SUMMARY
Glioblastoma is the most common malignant primary brain tumor. Cures are rare and median survival varies from several to 22 months. Standard treatment for good performance patients consists of maximal safe surgical resection followed by radiotherapy with concurrent temozolomide (TMZ) chemotherapy and six cycles of postradiotherapy TMZ. At recurrence, treatment options include repeat surgery (with or without Gliadel wafer placement), reirradiation or systemic therapy. Most patients with good performance status are treated with cytotoxic chemotherapy or targeted biologic therapy following or in lieu of repeat surgery. Cytotoxic chemotherapy options include nitrosoureas, rechallenge with TMZ, platins, phophoramides and topoisomerase inhibitors, although efficacy is limited. Despite the intense effort of developing biologic agents that target angiogenesis and growth and proliferative pathways, bevacizumab is the only agent that has shown efficacy in clinical trials. It was awarded accelerated approval in the USA after demonstrating an impressive radiographic response in two open-label, prospective Phase II studies. Two randomized, Phase III trials of upfront bevacizumab have completed and may demonstrate survival benefit; however, results are pending at this time. Given the limited treatment options at tumor recurrence, consideration for enrollment on a clinical trial is encouraged.
Practice Points.
Median survival of clinical trial-eligible (age <71 years and Karnofsky Performance Status >60), US glioblastoma (GB) patients is 18–20 months, which is partly a reflection of improved supportive care, recognition of pseudoprogression and availability of bevacizumab for recurrent disease.
-
GB may be subdivided into two prognostic groups based on MGMT promoter methylation:
Methylated: relatively responsive to alkylating chemotherapy (such as temozolomide [TMZ]), better prognosis;
Unmethylated: relatively unresponsive to alkylating chemotherapy, worse prognosis.
Recommended initial treatment for patients with good performance status consists of maximal safe surgical resection followed by adjuvant external-beam radiotherapy with concurrent TMZ chemotherapy and postradiotherapy TMZ chemotherapy for 6 months.
-
Recommended treatment options in elderly GB patients:
Physiologically young: standard radiotherapy/TMZ;
Poor performance status: hypofractionated radiotherapy, primary TMZ chemotherapy or no treatment;
Frail elderly with good performance status: hypofractionated radiotherapy or primary TMZ chemotherapy.
Consideration of enrollment on a clinical trial at disease presentation or recurrence is strongly recommended in appropriate patients.
Filadel, lomustine and bevacizumab are the only three agents that can be recommended for recurrent GB as the vast majority of cytotoxic chemotherapeutic and biologic targeted agents have not shown benefit in controlled clinical trials.
Glioblastoma (GB; WHO grade 4 glioma) is the most common malignant primary brain tumor with an annual incidence of 12,943 cases in the USA [1]. It is a tumor of the elderly with a median age of onset of 64 years, although children and young adults are also affected. GB is associated with a poor prognosis; despite best treatment, most community-based patients will not survive 1 year [1]. Cures are rare and overall survival rates at 2 and 5 years are 26–48% and 12%, respectively, in highly selected, contemporary, clinical trial-eligible patients [2,3]. For protocol-eligible US patients, the median survival is 18–20 months, which is partly a reflection of improved supportive care, recognition of pseudoprogression and availability of bevacizumab at recurrence [3,4]. The exact nature of GB's cell of origin is unclear, but is thought to be an astrocyte because of histologic tumor staining for glial fibrillary acidic protein. The mechanism by which an astrocyte transforms into GB is unknown, but is thought to result from sequential aberrant genetic and epigentic alterations of oncogenes and tumor suppressor genes. Based on molecular analysis, GB is comprised of four subtypes (neuronal, proneural, classic and mesenchymal); however, the relevance to outcome and treatment remains to be determined [5]. This review focuses on the treatment of GB, particularly the use of chemotherapy and targeted agents.
Treatment at diagnosis
Initial treatment for patients with high performance (Karnofsky Performance Status [KPS] >60 and age <71 years) consists of maximal safe surgical resection followed by adjuvant focal, external-beam radiotherapy (RT) with concurrent temozolomide (TMZ) chemotherapy and post-RT TMZ for 6 months [2,6,7]. It should be noted that it is convention to use the term ‘adjuvant’ to describe postsurgical RT and chemotherapy even though complete resection of the tumor is not usually possible given the infiltrative nature of GB. TMZ and carmustine biodegradable wafer (Gliadel®) are the only adjuvant chemotherapies that have improved survival in randomized GB clinical trials [2,8]. The standard treatment is based upon a European Organization for Research and Treatment of Cancer (EORTC) and National Cancer Institute of Canada (NCIC) randomized, Phase III trial of 573 patients with newly diagnosed GB (age 19–71 years and WHO Performance Status ≤2) that compared RT alone (total dose 60 Gy) to TMZ chemotherapy in combination with RT (total 60 Gy), followed by 6 months of post-RT TMZ. Patients on the experimental arm received TMZ daily during RT (because of its purported radiosensitization effect in preclinical studies) at a dose of 75 mg/m2, followed by monthly TMZ at a dose of 150–200 mg/m2/day on a 5/28-day schedule for six cycles.
The RT/TMZ arm had a median overall survival (OS) of 14.6 months compared with 12.1 months for RT alone. The 2-year survival (not a prespecified end point) of patients treated with RT plus TMZ was 26.5% as compared with 10.4% for radiation alone. This impressive increase in 2-year survival with minor improvement in overall survival suggested that a subpopulation of patients benefited from the addition of TMZ.
The survival benefit of TMZ was subsequently demonstrated for at least 5 years following treatment [7]. The 5-year OS was 9.8% for patients who received combined TMZ and RT compared with 1.9% for RT alone.
Building upon the EORTC/NCIC treatment platform, the Radiation Treatment Oncology Group (RTOG) completed a 1100 patient, randomized, Phase III upfront GB trial (RTOG 0525) comparing standard post-RT TMZ with dose-dense TMZ [4]. Patients were randomized to receive either standard therapy (TMZ plus RT followed by 6–12 cycles of TMZ at a dose of 150–200 mg/m2/day on a 5/28-day schedule) versus dose-dense TMZ (TMZ plus RT followed by 6–12 cycles of TMZ at a dose of 100 mg/m2/day on a 21/28-day schedule). Median and 2-year survivals were 14 and 26%, respectively, for both the experimental and control arms, indicating no additional benefit from dose-dense TMZ. The dose-dense arm resulted in increased hematologic toxicity. Thus, there is no role for dose-dense TMZ for newly diagnosed GB.
The identification of predictive markers of TMZ responsiveness is needed to avoid exposing patients with TMZ-resistant tumors to the toxicity of an ineffective therapy. Epigenetic silencing of the MGMT DNA repair gene by promoter methylation has been associated with longer survival for GB patients treated with alkylating agents [9]. A retrospective analysis of assessable cases (approximately 40% of total patients) from the EORTC/NCIC TMZ study demonstrated a survival benefit for patients treated with TMZ and RT if their tumor contained a methylated MGMT promoter (median 21.7 months) as compared with patients with a nonmethylated MGMT promoter (median 12.7 months) [10]. Even patients in the RT only treatment arm had a greater OS if their tumor had a methylated MGMT promoter (median 15.3 months) as compared with those that were nonmethylated (median 11.8 months), suggesting that MGMT could be a prognostic, if not predictive, marker. The authors of this retrospective analysis concluded that there appeared to be no benefit of TMZ in patients with unmethylated MGMT (notwithstanding the modest improvement in 2-year survival, a nonspecified end point) when compared with treatment with RT only.
Prospective data corroborating the prognostic value of MGMT promoter methylation was obtained in RTOG 0525. MGMT methylation (35% of all patients) was associated with improved median OS (21.2 months as compared with 14 months in unmethylated tumors), median progression-free survival (PFS; 8.7 vs 5.7 months) and radiographic response [4]. Since RTOG 0525 allocated all patients to TMZ treatment, there was no opportunity to corroborate the lack of benefit of TMZ in unmethylated GB as demonstrated in the EORTC/NCIC trial.
Current upfront GB studies focus on adding investigational agents to the TMZ/RT regimen. The addition of bevacizumab, an effective targeted agent for recurrent GB (discussed below), to the TMZ/RT regimen was investigated in two Phase II studies [11,12]. These studies demonstrated an improvement in median PFS as compared with the EORTC/NCIC historical controls (13.6 vs 7.6 months [11] and 13.8 vs 6.9 months [12]), without an improvement in OS, as compared with historical controls in which bevacizumab was utilized at progression. Two Phase III, randomized clinical trials investigating the addition of bevacizumab to the EORTC/NCIC regimen have completed accrual; the results are pending (Tables 1 & 2).
Table 1. . Important ongoing Phase II and III clinical trials utilizing targeted therapy or immunotherapy for newly diagnosed glioblastoma.
| Study | Phase | Agent added to standard RT/TMZ | Accrual/randomization (drug:placebo) | Primary end point |
|---|---|---|---|---|
| Targeted therapies | ||||
| Randomized, Phase Il, Double-Blind, Placebo-Controlled Trial of Conventional RT/TMZ Plus Cediranib Versus Conventional RT/TMZ Plus Placebo in Newly Diagnosed Glioblastoma | II | Cediranib (20 mg q.d.) | 150/2:1 | 6-month PFS rate |
| Radiation Therapy and Concurrent Plus Adjuvant Temsirolimus Versus Chemo-Irradiation with Temozolomide in Newly Diagnosed Glioblastoma without Methylation of the MGMT Gene Promoter – a Randomized Multicenter, Open-Label, Phase II Study | II | Temsirolimus (25 mg iv. qweek) | Probability of survival at 12 months | |
| Phase III Double-Blind Placebo-Controlled Trial of Conventional RT/TMZ Plus Bevacizumab Versus Conventional RT/TMZ Plus Placebo in Newly Diagnosed Glioblastoma | III | Bevacizumab 10 mg/kg q2 week | 720/1:1 | PFS and OS |
| AvaGlio: Phase III Trial of Bevacizumab Plus TMZ/RT in Newly Diagnosed Glioblastoma Multiforme | III | Bevacizumab (10 mg/kg q2 week) during RT/TMZ and adjuvant TMZ, then bevacizumab (15 mg/kg q3 week) monotherapy | 920/1:1 | PFS and OS |
| Cilengitide in Newly Diagnosed Glioblastoma with MGMT Promoter Methylation: a Multicenter, Randomized, Open-Label, Controlled Phase III Trial (CENTRIC) | III | Cilengitide (2000 mg twice weekly) | 504/1:1 | OS |
| Immunotherapy | ||||
| Randomized, Double-blind, Controlled Phase III Trial of Rindopepimut in Patients with Surgically Resected EGF variant III (EGFRvIII)-Positive Glioblastoma, the ‘ACT IV Study’ | III | Rindopepimut (EGFRvIII-targeted peptide vaccine) | 440/1:1 | OS |
| A Phase II Clinical Trial Evaluating DCVax®-Brain, Autologous Dendritic Cells Pulsed with Tumor Lysate Antigen for the Treatment of Glioblastoma Multiforme | II | Autologous tumor lysate-pulsed dendritic cell vaccine | 240/2:1 | OS |
| A Randomized-Double-Blind, Controlled Phase IIb Study of the Safety and Efficacy of ICT-107 in Newly Diagnosed Patients with Stage IV Glioblastoma Multiforme (GBM) Following Resection and Chemoradiation | II | Dendritic cells, prepared from autologous mononuclear cells that are pulsed with six synthetic peptides derived from: MAGE-1, HER-2, AIM-2 TRP-2, gp100 and IL-13Rα2 | 102/2:1 | PFS and OS |
| Phase II, Multicenter, Single Arm Investigation of HSPPC-96 Vaccine with Temozolomide in Patients with Newly Diagnosed Glioblastoma Multiforme | II | HSPPC-96 (is an autologous, tumor-derived HSP [glycoprotein 96]-peptide complex vaccine that is individually prepared from the patient's tumor) | 55 | OS |
AIM-2: Antigen isolated from immunoselected melanoma-2; D: Day; EGFR: EGF receptor; HSP: Heat shock protein; iv.: Intravenously; OS: Overall survival; PFS: Progression-free survival; q2 week: Once every 2 weeks; q3 week: Once every 3 weeks; q.d.: Daily; qweek: One per week; RT: Radiotherapy; TMZ: Temozolomide.
Table 2. . Comparison of the large, randomized upfront bevacizumab glioblastoma trials.
| AvaGlio | RTOG 0825 | |
|---|---|---|
| Patient eligibility | Biopsy allowed | Partial or gross total resection (biopsy excluded) |
| Stratification factors | RPA class | MGMT Molecular |
| Start of bevacizumab | With CRT | 3 weeks after the onset of CRT |
| Duration of therapy | Six cycles of standard TMZ bevacizumab until PD | 12 cycles of standard TMZ plus bevacizumab |
| Blinding | Unblinding if necessary | All unblinded at PD (allows crossover) |
CRT: Chemoradiotherapy; PD: Disease progression; RPA: Recursive partitioning analysis; TMZ: Temozolomide.
Other recently completed Phase II, upfront GB trials are summarized in Table 3. These studies demonstrated similar improvements in median OS to the two upfront bevacizumab combination studies, suggesting no benefit from the added investigational agents but rather improvement in supportive care, recognition of pseudoprogression and the use of bevacizumab for recurrent disease.
Table 3. . Summary of recently completed Phase II clinical trials utilizing targeted agents or immunotherapy for newly diagnosed glioblastoma.
| Study (year) | Agent added to standard RT/TMZ | Patients (n) | Results | Ref. |
|---|---|---|---|---|
| Brown et al. (2008) | Erlotinib (150 mg q.d.) alone x 1 week, then concurrently with TMZ/RT and adjuvant TMZ | 97 | mPFS 7.2 months mOS 15.3 months |
[102] |
| Prados et al. (2009) | Erlotinib (100–200 mg q.d. during RT and 150–300 mg q.d. after RT) | 65 | mPFS 8.2 months mOS 19.3 months |
[104] |
| Grossman et al. (2009) | Talampanel (25–75 mg t.i.d.) | 72 | mOS 18.3 months (95% CI: 14.6–22.5 months) 2-year survival rate 41.7% |
[118] |
| Peereboom et al. (2010) | Erlotinib 50 mg/day, increased by 50 mg every 2 weeks until occurrence of grade 2 rash or maximum of 150 mg/day | 27 | mPFS 2.8 months mOS 8.6 months |
[103] |
| Stupp et al. (2010) | Cilengitide (500 mg twice weekly) | 52 | mPFS 8 months (95% CI: 6.0–10.7 months) mOS 16.1 months (95% CI: 13.1–23.2 months) 2-year survival rate: 35% |
[119] |
| Rosenfield et al.(2010) | im. poly-ICLC (20 mg/kg/dose given three-times per week for weeks 2–8) | 97 | mOS 17.2 months (95% CI: 15.4–19.3 months) 2-year survival rate: 23% |
[120] |
| Hainsworth et al. (2010) | Sorafenib 400 mg twice daily during adjuvant TMZ | 47 | mPFS 6 months (95% CI: 3.7–7 months) mOS 12 months (95% CI: 7.2–16 months) |
[121] |
| Uhm et al. (2011) | Gefitinib 500–1000 mg q.d. | 98 | mOS 12 months | [105] |
| Butowski et al. (2011) | Enzastaurin (250 mg q.d.) | 66 | mPFS 36 wk (95% CI: 30–49 weeks) mOS 74 wk (95% CI: 62–83 weeks) |
[113] |
| Lai et al. (2010) | Bevacizumab 10 mg/kg q2 week | 70 | mPFS 13.6 months mOS 19.6 months |
[11] |
| Vredenburgh et al. (2011) | Bevacizumab 10 mg/kg q2 week during standard RT/TMZ and bevacizumab 10 mg/kg q2 week plus irinotecan 125 mg/m2 q2 week during adjuvant TMZ | 75 | mPFS 14.2 months (95% CI: 12–16 months) mOS 21.2 months (95% CI: 17.2–25.4 months) |
[122] |
| Lai et al. (2011) | Rindopepimut (vaccine consisting of the EGFRvIII antigen chemically conjugated to Keyhole Limpet Hemocyanin) 500 µg id. q2 week first month, then monthly during adjuvant RT | 65 | mPFS 12.3 months mOS 24.6 months 2-year survival rate: 52% |
[123] |
EGFR: EGF receptor; GB: Glioblastoma; id.: Intradermal; im.: Intramuscular; mOS: Median overall survival; mPFS: Median progression-free survival; poly-ICLC: poly-L-lysine and carboxymethyl cellulose; q.d.: Daily; q2 week: Once every 2 weeks; RT: Radiotherapy; t.i.d.: Three times daily; TMZ: Temozolomide.
Table 1 summarizes important ongoing Phase II and III upfront clinical trials that utilize targeted therapy and immunotherapy in conjunction with standard RT/TMZ. Note that the Cilengitide Centric and CORE studies (patients with methylated or unmethylated MGMT promoter gene) and the EORTC temsirolimus study (patients with an umethylated MGMT promoter gene) are the first to limit enrollment by MGMT status, a trend that will likely continue in the future, even though issues remain, since the test utilized for MGMT methylation has never been validated.
Three patient subgroups: elderly (age >70 years), those with poor performance status and unmethylated MGMT promoter deserve additional discussion as no standard care exists. The elderly comprise at least 20% of newly diagnosed GB patients. The percentage of elderly GB patients is anticipated to increase as a result of demographic shifts in the USA and Europe [13]. There is no consensus as to the appropriate care of elderly GB patients. Elderly patients are usually excluded from clinical trials (e.g., the EORTC/NCIC TMZ study) and there is a perception that they have more difficulty tolerating treatment than young patients. Treatment options for elderly patients have included standard RT/TMZ (for fit, otherwise healthy elderly patients), accelerated hypofractionated RT (34–40 Gy in 10–15 fractions) without TMZ (for the majority of treatment-eligible elderly patients) and primary TMZ chemotherapy with deferred RT. Malmstrom et al. conducted a Phase III trial of 291 GB patients aged ≥60 (median 70) years who were randomized to either standard RT (60 Gy in 2 Gy fractions), hypofractionated RT (34 Gy in 3.4 Gy fractions) or six cycles of TMZ (200 mg/m2/day, 5/28 days) [14]. There was no significant difference in OS between the three treatment arms (median OS 8 months for TMZ, 7.5 months for hypofractionated RT and 6 months for standard RT), suggesting that all were reasonable options. The NOA-08 trial randomized 373 patients, aged >65 years, with anaplastic astrocytoma or GB to standard postsurgical RT (54–60 Gy) versus TMZ (100 mg/m2/day, 1-week-on/1-week-off) [15]. Similar to Malmstrom's study, patients in the TMZ arm had a similar outcome, suggesting that RT may be deferred in the treatment of elderly GB patients.
An ongoing NCIC/EORTC Phase III study of newly diagnosed GB patients ≥65 years is randomizing patients to either RT (40 Gy in 15 fractions) or RT (40 Gy in 15 fractions) and concurrent TMZ (75 mg/m2/day) followed by TMZ 150–200 mg/m2/day on a 5/28 schedule for a maximum of 12 cycles. The primary end point is OS.
Optimal treatment for patients with unmethylated MGMT promoter is not defined. The benefit of concurrent and adjuvant TMZ is likely minimal, although patients with an unmethylated MGMT promoter treated with TMZ on the EORTC/NCIC study had an improved 2-year survival rate (not a prespecified end point) compared with those who received RT alone (13.8 vs <2%) [2]. There was no benefit of dose-dense TMZ (100 mg/m2 days 1–21/28 days) as compared with standard TMZ dosing (150–200 mg/m2 days 1–5/28) for unmethylated MGMT promoter patients in the RTOG 0525 study [4]. The use of non-TMZ treatment regimens for GB patients with unmethylated MGMT promoter will need to be addressed in future prospective studies. To date, only one published study has explored a non-TMZ regimen in GB with an unmethylated MGMT promoter [16].
Treatment options at recurrence
Given the modest efficacy of salvage therapy and the need to define new GB treatments, enrollment on a clinical trial is encouraged at tumor recurrence in eligible patients. Re-resection with or without Gliadel [17] can be helpful in selected patients, particularly those symptomatic from tumor mass effect and with tumors in noneloquent brain. Although surgery may improve performance, the benefit with respect to survival is less clear [18]. Low performance status (KPS ≤80), large tumor size (tumor volume ≥50 cm3) and tumor involvement of eloquent brain were associated with poorer postoperative survival in a recurrent GB study [19].
Reirradiation is less well studied; at present there are no large prospective clinical trials in recurrent GB. Three single institutional trials in highly selected patients suggest that reradiation may provide significant palliation; however, all suffer from the retrospective nature of the study and probably selection bias [20–22].
Nonetheless, most patients with recurrent GB are not eligible for additional RT, and consequently are offered one of several chemotherapeutic options with, at best, modest responses. Molecular and genetic heterogeneity, complex and redundant activation of intracellular signaling pathways (that regulate proliferation, invasion, angiogenesis and survival), genetic instability leading to de novo (primary) and acquired (secondary) resistance, restricted delivery of agents into the CNS due to the blood–brain barrier (BBB) and increased interstitial fluid pressure within the tumor, and variable end points makes the use and assessment of pharmacological agents in recurrent GB particularly challenging.
Most chemotherapy efficacy data in recurrent GB has come from single-arm, nonrandomized Phase II studies with well-recognized inherent limitations. The 6-month progression-free survival (PFS6) was validated as an end point for recurrent GB trials based on three aggregate Phase II studies by Wong et al. [23] (pre-TMZ era), Ballman et al. [24] (post-TMZ era) and Lamborn et al. [25] (post-TMZ era) (Table 4). In eight consecutive Phase II recurrent GB studies that included 225 patients, Wong reported a PFS6 of 15%. Similarly, Lamborn reported a PFS6 of 16% in 12 consecutive Phase II clinical trials that included 437 recurrent GB patients. In a subgroup analysis of 146 recurrent GB patients who received TMZ at recurrence, the PFS6 was 28%. PFS6 was a strong predictor of survival, suggesting that it is a valid end point for recurrent GB trials. Ballman assessed the relationship between PFS6 and 12-month OS on pooled data from 16 North Central Cancer Treatment Group recurrent GB (n = 345 patients) trials. PFS6 and 12-month OS were 9 and 14%, respectively [24]. The study decisions made using PFS6 and 12-month OS were in agreement 90% of the time, supporting a strong correlation. The lomustine control arm from a recent Phase III recurrent GB study reported similar results [26]. Based on this data, a 10% improvement in PFS6 (PFS6 ≥25%) is required for a therapeutic agent to be considered of interest for further study.
Table 4. . Response rate and 6-month progression-free survival in pooled analyses of trials for recurrent GB.
| Publication | Sample size | Response rate (%) | PFS6 (%) | OS (months) | 1-year survival (%) | Ref. |
|---|---|---|---|---|---|---|
| 8 MD Anderson trials 1986–1995 | 225 | 6 | 15 | 5.7 | 21 | [23] |
| 16 NCCTG trials | 345 | N/A | 9 | 5.1 | 14 | [24] |
| 12 NABTC trials | 437 | 7 | 16 | 6.9 | 25 | [25] |
N/A: Not applicable, NABTC: North American Brain Tumor Coalition; NCCTG: North Central Cancer Treatment Group; OS: Overall survival; PFS6: 6-month progression-free survival.
Accurately and reproducibly assessing response to therapy and tumor progression by imaging is particularly challenging in GB. Traditionally clinical trials utilized the ‘MacDonald Criteria’, which was based on bidimensional measurements of contrast-enhancing tumor on imaging studies [27]. The limitations of the criteria, summarized by Wen et al. [28], include difficulty measuring irregularly shaped tumors, inter-observer variability, the lack of assessment of nonenhancing tumor, lack of guidance for the assessment of multifocal tumors and the difficulty in measuring enhancing lesions in the wall of cystic or surgical cavities because the cyst/cavity itself may be included in the tumor measurement. The limitation of failing to account for progression of nonenchancing tumor became particularly apparent and of increased importance with the introduction of the antiangiogenic agents discussed in the text below. The Response Assessment in Neuro-Oncology (RANO) Working Groups was organized to address these limitations [28]. The proposed RANO criteria are summarized in Table 5.
Table 5. . Summary of proposed Response Assessment in Neuro-Oncology criteria.
| T1-postcontrast MRI | FLAIR | New lesion | Clinical status | |
|---|---|---|---|---|
| Progressive disease: | ||||
| <12 weeks after completion of chemoradiotherapy | New enhancing lesion outside the RT field or unequivocal evidences of tumor on histopathologic sampling | Not sufficient alone | ||
| ≥12 weeks after completion of chemoradiotherapy | Increase by ≥25% in sum of the products of perpendicular diameters between the first post-RT scan, or subsequent scan with smaller tumor size, and the scan at 12 weeks or later on stable or increasing steroid dose | For patients receiving antiangiogenic therapy, significant increase in T2/FLAIR nonenhancing lesion Increased T2/FLAIR must occur on stable or increasing dose of steroids as compared with baseline or best response scan |
New enhancing lesion outside of RT field on decreasing, stable or increasing dose of steroids | Sufficient, if not attributable to concurrent medications or treatment |
| PR | ≥50% decrease of products of perpendicular diameters for at least 4 weeks as compared with baseline scan No progression of nonmeasurable disease |
Stable or improved nonenhancing lesions on same or lower dose of corticosteroids compared with baseline scan | No new lesions | Stable or improved |
| CR | Disappearance of all measurable and nonmeasurable disease for at least 4 weeks | Nonenhancing T2/FLAIR lesions stable or improved | No new lesions | Stable or improved |
| Stable disease | Does not qualify for PD, PR or CR | Stable nonenhancing lesions on same or lower dose of steroids compared with baseline scan. If steroid dose was increased for new symptoms and signs without confirmation of disease progression on imaging | In the event that steroids were increased for new symptoms/signs, without confirmation of progression on imaging, and subsequent imaging shows that increase in steroids was required for disease progression, the last scan considered to show stable disease will be the scan where the steroids dose was equivalent to the baseline dose | |
CR: Complete response; FLAIR: Fluid-attenuated inversion recovery; PD: Disease progression; PR: Partial response; RT: Radiotherapy.
Data taken from [28].
Cytotoxic chemotherapy options at recurrence
▪ Nitrosoureas
Nitrosoureas (carmustine, lomustine, fotemustine and bendamustine) are highly lipid-soluble agents that easily cross the BBB. They alkylate the O 6 position of guanine in DNA leading to single and double strand DNA breaks.
Carmustine and lomustine are the only US-approved cytotoxic agents for the treatment of recurrent GB, although data regarding efficacy is limited [29]. More contemporary data were obtained in randomized controlled Phase III studies that utilized lomustine as the control agent [28–30]. Wick (comparing enzastaurin to lomustine) and Batchelor (comparing cediranib to lomustine) reported a PFS6 of 19 (at first or second recurrence) and 24.5% (first recurrence), respectively, for single-agent lomustine. In both studies, lomustine was as or more effective than the investigational agent.
Fotemustine has been investigated in multiple, nonrandomized Phase II GB studies at first relapse; PFS6 ranged from 21–52% [31–33]. Bendamustine, a bifunctional alkylating agent, failed to meet criteria for efficacy in a single institution Phase II recurrent GB study [34]. Only 6% were progression-free at 6 months, triggering an early stopping rule for futility.
In summary, lomustine has activity in recurrent GB based on two prospective, randomized studies. Whether other nitrosourea agents offer improvement or benefit relative to oral lomustine is uncertain.
▪ TMZ
Multiple investigators have explored dose-dense TMZ schedules as MGMT-depleting strategies in recurrent GB [35–38]. TMZ retreatment has been evaluated in three distinct recurrent GB groups: no prior history of TMZ exposure; progression occurring during a TMZ-free interval after initial disease stabilization (previous TMZ responders); and progression occurring during standard post-RT TMZ therapy. Brada conducted a randomized trial of two TMZ schedules (dose-dense and standard dose) versus procarbazine, lomustine and vincristine in recurrent GB patients with no prior chemotherapy exposure (prior treatment surgery and RT only) [39]. No difference in response was seen, suggesting limited benefit to dose-dense TMZ and again attesting to the activity of lomustine in recurrent GB. Perry conducted a single arm, Phase II trial of recurrent malignant gliomas treated with continuous dose-dense TMZ (50 mg/m2/day) [37]. The hypothesis was that protracted TMZ dosing would overcome drug resistance by reducing intratumoral MGMT activity and provide an antiangiogenic effect (limit endothelial cell recovery, inhibit the activity of circulating endothelial precursors and upregulate thrombospondin-1). Patients were separated into four groups: group 1 anaplastic glioma (n = 29); group 2 GB with progression while receiving adjuvant TMZ, but before completion of six post-RT TMZ cycles (n = 31); group 3 GB with progression while receiving extended adjuvant TMZ beyond the standard six post-RT TMZ cycles, but before completion of adjuvant treatment (n = 29); and group 4 GB with progression after completion of adjuvant treatment and a treatment-free interval of greater than 2 months (n = 29). The PFS6 rates in patients with recurrent GB were 27.3% in group 2, 7.4% in group 3 and 35.7% in group 4. Based on these results, protracted, low-dose TMZ may be an option for recurrent GB patients with early progression (before completion of six cycles of adjuvant therapy, although it is unclear how many responders in this cohort may have manifested pseudoprogression) and in previous responders (those who progressed more than 2 months after completing adjuvant therapy). It remains uncertain whether metronomic TMZ is more effective than re-challenge with standard dose TMZ in previous responders. The DIRECTOR trial, currently in progress in German-speaking countries, is comparing a 21 of 28 days regimen with a 7 days on/7 days off regimen. Dose-dense TMZ has been associated with increased toxicity as compared with standard dosing [4].
RTOG 0929 is another ongoing study exploring TMZ rechallenge in combination with a PARP inhibitor (ABT-888). The rationale behind this combination is that base excision repair, which utilizes PARP, repairs TMZ-induced alkylation of the N-7 position of guanine and N-3 position of adenine. By inhibiting PARP and thereby base excision repair, these adducts become cytotoxic and amplify TMZ DNA damage.
▪ Platins
Platinum-based chemotherapy agents (cisplatin, carboplatin and oxaliplatin) covalently bind to DNA bases and thus disrupt DNA function. Carboplatin achieves the highest CNS concentration, although all platins cross the BBB poorly [40]. Multiple Phase II trials have investigated the use of carboplatin as a single agent or combination therapy for malignant gliomas [41–52]. The results suggest minimal efficacy.
▪ Phosphoramides
Cyclophosphamide, ifosfamide and glufosfamide are alklyating agents that have been used in recurrent GB. A Phase II study of salvage cyclophosphamide (750 mg/m2 per day on 2 consecutive days every 4 weeks) in 40 temozolomide-refractory, recurrent GB patients was conducted by Chamberlain [53]. The PFS6 (primary end point) was 20%. A total of 17.5% of patients had a partial radiographic response. The median PFS and median OS were 2 and 4 months, respectively.
The combination of ifosfamide, carboplatin and etoposide has been investigated in several recurrent GB studies [54–56]. A prospective Phase II study of GB at first recurrence reported a PFS6 of 35%, median PFS 17 weeks and 25% radiographic response rate [54].
A Phase II study of 31 recurrent GB patients evaluated the use of glufosfamide (5000 mg/m2 every 3 weeks) [57]. PFS6 was 3% and there were no radiographic responses. Given the lack of efficacy data, the phosphoramides are not recommended for recurrent GB.
▪ Topoisomerase inhibitors
Topotecan and irinotecan (CPT-11) are topoisomerase I inhibitors that readily cross the BBB. Preclinical and clinical data suggest that toposiomerase I inhibitors may be synergistic with aklyating agents [58]. Single agent CPT-11 has demonstrated minimal activity in multiple Phase II recurrent GB studies (Table 6) [58–64]. Similarly, topotecan demonstrated limited activity in two Phase II studies [65,66], suggesting little or no role for topoisomerase I inhibitors in recurrent GB.
Table 6. . Summary of Phase II clinical trials utilizing intravenous CPT-11 for the treatment of recurrent malignant glioma (excluding bevacizumab combinations).
| Study (year) | Regimen | Patients (n) | Results | Ref. |
|---|---|---|---|---|
| Friedman et al. (1999) | CPT-11 (125 mg/m2 q2 week) | 60 MG | 9 (15%, 95% CI: 6–24) PR | [58] |
| Chamberlain (2002) | CPT-11 (400 mg/m2, 500 mg/m2 3 weeks later) | 40 GB | No responses. mOS 4 months | [59] |
| Cloughesy et al. (2003) | CPT-11 (300 mg/m2 q3week x 2, then 350 mg/m2 q3 week) CPT-11 (350–400 mg/m2 q3 week, increasing qcycle by 100 mg/m2 with EIASD or 50 mg/m2 without EIASD) |
14 MG (13 on EIASD) 26 GB 9 AG |
2 PR, mPFS 6 weeks, mOS 24 weeks 3 PR, mPFS 2.7 months, mOS 8.5 months |
[60] |
| Batchelor et al. (2004) | CPT-11 (411 mg/m2 qweek for 4/6 weeks with EIASD, or 117 mg/m2 qweek without EIASD) | 18 AG (12 EIASD, 6 no EIASD) | 1 CR, 0 PR | [64] |
| Prados et al. (2006) | CPT-11 (350 mg/m2 3 weeks without EIASD, 750 mg/m2 q3 week with EIASD) | 38 GB 13 AG |
PFS6 17.6% (entire cohort) PFS6 15.7% (GB, 95% CI: 7–31) PFS6 23% (AG, 95% CI: 7-52) |
[62] |
AG: Anaplastic glioma; CR: Complete response; EIASD: Enzyme-inducing antiseizure drug; GB: Glioblastoma; MG: Malignant glioma; mOS: Median overall survival; mPFS: Median progression-free survival; PFS6: Percentage of patients progression-free at 6 months; PR: Partial response; q2 week: Once every 2 weeks; q3 week: Once every 3 weeks; qweek: One per week; SD: Stable disease.
Etoposide is a synthetic derivative of Epipodophyllotoxin that acts on topoisomerase II. The drug penetrates the cerebrospinal fluid poorly. A Phase II study in 46 recurrent malignant glioma patients of continuous etoposide (50 mg/m2) demonstrated median PFS of 7.5 weeks for GB (n = 21) [67].
Targeted biologic therapy
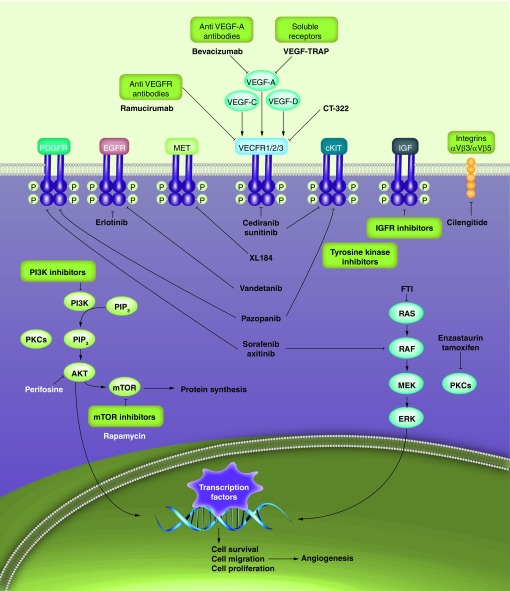
Although no single GB-defining genetic mutation has been identified, several key proliferation and survival signaling pathways appear to be important (Figure 1) [68,69]. The VEGF, EGF, PDGF, HGF and IGF pathways are thought to be particularly relevant in the growth and proliferation of GB. These pathways are characterized by receptors associated with tyrosine kinase activities and share common mechanisms of pathway activation and intracellular signaling [70]. Overexpression or mutations of receptors and intracellular downstream effectors have been identified in GB, leading to constitutive activation of signaling pathways, resulting in uncontrolled cellular proliferation, survival and invasion. The Cancer Genome Atlas Research Network demonstrated that 88% of primary GBs have deregulation in the receptor tyrosine kinases and downstream RAS or PI3K pathways [70]. Laboratory and clinical trials have focused on targeting these pathways with a variety of agents, including monoclonal antibodies, low-molecular weight tyrosine kinase inhibitors and ligand-toxin conjugates. At the present time, the VEGF pathway inhibitor bevacizumab is the only targeted agent approved for GB. The efficacy of others has been overwhelmingly disappointing in GB.
Figure 1. Targeted antiangiogenic therapies and relevant signaling pathways in glioblastoma.
EGFR: EGF receptor; IGFR: IGF receptor; PDGFR: PDGF receptor; PIP2: Phosphatidylinositol 4, 5 bisphosphate; PIP3: Phosphatidylinositol (3,4,5) -triphosphate; VEGFR: VEGF receptor.
Reproduced with permission from [69].
VEGF pathway inhibitors
GB is a highly vascular tumor that depends on angiogenesis for growth and proliferation. VEGF pathway inhibitors were developed to inhibit tumor angiogenesis. The VEGF pathway can be inhibited by either blocking the ligand (VEGF) or the receptor (VEGFR). Inhibition of the VEGF pathway affects the permeability of the BBB, which often results in decreased tumor contrast enhancement on imaging, even in obvious cases of disease progression evident on T2-based MRI. As discussed in a preceding section, changes to the imaging response criteria in GB clinical trials have been proposed to account for this ‘pseudoresponse’ and nonenhancing tumor progression [28].
VEGF ligand inhibitors
Bevacizumab is a humanized, anti-VEGF monoclonal antibody that was granted accelerated approval by the US FDA in March 2009 for single-agent treatment of recurrent GB. The approval was based on the high radiologic response rate (in an independent radiology review) demonstrated in two Phase II clinical trials [71,72]. A summary of the multiple small studies that have utilized bevacizumab for recurrent GB is displayed in Table 7 [71–81].
Table 7. . Summary of Phase II clinical trials utilizing bevacizumab-based regimens for the treatment of recurrent glioblastoma.
| Study (year) | Regimen | Patients (n) | Results | Ref. |
|---|---|---|---|---|
| Vredenburgh et al. (2007) | Cohort 1: Bev 10 mg/kg q2 week Irinotecan (125 or 340 mg/m2) q2 week Cohort 2: Bev 15 mg/kg q21 days Irinotecan days 1, 8, 22 and 29 |
23 GBM 12 GBM |
PFS6: 46% (95% CI: 32–66%) for all 6-month OS was 77% (95% CI: 64–92%) 57% PR (95% CI: 39–74%) One CNS hemorrhage. Four thromboembolic complications (deep venous thrombosis and/or pulmonary emboli) |
[78] |
| Kreisl et al. (2009) | Bev (10 mg/kg q2 week) plus irinotecan (125 or 340 mg/m2 q2 week) at progression |
48 GBM | PFS6: 29% (95% CI: 18–48%) 71% PR (Levin Criteria) 35% PR (Macdonald criteria) Median PFS was 16 weeks (95% CI: 12–26 weeks) Median OS was 31 weeks (95% CI: 21–54 weeks) Six were removed from study for toxicity (five thromboembolic events and one bowel perforation) |
[72] |
| Gutin et al. (2009) | Bev (10 mg/kg q2 week) 30 Gy HFSRT in five fractions after first Bev cycle |
20 GBM 5 AG |
Three discontinued treatment for toxicity (intratumoral hemorrhage, wound dehiscence, bowel perforation) 50% radiographic response rate PFS6: 65% Median OS: 12.5 m, 1-year survival: 54% |
[80] |
| Friedman et al. (2009) | Bev (10 mg/kg q2 week) Bev (10 mg/kg q2 week) irinotecan (125 or 340 mg/m2 q2 week) |
78 GBM 7 AA 76 GBM 6 AA |
PFS6 = 42.6% (97.5% CI: 29.6–55.5%) 28.2% PR (97.5% CI: 18.5–40.3%) Median PFS: 4.2 months (95% CI: 2.9–5.8) Median OS: 9.2 months (95% CI: 8.2–10.7) PFS6: 50.3% (97.5% CI: 36.8–63.9%) 37.8% PR (97.5% CI: 26.5–50.8%) Median PFS: 5.6 months (95% CI: 4.4–6.2) Median OS: 8.7 months (95% CI: 7.8–10.9) |
[71] |
| Reardon et al. (2009) | Bev (10 mg/kg q2 week) Etoposide (50 mg/m2 days 1–21/28) |
27 GBM 32 MG |
PFS6 40.6% (MG) and 44.4% (GBM) RRR 22% (MG) and 37% (GBM) Median OS: 63.1 weeks (MG) Median OS: 44.4 weeks (GBM) Two had asymptomatic grade I hemorrhage One on-study death occurred because of pulmonary embolus |
[77] |
| Reardon et al. (2010) | Bev (10 mg/kg q2 week) Erlotinib (150 mg or 450 mg q.d.) Sirolimus (5 mg or 10 mg q.d.) |
32 GBM (28% had prior Bev) | PFS6: 3.1% No patients achieved CR or PR |
[76] |
| Hasselbalch et al. (2010) | Bev (5–10 mg/kg q2 week) Irinotecan (340 or 125 mg/m2 q2 week) Cetuximab (400 mg/m2 as load, followed by 250 mg/m2 weekly) |
43 GBM (32 available for response) |
34% RRR (2 CR, 9 PR) PFS6: 30% Median OS 29 weeks (95% CI: 23–37) |
[73] |
| Sathornsumetee et al. (2010) | Bev (10 mg/kg q2 week) Erlotinib (200 or 500 mg q.d.) |
25 GBM 32 AG |
PFS6: 28%, median OS 42 weeks 48% RRR PFS6: 44%, median OS 71 weeks 31% RRR |
[81] |
| Raizer et al. (2010) | Bev (15 mg/kg q3 week) | 50 GBM 11 AG |
PFS6: 25%, median OS 25.6 weeks, 24.5% PR | [74] |
| Reardon et al. (2011) | Bev (10 mg/kg q2 wk) Carboplatin (AUC 4 mg/ml-min, q28 days) Irinotecan (340 mg/m2 or 125 mg/m2 q2 week) |
40 GBM | PFS6: 46.5% (95% CI: 30.4–61%) Median OS 8.3 months (95% CI: 5.9–10.7) One patient died due to treatment-related intestinal perforation |
[75] |
| Desjardins et al. (2012) | Bev (10 mg/kg q2 week) TMZ (50 mg/m2 q.d.) |
32 GBM | PFS6: 18.8% (95% CI: 7.6–33.7%) median PFS 15.8 weeks, median OS 37 week 28% RRR |
[79] |
AA: Anaplastic astrocytoma; AG: Anaplastic glioma; AUC: Area under the curve; Bev: Bevacizumab; CR: Complete response; GBM: Glioblastoma; HFSRT: Hypofractionated stereotactic radiotherapy; MG: Malignant glioma; mPFS: Median progression free survival; OS: Overall survival; PFS6: Percentage of patients progression-free at 6 months; PR: Partial response; RRR: Radiographic response rate; SD: Stable disease.
Friedman et al. performed a multicenter, open-label Phase II study of patients with recurrent GB after standard RT/TMZ. In total, 167 patients were randomized to receive bevacizumab (10 mg/kg) alone or bevacizumab (10 mg/kg) with irinotecan (340 mg/m2 or 125 mg/m2) every 2 weeks. The study was not designed to compare differences in response or survival between the two treatment arms. In the single-agent bevacizumab group, PFS6, objective radiographic response rate and median OS were 42.6%, 28.2% and 9.2 months, respectively.
Kreisl performed a nonrandomized, Phase II study of single-agent bevacizumab in 48 patients with recurrent GB. All patients received initial standard treatment with RT/TMZ. PFS6, median PFS and median OS were 29%, 16 weeks and 31 weeks, respectively. In total, 58% of patients receiving corticosteroids at the start of treatment were able to decrease their steroid requirement by an average of 59%. The overall radiographic response rate was 35% (one complete and 16 partial responses).
The approval of bevacizumab has been controversial despite the impressive radiographic response. It is argued that neutralization of VEGF results in stabilization of the BBB with a resultant decrease in vascular permeability, leading to an improvement in edema and a decrease in enhancement on imaging. Regardless, as per accelerated approval regulations, a Phase III randomized, placebo-controlled study of bevacizumab in combination with RT and TMZ in newly diagnosed GB (AVAglio Study) is being conducted to evaluate for a bevacizumab survival benefit [82]. The coprimary end points are PFS and OS. The study has completed accrual, although the primary end points have not been reached.
RTOG 0825 is another large Phase III, randomized, double-blind placebo-controlled trial of bevacizumab in combination with standard therapy for newly diagnosed GBM. The study has completed accrual but not reached the primary end points. Early results of these two studies should be available next year. A comparison of these two studies is summarized in Table 6.
It is still unclear whether bevacizumab should be combined with a cytotoxic chemotherapy, as is the practice with other solid tumors (employing so-called chemosynergy) and if so, the identity of optimal combinational treatment. Many of the initial bevacizumab GB studies combined treatment with CPT-11, despite the fact that CPT-11 has minimal single-agent activity in GB (see above). In addition, the optimal treatment dose, schedule and duration of bevacizumab treatment has not been determined [83]. Finally, the timing of introducing bevacizumab remains an area of controversy. Notably, there are reports that some bevacizumab-treated patients develop an aggressive, invasive ‘gliomatosis’ pattern of recurrence that is unresponsive to subsequent therapy. It has been hypothesized that by inhibiting VEGF, bevacizumab induces a phenotypic tumor change into an aggressive, invasive variant that co-opts available vessels rather than initiating angiogenesis. Whether this is the case, or the diffuse brain invasion is the natural history of GB in patients who do not succumb to tumor mass effect and edema, is not entirely clear. Current data suggest a diffuse disease pattern is more common over time, irrespective of treatment [84–86]. EORTC 26101 (bevacizumab vs lomustine in recurrent GB at first recurrence) is a planned Phase II study designed to determine the therapeutic role of bevacizumab and optimization of sequencing the combination of bevacizumab and lomustine in GB at first recurrence.
Aflibercept (VEGF Trap) is a recombinant fusion protein that scavenges both VEGF and placental growth factor. de Groot conducted a multicenter, single-arm, Phase II study to evaluate the efficacy of aflibercept (4 mg/kg intravenous once every 2 weeks) in recurrent GB (n = 42) and anaplastic glioma (n = 16) [87]. All patients were enrolled at first relapse after standard concurrent RT/TMZ followed by adjuvant TMZ. The PFS6 was 7.7 and 25% for patients with GB and anaplastic glioma, respectively. Overall radiographic response rate was 24% (18% for GBM and 44% for anaplastic glioma). Median PFS was 12 weeks for GB. In total, 25% of the patients were removed from the study for toxicity, on average less than 2 months from treatment initiation. An Adult Brain Tumor Consortium study (presently underway) is evaluating VEGF Trap in the upfront setting notwithstanding the very modest activity and toxicity seen with treatment at recurrence.
VEGFR inhibitors
The VEGFR can be inhibited by blocking the ligand binding site of the VEGFR with either monoclonal antibodies or genetically engineered peptides or blocking the tyrosine kinase activation site of VEGFR with small-molecule inhibitors (tyrosine kinase inhibitors) [88].
Ramucirumab is a fully human, monoclonal antibody directed against VEGFR-2 [89]. An open-label, Phase II study is planned to investigate ramucirumab in recurrent GB.
Cediranib is an orally available, pan-VEGFR tyrosine kinase inhibitor with additional activity against PDGFβ and c-Kit [90]. A Phase II study of single-agent cediranib in 31 recurrent GB patients was associated with a compelling radiographic response (56.7%) and PFS6 (25.8%) [90]. Given the encouraging Phase II results, a multicenter, randomized, parallel-group, Phase III recurrent GB trial (REGAL) was conducted to compare cediranib (as monotherapy and in combination with lomustine) with lomustine alone [30]. Patients were randomized (ratio 2:2:1), to cediranib monotherapy (30 mg/day; n = 131), a combination of cediranib (20 mg/day) plus lomustine (110 mg/m2 every 6 weeks; n = 129) or a control group of lomustine (110 mg/m2 every 6 weeks) and placebo (n = 65). There was no statistical difference in the median PFS (primary end point) of 92 days in the cediranib monotherapy arm, 125 days in the combination arm compared with 82 days in the lomustine control arm. PFS6 was 16% in the monotherapy arm, 34.5% in the combination arm and 24.5% in the control arm. The under-appreciation of lomustine activity in recurrent GB and the low cediranib dosing on both arms (less than that used in the prior single-arm Phase II study) were thought to be possible reasons for the negative Phase III study.
Cediranib is being investigated with concurrent RT/TMZ in newly diagnosed GB (RTOG 0837). The study is a Phase II, double-blind, placebo-controlled, randomized study that stratifies patients based on recursive partitioning analysis class and MGMT methylation status. Patients randomized to the experimental arm receive cediranib for 3 days prior to RT, during RT and for up to a year after RT.
Pazopanib, a multitargeted tyrosine kinase inhibitor against VEGFR 1, 2 and 3, PDGFR α and β, and c-Kit, was evaluated in a Phase II recurrent GB trial (no prior exposure to VEGF pathway inhibitors, ≤2 relapses) [91]. Thirty five patients were treated with pazopanib (800 mg daily). PFS6, the primary end point, was 3%. There were two partial radiographic responses by standard measurements and nine patients with decreased contrast enhancement and vasogenic edema, but less than 50% reduction in bidimensional measurement. Median PFS and OS were 12 weeks and 35 weeks, respectively.
Sunitinib, a multitargeted tyrosine kinase inhibitor against VEGFR2, c-Kit and PDGFR-α, was evaluated in a Phase II study in recurrent malignant glioma [92]. Twenty-one patients (16 GB) with recurrent malignant glioma were treated with sunitinib (37.5 mg daily) until disease progression. There were no objective responses. Median PFS and OS were 1.6 and 3.8 months, respectively. There was no correlation between VEGFR2, PDGFR-α and KIT gene copy numbers or protein expression and the effects of sunitinib.
XL-184 is a multitargeted tyrosine kinase inhibitor with activity against MET, VEGFR2 and RET. Wen conducted an open-label Phase II recurrent GB trial (n = 124) of XL-184 (175 mg/day) or XL-184 (125 mg/day) [93]. Patients with (n = 42) or without prior antiangiogenic therapy were included. In the 175 mg cohort (n = 46), PFS6 (primary end point) was 21%. The objective radiographic response rate in patients with (n = 12) and without (n = 34) prior antiangiogenic exposure was 8 and 12%, respectively. At the time of data presentation, the primary end point was not reached for the 125 mg cohort.
Other agents that have antiangiogenic properties include AMG 386 (an engineered peptibody composed of truncated human IgG1 Fc domain covalently linked to two copies of synthetic antiangiopeptin peptide), cilengitide (integrin inhibitor, see below), enzastaurin (protein kinase C inhibitor, see below), thalidomide (FGF inhibitor) and imatinib (PDGF inhibitor) [69,88].
EGF pathway inhibitors
EGFR is amplified in approximately 45% of GB, 40% of those express a mutant receptor (EGFRvIII) that has a deletion of exons 2–7, causing a defect in the extracellular ligand-binding domain, resulting in constitutive activation in a ligand-independent manner [70]. EGFRvIII positivity may be an independent prognostic factor for poor survival [94]. As such, it may play an important role in GB pathogenesis and may serve as a targeted treatment option. Several small clinical trials have investigated the inhibition of EGFR with small molecule inhibitors either alone or with combination therapy (Table 8) [42,81,76,95–101]. In summary, none of the Phase II studies of small molecule EGFR inhibitors have been successful, even in patients with EGFR amplification or the EGFRvIII mutant. In addition, small molecule EGFR inhibitors did not improve outcome over historic controls in newly diagnosed GB studies [102–105].
Table 8. . Summary of Phase II clinical trials utilizing EGFR inhibition for the treatment of recurrent malignant glioma and newly diagnosed glioblastoma.
| Study (year) | Regimen | Patients (n) | Results | Ref. |
|---|---|---|---|---|
| Recurrent malignant glioma studies | ||||
| Rich et al. (2004) | Gefitinib (500 mg q.d., escalated to 750–1000 mg q.d.) | 53 GB | No objective tumor response PFS6: 13%, mPFS 8.1 weeks, mOS 39.4 weeks |
[100] |
| Franceschi et al. (2007) | Gefitinib (250 mg q.d.) | 16 GB 9 AA 3 AO |
PFS: 14.3% (95% CI: 4–32.7%) for the whole cohort. EGFR expression or gene status, and p-Akt expression did not predict activity | [98] |
| Kreisl et al. (2009) | Gefitinib 250 mg q.d. Everolimus 70 mg qweek |
20 GB | 3 PR (14%), PFS6: 5% mPFS 2.6 months, mOS 5.8 months |
[99] |
| de Groot et al. (2008) | Carboplatin (AUC 6 mg x ml/min d1) q28 days Erlotinib (150 mg/day dose escalated to 200 mg/day as tolerated) |
43 GB | PFS6: 14% 1 PR 20 SD mFS 9 weeks, mOS 30 weeks |
[42] |
| van den Bent et al. (2009) | Erlotinib (150 mg/day dose escalated to 200 mg/day (no EIASD) or 300 mg/day dose escalated to 500 mg/day (EIASD) – experimental arm TMZ or Carmustine – control arm |
110 GB | PFS6: 11.4% (95% CI: 4.6–21.5%) – experimental arm 0/8 patients with EGFRvIII mutant presence and PTEN expression had 6-month PFS PFS6: 24% control arm |
[96] |
| Raizer et al. (2010) | Erlotinib (150 mg/day, no EIASD) | 53 recurrent MG | PFS6: 3%, median PFS 2 months for recurrent GB No effect on EGFR or intratumoral signaling was seen |
[95] |
| Reardon et al. (2010) | Erlotinib (150 mg q.d.) Sirolimus (5 mg q.d.) – no EIASD Erlotinib (450 mg q.d.) Sirolimus (10 mg q.d.) – EIASD |
32 GB | No CR or PR PFS6: 3.1% |
[76] |
| Yung et al. (2010) | Erlotinib (150 mg q.d.) – no EIASD Erlotinib (300 mg q.d.) – EIASD |
48 GB | 1 CR, 2 PR, mOS 9.7 months (95% CI: 5.9–11.6 months) PFS6: 20% (95% CI: 10–32.4%) Outcomes not related to EGFR amplification or EIASD status |
[97] |
| Sathornsumetee et al. (2010) | Erlotinib (200 mg/day) – no EIASD Erlotinib (500 mg/day) – EIASD Bevacizumab (10 mg/kg q2 weeks) |
25 GB 32 AG |
PFS6: 28%, mOS 42 weeks – GB PFS6: 44%, mOS 71 weeks – AG Similar PFS benefit and radiographic response as compared with historical bevacizumab-containing regimens |
[81] |
| Hegi et al. (2011) | Gefitinib 500 mg q.d. prior to surgery, then postoperative until recurrence | 22 GB | Resected tissue had high concentrations of gefitinib (median 4.1 µg/g). EGFR was efficiently dephosphorylated in treated patients compared with control No effect on 12 pathway constituents. Thus, regulation of downstream signal transducers in the EGFR pathway seems to be dominated by regulatory circuits independent of EGFR phosphorylation |
[101] |
| Newly diagnosed GB studies | ||||
| Brown et al. (2008) | Erlotinib (150 mg daily) x 1 week, then concurrently with TMZ (75 mg/m2/day) and RT (60 Gy), then concurrently with TMZ (200 mg/m2 5 days every 28 day) | 97 GB | mOS 15.3 months No benefit compared with historical TMZ controls Presence of rash, diarrhea, EGFRvIII, combination EGFR and PTEN, and EGFR amplification status were not predictive of survival |
[102] |
| Prados et al. (2009) | Erlotinib (100 mg/day during RT and 150 mg/day after RT) – non-EIASD Erlotinib (200 mg/day during RT and 300 mg/day after RT) All patients received standard RT/TMZ |
65 GB | mOS 19.3 months | [104] |
| Peereboom et al. (2010) | Erlotinib 50 mg/day, increased by 50 mg/day every 2 weeks until grade 2 rash or maximum Erlotinib 150 mg/day All received standard RT/TMZ |
27 GB | mOS 8.6 months, mPFS 2.8 months | [103] |
| Uhm et al. (2011) | Gefitinib 500 mg/day Gefitinib 1000 mg/day – EIASD or dexamethasone |
100 GB | 1-year OS 54.2% (not statistically different from historical controls) Clinical outcome was not affected by EGFR status (amplification or vIII mutation) |
[105] |
AA: Anaplastic astrocytoma; AG: Anaplastic glioma; AO: Anaplastic oligodendroglioma; CR: Complete response; EIASD: Enzyme-inducing antiseizure drug; GB: Glioblastoma; MG: Malignant glioma; mOS: Median overall survival; mPFS: Median progression-free survival; PFS6: Percentage of patients progression-free at 6 months; PR: Partial response; q2 week: Once every 2 weeks; q.d.: Daily; qweek: One per week; RT: Radiotherapy; SD: Stable disease; TMZ: Temozolomide.
Integrin inhibitors
Integrins are transmembrane cell surface receptors that play a key role in cancer initiation and progression by providing adhesive, migratory and survival cues to tumor cells and to cells of the microenvironment [106].
▪ Integrins
Overexpression of the integrin αvβ3 has been documented. Reardon conducted a randomized, Phase II study of cilengitide (an inhibitor of αvβ3 and αvβ5 integrin receptors) in recurrent GB [107]. Eighty one patients were randomized to cilengitide (500 mg twice weekly; n = 41) or cilengitide (2000 mg twice weekly; n = 40). PFS6 (the primary end point) was 15% and median OS was 9.9 months. The authors did not report how many patients were treated with bevacizumab after cilengitide failure. Gilbert conducted a Phase II study to evaluate the efficacy and tumor delivery of cilengitide in recurrent GB [108]. Thirty recurrent GB patients, scheduled to undergo surgery for optimal care, received three doses of cilengitide at 500–2000 mg prior to tumor resection. After surgery, patients received cilengitide (2000 mg twice weekly) until disease progression. Cilengitide was detected in all tumor specimens in association with corresponding low plasma levels, confirming drug delivery. PFS6 was 12%. Based on these two studies, cilengitide has minimal single-agent activity in recurrent GB despite the fact that it was demonstrated to accumulate in tumor tissue.
Despite the underwhelming response in recurrent disease, cilengitide is being investigated as an add-on to standard (RT/TMZ) newly diagnosed GB treatment based upon a single prospective Phase II trial [109]. CENTRIC, a randomized, open-label, Phase III study to evaluate the efficacy and safety of cilengitide in combination with standard RT/TMZ (experimental arm) versus standard RT/TMZ alone (control arm) in GB with a methylated MGMT gene promoter, has completed enrollment [110]. A companion randomized, Phase II trial (CORE Study) to evaluate the addition of two different cilengitide dosing regimens to standard RT/TMZ in newly diagnosed GB with an unmethylated MGMT gene promoter is underway [111].
Protein kinase C inhibitors
Protein kinase C (PKC) is a serine/threonine kinase that sits at the nexus of the RAF-MEK-ERK and PI3K/AKT pathways and thus is activated by upstream growth factor receptors and regulates cell proliferation, invasion and angiogenesis [70]. Enzastaurin is a potent PKC-β inhibitor that had direct cytotoxic activity against glioma cells in preclinical studies [112]. Kreisl conducted a 118 patient, Phase II study of enzastaurin in recurrent high-grade glioma. In total, 25% had an objective radiographic response. PFS6 was 7 and 16% for glioblastoma and anaplastic astrocytoma, respectively. Wick conducted a randomized, open-label study in patients with recurrent GB [26]. Two hundred and sixty six patients were randomized (2:1) to receive enzastaurin (n = 174) or lomustine (n = 92). Median PFS (1.5 vs 1.6 months), OS (6.6 vs 7.1 months) and PFS6 did not differ significantly between the two groups. Objective responses occurred in 2.9% (enzastaurin) versus 4.3% (lomustine). Based on these two studies, single-agent enzastaurin has minimal activity in recurrent GB. Wick's study nonetheless confirmed the modest activity of lomustine in recurrent GB, similar to that seen in the cediranib trial discussed above. Butowski conducted a 66 patient, open-label, single-arm, Phase II study of enzastaurin in combination with standard RT/TMZ. Median OS and median PFS were 74 and 36 weeks, respectively [113].
Histone deacetlylase inhibitors
Histone deacetlylase (HDAC) inhibitors (vorinostat and romidepsin) target cancer by preventing gene transcription, resulting in cell cycle arrest, differentiation and/or apoptosis. A Phase II study of 66 recurrent GB patients treated with vorinostat demonstrated PFS6 of 17% and median OS of 5.7 months [114]. Another Phase II trial of 35 patients investigated romidepsin in recurrent GB [115]. There were no objective radiographic responses. The median PFS and PFS6 were 8 weeks and 3%, respectively. Thus, neither single agent vorinostat nor romidepsin are effective in recurrent GB. Friday conducted a Phase II study of vorinostat in combination with bortezomib (proteosome inhibitor) [116]. Thirty seven recurrent GB patients were treated with vorinostat (400 mg daily for 14/21 days) and bortezomib (1.3 mg/m2 on days 1, 4, 8 and 11). PFS6 was 0%. One patient achieved a partial response. Thus the combination of vorinostat with bortezomib is not effective. Notwithstanding minimal single-agent activity of HDAC inhibitors, a consortium trial is accruing utilizing standard care and vorinostat in newly diagnosed GB.
Immunotherapy
Investigators have studied various adoptive and active immunotherapies in the treatment of GB [117]. Despite limited success, there has been renewed interest recently with the development of tumor-specific vaccines and the approval of new agents for other solid tumors (such as ipilimumab for melanoma). The use of an active, tumor-specific immunotherapy is appealing as it could potentially maintain long-term antitumor response while limiting toxicity. Although immunotherapy has been investigated in new and recurrent GB, the majority of current studies have been in the upfront setting. Ongoing clinical trials utilizing rindopepimut (EGFRvIII-targeted peptide vaccine), DC Vax (autologous tumor lysate-pulsed dendritic cell vaccine), ICT-107 (dendritic cells prepared from autologous mononuclear cells that are pulsed with six synthetic peptides) and HSPPC-96 (autologous tumor-derived heat shock protein [glycoprotein 96]) are summarized in Table 1. The combination of rindopepimut and bevacizumab is being investigated in a double-blind, Phase II recurrent GB trial.
Conclusion & future perspective
The addition of TMZ to RT has improved GB survival, particularly in those with MGMT promoter gene methylation. The questions of which aspect of the EORTC/NCIC regimen (TMZ concurrent with RT, TMZ after RT or the combination of both) is beneficial and how long TMZ should be continued after RT will likely never be answered in prospective clinical trials. However, the majority of patients with newly diagnosed GB (approximately 70% in the RTOG 0525 trial) obtain little to no benefit from TMZ-based chemoradiotherapy. Nonetheless, these patients presently defined by an unmethylated MGMT promoter, continue to receive TMZ as upfront therapy, reflecting the lack of an alternative therapy aside from surgery and RT only. An alternative therapy that provides similar or improved outcomes to that seen in patients with a methylated MGMT promoter is an unmet need in this large category. Few trials have been designed specifically for this patient population. It is possible that improved survival and quality of life may be achieved with either upfront bevacizumab (assessed but not yet reported in RTOG 0825 and AVAglio studies) or cilengitide (CORE) trials, since mechanistically these agents have no relationship with DNA enzyme repair systems. Alternatively the MGMT unmethylated cohort may benefit from an immunotherapy approach, such as the EGFRVIII peptide vaccine, a novel therapy not dependent upon alkylator-based chemotherapy. Future success will hopefully be achieved with the addition of as yet unidentified targeted agent(s).
The results of the two Phase III, upfront bevacizumab trials (AVAglio and RTOG 0825) could significantly change the initial and recurrent treatment of GB. More likely, the results will confirm the preliminary data from Lai [11] that suggests that bevacizumab has greatest utility in treating GB at recurrence. It is anticipated that GB patients with unmethylated MGMT will increasingly be treated with non-TMZ-based therapies in upfront clinical trials. Critical to this realization, however, is demonstrating activity of a novel class of targeted therapy in the recurrent setting, which has remained an elusive goal despite a panoply of unsuccessful single-agent targeted Phase II trials. Novel agents that target glioma stem cells (such as STAT3 and NOTCH inhibitors) appear promising in vitro. An increasingly common paradigm in upfront GB trials is to utilize targeted agents that have had little or no success in the recurrent setting with the rationale that the agent is synergistic with either TMZ or RT and has a novel mechanism of action. Based on the perspective gained in other solid cancers (e.g., lung or breast cancer) this paradigm is unlikely to succeed.
Despite the initial promise and excitement of targeted therapy, only bevacizumab has demonstrated efficacy (notably as a single agent in the recurrent setting). Confirmation of a survival benefit in a randomized Phase III trial is pending, albeit in a different patient population (i.e., newly diagnosed GB). It is anticipated that enrichment trials, wherein patients with the molecular target of interest are enrolled, will become standard in the investigation of targeted agents. Identifying subsets of GBs with apparent oncogene addiction may permit improved efficacy of available targeted therapies. Additionally, the use of correlative studies that utilize post-treatment surgical specimens to determine whether the targeted agent of interest enters the tumor and affects the targeted signaling pathway (as in the study by Gilbert et al. [108]) are expected to increase. Future treatments will emerge as the molecular biology of GB is further defined and perhaps permit treatment allocation based upon molecular and genetic characterization. One cautionary note, however, in further subdividing GB based upon molecular and gene expression, is creating an ever smaller subset of patients that benefit from current therapy with few to no alternatives for molecularly unfavorable GB subtypes (e.g., the mesenchymal subtype).
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Central Brain Tumor Registry of the USA. Central Brain Tumor Registry of the USA; IL, USA: 2011. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States, 2004–2007. [Google Scholar]
- 2.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]; ▪▪ Established the current upfront treatment regimen for glioblastoma (GB) following initial surgery.
- 3.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin. Cancer Res. 2010;16(8):2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Study suggesting improved survival of newly diagnosed, protocol-eligible patients as compared with historical controls, which is partly a reflection of improved supportive care, recognition of pseudoprogression and availability of bevacizumab for recurrent disease.
- 4.Gilbert MR. RTOG 0525: a randomized Phase III trial comparing standard adjuvant temozolomide with a dose-dense schedule in newly diagnosed glioblastoma. J. Clin. Oncol. 2011;29(Suppl.) Abstract 2006. [Google Scholar]; ▪▪ Large, randomized, Phase III clinical trial that demonstrated no benefit of dose-dense temozolomide (TMZ) over standard TMZ in newly diagnosed GB with methylated or unmethylated promoters. The study also prospectively validated MGMT as a prognostic factor.
- 5.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Brem SS, Bierman PJ, Brem H, et al. Central nervous system cancers. J. Nat. Compr. Cancer Netw. 2011;9(4):352–400. doi: 10.6004/jnccn.2011.0036. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 8.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochirurgica. 2006;148(3):269–275. doi: 10.1007/s00701-005-0707-z. Discussion 275. [DOI] [PubMed] [Google Scholar]
- 9.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 2000;343(19):1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 10.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]; ▪ Retrospective study demonstrating the prognostic value of MGMT promoter region methylation in GB.
- 11.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2010;29(2):142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ First trial using bevacizumab in newly diagnosed GB in conjunction with standard radiotherapy (RT)/TMZ.
- 12.Vredenburgh JJ, Desjardins A, Kirkpatrick JP, et al. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2010;82(1):58–66. doi: 10.1016/j.ijrobp.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 13.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101(10):2293–2299. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 14.Malmstrom A, Grønberg BH, Stupp R, et al. Glioblastoma in elderly patients: a randomized Phase III trial comparing survival in patients treated with 6-week radiotherapy (RT) versus hypofractionated RT over 2 weeks versus temozolomide single-agent chemotherapy. J. Clin. Oncol. 2010;28(Suppl.):18s. Abstract LBA2002. [Google Scholar]
- 15.Wick WEC, Combs SE, Nikkhak G, et al. NOA-08 randomized Phase III trial of 1-week-on/1-week-off temozolomide versus involved-field radiotherapy in elderly (older than age 65) patients with newly diagnosed anaplastic astrocytoma or glioblastoma. J. Clin. Oncol. 2010;18(Suppl.) 28. Abstract LBA2001. [Google Scholar]
- 16.Wick WSJ, Combs SE, Platten M, et al. American Society of Clinical Oncology Annual Meeting. Chicago, IL, USA: 3–7 June 2011. Enzastaurin (ENZ) before and concomitant with radiation therapy followed by ENZ maintenance therapy in patients with newly diagnosed glioblastoma without hypermethylation of the MGMT promoter: a multicenter, open-label, uncontrolled Phase II study. Presented at. [Google Scholar]
- 17.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 18.Clarke JL, Ennis MM, Yung WK, et al. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro-oncology. 2011;13(10):1118–1124. doi: 10.1093/neuonc/nor110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JK, Hodges T, Arko L, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J. Clin. Oncol. 2010;28(24):3838–3843. doi: 10.1200/JCO.2010.30.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adkison JB, Tome W, Seo S, et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(3):835–841. doi: 10.1016/j.ijrobp.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 21.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J. Clin. Oncol. 2010;28(18):3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd SF, Laing RW, Cosgrove VP, et al. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int. J. Radiat. Oncol. Biol. Phys. 1997;37(2):393–398. doi: 10.1016/s0360-3016(96)00455-5. [DOI] [PubMed] [Google Scholar]
- 23.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto Phase II clinical trials. J. Clin. Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]; ▪ Reference study establishing median, 6-month and overall survival in adults with recurrent GB treated with salvage therapy.
- 24.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for Phase II trials in patients with glioblastoma multiforme. Neuro-oncology. 2007;9(1):29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro. Oncol. 2008;10(2):162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J. Clin. Oncol. 2010;28(7):1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdonald D, Cascino T, Schold SJ, Cairncross J. Response criteria for Phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 28.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment in gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]; ▪▪ Describes the current recommendations for response assessment in high-grade gliomas.
- 29.Levin VA, Wilson CB. Nitrosourea chemotherapy for primary malignant gliomas. Cancer Treat. Reports. 1976;60(6):719–724. [PubMed] [Google Scholar]
- 30.Batchelor T, Mulholland P, Neyns B, et al. A Phase III randomized study comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, with lomustine alone in recurrent glioblastoma patients. Ann. Oncol. 2010;21(Suppl. 8) doi: 10.1200/JCO.2012.47.2464. Abstract LBA7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandes AA, Tosoni A, Franceschi E, et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a Phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Cancer Chemother. Pharmacol. 2009;64(4):769–775. doi: 10.1007/s00280-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabrini MG, Silvano G, Lolli I, et al. A multi-institutional Phase II study on second-line Fotemustine chemotherapy in recurrent glioblastoma. J. Neurooncol. 2009;92(1):79–86. doi: 10.1007/s11060-008-9739-6. [DOI] [PubMed] [Google Scholar]
- 33.Scoccianti S, Detti B, Sardaro A, et al. Second-line chemotherapy with fotemustine in temozolomide-pretreated patients with relapsing glioblastoma: a single institution experience. Anti-Cancer Drugs. 2008;19(6):613–620. doi: 10.1097/CAD.0b013e3283005075. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlain MC, Johnston SK. Salvage therapy with single agent bendamustine for recurrent glioblastoma. J. Neurooncol. 2011;105(3):523–530. doi: 10.1007/s11060-011-0612-7. [DOI] [PubMed] [Google Scholar]
- 35.Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: Phase II study from Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br. J. Cancer. 2006;95(9):1155–1160. doi: 10.1038/sj.bjc.6603376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franceschi E, Omuro AM, Lassman AB, Demopoulos A, Nolan C, Abrey LE. Salvage temozolomide for prior temozolomide responders. Cancer. 2005;104(11):2473–2476. doi: 10.1002/cncr.21564. [DOI] [PubMed] [Google Scholar]
- 37.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J. Clin. Oncol. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]; ▪ Study that established the utility of TMZ rechallenge in recurrent GB.
- 38.Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J. Clin. Oncol. 2007;25(22):3357–3361. doi: 10.1200/JCO.2007.10.7722. [DOI] [PubMed] [Google Scholar]
- 39.Brada M, Stenning S, Gabe R, et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J. Clin. Oncol. 2010;28(30):4601–4608. doi: 10.1200/JCO.2009.27.1932. [DOI] [PubMed] [Google Scholar]
- 40.Riccardi R, Riccardi A, Di Rocco C, et al. Cerebrospinal fluid pharmacokinetics of carboplatin in children with brain tumors. Cancer Chemother. Pharmacol. 1992;30(1):21–24. doi: 10.1007/BF00686480. [DOI] [PubMed] [Google Scholar]
- 41.Aoki T, Mizutani T, Nojima K, et al. Phase II study of ifosfamide, carboplatin, and etoposide in patients with a first recurrence of glioblastoma multiforme. J. Neurosurg. 2010;112(1):50–56. doi: 10.3171/2009.5.JNS081738. [DOI] [PubMed] [Google Scholar]
- 42.De Groot JF, Gilbert MR, Aldape K, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J. Neurooncol. 2008;90(1):89–97. doi: 10.1007/s11060-008-9637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franceschi E, Cavallo G, Scopece L, et al. Phase II trial of carboplatin and etoposide for patients with recurrent high-grade glioma. Br. J. Cancer. 2004;91(6):1038–1044. doi: 10.1038/sj.bjc.6602105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeremic B, Grujicic D, Jevremovic S, et al. Carboplatin and etoposide chemotherapy regimen for recurrent malignant glioma: a Phase II study. J. Clin. Oncol. 1992;10(7):1074–1077. doi: 10.1200/JCO.1992.10.7.1074. [DOI] [PubMed] [Google Scholar]
- 45.Poisson M, Pereon Y, Chiras J, Delattre JY. Treatment of recurrent malignant supratentorial gliomas with carboplatin (CBDCA) J. Neurooncol. 1991;10(2):139–144. doi: 10.1007/BF00146875. [DOI] [PubMed] [Google Scholar]
- 46.Prados MD, Schold SJS, Fine HA, et al. A randomized, double-blind, placebo-controlled, Phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro. Oncol. 2003;5(2):96–103. doi: 10.1093/neuonc/5.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prados MD, Warnick RE, Mack EE, et al. Intravenous carboplatin for recurrent gliomas. A dose-escalating Phase II trial. Am. J. Clin. Oncol. 1996;19(6):609–612. doi: 10.1097/00000421-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Robins HI, Chang SM, Prados MD, et al. A Phase II trial of thymidine and carboplatin for recurrent malignant glioma: a North American Brain Tumor Consortium Study. Neuro. Oncol. 2002;4(2):109–114. [PMC free article] [PubMed] [Google Scholar]
- 49.Schafer N, Tichy J, Thanendrarajan S, et al. Ifosfamide, carboplatin and etoposide in recurrent malignant glioma. Oncology. 2011;80(5–6):330–332. doi: 10.1159/000330358. [DOI] [PubMed] [Google Scholar]
- 50.Tang P, Roldan G, Brasher PM, et al. A Phase II study of carboplatin and chronic high-dose tamoxifen in patients with recurrent malignant glioma. J. Neurooncol. 2006;78(3):311–316. doi: 10.1007/s11060-005-9104-y. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe K, Kanaya H, Fujiyama Y, Kim P. Combination chemotherapy using carboplatin (JM-8) and etoposide (JET therapy) for recurrent malignant gliomas: a Phase II study. Acta Neurochirurgica. 2002;144(12):1265–1270. doi: 10.1007/s00701-002-1023-5. Discussion 1270. [DOI] [PubMed] [Google Scholar]
- 52.Yung WK, Mechtler L, Gleason MJ. Intravenous carboplatin for recurrent malignant glioma: a Phase II study. J. Clin. Oncol. 1991;9(5):860–864. doi: 10.1200/JCO.1991.9.5.860. [DOI] [PubMed] [Google Scholar]
- 53.Chamberlain MC, Tsao-Wei DD. Salvage chemotherapy with cyclophosphamide for recurrent, temozolomide-refractory glioblastoma multiforme. Cancer. 2004;100(6):1213–1220. doi: 10.1002/cncr.20072. [DOI] [PubMed] [Google Scholar]
- 54.Aoki T, Mizutani T, Nojima K, et al. Phase II study of ifosfamide, carboplatin, and etoposide in patients with a first recurrence of glioblastoma multiforme. J. Neurosurg. 2010;112(1):50–56. doi: 10.3171/2009.5.JNS081738. [DOI] [PubMed] [Google Scholar]
- 55.Sanson M, Ameri A, Monjour A, et al. Treatment of recurrent malignant supratentorial gliomas with ifosfamide, carboplatin and etoposide: a Phase II study. Eur. J. Cancer. 1996;32A(13):2229–2235. doi: 10.1016/s0959-8049(96)00299-7. [DOI] [PubMed] [Google Scholar]
- 56.Schafer N, Tichy J, Thanendrarajan S, et al. Ifosfamide, carboplatin and etoposide in recurrent malignant glioma. Oncology. 2011;80(5–6):330–332. doi: 10.1159/000330358. [DOI] [PubMed] [Google Scholar]
- 57.Van Den Bent MJ, Grisold W, Frappaz D, et al. European Organization for Research and Treatment of Cancer (EORTC) open label Phase II study on glufosfamide administered as a 60-mininfusion every 3 weeks in recurrent glioblastoma multiforme. Ann. Oncol. 2003;14(12):1732–1734. doi: 10.1093/annonc/mdg491. [DOI] [PubMed] [Google Scholar]
- 58.Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS. Experience with irinotecan for the treatment of malignant glioma. Neuro. Oncol. 2009;11(1):80–91. doi: 10.1215/15228517-2008-075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamberlain MC. Salvage chemotherapy with CPT-11 for recurrent glioblastoma multiforme. J. Neurooncol. 2002;56(2):183–188. doi: 10.1023/a:1014532202188. [DOI] [PubMed] [Google Scholar]
- 60.Cloughesy TF, Filka E, Kuhn J, et al. Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer. 2003;97(Suppl. 9):S2381–S2386. doi: 10.1002/cncr.11306. [DOI] [PubMed] [Google Scholar]
- 61.Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J. Clin. Oncol. 1999;17(5):1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- 62.Prados MD, Lamborn K, Yung WK, et al. A Phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro. Oncol. 2006;8(2):189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reardon DA, Friedman HS, Powell JB, Jr, Gilbert M, Yung WK. Irinotecan: promising activity in the treatment of malignant glioma. Oncol. Williston Park, N.Y. 2003;17(5 Suppl. 5):9–14. [PubMed] [Google Scholar]
- 64.Batchelor TT, Gilbert MR, Supko JG, et al. Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97–11. Neuro. Oncol. 2004;6(1):21–27. doi: 10.1215/S1152851703000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedman HS, Kerby T, Fields S, et al. Topotecan treatment of adults with primary malignant glioma. The brain tumor center at Duke. Cancer. 1999;85(5):1160–1165. [PubMed] [Google Scholar]
- 66.Macdonald D, Cairncross G, Stewart D, et al. Phase II study of topotecan in patients with recurrent malignant glioma. National Clinical Institute of Canada Clinical Trials Group. Ann. Oncol. 1996;7(2):205–207. doi: 10.1093/oxfordjournals.annonc.a010550. [DOI] [PubMed] [Google Scholar]
- 67.Fulton D, Urtasun R, Forsyth P. Phase II study of prolonged oral therapy with etoposide (VP16) for patients with recurrent malignant glioma. J. Neurooncol. 1996;27(2):149–155. doi: 10.1007/BF00177478. [DOI] [PubMed] [Google Scholar]
- 68.Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN. Molecularly targeted therapy for malignant glioma. Cancer. 2007;110(1):13–24. doi: 10.1002/cncr.22741. [DOI] [PubMed] [Google Scholar]
- 69.Chamberlain M. Evolving strategies: future treatment of glioblastoma. Expert Rev. Neurother. 2011;11(4):519–532. doi: 10.1586/ern.11.30. [DOI] [PubMed] [Google Scholar]
- 70.Sathornsumetee S, Rich JN. Vinken PJ, Bruyn GW. Handbook of Clinical Neurology. Elsevier; Amsterdam, The Netherlands: 2012. Molecularly targeted therapy in neuro-oncology; pp. 255–278. [DOI] [PubMed] [Google Scholar]
- 71.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]; ▪▪ One of two open-label trials that established the benefit of single-agent bevacizumab for recurrent GB.
- 72.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ One of two open-label trials that established the benefit of single-agent bevacizumab for recurrent GB.
- 73.Hasselbalch B, Lassen U, Hansen S, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a Phase II trial. Neuro. Oncol. 2010;12(5):508–516. doi: 10.1093/neuonc/nop063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raizer JJ, Grimm S, Chamberlain MC, et al. A Phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–5305. doi: 10.1002/cncr.25462. [DOI] [PubMed] [Google Scholar]
- 75.Reardon DA, Desjardins A, Peters KB, et al. Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer. 2011;117(23):5351–5358. doi: 10.1002/cncr.26188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J. Neurooncol. 2010;96(2):219–230. doi: 10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a Phase II study. Br. J. Cancer. 2009;101(12):1986–1994. doi: 10.1038/sj.bjc.6605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 79.Desjardins A, Reardon DA, Coan A, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118(5):1302–1312. doi: 10.1002/cncr.26381. [DOI] [PubMed] [Google Scholar]
- 80.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2009;75(1):156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sathornsumetee S, Desjardins A, Vredenburgh JJ, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro. Oncol. 2010;12(12):1300–1310. doi: 10.1093/neuonc/noq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chinot OlWW, Saran F, Mason W, et al. 2011 ASCO Annual Meeting. Chicago, IL, USA: 3–7 June 2011. AVAglio: a Phase III trial of bevacizumab added to standard radiotherapy and temozolomide in patients with newly diagnosed glioblastoma. Presented at. [Google Scholar]
- 83.Wong ET, Brem S. Taming glioblastoma by targeting angiogenesis: 3 years later. J. Clin. Oncol. 2010;29(2):124–126. doi: 10.1200/JCO.2010.32.5282. [DOI] [PubMed] [Google Scholar]
- 84.Chamberlain MC. Radiographic patterns of relapse in glioblastoma. J. Neurooncol. 2011;101(2):319–323. doi: 10.1007/s11060-010-0251-4. [DOI] [PubMed] [Google Scholar]
- 85.Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011;76(5):432–437. doi: 10.1212/WNL.0b013e31820a0a8a. [DOI] [PubMed] [Google Scholar]; ▪ Retrospective study suggesting that angiogenic inhibition does not promote GB brain invasion as determined by MRI-based radiology.
- 86.Wick A, Dorner N, Schafer N, et al. Bevacizumab does not increase the risk of remote relapse in malignant glioma. Ann. Neurol. 2011;69(3):586–592. doi: 10.1002/ana.22336. [DOI] [PubMed] [Google Scholar]
- 87.De Groot JF, Lamborn KR, Chang SM, et al. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J. Clin. Oncol. 2011;29(19):2689–2695. doi: 10.1200/JCO.2010.34.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reardon DA, Turner S, Peters KB, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J. Natl Compr. Cancer Netw. 2011;9(4):414–427. doi: 10.6004/jnccn.2011.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsu JY, Wakelee HA. Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy. Biodrugs. 2009;23(5):289–304. doi: 10.2165/11317600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 90.Batchelor TT, Duda DG, Di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwamoto FM, Lamborn KR, Robins HI, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06–02) Neuro. Oncol. 2010;12(8):855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neyns B, Sadones J, Chaskis C, et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J. Neurooncol. 2011;103(3):491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 93.Wen PYPM, Schiff D, Reardon T, et al. American Society of Clinical Oncology Annual Meeting. Chicago, IL, USA: 4–8 June 2010. Phase II study of XL184 (BMS 907351), an inhibitor of MET, VEGFR2, and RET in patients with progressive glioblastoma. Presented at. [Google Scholar]
- 94.Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J. Clin. Oncol. 2007;25(16):2288–2294. doi: 10.1200/JCO.2006.08.0705. [DOI] [PubMed] [Google Scholar]
- 95.Raizer JJ, Abrey LE, Lassman AB, et al. A Phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro. Oncol. 2010;12(1):95–103. doi: 10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized Phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 2009;27(8):1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Randomized study demonstrating greater efficacy of lomustine in the comparison of the lomustine arm to erlotinib treatment arm for recurrent GB.
- 97.Yung WK, Vredenburgh JJ, Cloughesy TF, et al. Safety and efficacy of erlotinib in first-relapse glioblastoma: a Phase II open-label study. Neuro. Oncol. 2010;12(10):1061–1070. doi: 10.1093/neuonc/noq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre Phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br. J. Cancer. 2007;96(7):1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kreisl TN, Lassman AB, Mischel PS, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM) J. Neurooncol. 2009;92(1):99–105. doi: 10.1007/s11060-008-9741-z. [DOI] [PubMed] [Google Scholar]
- 100.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J. Clin. Oncol. 2004;22(1):133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 101.Hegi ME, Diserens AC, Bady P, et al. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib – a Phase II trial. Mol. Cancer Ther. 2011;10(6):1102–1112. doi: 10.1158/1535-7163.MCT-11-0048. [DOI] [PubMed] [Google Scholar]
- 102.Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J. Clin. Oncol. 2008;26(34):5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J. Neurooncol. 2010;98(1):93–99. doi: 10.1007/s11060-009-0067-2. [DOI] [PubMed] [Google Scholar]
- 104.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J. Clin. Oncol. 2009;27(4):579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uhm JH, Ballman KV, Wu W, et al. Phase II evaluation of gefitinib in patients with newly diagnosed Grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int. J. Radiat. Oncol. Biol. Phys. 2011;80(2):347–353. doi: 10.1016/j.ijrobp.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tabatabai G, Weller M, Nabors B, et al. Targeting integrins in malignant glioma. Targeted Oncol. 2010;5(3):175–181. doi: 10.1007/s11523-010-0156-3. [DOI] [PubMed] [Google Scholar]
- 107.Reardon DA, Fink KL, Mikkelsen T, et al. Randomized Phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J. Clin. Oncol. 2008;26(34):5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 108.Gilbert MR, Kuhn J, Lamborn KR, et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03–02, a Phase II trial with measures of treatment delivery. J. Neurooncol. 2012;106(1):147–153. doi: 10.1007/s11060-011-0650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stupp R, Hegi ME, Neyns B, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010;28(16):2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 110.Stupp R, Van Den Bent MJ, Erridge SC, et al. Cilengitide in newly diagnosed glioblastoma with MGMT promoter methylation: protocol of a multicenter, randomized, open-label, controlled Phase III trial (CENTRIC) J. Clin. Oncol. 2010;28:15s. [Google Scholar]
- 111.Nabors LB, Fink F, Reardon DA, et al. American Society of Clinical Oncology. Chicago, IL, USA: 4–8 June 2010. Cilengitided in patients with newly diagnosed glioblastoma multiforme and unmethylated MGMT gene promoter: protocol of a multicenter, randomized, open-label, controlled Phase II study. Presented at. [Google Scholar]
- 112.Kreisl TN, Kotliarova S, Butman JA, et al. A Phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro. Oncol. 2010;12(2):181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Butowski N, Chang SM, Lamborn KR, et al. Phase II and pharmacogenomics study of enzastaurin plus temozolomide during and following radiation therapy in patients with newly diagnosed glioblastoma multiforme and gliosarcoma. Neuro. Oncol. 2011;13(12):1331–1338. doi: 10.1093/neuonc/nor130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J. Clin. Oncol. 2009;27(12):2052–2058. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iwamoto FM, Lamborn KR, Kuhn JG, et al. A Phase I/II trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03–03. Neuro. Oncol. 2011;13(5):509–516. doi: 10.1093/neuonc/nor017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Friday BB, Anderson SK, Buckner J, et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro. Oncol. 2012;14(2):215–221. doi: 10.1093/neuonc/nor198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vauleon E, Avril T, Collet B, Mosser J, Quillien V. Overview of cellular immunotherapy for patients with glioblastoma. Clin. Dev. Immun. 2010;2010 doi: 10.1155/2010/689171. pii:689171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grossman SA, Ye X, Chamberlain M, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter Phase II trial. J. Clin. Oncol. 2009;27(25):4155–4161. doi: 10.1200/JCO.2008.21.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stupp R, Hegi ME, Neyns B, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010;28(16):2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 120.Rosenfeld MR, Chamberlain MC, Grossman SA, et al. A multi-institution Phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro. Oncol. 2010;12(10):1071–1077. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hainsworth JD, Ervin T, Friedman E, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116(15):3663–3669. doi: 10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 122.Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin. Cancer Res. 2011;17(12):4119–4124. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lai RK, Recht LD, Reardon DA, et al. Society for Neuro-Oncology Annual Scientific Meeting. Orange County, CA, USA: 17–20 November 2011. Long-term follow-up of ACT III: a Phase II trial of Rindopepimut (CDX-110) in newly diagnosed glioblatoma. Presented at. [DOI] [PMC free article] [PubMed] [Google Scholar]