Abstract
This report evaluates whether a rat releasing a trapped rat from a restraint tube is better explained as due to its empathic motivation or to the pursuit of social contact. In the first condition, each of six rats chose in an E maze between entering an empty goal box vs. entering a goal box where its entrance caused a rat trapped in a restraint tube to be released. Rats preferred the goal box with the trapped rat over the empty goal box. In the second condition, these rats chose between releasing a restraint-tube-trapped rat in one goal box and another rat in the second goal box that was not locked into its restraint tube. Rats showed no preference between alternatives. In the third condition, rats chose between a goal box containing a rat with an open restraint tube and an empty goal box. Rats preferred the rat with the open restraint tube over the empty goal box. These results support attributing the response of releasing a rat from a restraint tube to the reinforcing power of social contact (Silberberg et al., 2014) rather than interpreting this response as empathically motivated (Ben-Ami Bartal et al., 2011).
Keywords: rat empathy, social-contact hypothesis, restraint tube, rats, maze
Empathic action is so pervasive a human attribute that its absence is often diagnostic of maladies such as autism, sociopathy, social avoidance and social phobia (Belzung, Leman, Vourc’h, & Andres, 2005; Lukas, Toth, Reber, Slattery, Veenema, & Neumann, 2011; Preston, Bechara, Damaslio, Grabowsk, & Stansfield, 2007). Researchers have sought animal models of human empathy because of the clinical importance of addressing cases where human empathy is absent. Their goal has been to elucidate the affective/cognitive/biological substrate that motivates empathic action so that ameliorative strategies can be created for those in need. If approximations to these disorders can be created in rodents or other animals, the environmental and biological factors that underlie these maladies may be uncovered. Some elements of human empathy—for example, emotional contagion (e.g., one baby cries, other babies cry as well)—have been convincingly demonstrated in rodents (e.g., Langford, Crager, Shehzad, Smith, Sotocinal, Levenstadt, … & Mogil, 2006). Moreover, higher-order, multi-component models have been constructed that include emotional contagion and other processes (e.g., perspective taking and consolation) as building blocks of a perception-action theory of empathy (de Waal, 2008).
Although these accomplishments are noteworthy, there is concern that some of the claimed homologies between animal and human empathy are doubtful. To offer one illustration, Nowbahari, Scohier, Durand, and Hollis’ (2009) have shown that unconstrained ants will cut a nylon string entrapping a nestmate. On its face, such behavior arguably qualifies as empathic action—that is, behavior in a rescuer intended solely to relieve distress in another. However, application of notions such as emotional contagion and the like—presumed motivators of empathy in rats and humans—strains credulity if applied to ants. As these authors note, if “intention” and “empathic motivation”—key elements of any definition of human empathy—seem implausible if assumed operative in ants, why are these processes assumed operative in rats? Vasconcelos, Hollis, Nowbahari, and Kacelnik (2012) support this perspective by delineating control procedures not yet conducted but necessary to show empathic motivation in rats.
This report addresses whether results to date support the view that rats can serve as a plausible model of human empathy. We begin with a review of three different designs that claim to have demonstrated empathic action in rats. As will be apparent, each of these tests has been subsequently criticized in another study.
First design: Rice & Gainer vs. Lavery & Foley
Rice and Gainer (1962) induced squealing in a rat by lifting it into the air with a hoist and, if necessary, touching it. Bar presses by a second rat rescued the distressed rat by lowering it to the ground. Rice and Gainer theorized that this bar pressing could be viewed as empathic action by the rescuing rat. However, Lavery and Foley (1963) questioned this interpretation based on their finding that rats will bar press to stop recorded rat squeals or the presentation of white noise. Possibly, bar pressing was escape behavior, terminating the presence of aversive sounds.
Second design: Ben-Ami Bartal et al. vs. Silberberg et al.
Ben-Ami Bartal, Decety and Mason (2011) trapped a rat in a restraint tube that could be opened by a second rat that moved freely within the chamber. They recorded the likelihood of ultrasonic vocalizations in the trapped rats and found they were elevated during initial sessions when they were not quickly released. They viewed these elevated vocalizations as evidence that being in the restraint tube was stressful. They also demonstrated that a free rat would learn to push open the proximal door of the restraint tube (the proximal door led into the chamber with the rescuing rat; the distal door opened into an adjacent chamber) to free the trapped rat to join it, and that this behavior persisted when the door-opening response only opened a distal door at the other end of the restraint tube, freeing the trapped rat into a second chamber that was inaccessible to the free rat. They viewed the door-opening response as an empathic act and its persistence when the trapped rat was freed into a distal chamber as evidence this behavior is not due to the pursuit of social contact.
Silberberg, Allouch, Sandfort, Kearns, Karpel and Slotnick (2014) conducted a systematic replication (Sidman, 1960) of the Ben-Ami Bartal et al. (2011) procedure. The goal of a systematic replication is not a strict reproduction of an earlier procedure. Instead, it tests the robustness of an effect by varying a procedure in ways that should not compromise the conclusions of the target study if the processes attributed to it are veridical. Silberberg et al. reported several effects that weakened confidence in Ben-Ami Bartal et al.’s empathy account: (a) a free rat will not learn to contact the proximal door of the restraint tube to free the trapped rat into a distal chamber if it is the first condition of the experiment; (b) once it has learned the response, both rats will often enter the opened restraint tube, an outcome that calls into question that the restraint tube is, in fact, aversive; and (c) a free rat that previously learned to open a tube door to release a trapped rat into its chamber will continue to make this response even if the response no longer works. They attributed their and Ben-Ami Bartal et al.’s results to pursuit of social contact, not empathy.
Third design: Sato et al. vs. Schwartz et al.
The final design used to test for empathic action in rats is from Sato, Tan, Tate, and Okada (2015). In their study, a free rat could free a stressed rat trapped in a pool of water. They demonstrated their effects in two different apparatuses. In their first apparatus, they found a free rat would frequently open a circular door to enable a water-trapped rat to join it on dry ground; however, in their second apparatus, a dry rat trapped in a chamber was seldom released by the free rat. In this context, a pursuit-of-social-contact hypothesis predicts equal release frequencies in both apparatuses whereas an empathic-action account predicts releasing behavior only when the trapped rat was in a pool of water. Given their data, they interpreted their results as demonstrating empathically motivated behavior.
Schwartz, Silberberg, Casey, Kearns, and Slotnick (2017) questioned Sato et al. (2015) for failing to demonstrate that the second apparatus led to wet-rat release frequencies like those seen in their wet-rat test from the first apparatus. Such a comparison was necessitated by the fact that the apparatus not only differed in several design features from the first apparatus, but also because it may be the case that the circular door blocking trapped rat egress in the second apparatus was heavier than the door used in the first apparatus (see discussion in Schwartz et al.). If so, the reduced frequency of door opening in the second apparatus may have been due to the door being heavier rather than to the absence of the empathy putatively present when the trapped rat was wet.
Based on this criticism, Schwartz et al. (2017) systematically replicated Sato et al. (2015) in six different experiments in an E maze. The free rat was always placed in the center arm of the E maze. It could choose between alternative outcomes by turning either left or right in the maze. Depending on the experiment, the free rat chose between (a) a wet and a dry rat, (b) a pool of water and dry ground, (c) a pool of water and dry ground where access was blocked by a transparent door, and (d) a dry rat and an empty goal box. They demonstrated that both access to a trapped rat and access to a pool of water were individually reinforcing for the free rat. They found that free rats preferred releasing a wet rat over a dry rat; however, this preference need not due to empathic motivation. Instead, it could be due to the separable reinforcing effects of a rat and a pool of water combining to be more reinforcing than a rat standing on dry ground. They interpreted their data in terms of pursuit of social contact, not empathy.
Research plan
In Silberberg et al. (2014) and Schwartz et al. (2017) we have argued that a pursuit-of-social-contact account of the results of Ben-Ami Bartal (2011) and Sato et al. (2015) is more credible than the empathic-action account they favor. Nevertheless, additional confirmation of our claims is necessary. Toward this end we have modified the E maze used in Schwartz et al. by enlarging the size of the goal boxes so that the restraint tube used by Ben-Ami Bartal and a rat not locked in that tube could both fit in each goal box. In the present experiment, the first condition gives a free rat a choice between a rat trapped inside a restraint tube and an empty goal box. The third condition presents the complementary test--the free rat chooses between an empty goal box and a goal box containing not only a rat, but also a restraint tube that has its door open (there is enough room in the goal box to let the unlocked rat walk out of the restraint tube). The second condition differs from these other conditions in that both goal boxes are occupied, one with a rat trapped in the restraint tube in one goal box and the other with an unrestrained rat capable of stepping out of the restraint tube if it so wishes while remaining in the goal box. The predictions of pursuit of social contact are clear-cut. In the first and third conditions, the free rat should prefer the rat with the restraint tube whether that goal-box rat is locked in the tube or not. Regarding the second condition, a free rat in pursuit of social contact should have no preference because both goal boxes offer a rat as a companion.
The predictions of an empathic-action account are less clear-cut because it is silent about free-rat preference when choosing between an empty goal box and a second goal box containing a rat and an open restraint tube. There is no need for an empathically motivated response because the rat is already free to walk out of the restraint tube. For this reason, we accept any outcome in this, the third condition, as compatible with the predictions of an empathic-action account. Regarding the first condition, an empathically motivated rat would be expected to prefer the rat locked in the restraint tube over the empty goal box. This, of course, is the same prediction as the social-contact account. The critical test between accounts is in the second condition. As noted earlier, a socially motivated free rat should be indifferent between goal boxes. An empathically motivated free rat, on the other hand, should prefer the goal box with the rat trapped in the restraint tube.
METHOD
Subjects
Eighteen male Sprague-Dawley rats (Harlan Laboratories, USA) approximately 2 weeks of age at the time of their arrival in the animal colony served as subjects. They were housed as triplets in six home cages with unrestricted access to food and water for 10 weeks before the experiment began. The colony was maintained on a 12-h light, 12-h dark cycle, lights on at 8 AM.
Apparatus
Figure 1 illustrates the design of the E maze, restraint tube and restraint-tube door used in this experiment. The maze was constructed from polyvinyl chloride, with a Plexiglas ceiling. Two polyvinyl chloride panels (Door A in Figure 1) could block movement through the alley when lowered through a slit in the Plexiglas ceiling that covered the entire maze. A second pair of sliding polyvinyl chloride panels (Door B in Figure 1) could block access to a chosen alley. Door C, also made of polyvinyl chloride, was removed to place the free rat in the maze. Once the free rat was in place, this door was closed. All latencies, trial durations, and time inside a goal box were measured by a stopwatch with 1-s resolution.
Figure 1.
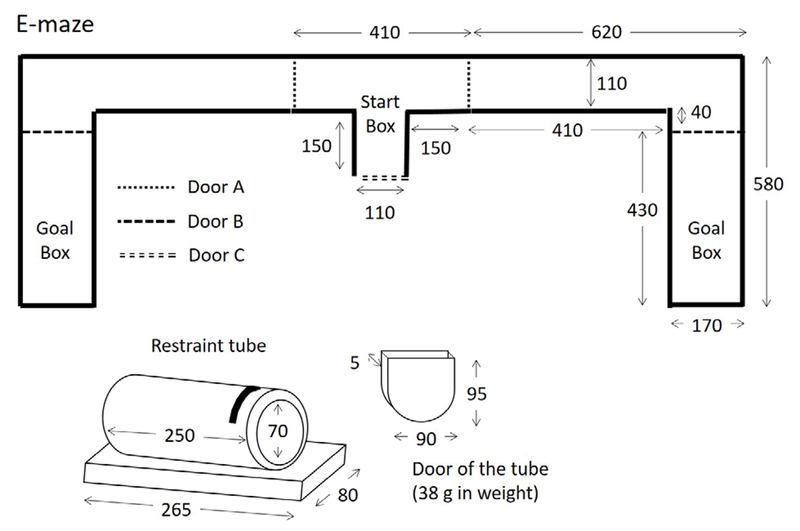
Schematic of E maze, restraint tube, and restraint-tube door drawn to scale. All measures of length are in mm. The height of the maze was 110 mm.
Procedure
For two weeks prior to beginning the experiment, each rat was handled by the experimenter for 5 min daily so that the rat could acclimate to human contact. Thereafter, each rat in each home cage was randomly assigned the role of “free rat,” “open restraint-tube rat” or “closed restraint-tube rat.” Free rats began each trial in the start box and could choose either the left or right arm of the maze. The restraint-tube subjects were placed in the goal box with either a Plexiglas door locking the rat in the tube (closed restraint-tube rat) or not (open restraint-tube rat).
For three of the free rats in the first condition of the experiment, the left goal box was empty and the right goal box contained a rat in a closed restraint tube. For the remaining three free rats, these goal-box assignments were reversed. In Condition 2, both goal boxes were occupied, one with a rat in a closed tube and the other with a rat with the tube door removed. In this condition, the goal-box assignment of the closed tube was switched from that in Condition 1. In Condition 3, free rats chose between an empty goal box and the home-cage rat assigned to the open restraint tube. The goal-box assignment of this tube was reversed from what it had been in Condition 2.
Each session began with forced-choice trials, the order of which was randomized with the constraint that the free rat was required to go to the left and right maze arms an equal number of times. Trials began by removing the free rat from its carrying cage located near the E maze and placing it in the start box. The free rat could move through an already-open door A (the other door A was closed). If the free rat did not go within 3 cm of door B within 3 min, the trial was terminated, and the free rat was placed in its cage for 20 s to await the next trial. When the free rat made the criterion door-B response, the Plexiglas door separating it from the goal box was removed and the free rat could enter the goal box. As described above, the goal box was either empty or contained a rat in an open or closed restraint tube; if the tube was closed, it was opened synchronously with the removal of door B. The free rat could stay in the proximity of the goal box for 1 min after which the trial ended. Then the free rat was returned to its carrying cage; and if a goal-box rat was released on that trial, it was returned to its goal-box assignment (either locked in a tube or with the tube door absent) and its door B was closed. After a 20-s intertrial interval, the next trial began with the reintroduction of the free rat to its start box. After six forced-choice trials, the free-choice trials began. All manipulations were done manually.
The six free-choice trials were the same as the forced trials except that both A doors were already open, permitting the free rat to choose either goal box. In all other ways, free-choice trials were the same as forced-choice trials. Sessions were conducted five days per week. Each experimental condition continued for eight sessions.
RESULTS
Figure 2 presents the proportion of choices of individual subjects during the last two sessions of each condition. We interpret this Figure by making pairwise comparisons between the first and third conditions against the second condition. Except for S4, all subjects preferred to go to the rat locked in its restraint tube more frequently when the other goal box was empty (Condition 1) than when the choice was between two rats, one locked in a tube and the other not (Condition 2). Next, we compare the third condition with the second condition. All rats preferred to go to a rat sitting outside its restraint tube (Condition 3) more frequently when the alternative was to go to an empty goal box than going to a rat locked in its restraint tube when the alternative was an already-released rat (Condition 2). A one-way, repeated measures ANOVA was conducted based on the proportions data in Figure 2. The factor of Conditions showed a significant difference (F(2,5) = 10.76, p = .003). Post analyses showed significant differences between Conditions 2 and 3 (t(5) = 5.22, p = .01) and Conditions 1 and 2 (t(5) = 3.06, p = .02), but not between Conditions 1 and 3 (t(5) = 0.89, p = .41).
Figure 2.
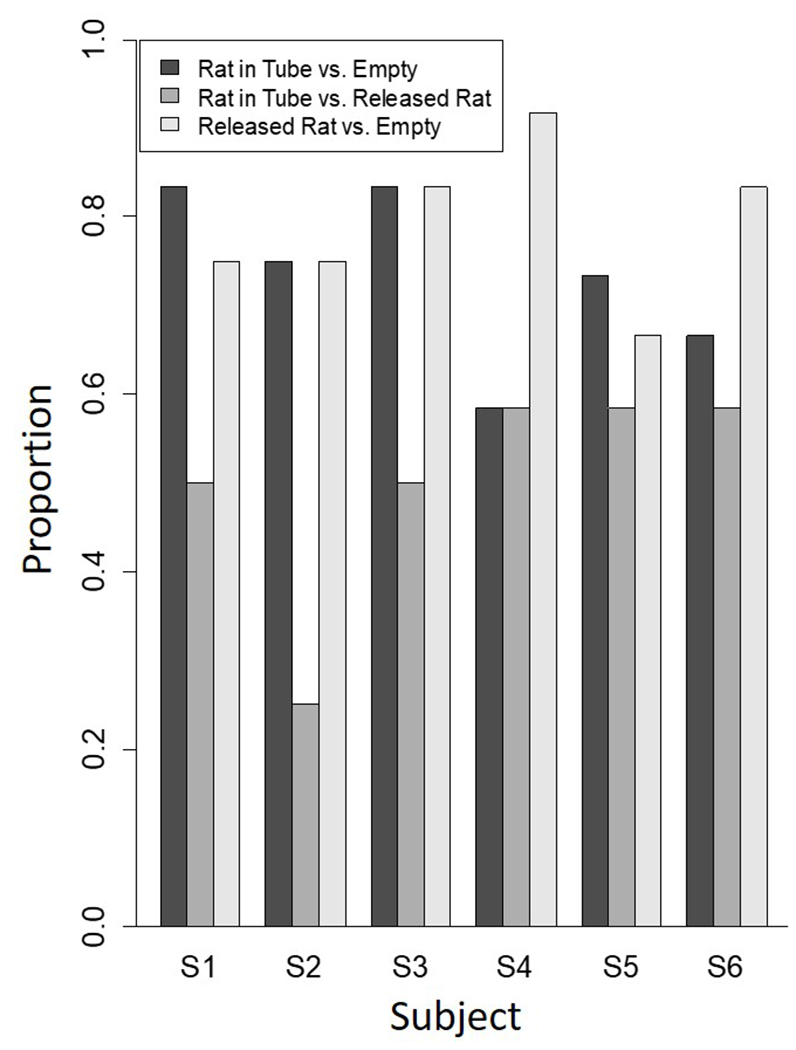
Free-choice preference data for individual subjects in each of the three conditions of the experiment during the last two sessions of the experiment. For Condition 1 (rat in tube vs. empty goal box) this measure is of choices of restrained rat divided by all choices. For Condition 2 (rat in tube vs. rat outside of tube) this measure is of choices of restrained rat divided by all choices. For Condition 3 (rat outside of tube vs. empty goal box) this measure is of choices of the released rat divided by all choices. S5 did not respond within the 3-min criterion during one trial in Condition 1. Therefore, his data are based on 11, not 12 trials.
The top panel of Figure 3 presents the frequency with which the free rat chose the goal box of the rat locked in a restraint tube divided by all choices over each of the eight sessions defining the first condition (choice between restrained rat and an empty goal box). The middle panel of Figure 3 presents the same measures as the top panel during the second condition of the experiment (choice between a rat locked in a restraint tube vs. one that is not locked in its tube). The bottom panel of Figure 3 presents the results of the third condition of this experiment. In this condition, free-rat choice is between a rat beside an open restraint tube and an empty goal box. We also tested for across-session trends by comparing mean choice proportions during the first two and last two sessions of each condition. A paired t-test established that preference for the open-vs.-empty manipulation (Condition 3) increased over sessions (t(5) = −3.75, p = .01), but did not attain significance in either of the other two conditions (restrained-vs.-empty manipulation (Condition 1, t(5) = −2.29, p = .07); restrained-vs.-open manipulation (Condition 2, t(5) = −0.18, p = .87).
Figure 3.
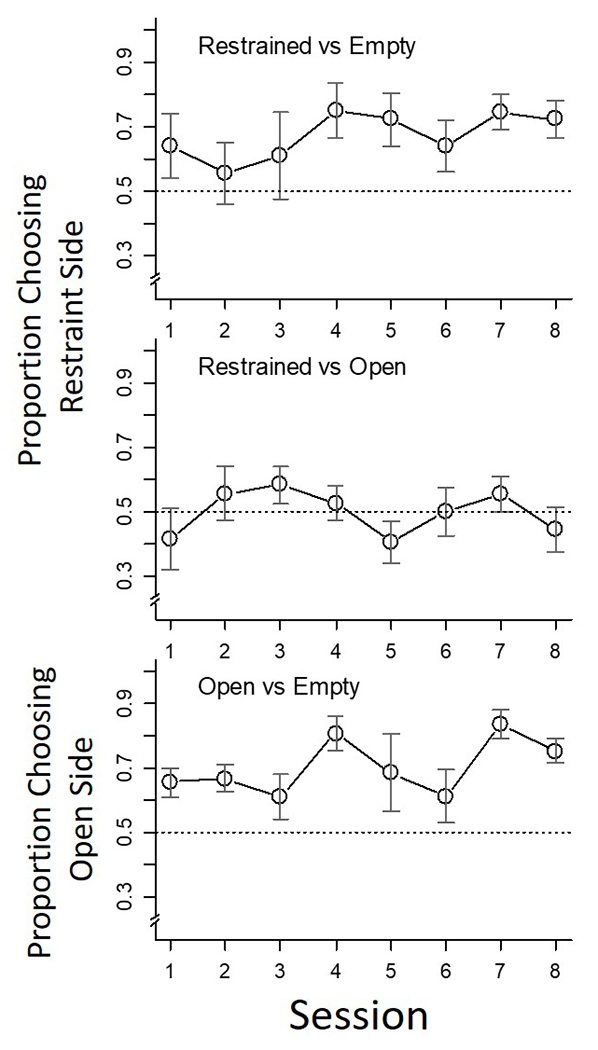
Group preference data for Conditions 1 (top panel), 2 (middle panel) and 3 (bottom panel). The dotted line defines a proportion of 0.5. The range bars through each data point define +1/−1 standard error of the mean. See text for other details.
Figure 4 presents group-mean latencies in s to make a choice (move from start box to within 3 cm of a B door) for each session of the experiment. Each datum was calculated by averaging the median latencies of each subject within a session. Trials in which no response occurred within the 3-min criterion were excluded from calculation (6 occurrences in 1728 trials). Choice-trial latencies in the restrained-vs.-empty condition (first row of panels) were shorter than in the choice trials of the second condition (rat in tube vs. rat outside of tube), especially in the last two sessions of the experiment.
Figure 4.
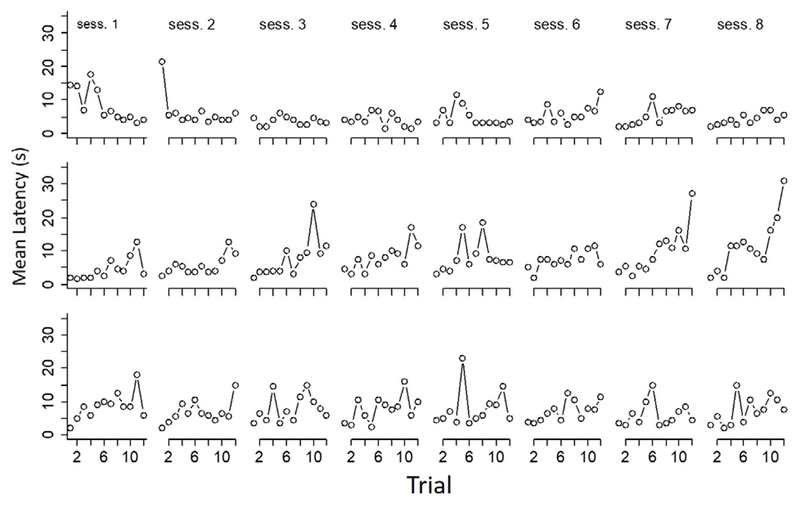
Mean response latency in s in each trial of each session of the three conditions of this study. The top, middle and bottom rows are, respectively, data from Conditions 1 (rat in tube vs. empty goal box), 2 (rat in tube vs. rat outside of tube) and 3 (rat outside of tube vs. empty goal box).
Figure 5 plots group-mean latency to respond during forced and choice trials averaged over the last two sessions of each condition. A two-way repeated measures ANOVA (Trial type × Condition) of these data showed significant effects of trial type (F(1, 5) = 15.20, p = .01), and condition (F(2, 10) = 8.29, p = .0075). The trial type x condition interaction was also significant (F(2, 10) = 9.40, p = .005). Post analyses showed the latency of choice trials in Condition 2 was longer than in Conditions 1 (t((5) = 3.09, p = .02) and 3 (t(5) = 3.83, p = .01). The comparison between forced and choice trials showed the latencies in choice trials were longer than forced trials in Conditions 1 (F(1, 5) = 6.99, p = .05) and 2 (F(1, 5) = 22.16, p = .005). Other comparisons were not significant.
Figure 5.
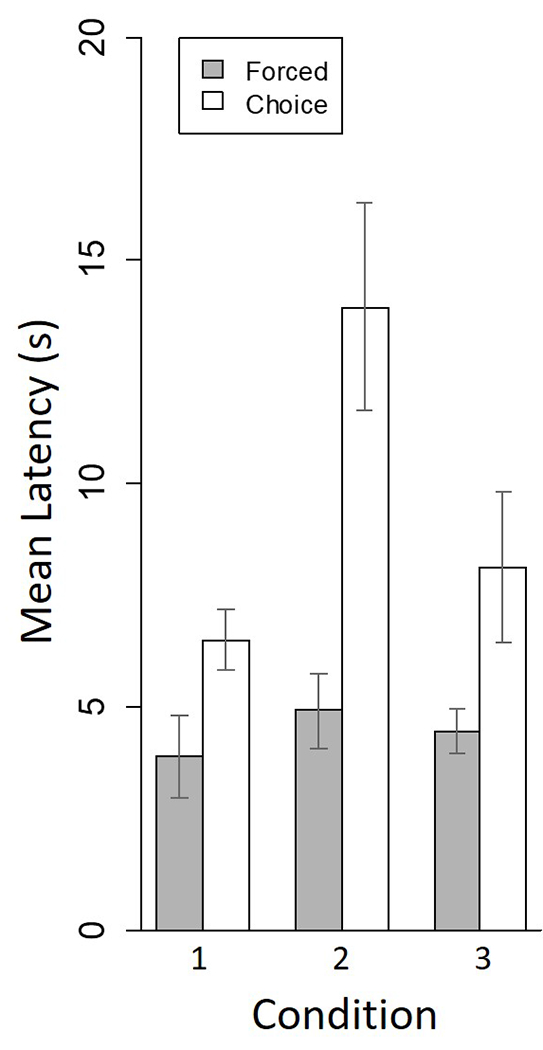
Mean group latency in s during the last two sessions of each condition of the experiment. Error bars define +/− one standard error of the mean.
DISCUSSION
In Condition 1, rats preferred to be in the presence of a rat locked in a restraint tube over an empty goal box. This result is compatible with both Ben-Ami Bartal et al.’s (2011) empathic-action hypothesis and Silberberg et al.’s (2014) social-contact hypothesis. From the perspective of the former account, this preference is because it relieves the presumed distress of the rat locked in the tube. From the perspective of the social-contact hypothesis, this preference is also sensible: It enables the free rat to have a companion whereas the empty goal-box alternative does not.
In Condition 3, rats preferred to be in the presence of a rat in an open restraint tube over an empty goal box. As noted earlier, an empathic-action account makes no prediction in this case. Nevertheless, the empathic-action hypothesis is not violated by this result. Perhaps a rat is motivated for both social contact and empathic action. If so, a free rat’s preference for the rat in the open restraint tube does not provide a critical test of these two accounts. Regarding a social-contact account, this result is obviously compatible with its predictions.
Matters differ for the data from Condition 2. In this condition, each free rat chooses between two rats, one locked in a restraint tube and the other not. Because there are prospective companions for the free rat regardless of its choice, the social-contact hypothesis predicts no preference, the result obtained. The empathic-action hypothesis, on the other hand, predicts a preference for the goal box containing the rat locked in its restraint tube because only such a choice can relieve the presumed distress of the restraint-tube occupant. The finding of indifference in choice between these two outcomes favors attributing the tube-releasing operant in Ben-Ami Bartal et al. (2011) and Silberberg et al. (2014) to the reinforcing properties of social contact rather than empathic action.
A habituation argument
Response latencies during choice trials increased between the restrained-vs.-empty condition (Condition 1) and the restrained-vs.-open condition (Condition 2; see Figure 5). This change in latency might be explained in two ways that are compatible with the idea that choice in our results is empathically motivated: First, the trapped rat may have habituated to the distress caused by the restraint tube over trials in the first condition and therefore was not in need of empathic action from the free rat in the second condition. Alternatively, the free rat’s motivation for empathic action may have weakened after the first condition of the experiment because of habituation. In consequence, the free rats were indifferent in the second condition between the trapped-rat and open-rat goal boxes.
A habituation interpretation presumes latency is continuously increasing across sessions. While this argument is generally consistent with outcomes when the first and second conditions are compared, it is violated when applied to the free-choice latencies in the second vs. third conditions of this study. As shown in Figure 5, free-choice latencies shortened from Condition 2 to Condition 3. Such an outcome would not be expected if habituation either to a trapped rat’s distress or to empathic action itself governed responding. A better explanation for these results is that in Condition 2, unlike Conditions 1 and 3, the free rat had a choice between other rats, and the large choice latencies in Condition 2 reflect not habituation, but latency to selection between two rats. The brief latencies during forced-choice trials is also consistent with this interpretation.
Conclusions
The present findings weaken confidence in Ben-Ami Bartal et al.’s (2011) claim to have demonstrated empathic action in rats and strengthens our earlier claim in Silberberg et al. (2014) that what Ben-Ami Bartal et al. demonstrated was not the operation of empathy, but of sociality. Despite this fact, we do not consider this research question resolved. This study, like Silberberg et al.’s, differed from Ben-Ami Bartal et al.’s experiment in ways that preserve the possibility that free rats could have been empathically motivated in Ben-Ami-Bartal et al. For example, on one hand, in the first few sessions of their study, restraint-tube rats were locked up for many min; in the present study, on the other hand, rats were typically locked in their restraint tubes for briefer periods. Possibly longer-term restraint is what is required to create true distress in these subjects that could then tap into the empathic motivation present in free rats. More work confirming the pursuit-of-social-contact hypothesis is needed before we can conclude their work merely demonstrates the reinforcing power of rat sociality.
Acknowledgments
This work was funded by NIH 1 R15 MH109922 to American University and by JSPS overseas research fellowships to Yosuke Hachiga.
References
- Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, … & Keysers C (2011). Experience modulates vicarious freezing in rats: a model for empathy. PloS One, 6, e21855. doi: 10.1371/journal.pone.0021855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartal IBA, Decety J, & Mason P (2011). Empathy and pro-social behavior in rats. Science, 334, 1427–1430. doi: 10.1126/science.1210789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Leman S, Vourc’h P, & Andres C (2005). Rodent models for autism: A critical review. Drug Discovery Today: Disease Models, 2, 93–101. doi: 10.1016/j.ddmod.2005.05.004 [DOI] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, … & Mogil JS (2006). Social modulation of pain as evidence for empathy in mice. Science, 312, 1967–1970. doi: 10.1126/science.1128322 [DOI] [PubMed] [Google Scholar]
- Lavery JJ, & Foley PJ (1963). Altruism or arousal in the rat? Science, 140, 172–173. DOI: 10.1126/science.140.3563.172 [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, & Neumann ID (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology, 36, 2159–2168. DOI: 10.1038/npp.2011.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowbahari E, Scohier A, Durand JL, & Hollis KL (2009). Ants, Cataglyphis cursor, use precisely directed rescue behavior to free entrapped relatives. PLoS One, 4, e6573 10.1371/journal.pone.0006573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, Bechara A, Damasio H, Grabowski TJ, Stansfield RB, Mehta S, & Damasio AR (2007). The neural substrates of cognitive empathy. Social Neuroscience, 2, 254–275. DOI: 10.1080/17470910701376902 [DOI] [PubMed] [Google Scholar]
- Rice GE, & Gainer P (1962). “ Altruism” in the albino rat. Journal of Comparative and Physiological Psychology, 55, 123–125. 10.1037/h0042276 [DOI] [PubMed] [Google Scholar]
- Sato N, Tan L, Tate K, & Okada M (2015). Rats demonstrate helping behavior toward a soaked conspecific. Animal Cognition, 18, 1039–1047. DOI: 10.1007/s10071-015-0872-2 [DOI] [PubMed] [Google Scholar]
- Schwartz LP, Silberberg A, Casey AH, Kearns DN, & Slotnick B (2017). Does a rat release a soaked conspecific due to empathy? Animal Cognition, 20, 299–308. 10.1007/s10071-016-1052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M (1960) Tactics of scientific research: evaluating experimental data in psychology. Basic Books, New York. [Google Scholar]
- Silberberg A, Allouch C, Sandfort S, Kearns D, Karpel H, & Slotnick B (2014). Desire for social contact, not empathy, may explain “rescue” behavior in rats. Animal Cognition, 17, 609–618. DOI: 10.1007/s10071-013-0692-1 [DOI] [PubMed] [Google Scholar]
- Vasconcelos M, Hollis K, Nowbahari E, & Kacelnik A (2012). Pro-sociality without empathy. Biology Letters, rsbl20120554. doi: 10.1098/rsbl.2012.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]


