Abstract
The meniscus is the most commonly injured structure in the human knee. Meniscus deficiency has been shown to lead to advanced osteoarthritis (OA) due to abnormal mechanical forces, and replacement strategies for this structure have lagged behind other tissue engineering endeavors. The challenges include the complex three-dimensional structure with individualized size parameters, the significant compressive, tensile and shear loads encountered, and the poor blood supply. In this progress report, we provide a review of the current clinical treatments for different types of meniscal injury. We discuss the state-of-the-art research in cellular therapies and novel cell-sources for these therapies. We present the clinically available cell-free biomaterial implants and the current progress on cell-free biomaterial implants and cell-based tissue engineering strategies for the repair and replacement of meniscus, and identify the current challenges. Tissue-engineered meniscal biocomposite implants may provide an alternative solution for the treatment of meniscal injury to prevent OA in the long run, because of the limitations of the existing therapies.
Keywords: tissue engineering, meniscus, regenerative medicine, biomaterials, progenitors
1. Introduction
The knee joint capsule contains three tissues that function in harmony to provide biomechanical support: ligaments, menisci and cartilage. Meniscus is a semi-lunar wedge shaped fibrocartilaginous tissue that absorbs shocks between the articular cartilage of the tibia and femur (Figure 1). The primary biomechanical function of meniscus comprises of providing shock absorption and overall mechanical stability in the knee [1].
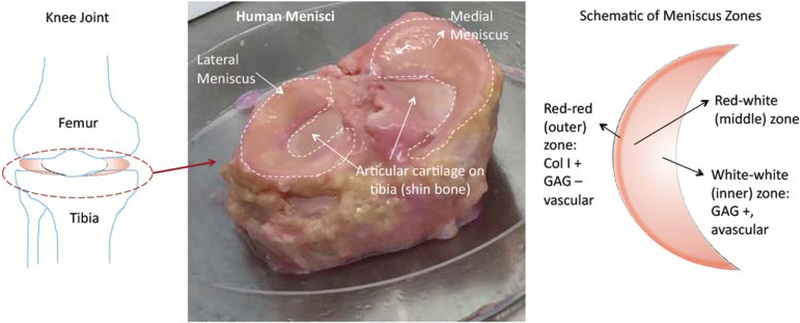
Figure 1.
Meniscus Structure: (Left) Location of menisci between the bones of the knee joint (femur and tibia) (Middle) This human cadaver knee joint photograph shows the medial and lateral menisci on the articular cartilage surface of the tibia (shin bone). (Right) A schematic of zones of meniscus depicts the inner white-white avascular zone, the middle red-white transition zone and the outer red-red vascular zones.
The biochemical composition of human meniscus is reported to vary between 72–78% water, 13–23% collagen (mostly type-I collagen), 0.4–0.5% glycosaminoglycans (GAGs) and about 0.12% DNA [2], depending on the location [3, 4]. Meniscus has a unique zonal organization and structure. The inner region is more cartilage-like and the outer region is more ligament-like; the cellular phenotype in the avascular inner region is chondrocyte-like, with glycosaminoglycan and type II collagen presence [3] (Figure 1). The outer (red-red) and the middle (red-white) regions have higher type I collagen content (80% dry weight) than the inner (white-white) region (70 % dry weight, both type I and II collagen). The thin outer red-red region is highly vascularized, therefore it has a higher healing capacity, whereas the inner region does not heal very well, not unlike articular cartilage [5]. The meniscus has a unique anisotropic collagen fiber and extracellular matrix organization which determines its mechanical function [3]. This unique structural and zonal organization has been a major challenge to match for biomedical scientists.
Knee injuries in young and active population often results in loss of tissue function, which leads to severe osteoarthritis, the most common form of arthritis, affecting 22.7% of adults in the USA [6]. Arthritis is the most common disability in the USA and the risk of osteoarthritis ([7] after knee injury is 57% [8]. In 2003, it was reported that the cost of OA is 128 billion US dollars in medical expenses and lost wages [9]. Trauma in the knee joint causes lesions that can lead to osteoarthritis. Meniscus injuries occur commonly in active populations and may lead to secondary joint complications [10, 11]. Smaller meniscal tears can be repaired to some extent by suturing or partial replacement of the meniscus using recently commercialized cell-free (acellular) biomaterials. These biomaterials are currently not suitable for total meniscusreplacements, where meniscus damage is severe. Irreparable meniscal tears are commonly treated by meniscectomy or meniscal allograft transplantation [10]. Meniscectomy is shown to disrupt joint biomechanics, hence causing osteoarthritis in the long term [12]. Common issues with the use of meniscal allografts include availability, graft sizing, sterilization and transplant remodeling [13]. There is a clinical need for more research in meniscus replacement options.
Here we provide a critical review of the current progress and identify the gaps in the field of regenerative therapies for meniscus repair and meniscus tissue engineering. The first section provides a brief review on the current challenges for the clinical treatments for different types of meniscal injury and outline the need for different repair options. The second section discusses the state-of-the-art of research in cellular therapies and novel cell-sources for these therapies. The third section reports the research on acellular biomaterial implants, which are being developed or in clinical trials. Finally, the combined approach of cell-based therapies and biomaterials are discussed in the Cellular Biomaterials section.
2. Clinical Treatments for Meniscal Injury
The meniscus is the most commonly injured structure in the human knee. While meniscus repair is an option in young patients with traumatic injuries, degenerative tears in older patients require meniscectomy – removal of the torn portion of the meniscus [14]. Even in younger patients, meniscus deficiency can result from failed meniscus repairs or meniscectomy, the natural history of a meniscectomized knee suggests that osteoarthritis ensues. In 1948, Fairbank described the characteristic changes in the knee joint following meniscectomy that now bear his name and are understood to be osteoarthritis [15].
To date, the approach to meniscus injuries in young patients has revolved around meniscus repair techniques. Suturing of the meniscus has been facilitated with advancements in knee arthroscopy, allowing for numerous suture configurations and the introduction of more biological repair strategies such as use of platelet rich plasma (PRP) or blood clot augmentation [16]. Until recently, there have been few options for the partial replacement of meniscus. One recent product, the collagen meniscus implant (CMI, also known as Menaflex) has seen promising results in Europe and has become available in the United States [17]. The challenge of partial replacement is finding a scaffold that is robust enough to allow strong suture repair yet compressible enough to avoid articular cartilage damage with weight bearing. In addition, the central portion of the meniscus has a poor blood supply, further hampering the success of graft incorporation and healing.
In cases of complete or subtotal meniscus deficiency, the only current option is the replacement of the total meniscus, using meniscus allograft transplantation, especially in the young and active patient population [10]. This complex procedure uses a once frozen meniscus from an organ donor. The graft is size matched based upon the tibial plateau anatomy and is sewn into position with or without the fixation of bone plugs at the roots. The graft sizing has been studied extensively, but despite this knowledge, intraoperative size issues often occur [18]. Over time these grafts have not been seen to decrease in size, but more often to become extruded from the knee joint, which may have more to do with the host mechanical environment and osteophyte formation between meniscus excision and replacement [19]. Graft storage, processing and sterilization are other concerns that tend to get overlooked, which affect the mechanical properties of the graft and the metabolic activity of the surviving cells after thawing [13, 20].
A retrospective review reported an overall failure rate of 29% of meniscal allograft transplantation (MAT) (4–14 year post-op) [21]. Another analysis of literature indicated that MAT seemed to have higher reoperation and failure rates than the available acellular meniscal scaffolds (Actifit polyurethane meniscal scaffold and CMI collagen meniscal implant) [22]. A gap exists where meniscus surgeons have few options for meniscus replacement in patients with meniscus deficiency. This need warrants the continuous work on viable tissue engineering constructs in this domain.
3. Cell Sources for Meniscus Regeneration and Repair
The limited vascularity of the meniscus contributes to its poor healing response. In particular, the inner meniscus referred to as the white-white (ww) region is devoid of blood vessels. Much like articular cartilage, the ww region of the meniscus is dependent on synovial fluid for nourishment. Given that inner peripheral meniscal tears present a significant clinical challenge, it is clear that neither the synovial fluid nor the neighboring tissues alone can stimulate an adequate repair response in the ww region. It is with this in mind that growth factors and platelet rich plasma have been used in past decades to try and augment peripheral healing. In the case of PRP specifically, although some studies have reported improvements in meniscal healing compared to PRP untreated controls [23], there are also studies that have reported minimal to no healing response [24, 25].
Comparatively, cell-based tissue regeneration has emerged as a promising avenue towards improving meniscus repair. An emerging strategy uses cells that can adhere to the meniscus and/or migrate to the area of injury in order to facilitate an improved repair response [26–28]. As one can imagine, this relies heavily on the reparative capabilities of the cells themselves and the efficiency of their method of delivery. As such, a considerable amount of ongoing effort by researchers focuses on finding an optimal cell source and delivery method to use in this strategy. Investigators have thus far explored the use of mesenchymal stem cells (MSCs) from several sources including bone marrow, adipose tissue, synovium and meniscus itself (see below sections in detail for each cell source). Furthermore, the use of non-load bearing cartilage derived MSCs in meniscus repair is promising and further investigation of their reparative potential is being explored in our laboratory. In this section, we will review the cell sources that have been explored to demonstrate potential for cell-based meniscus repair (Table 1).
Table 1.
Cells utilized for meniscus repair/regeneration and their tissue sources
| Cell Classification | Cell | Tissue Source | Studies |
|---|---|---|---|
| Mature Cells | Fibrochondrocytes | Meniscus | [73–76, 125] |
| Chondrocytes | Cartilage | [81] | |
| Stem/Progenitor/Multipotent Cells | Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Marrow | [25, 27, 43, 44, 45] |
| Synovium-derived mesenchymal stem cells (SMSCs) | Synovium | [54] | |
| Adipose-derived stem cells (ASCs) | Fat | [28, 56] | |
| Meniscus-derived mesenchymal stem cells (MMSCs) | Meniscus | [58, 59] | |
| Cartilage progenitor cells (CPCs) | Cartilage | [63] | |
| Myoblasts | Muscle | [68] | |
| Multipotent Vascular Endothelial Cells | Endothelium | [70] |
3.1. Stem cells
Although they are in short supply compared to fully differentiated cells, stem cells exhibit several key features that make them desirable for tissue repair. For instance, MSCs lack HLA class II expression rendering them effectively invisible to alloreactive lymphocytes [29] thus granting them reduced immunogenicity in comparison to mature cells. These cells also secrete immunoregulatory factors such as indoleamine 2,3-dioxygenase [30] and prostaglandin E2 (PGE2) which suppress the activation of immune cells [31]. Stem cells are also self-renewing and highly proliferative upon activation [32]. There are several sources of stem and progenitor cells that have demonstrated promise for use in meniscus repair.
3.1.1. Mesenchymal Stem Cells.
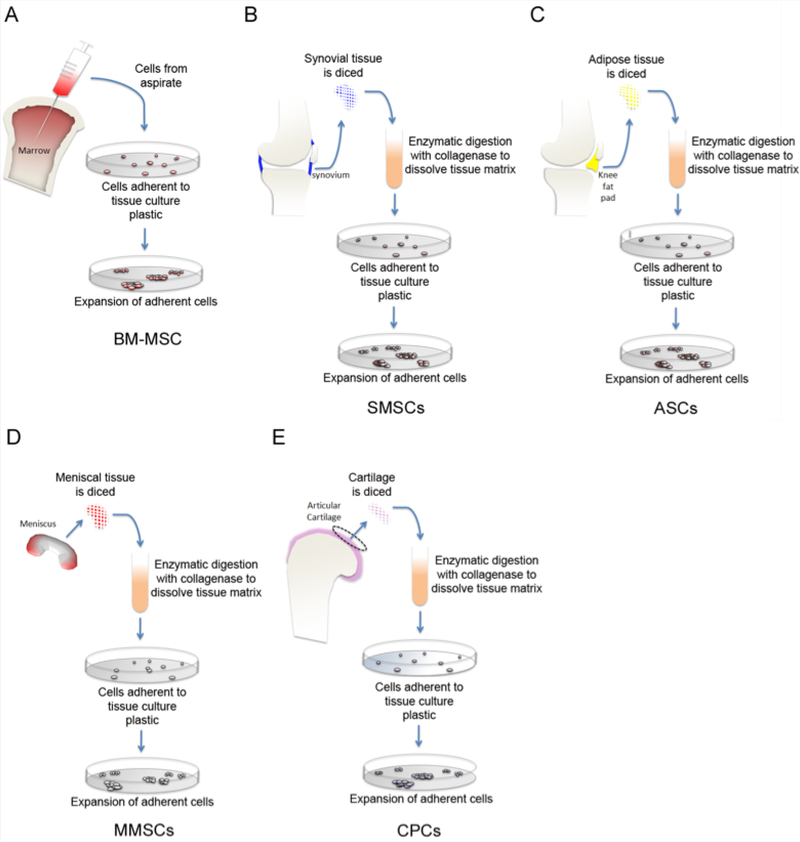
The most studied type of stem cell in the field of musculoskeletal research is the mesenchymal stem cell. MSCs are reported to exist in a wide variety of musculoskeletal tissues including bone marrow [33], cartilage [34], synovium [35], tendon [36], ligament [37], intrapatellar fat pad [38], and even meniscus itself [39]. Many of these tissues have been utilized as stem cell sources for cell-based meniscus repair strategies in animal models. Currently, bone marrow and synovium are the most commonly explored sources of MSCs.
Bone marrow –
Stromal cells from the bone marrow (BM-MSCs) were first discovered by Friedenstein et al. in 1968 [33]. While relatively easy to collect, they only make up 0.0017% – 0.0201% of bone marrow cells [40]. BM-MSCs are CD34-, CD44+, CD45-, CD54-, CD90+, CD105+, CD166+, CD271+ cells that exhibit the ability to differentiate along the chondrogenic, osteogenic and adipogenic lineage [41]. These cells can be extracted autologously through minimally invasive bone marrow aspiration procedure [42]. BM-MSCs have been utilized in many pre-clinical meniscal repair studies. They have been used in the repair of meniscal tears [27, 43] and meniscal defects [25], including partial meniscus transections [44, 45]. A reported disadvantage associated with the use of BM-MSCs for the purpose of tissue repair is their propensity for hypertrophic differentiation. Indeed, the occurrence of cellular hypertrophy of BM-MSCs has been brought to light in cartilage defect repair [46] and meniscus co-culture studies [47]. Currently there is a need to address this limitation - either through finding conditions that can prevent hypertrophy in BM-MSCs themselves or through the discovery of alternative cell sources that are more resistant to cellular hypertrophy altogether. With respect to the former, co-culturing BM-MSCs with meniscal cells from the outer meniscus has led to a reduction of hypertrophic differentiation of BM-MSCs in vitro [48]. Co-culturing a larger proportion of meniscus cells, relative to BM-MSCs, in a 3:1 ratio in the presence of TGF-beta resulted in a significant reduction of hypertrophic markers collagen × (COL10A1) and matrix metalloproteinase-13 (MMP-13), compared to BM-MSCs alone [49]. Culturing BM-MSCs embedded in powderized meniscal ECM components have also been reported to reduce hypertrophy [50]. With this being said, cellular hypertrophy remains the primary limitation that fuels criticism against utilizing BM-MSCs as a cell source for cartilage and meniscus repair.
Synovium –
Synovium derived mesenchymal stem cells (SMSCs) are a promising cell source for meniscus repair. These cells are reported to be chondrogenic and to form more colonies than BM-MSCs under monolayer culture conditions [35]. SMSCs have a similar gene expression profile to meniscus cells, as determined by cluster analysis [51]. In vitro culture expansion of synovial cells results in increasing percentage of SMSCs that express CD271 [52], a marker reported to be indicative of MSCs with enhanced differentiation potential [53]. Additionally, obtaining synovial tissue in order to isolate synovial cells does not compromise the articular surface and is therefore an attractive feature of their consideration for meniscus repair strategies. Meniscus repair in large animals has been investigated with moderate success using SMSCs [54]. While these studies brilliantly demonstrate that type II collagen is elevated in the SMSC treated menisci, relative to untreated controls, it would be beneficial for future studies to also measure Collagen × and other markers of cellular hypertrophy as well as degradative MMP levels.
Adipose tissue –
Adipose-derived stem cells (ASCs) from the infrapatellar fat pad of the knee is considered an alternative source of stem cells that are in consideration for emerging cell-based cartilage and meniscus repair strategies. Although these cells are isolated from fat tissue, they demonstrate the ability to undergo chondrogenic differentiation producing proteoglycans and type II collagen. Unlike bone marrow stromal cells, but similar to SMSCs, isolation of autologous ASCs requires initial surgery for tissue extraction. Studies have demonstrated the limited chondrogenic potential of ASCs in comparison to BM-MSCs [55] thereby dampening enthusiasm in their consideration for cartilaginous tissue repair. However, CD90 and CD49e double positive ASCs were reported in one study to improve healing of surgically created 5 mm meniscal lesions in the inner avascular region in a rabbit model. The authors demonstrate that the presence of a healing response is very much dependent on the size of the lesion and how long after the creation of the lesion the meniscus is treated with ASCs [56]. In another study, ASCs were isolated from subcutaneous neck fat in rats, labeled with iron oxide and localized to the site of a partial meniscal transection using magnets in order to evaluate the healing response of these cells when they are directed to the site of injury [28]. The authors demonstrated a notable increase in neomeniscal size in ASC treated groups at the 12-week time point.
Meniscus –
The use of meniscus derived MSCs (MMSCs) is one of the latest developments in cell-based meniscus repair/regeneration. Like most stem cells, MMSCs are reported to be highly adherent to tissue culture plastic. Isolation of MMSCs involves allowing these cells from either the vascularized red-red outer region or avascular white-white region to migrate out of meniscal tissue onto plastic (Figure 2A). Migrated individual cells that exhibit high colony forming efficiency (CFE) that can also undergo multi-lineage differentiation have been used for meniscus repair in ex vivo and live animal models [57–59]. A significant challenge in considering autologous MMSCs for cell-based tissue repair is that it seems inherently counterintuitive to extract cells from menisci in order to repair the same meniscal tissue. Instead, utilization of allogeneic MMSCs is a practical approach that has been demonstrated to promote meniscus healing in multiple animal models [58, 59]. Similar to MSCs from other tissue sources, MHC class II expression is lacking in MMSCs thereby minimizing the chance of allogeneic cell rejection [59]. MMSCs have been shown to be chemotactically attracted to SDF-1, the production of which is elevated in the meniscus up to 3-weeks following injury [58]. The involvement of the SDF-1/CXCR4 pathway in mediating MMSC migration to meniscal injury sites is being explored.
Figure 2.
Strategies for isolating mesenchymal stem/progenitor cells from their respective musculoskeletal tissue sources. Diagram of established isolation methods of mesenchymal stem cells from bone marrow (BM-MSC) (A), synovium (SMSC) (B), adipose tissue (ASC) (C), meniscus (MMSC) (D), and cartilage (CSC) (E).
Cartilage –
Similar to the aforementioned bone marrow, adipose tissue, synovium and meniscus; cartilage too harbors tissue specific mesenchymal stem cells [34, 60]. These cells are often referred to as cartilage-derived chondrogenic progenitor cells (CPCs). CPCs from cartilage can be effectively and inexpensively separated from mature chondrocytes and enriched through differential adhesion to fibronectin (Figure 4) as first demonstrated by Williams et al. [60]. These cells are reported to be highly proliferative and capable of tri-lineage differentiation along chondrogenic, osteogenic and adipogenic lineages [61]. Seol et al. demonstrated that CPCs are capable of mobilizing to sites of cartilage injury and/or trauma [62]. The authors reported that this migratory response to distress could be attenuated by inhibiting the receptor for advanced glycation end products (RAGE). CPCs from cartilage are fibrochondrocyte-like in appearance and they are recognized for their high colony forming efficiency and chondrogenic potential. It is for these reasons that they should not only be considered for cell-based cartilage defect repair but also meniscus repair. Cartilage CPCs can adhere and integrate into the avascular ww zone of the meniscus (Figure 3b, c). Furthermore, we show that these cells retain the ability to produce proteoglycans thereby remaining chondrogenic upon integration (Figure 3d). In comparison to BM-MSCs, CPCs from healthy cartilage tissue are resistant terminal differentiation and hypertrophy [63]. However, as is the case with synovium-derived mesenchymal stem cells, a challenge to using cartilage-derived CPCs in regenerative medicine is their bioavailability. CPCs are reported to make up less than 1% of all cells in articular cartilage [64]; although one study has reported 1.47 ± 0.16% fibronectin adherent progenitor cell colonies from normal healthy human cartilage [65]. Given their low natural abundance, their high colony forming efficiency becomes essential for expansion and their use in cell-based repair strategies. Indeed much like the established method of autologous chondrocyte implantation [66] in which healthy chondrocytes are relocated from non-load bearing regions of cartilage to a tissue defect, it should be possible to expand CPCs isolated from non-load bearing cartilage in order to use these cells autologously to repair meniscus tissue. Intriguingly, osteoarthritic (OA) cartilage harbors an even greater number of progenitor cells than found in normal healthy cartilage [65, 67]. Fellows et al. [65] recently demonstrated that there are OA CPCs that exhibit characteristics of early senescence as well as those that seem to retain normal proliferative capacity, giving the implication that the later may be utilized as a source of cell-based regenerative therapy. However, it must be taken into consideration that some OA CPCs also exhibit a propensity for premature hypertrophy and mineralization in culture (Jayasuriya et al. unpublished data). Hence, it is with the upmost care that risk factors be weighed before using these cells for therapeutic purposes.
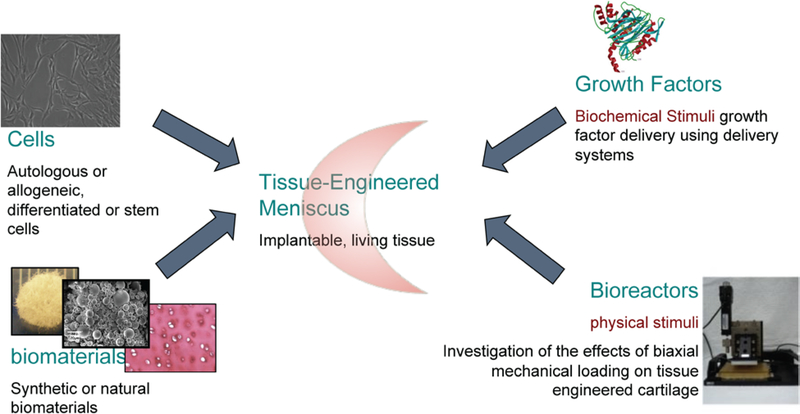
Figure 4.
Cell-based meniscus tissue engineering approach using cells, materials, bioactive growth factors, and in vitro bioreactors for pre-implantation testing and development.
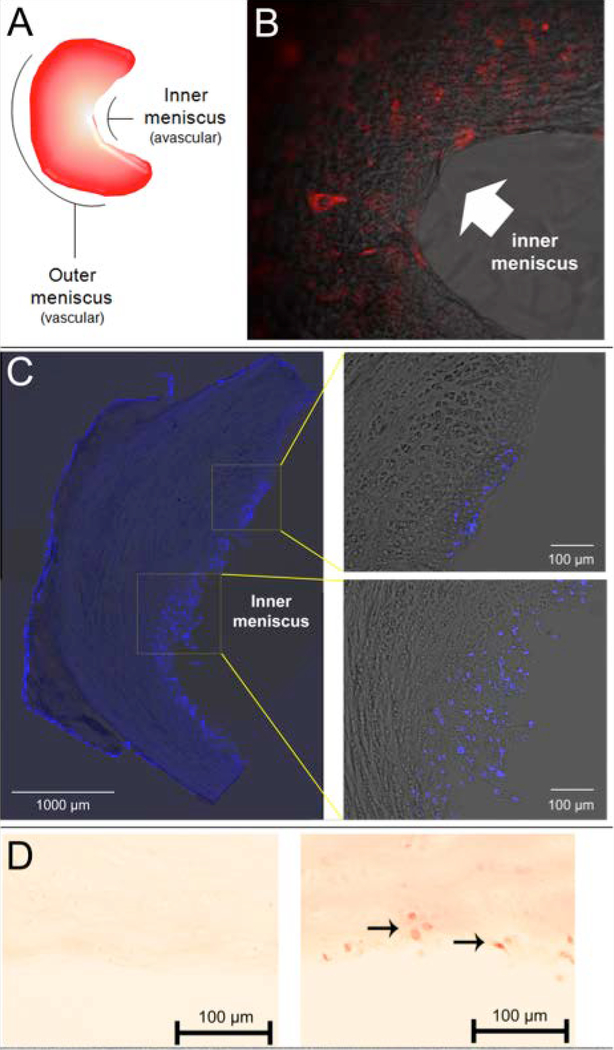
Figure 3.
Cartilage stem cells remain chondrogenic after integrating into the inner meniscus. Meniscus diagram depicts inner avascular white-white (ww) zone and outer vascular red-red (rr) zone (A). Image of fluorescently labeled cartilage-derived stem cells (1.0 × 105) cultured on a decellularized rat meniscus (B). Cartilage-derived stem cells exhibit strong adherence to the avascular inner meniscus following 4 days in culture (B). Image was obtained using an inverted microscope at 10x original magnification. Confocal microscope images of a sectioned decellularized rat meniscus 4 weeks after being seeded with cartilage-derived stem cells (C). DAPI nuclear staining (blue) indicates that the cells largely integrate into the inner meniscus. Safranin-O staining of the avascular region of sectioned decellularized rat meniscus 4 weeks after being seeded with CD90−/CD105+/CD166− cartilage stem cells. Left panel: No cell (control); Right panel: seeded with cartilage stem cells. The cells increase the proteoglycan content in the meniscus as indicated by the stronger staining in the right side panel, compared to left side control. Arrows signify stem cells that have integrated into the inner meniscus.
3.1.2. Myoblasts –
Few studies have explored the use of muscle-derived progenitors such as myosatellite cells and myoblasts for meniscus repair. Recently, Gu et al. [68] demonstrated that myoblasts subjected to chondrogenic differentiation in vitro can then be seeded into a polyglycolic acid (PLGA) mesh scaffold and used to try and repair a partially transected meniscus. The authors were able to achieve integration in all 12 canines implanted with the myoblast-seeded scaffold, 9 (75%) of which contained fibrocartilage tissue. Only 17% of animas implanted with cell-less composite scaffolds showed the presence of fibrocartilage tissue. In a later study, Zhu and colleagues utilize myoblasts genetically transfected to constitutively express cartilage-derived morphogenetic protein-2 (hCDMP-2) [69].
3.1.3. Multipotent Vascular Endothelial Cells –
As is the case with fat cells and muscle cells, isolating endothelial cells is convenient due to their abundance. With this consideration, multipotent progenitor cells from endothelial tissue has become an attractive cell-source to investigate for cell-based tissue repair. A recent study demonstrated the reparative capacity of CD34+, CD146+ multipotent vascular endothelial cells from human fetal menisci using an athymic rat model [70]. The authors reported that CD34+/CD146+ cells achieved more complete healing of a surgically created radial tear on the inner avascular region that ran two thirds of the way towards the back periphery, compared to cells that were double negative for these markers.
3.2. Mature cells
Some non-stem/progenitor cells have also been investigated for use in meniscus repair strategies including cells from cartilage, meniscus, synovium and adipose tissue [71, 72]. Meniscus repair was found to be least effective when using mature adipose cells, while chondrocytes and fibrochondrocytes have been reported to hold promise in multiple studies [71, 72]. However, it is worth noting that many of these studies would greatly benefit from exploring the quality of the resulting repair/regenerated tissue. For example, since a major limitation of utilizing BM-MSCs for cell-based meniscus repair is the occurrence of tissue hypertrophy, any alternative cell source (including mature cells) should also be tested for this outcome.
3.2.1. Meniscal Fibrochondrocytes –
Fibrochondrocytes from meniscus have been investigated as a cell source for meniscus repair and transplantation. Meniscal fibrochondrocytes are most commonly utilized as a cell source to seed meniscal scaffolds [73–75]. One study demonstrated that these cells help to form neo menisci when grown on a biodegradable scaffold and transplanted into rabbits [76]. However, there are not many studies that investigate the regenerative effects of these cells outside of a meniscal scaffold. In a 2015 study by Yuan et al., it was demonstrated that human umbilical vein endothelial cells (HUVECs) can regulate the regional migration of bovine fibrochondrocytes in an ex-vivo explant model of a cylindrical meniscal defect at full thickness [77]. Scaffolds were not used in this study. The authors reported that endothelial factor endothelin-1 increased fibrochondrocyte cell proliferation and migration to the interface of the defect thereby enhancing reintegration of cylindrically cut tissue core with the rest of the meniscus. Although it is true that there is a greater quantity of fibrochondrocytes in the meniscus than there are MMSCs, isolation of these fibrochondrocytes requires sacrificing healthy meniscal tissue. This drastically hinders the consideration of autologous fibrochondrocytes as a viable cell source for tissue engineering and regenerative medicine. This is why a prioritized emphasis should be placed on optimizing the usage of allogeneic donor-derived fibrochondrocytes for cell-based tissue repair instead.
3.2.2. Chondrocytes –
Chondrocytes from articular cartilage, costal cartilage and auricular cartilage have been explored for use in meniscal repair [71]. Out of these three sources, autologous and allogeneic chondrocytes from articular cartilage have been the most frequently used in conjunction with scaffolds to try and repair meniscal lesions and defects [78, 79]. A recent study reported that chondrocytes can be enhanced by treatment with PRP to improve cell attachment and meniscal tissue repair in a poly-lactic-co-glycolic acid scaffold [80]. As is the case with fibrochondrocytes, not many studies have utilized chondrocytes in the absence of some sort of scaffold or mesh. An earlier study reported the successful use of autologous articular chondrocytes in conjunction with allogeneic meniscal slices to repair a porcine model of a longitudinal meniscal tear in the avascular zone [81]. In the case of articular chondrocytes, the utilization of allogeneic cells is ultimately preferable to sacrificing healthy load bearing articular cartilage in order to extract cells for this purpose. However, if this is challenging, a viable alternative is to obtain autologous articular chondrocytes from non-load bearing regions of the joint.
3.3. Finding the “ideal” cell population for meniscus repair
While it is undeniable that advances in cell-based tissue repair are being made yearly, certain obstacles firmly remain. In the case of load-bearing tissue like meniscus and cartilage, a successful healing response must be capable of overcoming compressive and shearing motion that is commonplace in the knee. The cells in question must be capable of reaching the site of injury, adhering, and proliferating all while retaining a chondrogenic (or in the case of meniscus, fibrochondrogenic) cell phenotype. In many cases, the cells must be capable of filling and bridging large gaps by producing collagen matrices. The resulting repair tissue must be strong enough to remain intact as the joint is loaded and shear forces are applied to it.
4. Biomaterials for Meniscus Tissue Engineering
Biomaterials for meniscus tissue engineering can be synthesized from natural or synthetic components as raw materials[45, 82–86]. These biomaterials aim to provide 3D support and scaffolding, in addition to providing biomechanical support for this load-bearing tissue. Cell free biomaterials have certain advantages such as ease-of-use, increased shelf life, availability and cost. Non-biodegradable biomaterials can provide extended mechanical support, which is the most important function of meniscus. Forms of the commonly used biomaterials vary. Gel/fluid-like components could be injectable and thus could be preferred for smaller defects [83, 87, 88]. Solid stiff structures can provide three-dimensional support and mechanical scaffolding to enhance the meniscal mechanical function thereby supporting either pre-seeded cells or potential cell infiltration within the joint [86]. The biomaterial development for meniscus tissue engineering have been developing in parallel with tissue engineering efforts using cells and biomaterials together. Cell-laden biomaterials have the advantage of living cells replenishing any extracellular matrix that has been lost, and filling in as the biomaterials degrade over time [89].
4.1. Acellular Biomaterials
Cell-free biomaterials often provide 3D support and could be bioactive or bioinert. There are several clinically available scaffolds that are offered for partial meniscus repair.
Currently in the United States there is only one FDA-approved cell-free scaffold. Collagen meniscus implant (CMI®) is derived from bovine type I collagen and molded in the shape of meniscus that offers restoration of tissue function. This scaffold is porous and upon implantation, cell infiltration has been reported in clinical studies [90]. However, long-term follow-up studies have demonstrated significant shrinkage of the implant, possibly due to degradation, which would lead to decreased biomechanical function [91]. Due to these limitations, CMI is unable to support the meniscus by itself when total meniscus replacement is necessary. CMI is available off the shelf as a crescent-shaped device, which requires trimming by the surgeon to fit the partial meniscal defect. This could create potential size mismatch and thus change the mechanical environment in the knee joint potentially causing damage on the adjacent articular cartilage.
Non-degradable synthetic solid polyurethane scaffolds were originally designed as total meniscus replacements, however a 2- year follow up study using dogs indicated that the polyurethane implant was not chondroprotective [82], possibly due to the lack of scaffold degradation at 24 months [92]. Vrancken et al. tested a synthetic solid, non-degradable, anatomically shaped polycarbonate urethane scaffold and compared it to the use of MAT in a biomechanical human cadaver model [93] and then a goat animal model [94]. Twelve-month follow-up after total meniscal replacement indicated that while the implant could not prevent the cartilage degeneration, the progression of damage was similar to MAT, in keeping with the results from the ex vivo human cadaver study. This example emphasizes the role of the validation of the biomechanical function in a biomechanical human cadaver model in the translation potential.
Actifit ® is an improved version of the polyurethane scaffolds that is recently developed as a biodegradable composite scaffold consisting of synthetic polymers poly-ɛ-caprolactone (PCL) (80%) and polyurethane (20%), approved for use in Europe, with clinical results similar to CMI [95]. Baynat et al. used Actifit for partial meniscus replacement in 18 patients in France [96]. Their 2-year follow-up demonstrated cell infiltration into the porous polymer by chondrocytes and fibrochondrocytes and no adverse results were reported. However, only 50% of these young and active patients were able to return to their previous activity levels [96]. Leroy et al. reported a high failure rate and shrinkage in a 5-year follow up study using the Actifit polyurethane meniscal scaffold [97]. According to a recent systematic review, both Actifit and CMI meniscal scaffolds had lower reoperation and failure rates than MAT [22]. Both Actifit and CMI are somewhat effective in short-term restoration of function; however, shrinkage and irregularity in shape and dimension were observed in both [95, 98]. While these two biomaterials are promising, the clinically available cell-free implants are still not proven to function better than partial meniscectomy through randomized trials [92].
Recently, Elsner and colleagues have developed a cell-free anisotropic synthetic biomaterial (NUSurface®), which started phase I clinical trials as an alternative to meniscal allograft transplantation for total meniscus replacements. This polycarbonate urethane (PCU) implant, reinforced with polyethylene, was first tested on sheep using an anatomically shaped version with fixation bolts on both horns [7]. Currently the human clinical trials are conducted using a disc-shaped free-floating version of the implant, which is different from the version that was tested in sheep [99]. The method of fixation is instrumental for the faith of the implant as it can change the biomechanical environment in the joint.
Silk scaffolds are natural biomaterials that were proposed for the partial meniscal regeneration [100, 101]. When silk scaffolds were cultivated in vitro with human bone marrow stem cells, they exhibited improved mechanical properties, compared to acellular silk scaffolds [102]. The Fibrofix™ Meniscus scaffold, derived from silk is currently undergoing clinical trials. It was first tested in sheep for partial meniscus replacement model. Follow up after 3 and 6 months demonstrated chondroprotective effects of the silk scaffold [101].
Three-dimensional (3D) printing is very attractive as a method to fabricate biomaterials for meniscus repair because it could be customized for each patient to combat size-matching problems. A promising 3D printed fibrous polycaprolactone (PCL) scaffold was combined with growth factor release, including growth factor-encapsulating polymer microspheres to direct cell differentiation or to induce cellular homeostasis [86]. This study employed MSCs seeded on this PCL scaffold for in vitro studies to demonstrate their differentiation into fibrochondrocytes, while the scaffold was used cell-free in the large animal study. The partial meniscus replacement in sheep using the 3D-printed PCL scaffolds demonstrated zone-specific type I and II collagen deposition [86]. This study emphasized the importance of in situ growth factor delivery systems in the development of biomaterials for meniscal tissue engineering, in an attempt to recreate the zonal variation in meniscus.
Fibrous scaffolds including PCL polymers have been electrospun to make cell-free biodegradable biomaterials for meniscus tissue engineering and have promising results [103]. Their main advantage is that they can provide the mechanical function early on, and they can degrade slowly, allowing time for the regeneration of meniscus matrix.
Kobayashi et al. have proposed a non-biodegradable poly-vinyl alcohol (PVA) hydrogel for the replacement of cartilage and meniscus, matching the mechanical properties with promising 2-year follow up results using rabbits [104]. However, a 12-month large animal study using sheep demonstrated that the cell-free non-biodegradable PVA hydrogel caused more cartilage degeneration on the tibial plateau than the meniscal allograft transplantation [105]. These studies demonstrate the importance of using clinically relevant large animal models for testing alternatives for meniscal allograft transplantation.
An alternative approach for creating a biomimetic cell-free bioscaffold for meniscus repair is the decellularization of the intact meniscus tissue. Decellularization of native tissue has the advantages of preserving the unique zonal organization of its ultrastructure and the instructive extracellular matrix components. These scaffolds would present the closest match to the MAT allografts [106]. Despite these significant advantages and the low immunogenic reaction potential, one major challenge has been to facilitate cell infiltration into these scaffolds due to the dense extracellular matrix structure, which may be alleviated by manually injecting cells into the graft [107], introducing channels [108], or using these scaffolds coupled with chemotactic agents. Decellularized scaffolds have been proposed as either xenografts with low immunogenic risks or they could be an alternative to the cryopreserved allografts [107]. Currently there is no consensus in the optimum storage protocols for meniscal allografts. This variation in allograft storage protocols from different tissue banks leads to variation in cell viability in meniscus allografts, which could possibly affect the clinical outcomes. Frozen allografts often have dead cell debris which may have adverse effects [109]. The decellularization process allows for cleanup of cellular debris, which may be beneficial for the regeneration of new tissues.
In summary, acellular biomaterials have been developed from synthetic and natural materials, with demonstrated success in partial meniscus replacement. The major function of these biomaterials have been to provide 3D biomechanical support. Non-degradable materials have caused friction in the adjacent cartilage surfaces, therefore degradable biomaterials may have more potential for meniscus repair [85]. However degradable biomaterials need a regenerative strategy to prevent shrinkage. Most promising approaches possibly use cell-laden biomaterials (described in the following section) or use these biomaterials as a way to attract host cell infiltration, to ensure regeneration of meniscal extracellular matrix. Areas of improvement are the development of biomaterials that can be used for total meniscus replacement.
4.2. Cellular biomaterials
Cell laden biomaterials for meniscus tissue engineering vary in terms of their 3D structure. Injectable biomaterials are often used with cells, because of the ease with which cells can be embedded in hydrogels. While they do not have the advantage of a robust 3D structure, ease of use make injectables an attractive potential therapy for smaller meniscal defects. Decellularized extracellular matrix powders are often used to strengthen the biological activity of these hydrogels. Human bone marrow MSC were embedded in hydrogels containing powderized bovine decellularized meniscus ECM and type I bovine collagen [50]. Injection of this MSC-laden meniscal ECM-hydrogel in the articular joint space in an athymic nude rat model after meniscal injury was suggested to prevent hypertrophy when compared with MSC-laden type I-collagen hydrogels. Powderizing decellularized meniscal ECM has provided a biochemically inductive environment for the cells to differentiate or maintain their phenotype. Another major advantage is that because the intact ECM is dense and cell infiltration is limited, the digested ECM components can be homogeneously seeded with cells prior to injection. In addition, destroying the dense ECM structure enhances the host cell infiltration into the gels [87]. However these biomaterials lack the ultrastructure and zonal organization of the collagen fibers in meniscus.
Some of the aforementioned acellular meniscus scaffolds have also been tested with cell seeding prior to implantation. Short-term studies in sheep demonstrated that autologous fibrochondrocyte cell-seeded CMI demonstrated improved healing and size retention [73]. 3D printed PCL scaffolds were seeded with allogeneic bone marrow derived stem cells and cultured for 24 h prior to total meniscus replacement in a rabbit model [110]. Another rabbit study employed allogeneic fibrochondrocytes pre-seeded on PLDLA/PCL-T (poly(L-co-D,L-lactic acid)/poly(caprolactone-triol)) polymer scaffolds, cultured for 21 days prior to implantation [75]. Both studies showed that cell seeding seemed to improve the tissue-engineered meniscus properties after 12 and 24 weeks; however, these implants were not compared to MAT. In addition, the different biomechanics of the rabbit knee warrants more studies in large animal models that are necessary for clinical translation.
Most meniscus tissue engineering efforts use a homogeneous biomaterial approach to match the biochemical and mechanical properties of meniscus. One of the rarer approaches uses self-assembled allogeneic cells derived from the respective zones of meniscus to create a zonally differentiated tissue-engineered construct [111]. A similar approach uses co-cultures with different cell combinations (articular chondrocytes, MSC, meniscal fibrochondrocytes either 100% in each zone or in co-cultures, or co-cultures of chondrocytes and either meniscus tendon or ligament cells) in the different regions of meniscus [111, 112]. The use of self assembled co-cultures of zone-specific cells can create a zonally-organized matrix; however, possible drawbacks include the need for extremely high amount of cells and subsequent use of matrix-degrading enzymes to circumvent nutrient diffusion limitations, which could make the preclinical and clinical translation harder. A recent study demonstrated that the decellularized ECM fragments obtained from the inner and outer regions of meniscus could induce fibrocartilaginous and fibroblastic phenotype in hBMSCs, respectively [113]. These two approaches demonstrate that both different cell types and different matrix materials could be used for inducing the zonal variation in tissue-engineered meniscus.
Mechanical loading is another in vitro approach for the cultivation of functional tissue-engineered menisci with zonal variation. The meniscus is subject to strain levels ranging between 5–15% under physiologically relevant loading conditions [114]. The most commonly employed loading regimens include the application of dynamic compression at 10, 17, 18 or 15% strain [115] which have been shown to induce zonal differentiation in ECM production in tissue engineered meniscal constructs [116].
Here we reported research studies employing cell-based tissue engineering methods, mainly using allogeneic cells, which may alleviate availability and morbidity problems associated with autologous cell sources. The main concern with the use of allogeneic cell is the risk of immune response by the host. Chondrocytes have been reported to generate little or no immune response in animal studies [117]. Similarly, meniscal allografts containing viable cells have been successfully implanted with no adverse immune reaction [118]. In vitro co-cultures of articular chondrocytes or meniscus cells with peripheral blood mononuclear cells showed no proliferative response in the blood cells, which would be expected in the event of immune rejection [119]. Taken together, these studies imply that meniscus and cartilage cells share immuno-privileges, which means they could be used as allogeneic cell sources that cause little or no immune response when implanted.
Regardless of cellularity, functional implants for meniscal repair require validation using large animal models. While one year is typically recommended to observe whether implants cause joint degeneration, incidences of joint degeneration have been reported in cell-free meniscus implantation as early as after 4 months in animal models [79]. Functional improvement can be detected at 6 months as reported by different research groups [7, 101, 120]. While cartilage degeneration after meniscal injury in large animal models takes months to be detectable by histology, others have observed cartilage degeneration as soon as 28 days after meniscal resection, when daily (20 min) exercise (walking and climbing one step) was incorporated after surgery in minipigs [121]. It is important to consider meniscal allograft transplantation as a control group in these animal studies to assess if the proposed tissue engineered solution can provide a functional replacement, and an alternative to the current standard of care. Ex-vivo testing of the mechanical outcome of an implant is another way to ensure success in preclinical studies. Vrancken et al. [93] used human cadaveric knees to assess the effects of implants in comparison to total meniscectomy and allograft transplantation on knee kinematics. This type of assessment may provide guidance prior to in vivo animal experiments.
5. Conclusion
Meniscal repair or replacement should be decided on a case-by-case basis. In situations where isolated cuts/tears or small defect occur, the use of cell-based repair methods are more suitable over total meniscus replacement since significant tissue loss is not a factor that needs to be addressed. However, in situations where entire portions of tissue need to be surgically removed or reshaped, meniscus replacement is a justifiable and necessary therapeutic strategy. In the past decade, strategies that implement different cell types and newly developed novel biomaterials for meniscus repair and replacement have been designed and tested in animals. Now that partial meniscus replacements are available, research efforts have focused on developing functional total meniscus replacements. Matching the mechanical properties is a priority for tissues like meniscus and cartilage. Non-degradable polymers have been proposed as a mechanical replacement, however they present long-term problems such as adjacent cartilage damage, or integration issues due to lack of cell infiltration. Other important design considerations in tissue engineered meniscus replacements include tribological properties and mimicking the cell alignment in the direction of collagen [122]. In cases where meniscal replacement is necessary, we envision a “living” replacement that is an amalgamation of biomimetic scaffold and cells to have a longer and sustainable in vivo life. The size variation in human menisci [123] is an important parameter in designing functional implants. 3D printing techniques offer size matching using imaging such as MR. Our vision includes cells that could possibly include allogeneic sources, with anatomically shaped size-matched bioactive biomaterials (Figure 4). Growth factor delivery systems ensure the meniscal cellular phenotype to be preserved. An alternative approach is to use gene therapy to stimulate growth factor synthesis pathways for meniscal repair [124]. Using supportive biomaterials with a slow degradation rate could ensure mechanical function and provide a viable option using less cells than a cell-only approach. These biocomposites need to be assessed for biomechanical properties before the preclinical animal implantation studies.
In this progress report we discussed different approaches for meniscus tissue engineering and regeneration. The major hurdles in engineering a meniscus include the inability to mimic the native meniscus structure, and hence function – due to differences in cellular phenotypes, extracellular matrix (ECM) composition and mechanical properties spanning the zonal variations in the inner and outer regions of normal menisci. The design efforts have traditionally overlooked the zonal differentiation of the meniscus replacements. For cell-based meniscus tissue engineering, zonal approaches using different biomaterials in combination with different cell types (perhaps specialized cells (from these specific regions) or different cues (biochemical or material or mechanical cues) to differentiate into zonal phenotypes in meniscus. We envision that the design of zone-specific engineered tissues will lead to functional clinical therapies.
Biography
 Bahar Bilgen, PhD, is an Assistant Professor of Orthopaedics (Research) at the Warren Alpert Medical School of Brown University. Dr. Bilgen is a bioengineer whose research focuses on the elucidating the effects of biophysical stimuli on developing tissues and modulating the in vitro environment using instructive biomaterials and bioreactors for musculoskeletal tissue engineering of cartilage and meniscus. She is currently interested in developing a 3D-printed meniscus replacement.
Bahar Bilgen, PhD, is an Assistant Professor of Orthopaedics (Research) at the Warren Alpert Medical School of Brown University. Dr. Bilgen is a bioengineer whose research focuses on the elucidating the effects of biophysical stimuli on developing tissues and modulating the in vitro environment using instructive biomaterials and bioreactors for musculoskeletal tissue engineering of cartilage and meniscus. She is currently interested in developing a 3D-printed meniscus replacement.
 Chathuraka T. Jayasuriya, PhD, is an Assistant Professor of Orthopaedics (Research) at the Warren Alpert Medical School of Brown University and Rhode Island Hospital. His laboratory focuses on the development and application of cell-based strategies for joint and musculoskeletal tissue repair and regeneration. Ongoing projects in Dr. Jayasuriya’s laboratory include optimization of meniscal tissue repair strategies using mesenchymal stem cells from distinct tissue sources in tandem with growth factors and the matrilin family of chondrogenic ECM proteins.
Chathuraka T. Jayasuriya, PhD, is an Assistant Professor of Orthopaedics (Research) at the Warren Alpert Medical School of Brown University and Rhode Island Hospital. His laboratory focuses on the development and application of cell-based strategies for joint and musculoskeletal tissue repair and regeneration. Ongoing projects in Dr. Jayasuriya’s laboratory include optimization of meniscal tissue repair strategies using mesenchymal stem cells from distinct tissue sources in tandem with growth factors and the matrilin family of chondrogenic ECM proteins.
 Brett D. Owens, MD, is a board certified orthopaedic surgeon and a Professor of Orthopaedics at the Warren Alpert Medical School of Brown University and the Chief of Sports Medicine at the Miriam Hospital. He previously served as a Lieutenant Colonel in the US Army and Chief of Orthopaedics and Sports Medicine at Keller Army Hospital, West Point, NY. He is Associate Editor for the American Journal of Sports Medicine. His clinical practice focuses on complex knee, shoulder and cartilage surgeries.
Brett D. Owens, MD, is a board certified orthopaedic surgeon and a Professor of Orthopaedics at the Warren Alpert Medical School of Brown University and the Chief of Sports Medicine at the Miriam Hospital. He previously served as a Lieutenant Colonel in the US Army and Chief of Orthopaedics and Sports Medicine at Keller Army Hospital, West Point, NY. He is Associate Editor for the American Journal of Sports Medicine. His clinical practice focuses on complex knee, shoulder and cartilage surgeries.
6. References
- [1].Walker PS, Erkman MJ, Clin Orthop Relat Res 1975, 184. [DOI] [PubMed] [Google Scholar]
- [2].Herwig J, Egner E, Buddecke E, Ann. Rheum. Dis 1984, 43, 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fithian DC, Kelly MA, Mow VC, Clin Orthop Relat Res 1990, 252, 19. [PubMed] [Google Scholar]
- [4].Bursac P, York A, Kuznia P, Brown LM, Arnoczky SP, Am. J. Sports Med. 2009, 37, 884. [DOI] [PubMed] [Google Scholar]
- [5].Arnoczky SP, Warren RF, Am. J. Sports Med. 1982, 10, 90. [DOI] [PubMed] [Google Scholar]
- [6].CDC, Vol. 2016, 2017. [Google Scholar]
- [7].Zur G, Linder-Ganz E, Elsner JJ, Shani J, Brenner O, Agar G, Hershman EB, Arnoczky SP, Guilak F, Shterling A, Knee Surg. Sports Traumatol. Arthrosc 2011, 19, 255. [DOI] [PubMed] [Google Scholar]
- [8].Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM, Arthritis Rheum 2008, 59, 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG, Arthritis Rheum 2007, 56, 1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M, Am. J. Sports Med 2016, 44, 1237. [DOI] [PubMed] [Google Scholar]
- [11].Jones JC, Burks R, Owens BD, Sturdivant RX, Svoboda SJ, Cameron KL, Journal of athletic training 2012, 47, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hutchinson ID, Moran CJ, Potter HG, Warren RF, Rodeo SA, Am. J. Sports Med. 2014, 42, 987. [DOI] [PubMed] [Google Scholar]
- [13].Rosso F, Bisicchia S, Bonasia DE, Amendola A, Am. J. Sports Med 2015, 43, 998; [DOI] [PubMed] [Google Scholar]; Noyes FR, Barber-Westin SD, Am. J. Sports Med 2016, 44, 2330. [DOI] [PubMed] [Google Scholar]
- [14].Giuliani JR, Burns TC, Svoboda SJ, Cameron KL, Owens BD, The journal of knee surgery 2011, 24, 93. [DOI] [PubMed] [Google Scholar]
- [15].Fairbank TJ, J Bone Joint Surg Br 1948, 30B, 664. [PubMed] [Google Scholar]
- [16].Burns TC, Giuliani JR, Svoboda SJ, Owens BD, The journal of knee surgery 2011, 24, 167. [DOI] [PubMed] [Google Scholar]
- [17].Grassi A, Zaffagnini S, Marcheggiani Muccioli GM, Benzi A, Marcacci M, Int. Orthop 2014, 38, 1945. [DOI] [PubMed] [Google Scholar]
- [18].Kaleka CC, Netto AS, Silva JC, Toma MK, de Paula Leite Cury R, Severino NR, Santili C, Am. J. Sports Med 2016, 44, 2876. [DOI] [PubMed] [Google Scholar]
- [19].Jeon B, Kim JM, Kim JM, Lee CR, Kim KA, Bin SI, Am. J. Sports Med 2015, 43, 1215. [DOI] [PubMed] [Google Scholar]
- [20].McDermott ID, Cell and Tissue Banking 2010, 11, 75. [DOI] [PubMed] [Google Scholar]
- [21].Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC, Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 303. [DOI] [PubMed] [Google Scholar]
- [22].Dangelmajer S, Familiari F, Simonetta R, Kaymakoglu M, Huri G, Knee surgery & related research 2017, 29, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ishida K, Kuroda R, Miwa M, Tabata Y, Hokugo A, Kawamoto T, Sasaki K, Doita M, Kurosaka M, Tissue Eng 2007, 13, 1103; [DOI] [PubMed] [Google Scholar]; Pujol N, Salle De Chou E, Boisrenoult P, Beaufils P, Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 51. [DOI] [PubMed] [Google Scholar]
- [24].Griffin JW, Hadeed MM, Werner BC, Diduch DR, Carson EW, Miller MD, Clin Orthop Relat Res 2015, 473, 1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zellner J, Mueller M, Berner A, Dienstknecht T, Kujat R, Nerlich M, Hennemann B, Koller M, Prantl L, Angele M, Angele P, Journal of biomedical materials research. Part A 2010, 94, 1150. [DOI] [PubMed] [Google Scholar]
- [26].Mizuno K, Muneta T, Morito T, Ichinose S, Koga H, Nimura A, Mochizuki T, Sekiya I, J. Med. Dent. Sci 2008, 55, 101. [PubMed] [Google Scholar]
- [27].Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K, Knee Surg. Sports Traumatol. Arthrosc 2006, 14, 1307. [DOI] [PubMed] [Google Scholar]
- [28].Qi Y, Yang Z, Ding Q, Zhao T, Huang Z, Feng G, Experimental and therapeutic medicine 2016, 11, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O, Exp. Hematol 2003, 31, 890. [DOI] [PubMed] [Google Scholar]
- [30].Alini M, Kofsky Y, Wu W, Pidoux I, Poole AR, J Bone Miner Res 1996, 11, 105. [DOI] [PubMed] [Google Scholar]
- [31].Nauta AJ, Fibbe WE, Blood 2007, 110, 3499. [DOI] [PubMed] [Google Scholar]
- [32].Kolf CM, Cho E, Tuan RS, Arthritis research & therapy 2007, 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP, Transplantation 1968, 6, 230. [PubMed] [Google Scholar]
- [34].Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW, Cell Sci J. 2004, 117, 889. [DOI] [PubMed] [Google Scholar]
- [35].Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I, Cell Tissue Res 2007, 327, 449. [DOI] [PubMed] [Google Scholar]
- [36].Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF, Nat. Med 2007, 13, 1219. [DOI] [PubMed] [Google Scholar]
- [37].Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S, Lancet 2004, 364, 149. [DOI] [PubMed] [Google Scholar]
- [38].Koh YG, Choi YJ, The Knee 2012, 19, 902. [DOI] [PubMed] [Google Scholar]
- [39].Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, Ezura Y, Umezawa A, Sekiya I, J Orthop Res 2009, 27, 435. [DOI] [PubMed] [Google Scholar]
- [40].Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, Ferrero-Gutierrez A, Fernandez-Rodriguez MA, Gala J, Otero-Hernandez J, Transplant. Proc 2013, 45, 434. [DOI] [PubMed] [Google Scholar]
- [41].Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E, Cytotherapy 2006, 8, 315; [DOI] [PubMed] [Google Scholar]; Hass R, Kasper C, Bohm S, Jacobs R, Cell communication and signaling : CCS 2011, 9, 12; De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH, Cells Tissues Organs 2003, 174, 101,; Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR, Science 1999, 284, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Juneja SC, Viswanathan S, Ganguly M, Veillette C, Bone marrow research 2016, 2016, 3152065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hong JH, Park JI, Kim KH, Kim YM, Joo YB, Jeon YS, Knee surgery & related research 2011, 23, 164; Zhang H, Leng P, Zhang J, Clin Orthop Relat Res 2009, 467, 3165,; Al Faqeh H, Nor Hamdan BM, Chen HC, Aminuddin BS, Ruszymah BH, Exp. Gerontol 2012, 47, 458,; Caminal M, Fonseca C, Peris D, Moll X, Rabanal RM, Barrachina J, Codina D, Garcia F, Cairo JJ, Godia F, Pla A, Vives J, New biotechnology 2014, 31, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Horie M, Choi H, Lee RH, Reger RL, Ylostalo J, Muneta T, Sekiya I, Prockop DJ, Osteoarthritis Cartilage 2012, 20, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Walsh CJ, Goodman D, Caplan AI, Goldberg VM, Tissue Eng 1999, 5, 327. [DOI] [PubMed] [Google Scholar]
- [46].Somoza RA, Welter JF, Correa D, Caplan AI, Tissue engineering. Part B, Reviews 2014, 20, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van Arenthals J, Dekker N, Hanselaar T, Exalto N, Eur. J. Obstet. Gynecol. Reprod. Biol 1988, 28, 79; Chowdhury A, Bezuidenhout LW, Mulet-Sierra A, Jomha NM, Adesida AB, BMC musculoskeletal disorders 2013, 14, 216. [DOI] [PubMed] [Google Scholar]
- [48].Saliken DJ, Mulet-Sierra A, Jomha NM, Adesida AB, Arthritis research & therapy 2012, 14, R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cui X, Hasegawa A, Lotz M, D’Lima D, Biotechnol Bioeng 2012, 109, 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yuan X, Wei Y, Villasante A, Ng JJD, Arkonac DE, Chao PG, Vunjak-Novakovic G, Biomaterials 2017, 132, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Horie M, Sekiya I, Muneta T, Ichinose S, Matsumoto K, Saito H, Murakami T, Kobayashi E, Stem Cells 2009, 27, 878. [DOI] [PubMed] [Google Scholar]
- [52].Del Rey MJ, Fare R, Usategui A, Canete JD, Bravo B, Galindo M, Criado G, Pablos JL, Arthritis research & therapy 2016, 18, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jones E, English A, Churchman SM, Kouroupis D, Boxall SA, Kinsey S, Giannoudis PG, Emery P, McGonagle D, Arthritis Rheum 2010, 62, 1944. [DOI] [PubMed] [Google Scholar]
- [54].Nakagawa Y, Muneta T, Kondo S, Mizuno M, Takakuda K, Ichinose S, Tabuchi T, Koga H, Tsuji K, Sekiya I, Osteoarthritis Cartilage 2015, 23, 1007; Hatsushika D, Muneta T, Nakamura T, Horie M, Koga H, Nakagawa Y, Tsuji K, Hishikawa S, Kobayashi E, Sekiya I, Osteoarthritis Cartilage 2014, 22, 941. [DOI] [PubMed] [Google Scholar]
- [55].Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F, Proc. Natl. Acad. Sci. U. S. A 2012, 109, 19172; Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F, Tissue Eng Part A 2010, 16, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ruiz-Iban MA, Diaz-Heredia J, Garcia-Gomez I, Gonzalez-Lizan F, Elias-Martin E, Abraira V, Arthroscopy 2011, 27, 1688. [DOI] [PubMed] [Google Scholar]
- [57].Gui J, Zhang J, Huang H, Current stem cell research & therapy 2015, 10, 353. [PubMed] [Google Scholar]
- [58].Shen W, Chen J, Zhu T, Chen L, Zhang W, Fang Z, Heng BC, Yin Z, Chen X, Ji J, Chen W, Ouyang HW, Stem cells translational medicine 2014, 3, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Shen W, Chen J, Zhu T, Yin Z, Chen X, Chen L, Fang Z, Heng BC, Ji J, Chen W, Ouyang HW, Stem Cells Dev 2013, 22, 2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, Singhrao SK, Dowthwaite GP, Jones RE, Baird DM, Lewis H, Roberts S, Shaw HM, Dudhia J, Fairclough J, Briggs T, Archer CW, PloS one 2010, 5, e13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].deShazo RD, Mather P, Grant W, Carrington D, Frentz JM, Lueg M, Lauritano AA, Falholt K, Diabetes Care 1987, 10, 330. [DOI] [PubMed] [Google Scholar]
- [62].Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, Walter MW, Lehman AD, Ding L, Buckwalter JA, Martin JA, Arthritis Rheum 2012, 64, 3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jayasuriya CT, Owens B, Feltman P, Franco J, Twomey-Kozak J, Newberry J, Hansen H, Li N, Terek R, Chen Q, Ehrlich MG, “Cartilage Progenitor Cells Successfully Bridge Meniscal Tear and Resist Cellular Hypertrophy”, presented at Orthopaedic Research Society Annual Meeting, New Orleans, LA, March, 2018, 2018. [Google Scholar]
- [64].Hattori S, Oxford C, Reddi AH, Biochem Biophys Res Commun 2007, 358, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fellows CR, Williams R, Davies IR, Gohil K, Baird DM, Fairclough J, Rooney P, Archer CW, Khan IM, Scientific reports 2017, 7, 41421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].D’Angelo M, Pacifici M, J Bone Miner Res 1997, 12, 1368. [DOI] [PubMed] [Google Scholar]
- [67].Fickert S, Fiedler J, Brenner RE, Arthritis research & therapy 2004, 6, R422; Su X, Zuo W, Wu Z, Chen J, Wu N, Ma P, Xia Z, Jiang C, Ye Z, Liu S, Liu J, Zhou G, Wan C, Qiu G, J Orthop Res 2015, 33, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gu Y, Zhu W, Hao Y, Lu L, Chen Y, Wang Y, Experimental and therapeutic medicine 2012, 3, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhu WH, Wang YB, Wang L, Qiu GF, Lu LY, Molecular medicine reports 2014, 9, 1767. [DOI] [PubMed] [Google Scholar]
- [70].Osawa A, Harner CD, Gharaibeh B, Matsumoto T, Mifune Y, Kopf S, Ingham SJ, Schreiber V, Usas A, Huard J, Med. Sci. Sports Exerc 2013, 45, 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Weinand C, Peretti GM, Adams SB Jr., Randolph MA, Savvidis E, Gill TJ, Arch. Orthop. Trauma Surg 2006, 126, 599. [DOI] [PubMed] [Google Scholar]
- [72].Schwartz JA, Wang W, Goldstein T, Grande DA, Cartilage 2014, 5, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Martinek V, Ueblacker P, Braun K, Nitschke S, Mannhardt R, Specht K, Gansbacher B, Imhoff AB, Arch. Orthop. Trauma Surg 2006, 126, 228. [DOI] [PubMed] [Google Scholar]
- [74].Schoenfeld AJ, Jacquet R, Lowder E, Doherty A, Leeson MC, Landis WJ, Connect. Tissue Res 2009, 50, 307. [PubMed] [Google Scholar]
- [75].Esposito AR, Moda M, Cattani SM, de Santana GM, Barbieri JA, Munhoz MM, Cardoso TP, Barbo ML, Russo T, D’Amora U, Gloria A, Ambrosio L, Duek EA, BioResearch open access 2013, 2, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kang SW, Son SM, Lee JS, Lee ES, Lee KY, Park SG, Park JH, Kim BS, Journal of biomedical materials research. Part A 2006, 78, 659. [DOI] [PubMed] [Google Scholar]
- [77].Yuan X, Eng GM, Arkonac DE, Chao PH, Vunjak-Novakovic G, Arthritis & rheumatology (Hoboken, N.J.) 2015, 67, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Weinand C, Peretti GM, Adams SB Jr., Bonassar LJ, Randolph MA, Gill TJ, Am. J. Sports Med 2006, 34, 1779; Julke H, Mainil-Varlet P, Jakob RP, Brehm W, Schafer B, Nesic D, Cartilage 2015, 6, 20. [DOI] [PubMed] [Google Scholar]
- [79].Kon E, Chiari C, Marcacci M, Delcogliano M, Salter DM, Martin I, Ambrosio L, Fini M, Tschon M, Tognana E, Plasenzotti R, Nehrer S, Tissue Eng Part A 2008, 14, 1067. [DOI] [PubMed] [Google Scholar]
- [80].Kwak HS, Nam J, Lee JH, Kim HJ, Yoo JJ, J Tissue Eng Regen Med 2017, 11, 471. [DOI] [PubMed] [Google Scholar]
- [81].Peretti GM, Gill TJ, Xu JW, Randolph MA, Morse KR, Zaleske DJ, Am. J. Sports Med 2004, 32, 146. [DOI] [PubMed] [Google Scholar]
- [82].Welsing RT, van Tienen TG, Ramrattan N, Heijkants R, Schouten AJ, Veth RP, Buma P, Am. J. Sports Med 2008, 36, 1978. [DOI] [PubMed] [Google Scholar]
- [83].Rothrauff BB, Shimomura K, Gottardi R, Alexander PG, Tuan RS, Acta biomaterialia 2017, 49, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rey-Rico A, Cucchiarini M, Madry H, Connect. Tissue Res 2017, 58, 317. [DOI] [PubMed] [Google Scholar]
- [85].Shin YS, Lee HN, Sim HB, Kim HJ, Lee DH, Knee Surg. Sports Traumatol. Arthrosc 2017. [DOI] [PubMed] [Google Scholar]
- [86].Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ, Science translational medicine 2014, 6, 266ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wu J, Ding Q, Dutta A, Wang Y, Huang YH, Weng H, Tang L, Hong Y, Acta biomaterialia 2015, 16, 49. [DOI] [PubMed] [Google Scholar]
- [88].Monibi FA, Bozynski CC, Kuroki K, Stoker AM, Pfeiffer FM, Sherman SL, Cook JL, Tissue engineering. Part C, Methods 2016, 22, 1059. [DOI] [PubMed] [Google Scholar]
- [89].Angele P, Johnstone B, Kujat R, Zellner J, Nerlich M, Goldberg V, Yoo J, Journal of biomedical materials research. Part A 2008, 85, 445. [DOI] [PubMed] [Google Scholar]
- [90].Hansen R, Choi G, Bryk E, Vigorita V, Long J. Term Eff. Med. Implants 2011, 21, 321. [DOI] [PubMed] [Google Scholar]
- [91].Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, Bruni D, Giordano G, Ravazzolo G, Molinari M, Marcacci M, Am. J. Sports Med 2011, 39, 977; [DOI] [PubMed] [Google Scholar]; Zaffagnini S, Marcheggiani Muccioli GM, Bulgheroni P, Bulgheroni E, Grassi A, Bonanzinga T, Kon E, Filardo G, Busacca M, Marcacci M, Am. J. Sports Med 2012, 40, 2281. [DOI] [PubMed] [Google Scholar]
- [92].Vrancken AC, Buma P, van Tienen TG, Int. Orthop 2013, 37, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Vrancken AC, Eggermont F, van Tienen TG, Hannink G, Buma P, Janssen D, Verdonschot N, Knee Surg. Sports Traumatol. Arthrosc 2016, 24, 1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Vrancken AC, Madej W, Hannink G, Verdonschot N, van Tienen TG, Buma P, PloS one 2015, 10, e0133138; [DOI] [PMC free article] [PubMed] [Google Scholar]; Vrancken ACT, Hannink G, Madej W, Verdonschot N, van Tienen TG, Buma P, Vrancken AC, Madej W, Hannink G, Verdonschot N, van Tienen TG, Buma P, Vrancken AC, Eggermont F, van Tienen TG, Hannink G, Buma P, Janssen D, Verdonschot N, Vrancken AC, Crijns SP, Ploegmakers MJ, O’Kane C, van Tienen TG, Janssen D, Buma P, Verdonschot N, Vrancken AC, Buma P, van Tienen TG, Am. J. Sports Med 2017, 45, 2824. [DOI] [PubMed] [Google Scholar]
- [95].Bulgheroni E, Grassi A, Campagnolo M, Bulgheroni P, Mudhigere A, Gobbi A, Cartilage 2016, 7, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Baynat C, Andro C, Vincent JP, Schiele P, Buisson P, Dubrana F, Gunepin FX, Orthopaedics & traumatology, surgery & research : OTSR 2014, 100, S385. [DOI] [PubMed] [Google Scholar]
- [97].Leroy A, Beaufils P, Faivre B, Steltzlen C, Boisrenoult P, Pujol N, Orthopaedics & traumatology, surgery & research : OTSR 2017, 103, 609. [DOI] [PubMed] [Google Scholar]
- [98].Lin DD, Picardo NE, Adesida A, Khan WS, Current stem cell research & therapy 2016. [DOI] [PubMed] [Google Scholar]
- [99].Elsner JJ, Portnoy S, Zur G, Guilak F, Shterling A, Linder-Ganz E, J Biomech Eng 2010, 132, 095001. [DOI] [PubMed] [Google Scholar]
- [100].Mandal BB, Park SH, Gil ES, Kaplan DL, Biomaterials 2011, 32, 639; [DOI] [PMC free article] [PubMed] [Google Scholar]; Yan LP, Oliveira JM, Oliveira AL, Caridade SG, Mano JF, Reis RL, Acta biomaterialia 2012, 8, 289. [DOI] [PubMed] [Google Scholar]
- [101].Gruchenberg K, Ignatius A, Friemert B, von Lubken F, Skaer N, Gellynck K, Kessler O, Durselen L, Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mandal BB, Park SH, Gil ES, Kaplan DL, Tissue Eng Part A 2011, 17, 2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Fisher MB, Henning EA, Soegaard N, Bostrom M, Esterhai JL, Mauck RL, Biomech J. 2015, 48, 1412; [DOI] [PMC free article] [PubMed] [Google Scholar]; Martin JT, Milby AH, Ikuta K, Poudel S, Pfeifer CG, Elliott DM, Smith HE, Mauck RL, Acta biomaterialia 2015, 26, 97; [DOI] [PMC free article] [PubMed] [Google Scholar]; Shimomura K, Bean AC, Lin H, Nakamura N, Tuan RS, Tissue Eng Part A 2015, 21, 2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kobayashi M, Chang YS, Oka M, Biomaterials 2005, 26, 3243; [DOI] [PubMed] [Google Scholar]; Kobayashi M, Toguchida J, Oka M, The Knee 2003, 10, 53. [DOI] [PubMed] [Google Scholar]
- [105].Kelly BT, Robertson W, Potter HG, Deng XH, Turner AS, Lyman S, Warren RF, Rodeo SA, Am. J. Sports Med 2007, 35, 43. [DOI] [PubMed] [Google Scholar]
- [106].Stapleton TW, Ingram J, Fisher J, Ingham E, Tissue Eng Part A 2011, 17, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Maier D, Braeun K, Steinhauser E, Ueblacker P, Oberst M, Kreuz PC, Roos N, Martinek V, Imhoff AB, J Orthop Res 2007, 25, 1598. [DOI] [PubMed] [Google Scholar]
- [108].Bautista CA, Park HJ, Mazur CM, Aaron RK, Bilgen B, PloS one 2016, 11, e0158976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Londono R, Dziki JL, Haljasmaa E, Turner NJ, Leifer CA, Badylak SF, Journal of biomedical materials research. Part A 2017, 105, 2109. [DOI] [PubMed] [Google Scholar]
- [110].Zhang ZZ, Wang SJ, Zhang JY, Jiang WB, Huang AB, Qi YS, Ding JX, Chen XS, Jiang D, Yu JK, Am. J. Sports Med 2017, 45, 1497. [DOI] [PubMed] [Google Scholar]
- [111].Higashioka MM, Chen JA, Hu JC, Athanasiou KA, Tissue Eng Part A 2014, 20, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Huey DJ, Athanasiou KA, Biomaterials 2011, 32, 2052; [DOI] [PMC free article] [PubMed] [Google Scholar]; Huey DJ, Athanasiou KA, PloS one 2011, 6, e27857 Hadidi P, Paschos NK, Huang BJ, Aryaei A, Hu JC, Athanasiou KA, Osteoarthritis Cartilage 2016, 24, 2126.22114714 [Google Scholar]
- [113].Shimomura K, Rothrauff BB, Tuan RS, Am. J. Sports Med 2017, 45, 604. [DOI] [PubMed] [Google Scholar]
- [114].Freutel M, Seitz AM, Galbusera F, Bornstedt A, Rasche V, Knothe Tate ML, Ignatius A, Durselen L, J. Magn. Reson. Imaging 2014, 40, 1181. [DOI] [PubMed] [Google Scholar]
- [115].Ballyns JJ, Bonassar LJ, Biomech J. 2011, 44, 509. [DOI] [PubMed] [Google Scholar]
- [116].Puetzer JL, Bonassar LJ, Tissue Eng Part A 2016, 22, 907. [DOI] [PubMed] [Google Scholar]
- [117].Rahfoth B, Weisser J, Sternkopf F, Aigner T, von der Mark K, Brauer R, Osteoarthritis Cartilage 1998, 6, 50; [DOI] [PubMed] [Google Scholar]; Wakitani S, Kimura T, Hirooka A, Ochi T, Yoneda M, Yasui N, Owaki H, Ono K, J Bone Joint Surg Br 1989, 71, 74. [DOI] [PubMed] [Google Scholar]
- [118].Lee BS, Bin SI, Kim JM, Kim WK, Choi JW, Am. J. Sports Med. 2017, 45, 1095. [DOI] [PubMed] [Google Scholar]
- [119].Huey DJ, Sanchez-Adams J, Willard VP, Athanasiou KA, Tissue Eng Part A 2012, 18, 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Moriguchi Y, Tateishi K, Ando W, Shimomura K, Yonetani Y, Tanaka Y, Kita K, Hart DA, Gobbi A, Shino K, Yoshikawa H, Nakamura N, Biomaterials 2013, 34, 2185. [DOI] [PubMed] [Google Scholar]
- [121].Cruz R, Ramirez C, Rojas OI, Casas-Mejia O, Kouri JB, Vega-Lopez MA, Pathol. Res. Pract 2015, 211, 829. [DOI] [PubMed] [Google Scholar]
- [122].Warnecke D, Schild NB, Klose S, Joos H, Brenner RE, Kessler O, Skaer N, Walker R, Freutel M, Ignatius A, Durselen L, Tribology international 2017, 109, 586; [DOI] [PMC free article] [PubMed] [Google Scholar]; Nectow AR, Kilmer ME, Kaplan DL, Journal of biomedical materials research. Part A 2014, 102, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Vrancken AC, Crijns SP, Ploegmakers MJ, O’Kane C, van Tienen TG, Janssen D, Buma P, Verdonschot N, J Anat 2014, 225, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Cucchiarini M, McNulty AL, Mauck RL, Setton LA, Guilak F, Madry H, Osteoarthritis Cartilage 2016, 24, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zellner J, Pattappa G, Koch M, Lang S, Weber J, Pfeifer CG, Mueller MB, Kujat R, Nerlich M, Angele P, Stem cell research & therapy 2017, 8, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]