patients was analyzed for inflammatory cytokine levels, and the effects of these CSF samples on macrophage phenotype in vitro were determined. In addition, tumor macrophages in resected material from a Delta24-RGD–treated GBM patient were compared with 5 control GBM patient samples by flow cytometry.
Results
Human monocyte-derived M2 macrophages co-cultured with Delta24-RGD–infected GSCs shifted toward an M1-immunophenotype, coinciding with pro-inflammatory gene expression and cytokine production. This phenotypic switch was induced by the concerted effects of a change in tumor-produced soluble factors and the presence of viral particles. CSF samples from Delta24-RGD–treated GBM patients revealed cytokine levels indicative of a pro-inflammatory microenvironment. Furthermore, tumoral macrophages in a Delta24-RGD–treated patient showed significantly greater M1 characteristics than in control GBM tissue.
Conclusion
Together these in vitro and patient studies demonstrate that local Delta24-RGD therapy may provide a therapeutic tool to promote a prolonged shift in the protumoral M2 macrophages toward M1 in human GBM, inducing a pro-inflammatory and potentially tumor-detrimental microenvironment.
Keywords: glioblastoma, microenvironment, tumor macrophages, viral therapy
Importance of the study
Tumor macrophages are important for tumor progression and maintenance. This has led to a search for macrophage modulating therapeutic tools. Here we combine human in vitro and patient studies to demonstrate the glioblastoma macrophage modulating effect of the oncolytic adenovirus Delta24-RGD, leading to a pro-inflammatory and tumor-detrimental microenvironment. To our knowledge, this is the first study to describe an oncolytic virus–induced prolonged M2 to M1 tumor macrophage phenotype shift in human GBM patients. Since this shift disables a major GBM-maintaining mechanism and disrupts indirect local GBM-induced immune suppression, this effect may play a significant role in oncolytic virotherapy in humans.
Tumor macrophages have an important role in cancer pathogenesis. Within aggressive tumors such as glioblastoma (GBM), melanoma, and lung carcinoma, tumor-supportive macrophages have been found to inhibit the antitumor immune response and promote angiogenesis, tumor growth, and therapy resistance.1–5 These tumor-supportive macrophages, known as M2 macrophages, are one phenotype on the phenotypic spectrum that macrophages acquire through microenvironmental signaling.6–8
M2 macrophages provide local immune suppression, promote a T helper cell (Th) type 2 response, and are involved in parasite clearance, tissue remodeling, and tumor development through production of interleukin (IL)-1RA, IL-10, vascular endothelial growth factor, and transforming growth factor-β, regulated by interferon regulatory factor 4 (IRF4)‒signal transducer and activator of transcription 6 (STAT6) pathway.7,9–13 At the opposite end of the spectrum are M1 macrophages with pro-inflammatory properties. These macrophages kill intracellular pathogens and promote a Th1 response, as well as mediate an antitumor immune response by pro-inflammatory cytokine production such as tumor necrosis factor alpha (TNF-α), IL-1B, IL-6, IL-8, IL-12, and IL-23, regulated by the nuclear factor-kappaB (NF-κβ) pathway.7,14,15 However, the M1 and M2 dichotomy used to simplify description of polarized macrophages in vitro are phenotypic extremes and therefore do not fully resemble in vivo macrophage phenotypes, which show less pronounced M1/M2 phenotypes and functional plasticity.7 Both phenotypes are primarily regulated through IRF4 (M2) and IRF5 (M1) competing for the myeloid differentiation primary response gene 88 (MyD88) complex involved in Toll-like receptor (TLR) signaling.16,17 Therefore, the in vivo phenotype is based on the dominant cytokines, genes, and proteins expressed.
Within GBM, a malignant brain tumor known for its heterogeneity and dismal prognosis, M2-like macrophages are abundant and their presence is negatively correlated with patients’ progression-free survival and overall survival.18–20 Moreover, in vitro models have shown that glioma stem-like cells (GSCs),21 the current gold standard for glioma cultures, induce the M2 phenotype in macrophages, suppressing an immune response and enabling tumor evasion from immune cell clearance.22,23
A new and promising treatment for GBM consists of oncolytic viruses (OVs), which combine direct cancer cell lysis with systemic antitumor immunity induction.24 The oncolytic adenovirus Delta24-RGD, also known as DNX-2401, was designed to replicate selectively in cells harboring retinoblastoma gene or pathway mutations, currently under investigation in clinical phase II trials for GBM.25 We previously demonstrated that intratumoral injections of this virus in murine GBM leads to increased intratumoral macrophage numbers.26 Recently Saha et al demonstrated in murine models that tumoral macrophages play a crucial role in herpes simplex oncolytic virotherapy and that the virus shifts the murine GBM macrophage phenotype from M2 to M1,27 suggesting that OVs can be used as inducers of pro-inflammatory signaling in macrophages and may offer a tool to shift macrophage phenotype in human tumors.28
As clinical trial material is limited, this OV macrophage modulating effect has thus far not been studied in humans. To elucidate OV effects on behavior and function of human GBM macrophages we investigated macrophage phenotype and function upon Delta24-RGD treatment in vitro and in patients, including extensive analysis of GBM resection material from a Delta24-RGD–treated patient and cerebrospinal fluid (CSF) from 18 Delta24-RGD–treated patients. Together, our results demonstrate that Delta24-RGD therapy changes the GBM macrophage phenotype from protumoral M2 toward the antitumoral and pro-inflammatory M1 phenotype, thereby disabling a major tumor-maintaining mechanism.
Materials and Methods
Patient Material
Tumor tissue was obtained from GBM patients undergoing a routine tumor resection procedure at primary diagnosis. These tumor samples were either used to establish GSC cultures, according to Balvers et al,29 or used for flow cytometric analysis. Tumor tissue was also obtained from 2 patients enrolled in a clinical phase I/II study testing convection-enhanced delivery of Delta24-RGD virus (ClinicalTrials.gov: NCT01582516). This was an open-label nonrandomized 2-center study for which 19 patients were recruited. From Patients 12 and 16, tumor resection material was obtained at 8 weeks and 26 months, respectively, after receiving loco-regional infusion of 10^10 infectious viral particles. Stored material from Patient 12 was used for cryosectioning and immunohistochemistry, while tissue from Patient 16 was directly dissociated and analyzed by flow cytometry as described below.
CSF from 18 clinical trial patients was obtained through a subcutaneous ventricular catheter system enabling repetitive CSF sampling. Within one hour, cells and debris were removed and CSF was stored at −80°C. This trial was approved by the Central Committee on Research Involving Human Subjects and the Ministry of Health, Welfare, and Sport (VWS). All patient materials were obtained according to the local guidelines of the Medical Ethical Committees of Erasmus Medical Center Rotterdam and VU University Medical Center Amsterdam. Written informed consent was obtained from all participants.
Viruses
The conditionally replicating human adenoviruses Delta24-RGD and its derivative encoding green fluorescent protein (GFP) under the adenovirus major late promoter, Delta24-RGD-GFP, were described previously.30,31 The replication-deficient E1 deleted adenovirus expressing luciferase under the cytomegalovirus promoter, Ad.Luc.RGD, has been described previously.32
Immunohistochemistry
Fresh frozen tumor sample cryosections were fixed in acetone before Protein Block (Dako) was added, before staining with cluster of differentiation (CD)68 (clone KP-1, Dako), fluorescein isothiocyanate (FITC)–conjugated anti-hexon (Santa Cruz Biotechnology) and Alexa Fluor 568 rabbit anti-mouse immunoglobulin G (heavy + light chain) (Life Technologies Europe). Slides were mounted with 4′,6′-diamidino-2-phenylindole (Vectashield, Vector Laboratories) and imaged on a Leica DMRB microscope.
Macrophage Culture and Polarization
Human CD14-positive monocytes were isolated by CD14 MicroBeads (Miltenyi) from healthy donor buffy coats (project #NVT 0084.02, Sanquin). Monocytes were cultured for 4 days with Iscove’s modified Dulbecco’s medium (IMDM) (Thermo Fisher Scientific) containing 10% heat-inactivated fetal calf serum (Life Technologies), 5% penicillin/streptomycin (Life Technologies), and 5 ng/mL macrophage colony-stimulating factor (M-CSF) (R&D Systems). Then, IL-4, interferon (IFN)-γ (both 50 ng/mL, R&D Systems), or phosphate buffered saline (PBS) was added for 24 h.
GSCs were pre-infected at multiplicity of infection (MOI) 25 Delta24-RGD for 24 h then washed with PBS twice prior to being added to macrophages cultured and polarized using IL-4 (M2) as described above. All experiments were performed at least twice and in duplicate. Supernatants were stored at −20°C and cells were harvested for flow cytometry or mRNA isolation. Cytochalasin D (Sigma-Aldrich) was added to the appropriate cultures at 10 µM, 5 min prior to addition of infected GSCs.
Viral Infectivity and Replication Assay
Ad.Luc.RGD was added at MOI 25 to cultured M1 and M2 macrophages. After 24 h, intracellular luciferase was measured using the Promega Luciferase Assay according to the manufacturer’s instructions. Delta24-RGD-GFP was added at MOI 25 and macrophages were followed by time-lapse imaging with an IncuCyte system (Essen Bioscience).
Flow Cytometry
Scraped monolayer cells or dissociated tumor material was washed with with PBS+0.5% bovine serum albumin before 30 min staining with antibodies (Supplementary Table S1). Fix&Perm (Nordic MUbio) was used to fix and intracellularly stain with FITC-conjugated anti-hexon (Santa Cruz Biotechnology). Samples were acquired on a FACSCanto II (BD Biosciences) and analyzed using Infinicyt v1.8 (Cytognos) (see Supplementary Fig. S2 for gating strategy).
Gene Expression Analyses
RNA from cultured macrophages was isolated using the RNeasy mini kit (#74104, Qiagen). Primers and probes (Supplementary Table S1) were used to analyze cytokine expression, with the ABL gene as reference. Samples were run for 40 cycles on a 7900 HT Fast Real-Time PCR system (Applied Biosystems).
Cytokine Analysis
Cytokine levels in culture supernatants were measured using the Cytometric Bead Array Human Inflammatory Cytokines Kit (BD Bioscience), according to the manufacturer’s instructions with undiluted samples with technical replicates. Data analyses were done using FCAP Array v3.0 software (BD Bioscience).
Patient CSF cytokine levels were analyzed using MSD electrochemiluminescence panels and a SECTOR Imager 6000 Plate Reader according to the manufacturer’s instructions. Data were analyzed using MSD Discovery Workbench software (all MSD Mesoscale Discovery).
Conditioned Medium Experiments
Control GSCs and Delta24-RGD–infected GSCs (MOI 25) were cultured for 72 h in 5 ng/mL M-CSF in IMDM (10% fetal calf serum + 5% penicillin/streptomycin). The conditioned media (CM) were stored for up to 3 months at −80°. Thawed samples were added to M0 macrophages cultured as above with PBS. After 72 h, cells were harvested and stained for flow cytometric analyses as above. For Delta24-RGD spiking of control GSCs CM, MOI 25 relative to the monocytes was used. CM was filtered by Amicon Ultra-4 (100000 molecular weight cutoff) Centrifugal Filter Device (Millipore) at 4000 g for 15 minutes.
Macrophages Cultured in Patients’ CSF
CD14+ monocytes were cultured for 4 days in 5 ng/mL M-CSF and IMDM (10% fetal calf serum + 5% penicillin/streptomycin). Fresh medium was mixed (2:1) with patients’ CSF and added for 72 h prior to harvesting of cells and flow cytometric analysis.
Statistical Analysis
Statistical analysis was performed with SPSS Statistics 21 (IBM). Missing data points were excluded. Gaussian datasets were analyzed using one-way ANOVA and if P < 0.05 followed by Tukey’s multiple comparison test. Non-Gaussian distributed paired samples were analyzed using the Wilcoxon signed rank test. Differences were considered statistically significant when P < 0.05.
Results and Discussion
Tumor Macrophages Phagocytize Infected Tumor Cells
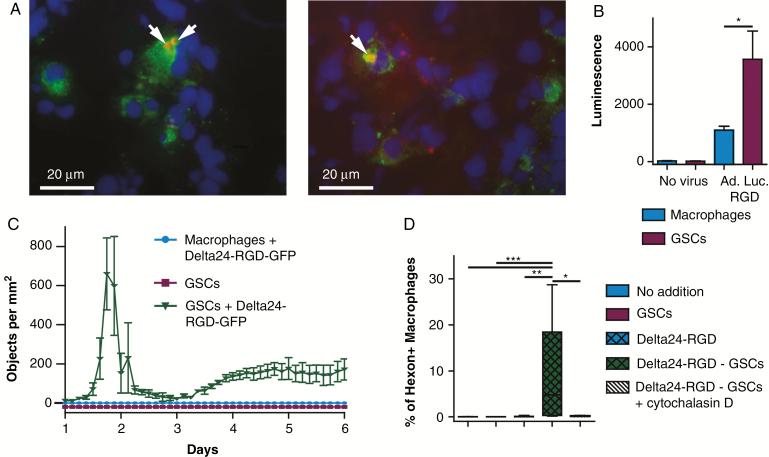
Cryosections of recurrent GBM tissue obtained from a Delta24-RGD–treated patient 8 weeks after virus infusion were stained for the macrophage marker CD68 and the adenoviral protein hexon to assess immune cell infiltration and virus-infected tumor cells. CD68-positive cells contained hexon-positive concentrations (Fig. 1A). We hypothesized that these hexon concentrations may be derived from either viral replication in macrophages or infected tumor cell phagocytosis by macrophages. To investigate this, we infected human macrophages and GSC cultures with the replication-defective adenoviral vector Ad.Luc.RGD. Significantly higher luciferase expression was found in the GSC cultures (ANOVA P = 0.003, Tukey P = 0.032, Fig. 1B), indicating that macrophages are susceptible to RGD-modified adenovirus infection, albeit to a lesser extent than tumor cells. Infection of GSCs and macrophages with the replication-competent adenovirus Delta24-RGD-GFP revealed abundant GFP expression in the infected GSCs, whereas no GFP was detected in the macrophages, indicating lack of adenoviral replication in macrophages (Fig. 1C and Supplementary Movie S1). The macrophage resistance to wild-type adenovirus replication has been shown previously,33 and our results demonstrate that also Delta24-RGD is not capable of replicating in these cells.
Fig. 1.
Tumor macrophages acquire hexon accumulations by phagocytosis of virus-infected tumor cells. (A) Delta24-RGD–treated patient tumor-resection material, stained for CD68 (green) and hexon (red). White bar indicates 20 μM. (B) Mean ± SEM luminescence of human macrophages and GSCs 24 h after mock or Ad.Luc.RGD infection. (C) GFP-positive objects per mm2 in 5-day culture of Delta24-RGD-GFP–infected macrophages and GSCs. (D) Mean ± SEM percentage of hexon-positive macrophages after 24 h of culture with GSCs, Delta24-RGD, or Delta24-RGD–infected GSCs with or without cytochalasin. (B–D) Data represent at least 2 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.005 (one-way ANOVA, Tukey post-hoc test).
To evaluate whether the observed hexon positivity in tumor macrophages is caused by phagocytosis, we cultured macrophages with Delta24-RGD, Delta24-RGD–infected human primary GSCs, or control human primary GSCs. Significantly increased amounts of hexon-positive macrophages were observed after 24 h in the co-cultures with Delta24-RGD–infected GSCs but not in co-cultures with Delta24-RGD (P = 0.001) or uninfected GSCs (P = 0.009) (Fig. 1D). Addition of cytochalasin D, which blocks actin polymerization required for phagocytosis,34 completely abrogated hexon positivity in macrophages (P = 0.011) (Fig. 1D). This indicates that the observed hexon-positive macrophages in the resected tumor have most likely acquired this through Delta24-RGD–infected tumor cell phagocytosis.
Phenotype, Cytokine Profile, and Phagocytosis Capacity of In Vitro Polarized Macrophages
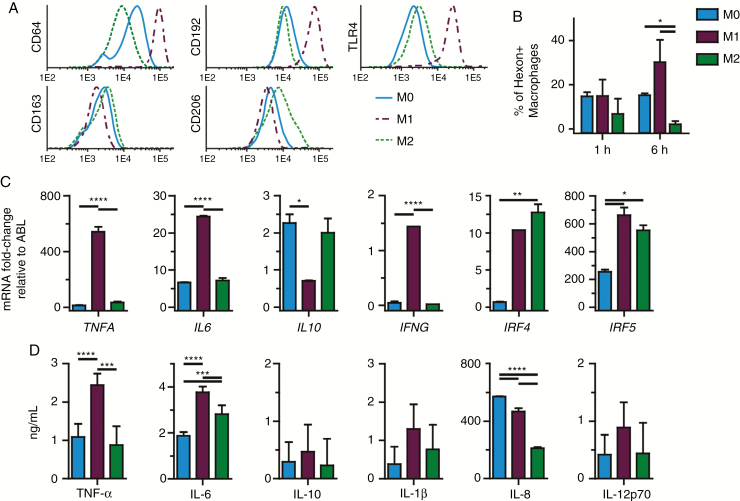
Most tumor macrophages are derived from blood monocytes,35,36 and their tumor promoting and immune-suppressive M2-like phenotype is induced by the tumor microenvironment. Therefore we used human monocytes to culture macrophages, which were polarized by IFN-γ (M1) or IL-4 (M2), or PBS (M0) as a control. To validate the macrophage phenotypes, differential expression of polarization markers CD64, CD192 (C-C chemokine receptor type 2), TLR4, CD163, and CD206 was assessed after 24 h. The selected markers were based on extensive in vitro immunophenotyping experiments.37 Macrophages cultured with IFN-γ increased expression of the M1 markers CD64, CD192, and TLR4 and downregulated CD163 and CD206, whereas macrophages cultured with IL-4 increased expression of CD163 and CD206 and downregulated CD64, confirming appropriate macrophage polarization (Fig. 2A).
Fig. 2.
Immunophenotyping, gene expression and cytokine production demonstrate in vitro macrophage polarization. (A) CD64, CD192, TLR4, CD163, and CD206 expression on cultured human macrophages after 24 h either unpolarized (M0) or polarized with IFN-γ or IL-4. (B) Mean ± SEM of hexon-positive macrophages per M0, M1, and M2 phenotype, indicative of phagocytosis efficiency at 1 h and 6 h (Student’s t-test). (C) Mean ± SEM of mRNA fold-change relative to ABL of TNFA, IL6, IL10, IFNG, IRF4, and IRF5 in M0 and M1 or M2 macrophages, 24 h after polarization. (D) Mean ± SEM of cytokine concentration of TNF-α, IL-6, IL-10, IL-1β, IL-8, and IL-12p70 in culture supernatants of M0, M1, and M2 macrophages. (B–D) Data represent at least 2 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001 (one-way ANOVA, Tukey post-hoc test).
Gene expression analysis showed that 24 h of M1 polarization significantly upregulated TNFA (P < 0.005), IL6 (P < 0.005), IFNG (P < 0.005), IRF4 (P < 0.01), and IRF5 (P < 0.05), and downregulated IL10 (P < 0.05) compared with M0, whereas M2 macrophages significantly upregulated IRF4 (P < 0.01) and IRF5 (P < 0.05). M2 macrophages had significantly lower TNFA (P < 0.001), IL6 (P < 0.001), and IFNG (P < 0.001) expression compared with M1 (Fig. 2B). Additionally, the supernatant cytokine profiles of the different phenotypes revealed increased levels of TNF-α (P < 0.01) and IL-6 (P < 0.01) in the M1 cultures. IL-8 was significantly decreased (P < 0.001) in the M2 culture. IL-10, IL-1β, and IL-12p70 levels did not significantly differ between phenotypes (Fig. 2C).
Co-cultures of the different macrophage phenotypes with Delta24-RGD–infected GSCs showed that M1 macrophages were more efficient in phagocytizing infected GSCs than the other phenotypes (P < 0.05) (Fig. 2D). Addition of the virus only to these cultures revealed no hexon-positive macrophages in any of the phenotypes up to 72 h (Supplementary Fig. S1), confirming that none of the phenotypes are permissive for adenoviral replication and that the observed hexon positivity was derived from phagocytosis of infected GSCs.
Delta24-RGD Shifts Macrophages from M2 to M1 Phenotype
As GBMs contain mostly protumoral M2-like macrophages19 and viruses can induce a pro-inflammatory microenvironment,27,28,38 we investigated whether Delta24-RGD was capable of shifting the phenotype of human macrophages from M2 to M1. M2 macrophages cultured with either Delta24-RGD or Delta24-RGD–infected GSCs for 72 h demonstrated upregulation of CD64 and downregulation of CD163, indicative of an immunophenotypic shift toward M1. This shift did not occur when M2 macrophages were cultured with uninfected GSCs (Fig. 3A).
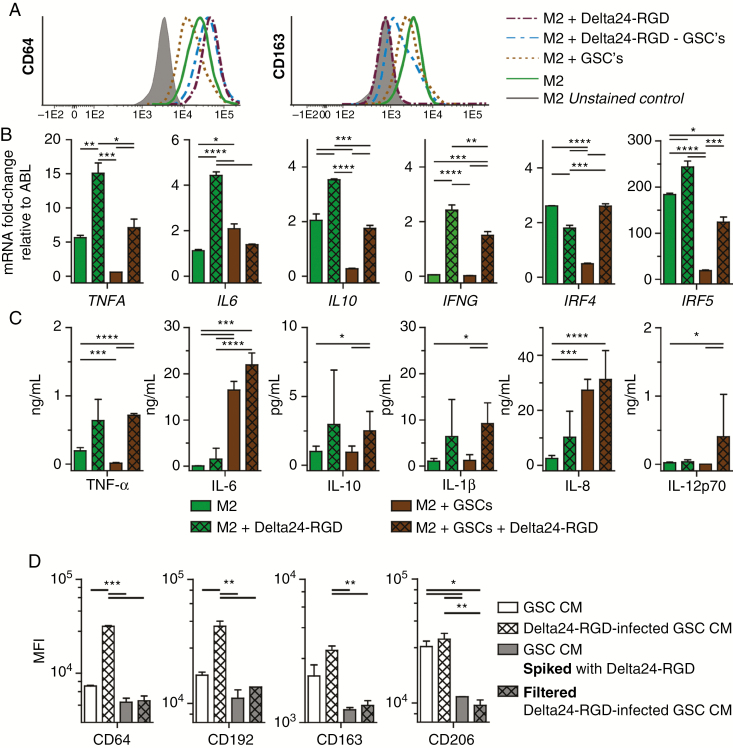
Fig. 3.
Delta24-RGD in vitro changes human macrophages’ genotype, immunophenotype, and cytokine profile. (A) CD64 and CD163 expression on M2 macrophages after 72 h of culturing with Delta24-RGD, GSCs, or Delta24-RGD–infected GSCs. (B) Mean ± SEM of mRNA fold-change relative to ABL of TNFA, IL6, IL10, IFNG, IRF4, and IRF5 in M2 macrophages after 72 h of culturing with Delta24-RGD, GSCs, or Delta24-RGD–infected GSCs. (C) Mean ± SEM cytokine concentration of TNF-α, IL-6, IL-10, IL-1β, IL-12p70 in supernatant of M2 macrophages co-cultured for 72 h with Delta24-RGD, GSCs, or Delta24-RGD–infected GSCs. (D) Mean fluorescence intensity (MFI) of CD64, CD192, CD163, CD206 on M0 macrophages cultured for 72 h with conditioned medium (CM) from Delta24-RGD–infected or control GSCs either with Delta24-RGD or filtered with a 100 kD filter. (B–D) Data represent at least 2 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001 (one-way ANOVA, Tukey post-hoc).
Furthermore, M2 macrophages cultured with Delta24-RGD for 72 h demonstrated significant upregulation of TNFA (P = 0.003), IL6 (P < 0.001), IL10 (P = 0.004), IFNG (P < 0.001), and IRF5 mRNA (P = 0.027) compared with cultures of M2 macrophages, while IRF4 was slightly downregulated (P = 0.003). A comparable change in gene expression was observed in the co-cultures with virus-infected GSCs compared with uninfected GSCs, where TNFA (P = 0.045), IL10 (P = 0.004), IFNG (P = 0.003), IRF4 (P < 0.001), and IRF5 (P = 0.003) were increased. Only the IL6 expression was decreased by infected GSCs compared with uninfected GSCs (P = 0.07) (Fig. 3B).
Cytokine levels in culture supernatants confirmed the gene expression results. TNF-α (P = 0.045), IL-10 (P = 0.04), IL-1β (P = 0.013), and IL-12p70 (P = 0.025) were increased in co-cultures with virus-infected GSCs. Contrary to the gene expression data, IL-6 production was significantly increased in conditions with GSCs compared with the conditions without GSCs (P = 0.003), but not by the virus alone (P = 0.68) (Fig. 3C). IL-6, under regulation of the M1-associated NF-κβ-pathway,39 has been ambiguously described as both tumor promoting as well as an initiator of antitumor immunity.40 Interestingly, among the evaluated cytokines, only IL-12p70 was increased with infected GSCs and not with Delta24-RGD alone, suggesting a combined effect of the presence of tumor cells and virus, leading to production of this anti-angiogenic and Th1-lymphocyte activating cytokine.41 The infected tumor cells themselves were not the source of this increase, since they stopped producing several of the evaluated cytokines, as shown by Supplementary Fig. S3). Altogether, a full phenotypic shift with complete loss of M2 traits was not observed, fitting with the notion that the defined M1 and M2 phenotypes are extremes on the polarization spectrum which cannot be obtained under physiological conditions. This is attributed to the complex changes necessary to shift phenotype as well as the many factors influencing macrophage heterogeneity in glioma.6 However, our results do demonstrate that in vitro Delta24-RGD shifts human macrophages from a more tumor-supportive toward a tumor-detrimental phenotype.
In the Tumor Environment Both GSC-Derived Factors and the Presence of Viral Particles Are Required to Induce M1 Polarization
To study whether in macrophage–tumor cell interactions (eg, phagocytosis), soluble factors or viral particles induce the macrophage phenotypic shift, we tested several conditions on unpolarized (M0) macrophages to allow the evaluation of small effects. Addition of conditioned medium from GSCs (GSC CM) to M0 macrophages significantly decreased CD64 expression (P < 0.001), whereas CD163 was not significantly affected, confirming the M2 polarizing effects of the tumor environment. CM from Delta24-RGD–infected GSCs led to increased M1 markers CD64 (P < 0.001) and CD192 expression (P = 0.005) compared with control GSC CM, whereas no significant effect was observed on CD163 and CD206 (Fig. 3D). This indicates that soluble factors from OV-infected GSCs can induce a phenotypic shift toward M1 without direct contact between macrophages and GSCs (eg, phagocytosis).
Whether Delta24-RGD particles in the tumor microenvironment exert these M1 polarizing effects was evaluated by spiking virus into GSC CM. Surprisingly, this did not alter CD64 and CD192 M1 marker expression compared with GSC CM, but did lead to decreased CD163 and CD206 M2 marker expression. Indeed, compared with Delta24-RGD–infected GSC CM, all markers remained lower. Together, these results suggest that local tumor cells need to be infected by the virus to allow M1 marker upregulation, and the viral particles in the tumor environment contribute to M2 marker downregulation, together inducing M1 polarization (Fig. 3D).
To gain insight into the nature of the secreted factors from infected cells that are involved in the M1 polarization, the Delta24-RGD–infected GSC CM was filtered using a 100 kD filter, which retains large structures, such as adenoviruses and extracellular vesicles, and allows small secreted and/or soluble factors such as cytokines to pass. This filtered CM did not induce the phenotypic shift observed with Delta24-RGD–infected GSC CM. The M1 marker increase induced by Delta24-RGD–infected GSC CM was not observed, while a decrease in the M2 markers CD163 and CD206 (P < 0.05) was observed. The latter is not unexpected, as it has been shown that larger-sized GSC-derived vesicles mediate M2 polarizing effects,21 although we cannot exclude that small molecules produced by the virus-infected cells also contribute to M1 polarization (Fig. 3D). We therefore conclude that within the tumor microenvironment, OV delivery leads to a phenotypic switch through the concerted action of a change in factors produced by OV-infected GSCs and the presence of viral particles, together contributing to an M1 macrophage phenotypic switch.
Delta24-RGD Treatment Markedly Changes Cytokine Profile in Patients’ CSF
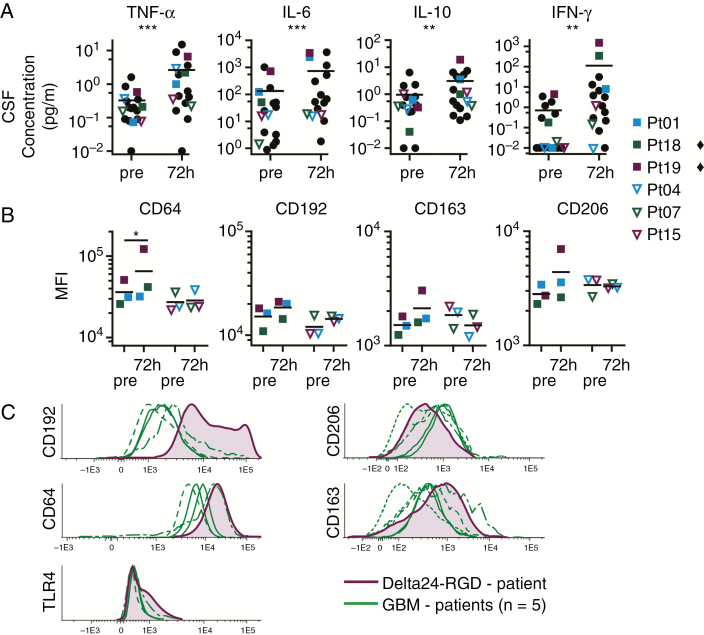
Unique CSF samples from patients enrolled in the phase I/II Delta24-RGD clinical trial at our institute provided the opportunity to explore the clinical relevance of our in vitro data. Poli et al previously showed that a tumor-detrimental microenvironment in mice can be detected through analysis of CSF cytokine levels.5 Indeed, analysis of post–Delta24-RGD treatment CSF samples revealed significantly increased levels of TNF-α (P = 0.002), IL-6 (P = 0.001), and IFN-γ (P = 0.005) (P < 0.01) (Fig. 4A). Additionally, IL-10 levels were also increased, corresponding with the virus-induced gene expression and cytokine profiles found in our in vitro experiments. This demonstrates that Delta24-RGD therapy in humans alters the tumor microenvironment toward a more pro-inflammatory state.
Fig. 4.
CSF cytokine profile and macrophage phenotype shift to pro-inflammatory under influence of Delta24-RGD treatment. (A) CSF cytokine concentration of TNF-α, IL-6, IL-10, and IFN-γ in 20 Delta24-RGD–treated patients (18 individual patients, 15 pairs) before and 72 h after viral infusion. Each dot represents one patient. Squares represent patients with increased cytokine concentrations. Open triangles represent patients with little cytokine changes upon treatment. Black line is the mean, **P < 0.01; ***P < 0.005 (Wilcoxon signed rank test). (B) CD64, CD163, CD192, and CD206 expression on macrophages cultured for 72 h with patient’s CSF both pretreatment and 72 h after start of treatment. Black line is the mean, each symbol represents mean of 2 separate experiments. The symbol ♦ marks patients with statistical significant changes, *P < 0.05 (one-way ANOVA, Tukey post-hoc). (C) CD206, CD163, CD192, CD64, and TLR4 expression on GBM macrophages, in untreated (N = 5) and Delta24-RGD–treated (N = 1) patients, *P < 0.05 (Student’s t-test).
Patients’ CSF Alters Macrophage Phenotypes
To assess whether the detected microenvironmental changes not only are hallmarks but also induce the macrophage phenotypic shift, we selected 3 CSF samples with a marked change in cytokine levels (Fig. 4A; full squares) and 3 samples with a limited change in cytokine levels (Fig. 4A; open triangles). Macrophages cultured for 72 h in markedly changed CSF revealed significant CD64 upregulation (Fig. 4B). Furthermore CD192, CD163, and CD206 were slightly upregulated with the posttherapy CSF. Interestingly, macrophages co-cultured with CSF samples with limitedly changed cytokine levels did not reveal changes in marker expression (Fig. 4B). This heterogeneity in macrophage response suggests that the conditions required to induce the immunophenotypic shift are present in some patients’ CSF, whereas in others it is not, a phenomenon that may be related to therapeutic efficacy of OV treatment.
Patients’ GBM-Macrophage Immunophenotype Shifts upon Delta24-RGD Treatment
Direct immunophenotypic analysis of tumor macrophages from OV-treated GBM patients is rarely possible. Only a limited number of clinical trials have been performed and secondary resections are uncommon. In the trial at our institute, a patient who had received Delta24-RGD treatment 26 months earlier underwent a second tumor resection due to recurrence. Considering the normal time to progression in recurrent GBM (mean 6.2 mo42), this was an exceptionally long progression-free survival. The resected tumor tissue was immunophenotyped and compared with 5 untreated GBMs. As GBM is notorious for intratumoral heterogeneity, the sampled tumor cannot represent the entire tumor, limiting result interpretation. Nevertheless, intratumoral macrophages in the virus-treated GBM demonstrated increased CD192 (P = 0.002), TLR4 (P = 0.008), and CD64 expression. Furthermore, we found decreased CD206 expression and increased levels of CD163 compared with the majority of untreated GBMs (Fig. 4C). Although CD163 was not decreased, as would be expected for an M1 phenotype, the other phenotype markers suggest OV treatment induced pro-inflammatory effects and prolonged phenotypic changes in tumor-associated macrophages. Whether this phenotypic shift is related to the patient’s delayed recurrence needs to be investigated in future studies.
The analyses of patient material and our in vitro experiments reveal that under the influence of Delta24-RGD, human (tumor) macrophages change phenotype, thereby modifying the microenvironment toward pro-inflammatory and antitumoral. Although the gene expression, immunophenotype, and cytokine profiles showed that M2 traits were not completely lost, M1 traits became dominant. This lack of a clear boundary between M1 and M2 macrophages in patient-derived material8 complicates result interpretation; however, as we used multiple macrophage traits to identify the prominent phenotype, a more general conclusion can be drawn. Furthermore, we demonstrated that a combination of factors is required to cause the observed phenotypic shift. Both direct macrophage–Delta24-RGD interaction as well as a Delta24-RGD–induced change in tumor-derived soluble factors are essential. The latter is most likely caused by virus-induced oncolysis and blocking or changing of the secretion of immune modulating factors. However, small molecular structures, such as cytokines from infected tumor cells alone, could not induce a switch to M1. This indicates that large soluble structures, such as extracellular vesicles, not only induce M2 macrophages within the tumor environment but may also contribute to the observed M1 shift upon viral infection of tumor cells.22,43Fig. 5 depicts a schematic representation of these processes. Most likely, macrophages need to be able to detect viral double-stranded DNA (via TLR9) or infected tumor cells (via TLR2), triggering the MyD88 complex and activating IRF5, which upregulates NF-κβ, IRF5, and IRF7, leading to M1-associated cytokine transcription (Fig. 5C–E).16,17,40,41
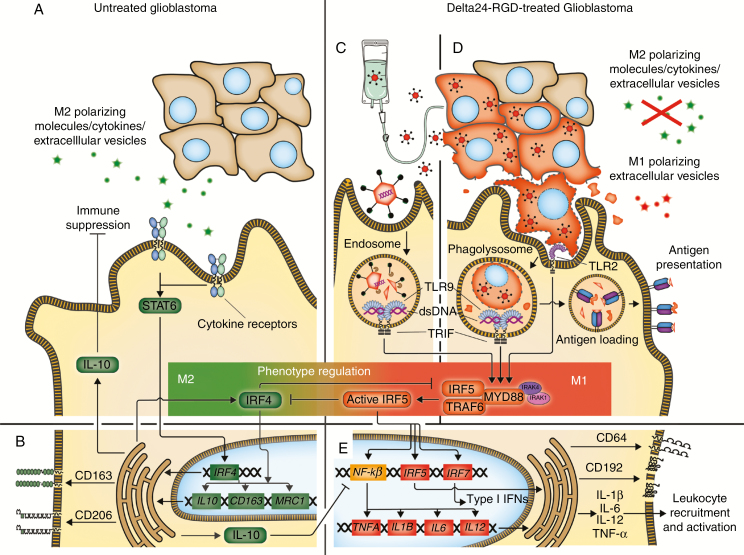
Fig. 5.
Macrophage phenotype regulation in Delta24-RGD therapy of glioblastoma. (A) Glioblastoma cells secrete M2 polarizing molecules, cytokines, and extracellular vesicles that primarily affect the macrophage IRF4-STAT6. (B) This induces M2-related gene transcription and M2 membrane marker upregulation. Furthermore IL-10, which suppresses leukocytes and the M1-associated NF-κβ pathway, and the MyD88–IRF5 complex inhibitor IRF4 are produced. (C) Delta24-RGD administration by direct infusion or released by infected tumor cells (as particles or in extracellular vesicles) leads to viral double-stranded DNA detection by TLR9 in macrophage endosomes, activating IRF5 via the MyD88–IRF5 complex. (D) Delta24-RGD infection of tumor cells inhibits M2 polarizing molecule production, allowing M1 polarization of macrophages and promoting tumor cell phagocytosis. Tumor cell digestion in the phagolysosome leads to virus detection by TLR2 and TLR9, activating IRF5 via the MyD88–IRF5 complex. Furthermore, digested infected tumor cells are loaded onto human leukocyte antigen class II for antigen presentation. (E) Active IRF5 inhibits IRF4 and the subsequent M2-related pathways and promotes NF-κβ, IRF5, and IRF7 transcription, leading to CD64 and CD192 upregulation, type I IFN production, and M1-associated gene transcription, together inducing inflammation, leukocyte recruitment, and activation.
Unfortunately, the limited availability of Delta24-RGD–treated patient material precluded more extensive analysis using larger panels of genes, markers, or timepoints, thereby limiting clinical effect evaluation of the phenotypic shift. In vivo studies in mice, however, may also have limited translational value with regard to macrophage plasticity as murine monocytes/macrophages differ from those in humans.44 More insight into the effect of phenotypic shifts on tumor cell clearing or antigen presentation upon OV therapy therefore requires further immune monitoring studies in clinical trials.
Taken together, we have shown that the antitumor effect of Delta24-RGD therapy is not merely restricted to oncolysis and the induction of a T cell–mediated antitumor response, but local macrophages also gain antitumoral properties. Our studies show that OV therapy may offer the highly sought after tool to modulate tumor-supportive M2 macrophages into antitumoral M1 macrophages in humans, providing a new therapeutic approach to change the tumor microenvironment and possibly improve patient survival.19,20,45
Funding
This work is supported by the Dutch Cancer Society (KWF) (project number: EMCR-2006–3696) and an Erasmus MC medical research grant (project code DRP337224).
Supplementary Material
Acknowledgments
We thank Daphne de Launay, Karin Josiassen, Sandra de Bruin-Versteeg, and Gita Naber for helping with the experiments; Wim Dik and Marjan Versnel for providing primers/probes.
Author contributions. W.vdB., A.K., C.T., J.V., C.E.T., J.vD., C.D., and M.L., designed and/or performed experiments. D.N., C.D. provided patient material. W.vdB., A.K., J.V., C.T., J.vD., C.D., and M.L. interpreted data. W.vdB. and A.K. drafted the manuscript; W.vdB., A.K., C.T., J.V., C.E.T., D.N., J.vD., C.D., and M.L. revised the manuscript.
Conflict of interest statement. J.vD. is chairman of the EuroFlow Scientific consortium, which receives royalties from licensed patents; this income is solely for continuation of the EuroFlow collaboration. Furthermore J.vD. has an Educational Service Agreement with BD Biosciences; income from this goes to the Leiden University Medical Center. C.D. and M.L. have consulted for DNAtrix Therapeutics Inc, for which Erasmus MC received compensation; no other relationships exist that could be perceived to have influenced the submitted work.
References
- 1. Hughes R, Qian BZ, Rowan C, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015;75(17):3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edin S, Wikberg ML, Dahlin AM, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7(10):e47045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. [DOI] [PubMed] [Google Scholar]
- 5. Poli A, Wang J, Domingues O, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4(9):1527–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowman RL, Klemm F, Akkari L, et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 2016;17(9):2445–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. [DOI] [PubMed] [Google Scholar]
- 8. Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72(21):4111–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. [DOI] [PubMed] [Google Scholar]
- 11. Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7(6):496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008; 7(6):467–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6(9):644–658. [DOI] [PubMed] [Google Scholar]
- 14. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rider P, Carmi Y, Guttman O, et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187(9):4835–4843. [DOI] [PubMed] [Google Scholar]
- 16. Takaoka A, Yanai H, Kondo S, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434(7030):243–249. [DOI] [PubMed] [Google Scholar]
- 17. Negishi H, Ohba Y, Yanai H, et al. Negative regulation of Toll-like-receptor signaling by IRF-4. Proc Natl Acad Sci U S A. 2005;102(44):15989–15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. [DOI] [PubMed] [Google Scholar]
- 19. Prosniak M, Harshyne LA, Andrews DW, et al. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19(14):3776–3786. [DOI] [PubMed] [Google Scholar]
- 20. Ding P, Wang W, Wang J, Yang Z, Xue L. Expression of tumor-associated macrophage in progression of human glioma. Cell Biochem Biophys. 2014;70(3):1625–1631. [DOI] [PubMed] [Google Scholar]
- 21. Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. [DOI] [PubMed] [Google Scholar]
- 22. de Vrij J, Maas SL, Kwappenberg KM, et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int J Cancer. 2015;137(7):1630–1642. [DOI] [PubMed] [Google Scholar]
- 23. Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11): 1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841-849. [DOI] [PubMed] [Google Scholar]
- 25. Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kleijn A, Kloezeman J, Treffers-Westerlaken E, et al. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS One. 2014;9(5):e97495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32(2):253–267.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Paolo NC, Miao EA, Iwakura Y, et al. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31(1):110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balvers RK, Kleijn A, Kloezeman JJ, et al. Serum-free culture success of glial tumors is related to specific molecular profiles and expression of extracellular matrix-associated gene modules. Neuro Oncol. 2013;15(12):1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balvers RK, Belcaid Z, van den Hengel SK, et al. Locally-delivered T-cell-derived cellular vehicles efficiently track and deliver adenovirus delta24-RGD to infiltrating glioma. Viruses. 2014;6(8):3080–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652–660. [DOI] [PubMed] [Google Scholar]
- 32. Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72(12):9706–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mercer J, Greber UF. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol. 2013;21(8):380–388. [DOI] [PubMed] [Google Scholar]
- 34. Lin DC, Tobin KD, Grumet M, Lin S. Cytochalasins inhibit nuclei-induced actin polymerization by blocking filament elongation. J Cell Biol. 1980;84(2):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shand FH, Ueha S, Otsuji M, et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc Natl Acad Sci U S A. 2014;111(21):7771–7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Dongen JJ, Lhermitte L, Böttcher S, et al. ; EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708) EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zsengellér Z, Otake K, Hossain SA, Berclaz PY, Trapnell BC. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J Virol. 2000;74(20):9655–9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10(5):2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benihoud K, Salone B, Esselin S, et al. The role of IL-6 in the inflammatory and humoral response to adenoviral vectors. J Gene Med. 2000;2(3):194–203. [DOI] [PubMed] [Google Scholar]
- 41. Bonapace L, Coissieux MM, Wyckoff J, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515(7525):130–133. [DOI] [PubMed] [Google Scholar]
- 42. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 43. van der Vos KE, Abels ER, Zhang X, et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol. 2016;18(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. [DOI] [PubMed] [Google Scholar]
- 45. Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6(8):e23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.