SUMMARY
The metabolism of the essential amino acid tryptophan is a key microenvironmental factor shaping the immunobiology of many tumor types. The current concept suggests that in the tumor microenvironment, tryptophan is metabolized by specialized dioxygenases, chiefly indoleamine-2,3-dioxygenase (IDO), which is expressed by tumor cells and antigen-presenting cells. High IDO activity leads to the depletion of tryptophan from the local microenvironment, while immediate tryptophan metabolites, particularly kynurenine, accumulate to high micromolar levels. Both the depletion of tryptophan and the accumulation of kynurenine lead to profound suppression of T-cell responses. Orally active IDO inhibitors are currently being explored in clinical trials for their efficacy in enhancing antitumor immune responses. Recent evidence points at alternative routes of tryptophan catabolism via tryptophan-2,3-dioxygenase, which is particularly expressed in malignant gliomas resulting in the production of high amounts of kynurenine. Tryptophan-2,3-dioxygenase-derived kynurenine in turn leads to the promotion of glioma growth and invasiveness and the suppression of antitumor immune responses by binding to the aryl hydrocarbon receptor expressed in glioma cells and glioma-infiltrating T cells. These new data open up novel therapeutic approaches to alleviate glioma-mediated immunosuppression. This review summarizes the current view on the relevance of tryptophan metabolism as an important immunosuppressive, proinvasive and growth-promoting metabolic pathway in malignant glioma.
Practice Points.
The metabolism of the essential amino acid tryptophan by indoleamine-2,3-dioxygenase (IDO) is an endogenous feedback loop restricting immune responses in tumors and sites of inflammation.
IDO inhibitors are currently tested in clinical trials in cancer patients with the aim of enhancing antitumor immune responses.
The conversion from tryptophan to kynurenine by tryptophan-2,3-dioxygenase (TDO) is an important microenvironmental factor in malignant glioma.
Kynurenine promotes glioma growth and invasiveness while suppressing antitumor immunity in glioma.
The growth promoting and immunosuppressive effects of kynurenine are mediated by the aryl hydrocarbon receptor (AHR) expressed in gliomas.
Therapeutic interference with tryptophan catabolism at the level of TDO and AHR is attractive in glioma.
As opposed to other tumor types, such as renal cell cancer and melanoma, gliomas are not considered immunogenic tumors per se. Despite this observation there is increasing evidence that gliomas express and present tumor-associated antigens resulting in natural T-cell responses detectable in the peripheral circulation of glioma patients [1]. This natural antitumor immunity, however, often does not translate into efficient antitumor immune responses, although current immunotherapeutic approaches, such as vaccination with autologous dendritic cells loaded with autologous tumor cell lysates or vaccination with tumor antigen or tumor-associated antigen peptides, are capable of inducing strong peripheral immune responses [2–4]. While some clinical trials suggest that the degree of peripheral immune responses correlates with clinical efficacy of the vaccine [2], solid evidence that this is due to strong immune-mediated tumor control is lacking. This question is particularly difficult to address in gliomas as tumor tissue for the analysis of intratumoral immune responses is not as easily obtained as in tumors growing at more accessible sites, such as the skin. Despite these uncertainties it is generally accepted that the tumor microenvironment – characterized by hypoxia and metabolic shifts – of malignant gliomas is particularly hostile for efficient antitumor immune responses [5]. This is not only an important factor limiting the efficacy of vaccination approaches. With increasing evidence that natural tumor immunity induced by conventional radiochemotherapy contributes to its therapeutic efficacy [6], the immunosuppressive tumor microenvironment has evolved as a key factor in therapy resistance in general.
The CNS is generally considered an immune privileged site, where antigen-specific T-cell responses are passively and actively limited by the restriction of pathogenic T-cell influx and by the prevention of expansion and activation of infiltrating pathogenic T cells through resident glial cells [7]. The immune privilege can, however, be overcome, as exemplified by inflammatory autoimmune diseases of the CNS, such as multiple sclerosis where the destruction of myelin sheaths and neurons is promoted by antigen-specific activation of pathogenic T cells within the CNS [8]. There is increasing evidence that the same cellular and molecular pathways that fail when autoimmunity in the CNS is initiated are overly active in the glioma microenvironment to prevent local antitumor immunity.
The common involvement of pathways in restricting tumor immunity and autoimmunity is illustrated by the metabolism of the essential amino acid tryptophan. This metabolic pathway is induced in the microenvironment of tumors or sites of inflammation, resulting in the depletion of tryptophan and the accumulation of kynurenine and downstream metabolites, leading to suppression of antigenic T-cell responses. After the demonstration of its important role in regulating immune responses in preclinical animal models of tumor immunity [9,10], autoimmunity [11], infection [12], transplant rejection [13] and allergy [14], and the discovery of its druggability [15], therapeutic strategies are currently being developed to interfere with or exploit this pathway in diseases with dysregulated immunity [16].
Immunological consequences of tryptophan catabolism
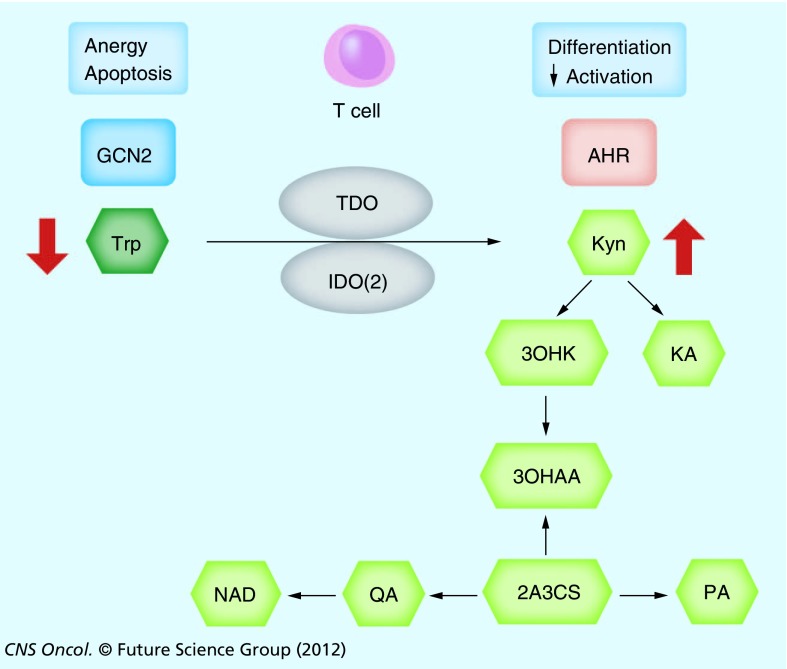
The vast majority of ingested tryptophan is catabolized along the kynurenine pathway. There are two major rate-limiting enzymes responsible for the first step of conversion: indoleamine-2,3-dioxygenase (IDO) and tryptophan dioxygenase (TDO). More recently an IDO isoform, termed IDO2, with unclear significance in humans has been described [17–19]. Tryptophan is constantly metabolized systemically along the kynurenine pathway through the hepatic activity of TDO to generate NAD as a source of energy. The conversion of kynurenine to NAD is enzymatically tightly controlled by several enzymes producing the intermediate metabolites kynurenic acid, 3-hydroxy-kynurenine, 3-hydroxy-anthranilic acid, anthranilic acid, 2-amino-3-carboxymuconate-semialdehyde, picolinic acid and quinolinic acid (Figure 1). Due to constant tryptophan intake, plasma levels of tryptophan are generally maintained at 50–60 µM, while kynurenine levels usually range between 1 and 2 µM. By contrast, in the microenvironment of tumors or sites of inflammation the activity of rate-limiting dioxygenases, chiefly IDO, can substantially deplete tryptophan well below physiological levels. This results in an imbalance of tryptophanyl tRNA synthases towards uncharged forms and subsequent activation of the amino acid starvation-sensing response pathway involving the broadly expressed general control non-derepressible kinase (GCN2) [20]. Activation of this pathway in T cells leads to anergy and subsequent cell death, which is the reason why local depletion of tryptophan by IDO in tumors and inflammatory sites has been proposed as the key ‘death by starvation’ mechanism of immunosuppression. T cells lacking GCN2 in mice are not susceptible to IDO-mediated anergy [21]. Of note, the GCN2 pathway is activated by the limitation of various amino acids and by endoplasmatic reticulum stress [22]. As tryptophan metabolism by IDO is induced by inflammatory mediators, notably IFN-γ, it is generally believed to represent an endogenous mechanism restricting excessive immune responses as evidenced in animal models of autoimmunity [11], allergy [14] and infection [12]. Importantly, this pathway appears to be a key factor in maintaining the immune privilege, for instance in the placenta [13]. In the context of cancer this feedback loop is exploited by IDO-expressing tumor cells and dendritic cells in tumor-draining lymph nodes, where antitumor immune responses are suppressed through the depletion of tryptophan [23]. The same metabolic consequences, particularly in glioma cells, may be achieved by constitutive activity of TDO [24,25].
Figure 1. The kynurenine pathway.
Trp is metabolized in the local microenvironment by TDO or IDO or IDO2, resulting in a depletion of Trp and an accumulation of Kyn. Trp depletion leads to T-cell anergy and apoptosis by activating the GCN2 pathway. Kyn alters T-cell differentiation and suppresses activation by binding the aryl hydrocarbon receptor AHR. Kyn is further metabolized by enzymatic steps to KA, 3OHKA, 3OHAA, 2A3CS, PA and QA, which is ultimately processed to NAD.
2A3CS: 2-amino-3-carboxymuconate-semialdehyde; 3OHAA: 3-hydroxy-anthranilic acid; 3OHKA: 3-hydroxy-kynurenine; AHR: Aryl hydrocarbon receptor; GCN2: General control nonderepressable 2; IDO: Indoleamine-2,3-dioxygenase; KA: Kynurenic acid; Kyn: Kynurenine; PA: Picolonic acid; QA: Quinolinic acid; TDO: Tryptophan-2,3-dioxygenase; Trp: Tryptophan.
In addition to tryptophan depletion, IDO and TDO activity result in the accumulation of immunosuppressive tryptophan metabolites, kynurenines (kynurenine, kynurenic acid and 3-hydroxykynurenine). These intermediate metabolites may individually and/or cooperatively result in T-cell death or anergy, or – at lower concentrations – skewing of T-cell responses to a regulatory phenotype [26]. With the discovery of receptor-mediated pathways activated by immunosuppressive kynurenines [27,28], the relevance for kynurenine accumulation in controlling tumor immune responses has been demonstrated in malignant glioma as a paradigmatic immunosuppressive tumor [24].
Tryptophan catabolism in the glioma microenvironment
Human gliomas take up and metabolize tryptophan as evidenced by PET [29]. The majority of tryptophan uptake is believed to be mediated by the large amino acid transport (LAT) system, particularly LAT1, which is expressed in gliomas and (even stronger) in the blood–brain barrier and associates with the CD98 heavy chain 4F2hc to transport branched amino acids and neurotransmitters and or their precursors such as L-DOPA [30]. LAT1 expression is associated with a malignant phenotype in gliomas [31–33]. Tryptophan metabolism in the glioma microenvironment is mediated through expression of IDO and TDO in tumor cells, infiltrating monocytes, resident microglial cells, endothelial cells and neurons. The contribution of these individual cell types to the net tryptophan metabolism is unclear, but studies with cultured and xenografted human gliomas suggest that TDO activity in glioma cells accounts for the majority of tryptophan metabolism, sufficient to deplete tryptophan from the serum of glioma patients [24]. In addition, tryptophan metabolites accumulate in the cerebrospinal fluid [34]. The kynurenine metabolite quinolinic acid (QA) also accumulates in gliomas [24]. While glioma cells themselves are not capable of generating QA from kynurenine, microglial cells readily produce QA from tryptophan after stimulation with proinflammatory mediators [35]. The pathophysiological relevance of QA accumulation in gliomas is currently being investigated.
Nonimmune consequences of kynurenine in gliomas
A potential explanation for the discrepancy between strong cancer-suppressive effects when IDO is inhibited pharmacologically versus the limited capability of IDO-deficient mice to control (reject) experimental (transplanted) cancer is that tryptophan metabolism drives tumor growth in a direct, autocrine fashion. In most preclinical cancer models, the tumor-suppressive effect of IDO or TDO inhibition is abrogated in immunodeficient hosts [9,10,25]. Biologically relevant levels of kynurenine accumulate in tumors constitutively expressing TDO [24,25], possibly because the effects are largely mediated by tryptophan depletion, to which T cells are particularly sensitive [21]. In gliomas, high levels of kynurenine (up to 50 µM) accumulate both in vitro and in vivo [24]. As opposed to immune cells, kynurenine is beneficial for glioma cells as it promotes clonogenic survival and enhances cell motility. As TDO is the major determinant for kynurenine production in gliomas, tumors constitutively expressing TDO grow faster in immunodeficient mice, even after the ablation of innate immune components [24]. It is tempting to speculate that the autocrine effects of kynurenine on clonogenic survival of glioma cells represent a unique feature of astrocytic brain tumors, which may have adapted to utilize the ability of kynurenine to activate cell survival pathways, while lacking the ability to further degrade kynurenine.
The aryl hydrocarbon receptor as a target of kynurenine
Recent studies have demonstrated that the mouse and human aryl hydrocarbon receptor (AHR) is a direct target of kynurenine [24,27,28]. The broadly expressed AHR belongs to the basic helix-loop-helix Per–Arnt–Sim transcription factor family. It has originally been identified as a nuclear transcription factor that is bound and activated by xenobiotics such as benzo[a]pyrene and 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD). The AHR function extends well beyond just eliminating xenobiotics from the body. Instead the AHR controls – in a cell-type-specific manner – the transcription of a multitude of genes that are involved in cell growth and differentiation, malignant transformation and cell death. Endogenously produced AHR ligands have subsequently been identified. Among these are arachidonic acid metabolites, bilirubin, cAMP, but also tryptophan metabolites such as tryptamine, kynurenic acid and 6-formylindolo[3,2-b]carbazole. A prominent role of the AHR in regulating immune responses has been uncovered with the discovery that it is enriched in IL-17-producing CD4+ T cells (TH17 cells) and that it controls the differentiation of naive CD4+ T cells to TH17 or Tregs depending on ligand avidity and affinity and the presence of additional signals [36]. In multiple sclerosis, the AHR appears to restrict autoimmunity by favoring the generation of Treg, although AHR-deficiency does not lead to an alteration of Treg number and/or function [37,38]. The role of the AHR in glioma immunity remains uncertain. While our studies indicate that immunosuppressive effects of kynurenine are mediated by the AHR and affect CD8+ T cells [24], it needs to be proven whether the AHR-mediated alteration of CD8+ T-cell function is a direct effect or mediated through T helper cells (or a distinct cellular population).
The AHR controls tumorigenesis in a broader context. While TCDD promotes cancer in an AHR-dependent manner [39], transgenic mice with a constitutively active AHR spontaneously develop tumors and the AHR repressor is a tumor suppressor in multiple human tumors [40,41]. The dichotomy between pro-survival signals in cancer cells and suppressive effects in immune cells is well established. The AHR function appears to be tightly controlled by the cell type and the local microenvironment, resulting in the activation of several pathways interfering with AHR signaling, such as the hypoxia pathway. Hypoxia-inducible factor 1β (HIF-1β) associates with the AHR to mediate nuclear translocation, hence it is also referred to as AHR nuclear translocator [42].
In glioma's the AHR is stongly expressed and promotes clonogenic survival and invasiveness [43]. AHR ablation suppresses glioma growth in immunocompromised hosts and the expression of AHR and its target genes in glioma tissue are prognosticators for poor survival [24]. Collectively these data indicate that the AHR promotes glioma growth both in a tumor cell autonomous fashion and by suppressing antitumor immune responses. While kynurenine generated by TDO expressed in glioma cells appears to be the major determinant driving constitutive AHR activity in glioma cells [24], other factors can certainly not be ruled out.
The AHR regulates a plethora of target genes in gliomas capable of driving glioma growth and invasiveness including TGF-β, an important proinvasive and immunosuppressive factor in gliomas [43], but also IL-1b, IL-6, IL-8, epiregulin and aldehyde dehydrogenase 1 family, member A3 [24]. The relevant downstream pathways are the subject of current investigation. It will also be important to delineate the role of hypoxia, which is a hallmark of the glioma microenvironment, for AHR signaling. It is tempting to speculate that these pathways interfere in gliomas as AHR and HIF-1α may compete for HIF-1β to deliver transcriptional activity with consequences for both AHR and HIF-1α signaling pathways.
Tryptophan catabolism as a therapeutic target in glioma
IDO has long been the focus of drug development, mainly in cancer, with the aim of enhancing antitumor immunity. Originally, the L-isomer of methylated tryptophan (1-L-MT), which is a competitive inhibitor of IDO, had been tested and proven efficacious in preclinical models of transplanted cancer in combination with preventive vaccination or chemotherapy [9,10]. Nevertheless (and surprisingly), the D-isomer 1-D-MT showed superior (IDO-dependent) activity in cancer animal models when combined with chemotherapy, although it does not block IDO directly but rather IDO2 activity [44]. Despite off-target effects of 1-D-MT, specifically its ability to induce MAPK and JNKs, potentially weakening its immunostimulatory effects in humans through transcriptional activation of IDO [45], 1-D-MT has been the first IDO inhibitor to enter Phase II clinical testing in patients with advanced solid cancer in conjunction with conventional chemotherapy or vaccination approaches. Meanwhile, several compounds with noncompetetive IDO inhibitory activity at much lower concentrations than methylated tryptophan have been identified and entered clinical trials [46]. The recent discovery of TDO as an alternative dioxygenase shaping the immunobiology of cancer, particularly malignant glioma, has sparked the search for TDO inhibitors. Indeed, with the known TDO inhibitor 3-(2-(pyridyl)ethenyl)indole (680C91) [47] as a backbone, an orally active, stable and nontoxic TDO inhibitor termed LM10 has recently been developed and shown to display immunostimulatory activity in preclinical cancer models, enhancing the efficacy of therapeutic vaccines [25,48]. Future studies will demonstrate whether TDO inhibition in general and LM10 in particular will be feasible for use in humans without generating liver toxicity. Preclinical studies indicate that pharmacological inhibition of TDO with LM10 does not result in relevant toxicity especially with respect to liver function [25]. One concern is a potential compensatory upregulation of systemic kynurenine levels as seen in the TDO-deficient mice [49]. Of note, while catalyzing the same enzymatic step, IDO and TDO are structurally distinct, which is the reason why most IDO inhibitors are not active against TDO and vice versa [50]. It is also conceivable that blocking TDO with a blood–brain barrier-permeable inhibitor could result in CNS side effects. Nevertheless current studies clearly demonstrate that IDO and TDO are druggable with compounds that are tolerated after systemic application, and that combining anti-IDO and anti-TDO therapies may increase the efficacy of reversing cancer-induced immunosuppression [25].
Conclusion & future perspective
With the increasing knowledge of the functional consequences of tryptophan catabolism for the microenvironment of tumors, particularly malignant gliomas, novel therapeutic options have opened. While a first-generation IDO inhibitor – 1-methyl-D-tryptophan – has entered Phase II clinical trials in patients with solid cancers, several newly identified compounds are now moving to early clinical testing. As malignant gliomas display strong TDO activity, compounds such as LM10 may be more promising [48]. LM10 is still in preclinical testing but did not show overt toxicity in mice [25]. Also, clinically approved drugs may exert antitumor function, in part through modulation of tryptophan catabolism as recently demonstrated for the tyrosine kinase inhibitor imatinib, which blocks IDO expression [51]. Based on evidence from genetic ablation in preclinical gliomas models, blocking the production of kynurenine would not only aim at restoring antitumor immunity but also suppress clonogenic growth and invasiveness. While the exact signaling events that mediate these effects are probably diverse, activation of the AHR is involved in both the paracrine immune-mediated and autocrine tumor cell intrinsic tumor-promoting effects of kynurenine (Figure 2). This opens an entirely new therapeutic perspective. Screens to identify specific antagonists blocking the kynurenine–AHR interaction are underway. Rational approaches, however, are hampered by the inability to crystallize the AHR and by the ligand promiscuity with respect to chemical structure. Nevertheless, several small molecules have been shown to block AHR activation, although target specificity remains an issue with the currently available compounds. More specific AHR antagonists may turn out to be superior in altering the immunsuppressive glioma microenvironment than IDO or TDO inhibitors.
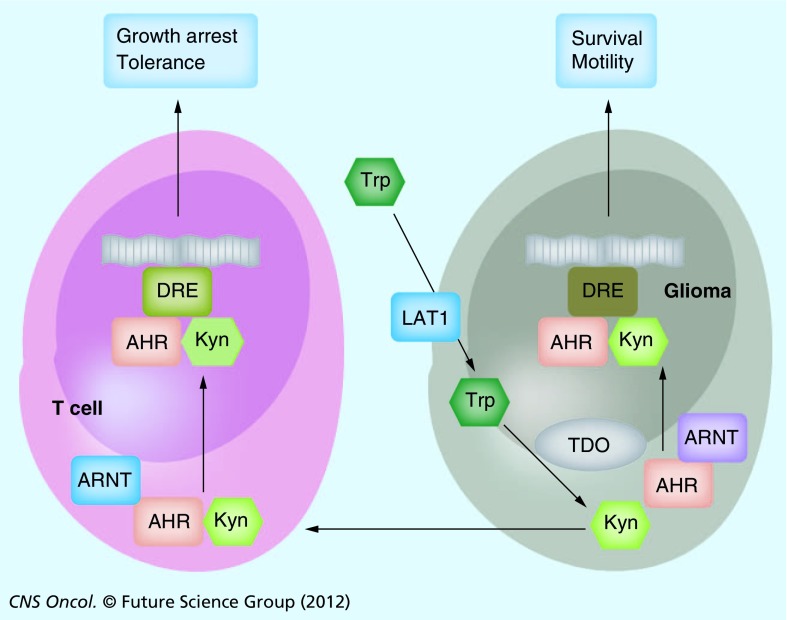
Figure 2. Tryptophan catabolism in the glioma microenvironment.
In glioma cells, Trp is transported from the extracellular space through the LAT1, where it is metabolized to Kyn by TDO. Intracellularly, Kyn binds to the AHR, which couples with AHR nuclear translocator ARNT to translocate to the nucleus where it activates cell type-specific target genes signaling cell survival and motility by binding DRE in the promoter sequences. Kyn is also excreted and taken up by neighboring T cells, where – through the same signaling cascade – target genes are activated, which signal growth arrest and tolerance (see Figure 1).
AHR: Aryl hydrocarbon receptor; ARNT: Aryl hydrocarbon receptor nuclear translocator; DRE: Dioxin responsive elements; Kyn: Kynurenine; LAT1: Large amino acid transport system 1; TDO: Tryptophan-2,3-dioxygenase; Trp: Tryptophan.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Dutoit V, Herold-Mende C, Hilf N, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135(Pt 4):1042–1054. doi: 10.1093/brain/aws042. [DOI] [PubMed] [Google Scholar]
- 2.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {α}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 2005;11(15):5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 5.Wei J, Wu A, Kong LY, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6(1):E16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat. Rev. Drug Discov. 2012;11(3):215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 7.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J. Clin. Invest. 2012;122(4):1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64(1):123–132. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]; ▪▪ First evidence for a key role of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism in antitumor immunity.
- 10.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005;11(3):312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 11.Platten M, Ho PP, Youssef S, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310(5749):850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 12.Romani L, Fallarino F, De Luca A, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451(7175):211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 13.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]; ▪▪ Identifies for the first time tryptophan degradation as a central metabolic pathway mediating immune tolerance in mammals.
- 14.Hayashi T, Beck L, Rossetto C, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J. Clin. Invest. 2004;114(2):270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller AJ, DuHadaway JB, Jaller D, Curtis P, Metz R, Prendergast GC. Immunotherapeutic suppression of indoleamine 2,3-dioxygenase and tumor growth with ethyl pyruvate. Cancer Res. 2010;70(5):1845–1853. doi: 10.1158/0008-5472.CAN-09-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Front. Biosci. (Elite Ed.) 2012;4:734–745. doi: 10.2741/e414. [DOI] [PubMed] [Google Scholar]
- 17.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 18.Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111(4):2152–2154. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 19.Meininger D, Zalameda L, Liu Y, et al. Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim. Biophys. Acta. 2011;1814(12):1947–1954. doi: 10.1016/j.bbapap.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell. 2000;6(2):269–279. doi: 10.1016/s1097-2765(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]; ▪ Identifies GCN2 as the key molecule mediating the immunosuppressive effects of tryptophan degradation.
- 22.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 23.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]; ▪▪ Demonstrates for the first time that tryptophan catabolism via tryptophan 2,3-dioxygenase (TDO) in human gliomas is capable of activating the aryl hydrocarbon receptor (AHR) and suppressing antitumor immune responses.
- 25.Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl Acad. Sci. USA. 2012;109(7):2497–2502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Demonstrates safety and efficacy of a novel TDO inhibitor in a preclinical cancer model as a cancer vaccine adjunct.
- 26.Opitz CA, Wick W, Steinman L, Platten M. Tryptophan degradation in autoimmune diseases. Cell. Mol. Life Sci. 2007;64(19–20):2542–2563. doi: 10.1007/s00018-007-7140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl Acad. Sci. USA. 2010;107(46):19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juhasz C, Muzik O, Chugani DC, et al. Differential kinetics of α-[(1)(1)C]methyl-L-tryptophan on PET in low-grade brain tumors. J. Neurooncol. 2011;102(3):409–415. doi: 10.1007/s11060-010-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc. Natl Acad. Sci. USA. 1999;96(21):12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haining Z, Kawai N, Miyake K, et al. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin. Pathol. 2012;12:4. doi: 10.1186/1472-6890-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi K, Ohnishi A, Promsuk J, et al. Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery. 2008;62(2):493–503. doi: 10.1227/01.neu.0000316018.51292.19. Discussion 503–504. [DOI] [PubMed] [Google Scholar]
- 33.Nawashiro H, Otani N, Shinomiya N, et al. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int. J. Cancer. 2006;119(3):484–492. doi: 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- 34.Locasale JW, Melman T, Song S, et al. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol. Cell Proteomics. 2012;11(6) doi: 10.1074/mcp.M111.014688. M111.014688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 36.Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin. Immunol. 2011;23(2):99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 38.Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 39.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor sans xenobiotics: endogenous function in genetic model systems. Mol. Pharmacol. 2007;72(3):487–498. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 40.Andersson P, McGuire J, Rubio C, et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc. Natl Acad. Sci. USA. 2002;99(15):9990–9995. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Demonstrates the ligand-independent tumor-promoting activity of the AHR.
- 41.Zudaire E, Cuesta N, Murty V, et al. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. J. Clin. Invest. 2008;118(2):640–650. doi: 10.1172/JCI30024. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Identifies the AHR repressor as a tumor suppressor.
- 42.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 43.Gramatzki D, Pantazis G, Schittenhelm J, et al. Aryl hydrocarbon receptor inhibition downregulates the TGF-β/Smad pathway in human glioblastoma cells. Oncogene. 2009;28(28):2593–2605. doi: 10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 44.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat. Rev. Cancer. 2009;9(6):445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 45.Opitz CA, Litzenburger UM, Opitz U, et al. The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells. PLoS One. 2011;6(5):E19823. doi: 10.1371/journal.pone.0019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koblish HK, Hansbury MJ, Bowman KJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol. Cancer Ther. 2010;9(2):489–498. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 47.Salter M, Hazelwood R, Pogson CI, Iyer R, Madge DJ. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem. Pharmacol. 1995;49(10):1435–1442. doi: 10.1016/0006-2952(95)00006-l. [DOI] [PubMed] [Google Scholar]
- 48.Dolusic E, Larrieu P, Moineaux L, et al. Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J. Med. Chem. 2011;54(15):5320–5334. doi: 10.1021/jm2006782. [DOI] [PubMed] [Google Scholar]
- 49.Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol. Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forouhar F, Anderson JL, Mowat CG, et al. Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc. Natl Acad. Sci. USA. 2007;104(2):473–478. doi: 10.1073/pnas.0610007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 2011;17(9):1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]