Abstract
Globin gene regulation occurs in the context of a maturing erythroid cell, which is undergoing significant changes in chromatin structure and gene expression. There are few model systems available that facilitate studies of globin gene regulation in the context of erythroid maturation. Extensively self-renewing erythroblasts (ESREs) are a nontransformed model of erythroid maturation derived from murine fetal liver or yolk sac. Imaging flow cytometry and RNA-seq studies demonstrate that ESREs functionally and molecularly model erythroid maturation. To address the need for a model system that also recapitulates human globin switching, ESREs were derived from mice transgenic for the complete human β-globin locus (β-yac ESREs). β-yac ESREs express β-globin from the transgenic human locus, with minimal γ-globin expression. When treated with hydroxyurea or inhibitors to histone deacetylases, DNA methyltransferases, or the histone demethylase lysine specific demethylase 1 (LSD1), β-Yac ESREs significantly increase their γ-globin expression, demonstrating their utility for studying agents that influence maturational globin switching. β-yac ESREs were further used to characterize the secondary effects of LSD1 inhibition on erythroid maturation, with inhibition of LSD1 resulting in altered cell and nuclear size, prolonged Kit expression, and decreased rates of enucleation consistent with impaired maturation. Taken together, these studies demonstrate that β-yac ESREs have significant utility for identifying modulators of maturational globin switching as well as for studying the broader role of those modulators in erythroid maturation.
The switch from γ-globin to β-globin has been intensely studied, with the hope of identifying novel therapies for sickle cell disease and β-thalassemia. Several chromatin modifiers that influence the γ- to β-globin switch have been identified, including histone deacetylases (HDACs) [1–3], DNA methyltransferases (DNMTs) [4,5], and the histone demethylase lysine specific demethylase 1 (LSD1) [6,7]. Compounds that inhibit these chromatin modifiers represent potential therapies for the β-globinopathies [5,6,8–10], however their effect in erythroid cells is not limited to globin switching. Globin gene regulation occurs in the context of a maturing erythroid cell, which is undergoing significant changes in gene expression and chromatin structure. Chromatin modifiers such as lysine specific demethylase 1 (LSD1) play a critical role in this process [11,12], and inhibition of these chromatin modifiers can affect erythroid maturation as well as globin gene expression [13]. Model systems that facilitate study of globin gene regulation in the context of a maturing erythroid cell are limited, since transformed cell lines, such as murine erythroleukemia (MEL) or K562, fail to accurately recapitulate erythroid maturation or globin switching [14] and have significant copy number and karyotypic abnormalities.
Extensively self-renewing erythroblasts (ESREs) provide a unique model system for studying the role of chromatin modifiers, such as LSD1, in erythroid maturation. Derived from murine yolk sac or fetal liver, ESREs are non-transformed cells that extensively proliferate the proerythroblast phase while retaining the ability to appropriately mature and enucleate in approximately 3–4 cell divisions [15]. They are synchronous and abundant, making them well suited for functional and genomic studies of erythroid maturation. Genomic and functional studies in this report demonstrate that ESREs have changes in morphology, cell surface marker, and RNA expression that are consistent with terminal erythroid maturation. In contrast to the widely used two-phase CD34+ culture system [16], ESREs proliferate as proerythroblasts, facilitating studies of the later stages of erythroid maturation, and do not require access to scarce human CD34+ hematopoietic stem and progenitor cells. Extensively self-renewing erythroblasts are excellent models of terminal erythroid maturation, but, because they are murine cells, they are limited in their utility for studies of γ-globin expression.
To create a model system that could be used to study both erythroid maturation and globin gene expression, ESREs were derived from transgenic mice with the complete human beta globin locus (β-yac mice), a model system that has been widely used to study the γ- to β-globin switch in vivo [17,18]. Extensively self-renewing erythroblasts derived from β-yac mice (β-yac ESREs) express significant amounts of human β-globin and little γ-globin from the transgenic locus. In contrast to other model systems that utilize a transgenic β-globin locus [19,20], treatment of β-yac ESREs with inhibitors to LSD1, HDACs, DNMTs, or hydroxyurea results in a significant increase in γ-globin expression, demonstrating that these cells can be used to study modulators of globin gene expression. Because the LSD1 inhibitor tranylcypromine (TCP) is a potential therapy for the β-globinopathies [6], β-yac ESREs were further used to study the effect of TCP on erythroid maturation. Consistent with previous studies [13], cells treated with TCP had alterations in multiple facets of erythroid maturation, including cell and nuclear size, cell surface marker expression, benzidine accumulation and enucleation. Taken together, these studies demonstrate that β-yac ESREs have significant utility for functional and molecular studies of both erythroid maturation and globin gene regulation.
Methods
Mice and tissues
The University of Rochester’s Committee on Animal Resources approved all experiments utilizing mice. Male mice hemizygous for four copies of the complete human β-globin locus (β-yac mice) were mated overnight with outbred ICR (Jackson Laboratories, Bar Harbor, Maine, USA) or C57BL/6 (Jackson Laboratories) mice, and vaginal plugs were checked after 12 hours (embryonic day 0.5). At various gestational ages, the mice were killed by CO2 narcosis, and the embryos harvested by dissection in PB2 (Dulbecco phosphate-buffered saline [Invitrogen, Grand Island, NY, USA] supplemented with 0.1% glucose [Invitrogen] and 0.3% bovine serum albumin [Fisher Scientific, Waltham, MA, USA]). Genotyping of resulting embryos was done using quantitative polymerase chain reaction. Primers are listed in Supplementary Table E1 (online only, available at www.exphem.org).
Erythroid expansion culture
Extensively self-renewing erythroblasts were derived as previously described [15]. They were maintained at a concentration of 1 × 106/mL in expansion media composed of StemSpan SFEM media (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with human recombinant erythropoietin 2U/mL, Stem Cell Factor 100 ng/mL (Pepro-Tech, Rocky Hill, NJ, USA), dexamethasone 10−6 mol/L (Sigma, St. Louis, MO, USA), insulin-like growth factor 1 40 ng/mL (Pepro Tech), penicillin/streptomycin (Invitrogen), and cholesterol mix to a final concentration of 0.4% (Sigma). Partial medium changes were done daily.
Erythroid cell maturation
Maturation of ESREs was initiated by placing them in dexamethasone-free medium, as previously described [15]. Briefly, the cells were washed in PB2 and suspended in maturation media, composed of Iscove’s modified Dulbecco’s medium (IMDM; Gibco, Grand Island, NY, USA), supplemented by 2U/mL human recombinant erythropoietin, 100 ng/mL stem cell factor (SCF), 10% serum replacement (Invitrogen), 5% plasma derived serum (PDS; Animal Technologies, Tyler, TX, USA), and glutamine.
Antibodies and inhibitors
Inhibitors utilized for studies of globin expression and erythroid maturation were 1 μmol/L TCP (Sigma), 0.75 μmol/L sodium butyrate (Sigma), 50 μmol/L hydroxyurea (HU) (Sigma) and 0.1 μmol/L decitabine (Selleck Chemicals, Houston, TX, USA). Antibodies used included CD71-FITC (eBioscience, San Diego, CA, USA), and Kit-FITC (eBioscience). Fetal globin detection was done using the Invitrogen Fetal Hemoglobin Test Kit (Invitrogen), according to the manufacturer’s instructions. An APC conjugated fetal hemoglobin antibody (Invitrogen) was substituted for the FITC-conjugated antibody provided in the kit to overcome the autofluorescence inherent in cultured cells.
Analysis of gene expression using quantitative reverse transcription PCR
RNA was isolated using Trizol, and cDNA was generated using the SuperScript III First Strand kit (Invitrogen). Quantitative reverse transcription polymerase chain reaction (qPCR) was used to assess the expression of the murine embryonic (βH1) and adult (β1 and β2) globins, as well as the human fetal (γ), and adult (β) globins. Primers are listed in Supplementary Table E1 (online only, available at www.exphem.org). Data were normalized to 18s expression, and p values were calculated using Student’s t test.
RNA-seq and bioinformatic analyses
RNA was isolated from self-renewing ESREs and ESREs following day 1 of maturation using RNAeasy kit (QIAGEN, Hilden, Germany) and subjected to polyA selection. Biological replicates were investigated at each time point. After fragmentation and reverse transcription, second-strand synthesis was used to create double-stranded cDNA fragments. The cDNA fragments were end repaired and Illumina-sequencing adaptors attached, followed by limited PCR amplification. After gel-based size selection, the resulting DNA was subjected to 65 bp of single-end sequencing. Image analysis and base-calling were done using Illumina Genome Analyzer Pipeline software (Illumina, San Diego, CA, USA). Sequence reads were aligned to the mouse reference genome (UCSC assembly mm9, NCBI build 37) using SHRiMP2 [21]. Differential expression was assessed using the Cuffdiff suite of software [22]. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO) [23] and are accessible through GEO Series accession number GSE54826 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE54826).
Imaging flow cytometry
Extensively self-renewing erythroblasts or β-yac ESREs were stained with CD71-FITC (eBioscience), c-Kit-PE (eBioscience), DAPI (Sigma-Aldrich, St Louis, MO, USA), and DRAQ5 (eBioscience) and run on the ImageStream (Amnis, Seattle, WA, USA). The data was analyzed with IDEAS software (Amnis) as previously published [24].
Results
Extensively self-renewing erythroblasts derived from transgenic mice with the human β-globin locus model terminal erythroid maturation and express β-globin from the transgenic locus
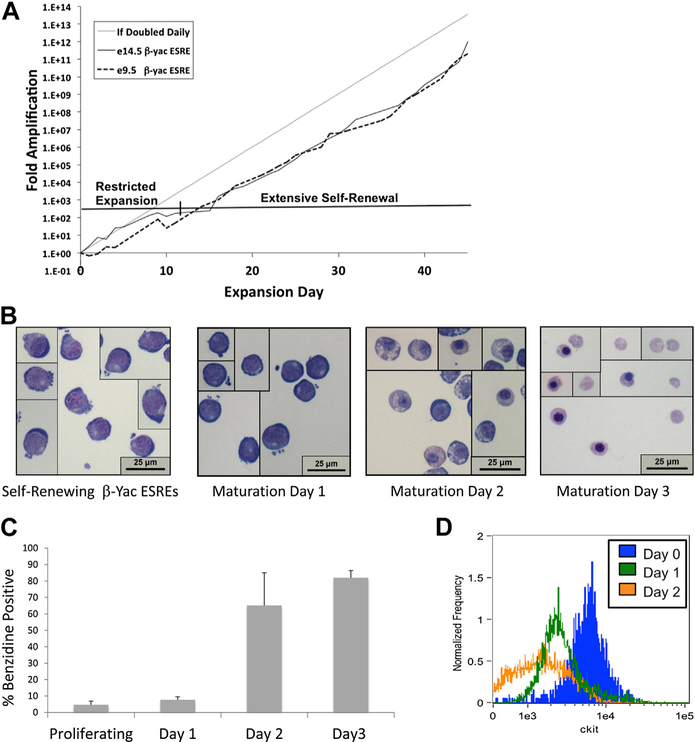
Extensively self-renewing erythroblasts were derived from mice transgenic for the complete human β-globin locus (β-yac ESREs) by culturing yolk sac or fetal liver in the presence of erythropoietin (2 U/mL), SCF (100 ng/mL), insulin-like growth factor 1 (IGF1; 40 ng/mL), and dexamethasone (10−6 mol/L) [15]. Consistent with previous studies [15], the cells initially underwent a 7–10 day period of restricted proliferation before the establishment of extensive ex vivo expansion (Fig. 1A). Once extensive ex vivo expansion was established, the β-yac ESREs doubled approximately once daily and had morphologic characteristics of proerythroblasts (Fig. 1B). The cultures were routinely maintained over 50 days.
Figure 1.
β-yac ESREs model terminal erythroid maturation. (A) Similar to nontransgenic ESREs, β-yac ESREs derived from both e9.5 yolk sac and e14.5 fetal liver demonstrate a period of restricted proliferation before the establishment of extensive self-renewal. (B) Photomicrographs of self-renewing and maturing β-ESREs. Self-renewing β-yac ESREs (left panel) resemble proerythroblasts. Middle panels are representative images of β-yac ESRE cultures during the first 2 days of maturation. After 3 days in maturation media (right panel), the culture is composed primarily of late-stage erythroid precursors and enucleated erythrocytes. (C) Benzidine staining of β-yac ESREs. After 3 days, the majority of the culture is benzidine positive. (D) Imaging flow cytometry demonstrates that maturation of β-yac ESREs is associated with loss of Kit expression. (E) Imaging flow cytometry demonstrates that maturation of β-yac ESREs is associated with a progressive decrease in cell and nuclear size. Upper panels are representative histograms. Lower panels represent mean and SEM of three independent experiments. (F) Percent of enucleated cells, determined by comparing DRAQ intensity to cell area. After 2 days of maturation, approximately 10% of cells are enucleated. After 3 days of maturation, approximately 40% of cells are enucleated. Data are mean and SEM of three independent experiments. Images of nucleated (center panel) and enucleated (right panel) cells from imaging flow cytometer are shown. Ter119 staining is yellow and DRAQ5 staining is red. BF = Bright Field; e = embryonic day.
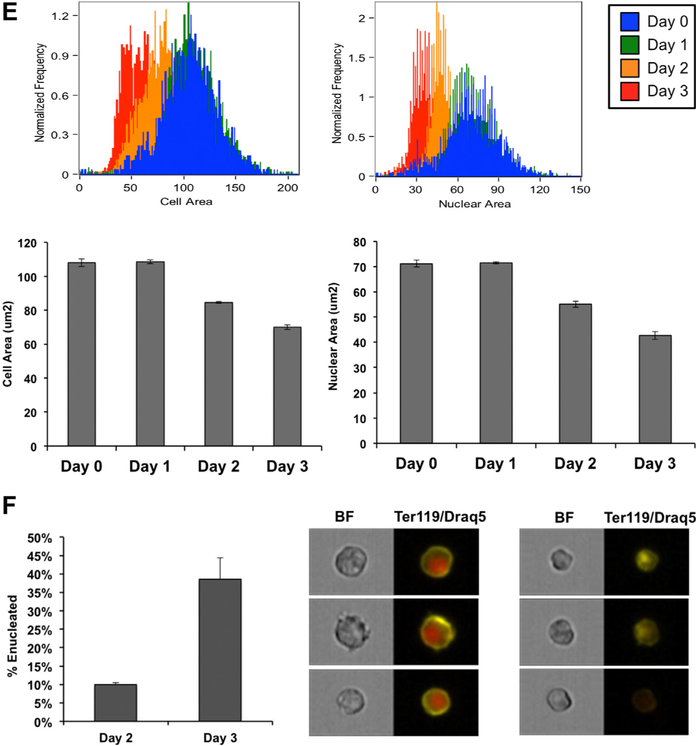
Erythroid maturation is characterized by a progressive decrease in cell and nuclear size, which is accompanied by changes in cell surface markers and ultimately followed by enucleation [25]. Despite their prolonged period of ex vivo self-renewal, β-yac ESREs maintained the ability to appropriately mature and enucleate as a semisynchronous cohort. After 3 days in maturation media, the culture was composed primarily of late stage erythroid precursors, enucleated reticulocytes, and pyrenocytes (Fig. 1B), and the majority of cells were benzidine positive (Fig. 1C). Imaging flow cytometry was used to further detail the phenotypic changes that occur during β-yac ESRE maturation. Before placement in maturation media, β-yac ESREs had a large nuclear-to-cytoplasmic ratio, as well as a Kit-high phenotype. During maturation, Kit expression was downregulated (Fig. 1D), and the cell and nuclear size became progressively smaller (Fig. 1E). β-yac ESREs enucleate in a semisynchronous manner, with 10% of cells enucleated on maturation day 2 and 40% of cells enucleated on maturation day 3 (Fig. 1F).
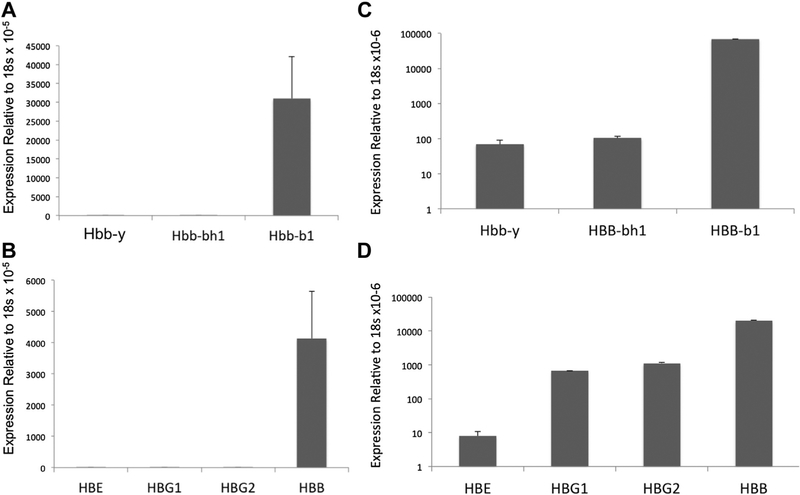
The globin expression profile of β-yac ESREs was assessed using qPCR. Similar to nontransgenic ESREs [15], β-yac ESREs express β major from the endogenous murine globin locus, with little βH1 expression (Fig. 2A). Importantly, β-yac ESREs also have an adult pattern of globin expression from the transgenic locus, expressing primarily β-globin (Fig. 2B). In contrast, in unsorted, uncultured β-yac fetal liver, both the murine and human embryonic and adult globins were detected (Fig. 2C and D). The pattern of globin expression did not vary between fetal liver–and yolk sac–derived β-yac ESREs (Supplementary Figure E1, online only, available at www.exphem.org).
Figure 2.
Quantitative PCR demonstrates that β-yac ESREs express adult globins, both from the endogenous murine beta globin locus and the transgenic human beta globin locus. Data on globin expression is presented from three independent transgenic cultures and from three independent cultures derived from nontransgenic littermate controls. (A) Murine globin expression in maturing β-yac ESREs. (B) Human globin expression in maturing β-yac ESREs. Only the adult globin, HBB, is expressed at significant levels. (C) At e12.5, the fetal liver contains maturing definitive erythroid cells, as well as circulating primitive erythrocytes [36]. In contrast to β-yac ESREs, quantitative PCR demonstrates that both embryonic and adult murine globins are detected in unsorted, uncultured e12.5 β-yac fetal liver. (D) The embryonic, fetal, and adult human globins are also detected in unsorted, uncultured e12.5 β-yac fetal liver. Hbb−y = hemoglobin Y, β-like embryonic chain; Hbb-bh1 = hemoglobin Z, β-like embryonic chain; Hbb-b1 = hemoglobin, β adult major chain; HBG1 = hemoglobin, Gamma A (γA); HBG2 = hemoglobin, Gamma G (γG).
Extensively self-renewing erythroblasts have mRNA expression changes consistent with erythroid maturation
Erythroid maturation is accompanied by characteristic changes in mRNA expression [26], with high-level expression of some erythroid-specific genes, as well as the silencing of many other genes [27] in preparation for nuclear condensation and enucleation. To further validate ESREs as a model of terminal erythroid maturation, RNA-seq was used to characterize changes in mRNA expression that occur during ESRE maturation. To simplify mapping of sequence reads to the murine reference genome, ESREs derived from nontransgenic mice were used for RNA-seq studies. Extensively self-renewing erythroblasts derived from nontransgenic mice behave similarly to β-yac ESREs. They are capable of extensive ex vivo expansion, doubling daily at the proerythroblast phase (Supplementary Figure E2, online only, available at www.exphme.org), while maintaining the ability to mature and enucleate (Supplementary Figures 2 and 3, online only, available at www.exphem.org) in a semisynchronous cohort. To further demonstrate the similarities between β-yac ESREs and nontransgenic ESREs, the expression of several key transcriptional regulators was found to be similar after assessment by qPCR in expanding ESREs and β-yac ESREs (Supplementary Figure E4, online only, available at www.exphem.org).
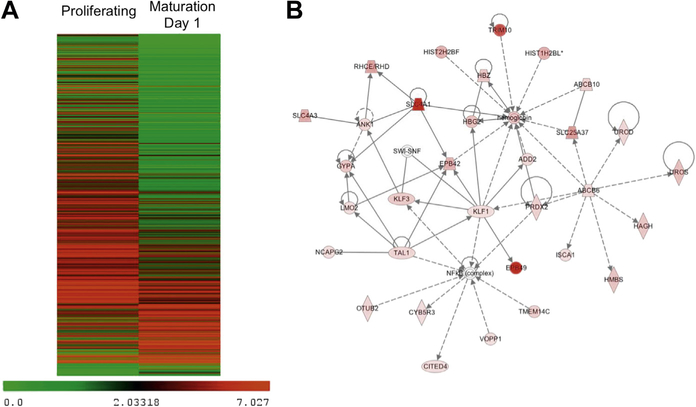
The transcriptome of expanding ESREs was compared with the transcriptome of ESREs after 1 day of maturation, when the cells have begun to mature, but not yet enucleate. Biological replicates were investigated at each time point. Although the cells underwent only 24 hours of maturation, over 1,600 genes were differentially expressed (p < 10−3, false discovery rate < 0.01) (Supplementary Table E2, online only, available at www.exphem.org). Differentially expressed genes included key erythroid transcription factors (Tal1, Klf1), membrane protein genes (EPB49, Band3, Gypa, and TMOD1) and several genes that play pivotal roles in globin switching (Myb, Sox6, and Klf1). In total, 354 genes were upregulated, 1,318 genes were downregulated, and 24,117 genes had no statistically significant changes in expression (Fig. 3A).
Figure 3.
ESREs have gene expression changes consistent with terminal erythroid maturation. (A) Heat map of genes with significant changes in expression during maturation of ESREs, demonstrating that the majority of genes are downregulated with maturation. (B) Top network identified by Ingenuity Pathway Analysis of genes upregulated during ESRE maturation.
Pathway analysis was performed with Ingenuity Pathway Analysis (Qiagen; www.ingenuity.com) on the 354 genes with a statistically significant increase in expression. Consistent with their phenotype as maturing erythroid cells, the most significant canonical pathway identified was heme biosynthe6sis (p = 5.3 × 10−6), and the top network identified was hematologic disease (score 62, focus molecules 32) (Fig. 3B). In addition, Gata1, Klf1, and erythropoietin were all predicated as upstream regulators, with p values of 2.8 × 10−11, 1.13 × 10−8, and 8.98 × 10−5, respectively.
The transcriptome of ESREs is similar to uncultured fetal liver–derived erythroid cells
To gain further insights into the validity of the ESRE culture system, the RNA expression profile of proliferating ERSEs was compared with the RNA expression profile of primary fetal liver–derived proerythroblasts and basophilic erythroblasts. Gene expression in the ESREs was determined using RNA-seq, as described above. Expression data on the primary uncultured fetal liver proerythroblasts and basophilic erythroblasts was attained from the Erythron Database [27], which contains Affymetrix expression data on well characterized populations of primary erythroid precursors. As expected, RNA-seq was significantly more sensitive at determining gene expression than microarray, with the detection of 21,278 transcripts by RNA-seq in proliferating ESREs, and 4,412 transcripts detected by microarray in uncultured proerythroblasts and basophilic erythroblasts. There was significant overlap in the gene expression patterns between the ESREs and uncultured erythroid precursors, with 93% of genes expressed in proerythroblasts and basophilic erythroblasts expressed in proliferating ESREs (Supplementary Figure E5A, online only, available at www.exphem.org). When applying a stringent expression cutoff of fragments per kilobase of exon per million fragments mapped (FPKM) > 0.5 to the RNA-seq data, there were 10, 384 transcripts detected in the expanding ESREs. Even with the stringent expression cutoff, 79% of genes expressed in the proerythroblasts and basophilic erythroblasts were also expressed in proliferating ESREs (Supplementary Figure E5B, online only, available at www.exphem.org).
Since the most clinically relevant regulation of γ-globin expression occurs in adult bone marrow, the transcriptome of proliferating ESREs was also compared with the transcriptome of uncultured adult bone marrow–derived erythroid precursors. We detected 7297 transcripts by Affymetrix array [27] in sorted adult bone marrow proerythroblasts and basophilic erythroblasts. Of those transcripts, 91% were also expressed in proliferating ESREs (Supplementary Figure E6A, online only, available at www.exphem.org). When an expression cutoff of FPKM > 0.5 was applied to the RNA-seq data, there was a 78% overlap in gene expression (Supplementary Figure E6B, online only, available at www.exphem.org).
Inhibition of chromatin modifiers alters the globin expression patterns of β-yac ESREs
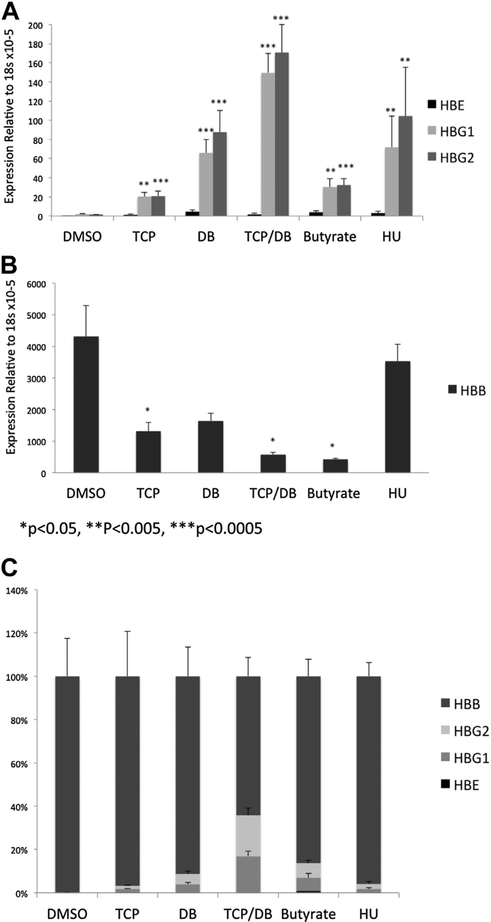
Inhibitors to HDACs, DNMTs, and LSD1 have been shown to increase γ-globin expression in some model systems [6,12,28–32], and some of these agents have been proposed as potential therapies for the β-globinopathies. To determine whether β-yac ESREs are a suitable model system for studying agents that alter γ-globin expression, β-yac ESREs were placed in maturation media and treated with either TCP (LSD1 inhibitor), sodium butyrate (HDAC inhibitor), decitabine (DNA methyltransferase inhibitor), HU, or a combination of decitabine and TCP for 24 hours. RNA was then harvested and mRNA expression of the murine and transgenic globin genes assessed. A significant increase in γ-globin expression was seen with all treatment conditions (Fig. 4A), demonstrating that β-yac ESREs can upregulate their γ-globin expression in response to a variety of agents with differing mechanisms of action. Consistent with a recent study [6], the largest increase in γ-globin expression was seen when the cells were treated with a combination of TCP and decitabine, with over a hundredfold increase in both γA− and γG-globin expression (p < 0.0005). For all treatment conditions, the increase in γ-globin expression was accompanied by a corresponding decline in β-globin expression (Fig 4B and C).
Figure 4.
Inhibitors to HDACs, DNMTs, and LSD1 alter globin expression in β-yac ESREs. Error bars represent standard error of a minimum of three independent experiments. (A) Inhibitors to chromatin modifiers induce γ-globin expression in β-yac ESREs. β-yac ESREs were placed in maturation media with either vehicle control (dimethyl sulfoxide), TCP, DB, a combination of TCP and DB (TCP/DB), sodium butyrate, or HU for 24 hours. Then, globin expression was assessed by quantitative PCR. Light grey bars represent HBG1 expression, dark grey bars represent HBG2 expression, and black bars represent HBE expression. A statistically significant increase in gamma globin expression was seen in all treatment conditions. (B) β-yac ESREs treated with TCP, DB, and sodium butyrate express less β-globin from the transgenic human locus than vehicle-treated controls. (C) Data are presented as percent of total human globin. Error bars represent SEM of a minimum of three independent experiments. DB = Decitabine; DMSO = dimethyl sulf-oxide; HBB = β-globin; HBE = hemoglobin E; HBG1 = hemoglobin, Gamma A (γA); HBG2 = hemoglobin, Gamma G (γG).
Treatment of β-yac ESREs with the inhibitors also affected globin expression from the endogenous murine locus. Consistent with previous studies suggesting an evolutionary relationship between γ-globin and the embryonic murine globin βH1 [33–36], βH1 is expressed at extremely low levels in β-yac ESREs. Treatment of β-yac ESREs with decitabine, TCP and decitabine, sodium butyrate, or HU resulted in a modest increase in βH1 expression (Supplementary Figure E7A, online only, available at www.exphem.org). Inhibitor treatment also influenced murine beta major expression (Supplementary Figure E7B, online only, available at www.exphem.org), but had no effect on α-globin expression (Supplementary Figure E7C, online only, available at www.exphem.org).
Flow cytometric (FACS) analyses were used to determine the fraction of cells expressing fetal hemoglobin. Before maturation, the β-yac ESREs did not express significant amounts of adult or fetal hemoglobin. Compared with vehicle control, a significant induction of fetal hemoglobin was detected when the cells were matured in the presence of decitabine, sodium butyrate, or hydroxyurea (Supplementary Figure E8, online only, available at www.exphem.org). Interestingly, the addition of TCP to the culture medium, either alone or in combination with decitabine, resulted in a significant overall decrease in the number of cells positive for both adult and fetal globin. Impaired or delayed erythroid maturation may have contributed to the decreased expression of both the adult and fetal globin in the TCP-treated samples.
Inhibition of the histone demethylase LSD1 leads to phenotypic changes in ESREs consistent with delayed erythroid maturation
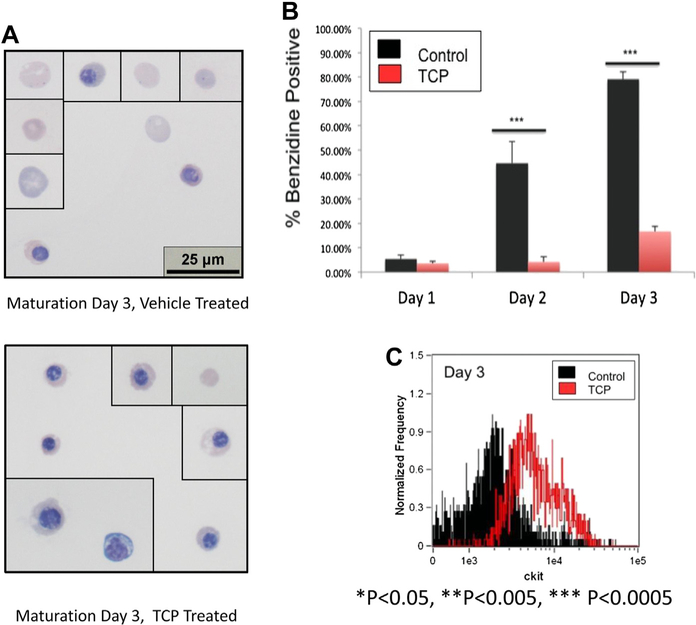
Multiple studies have suggested that LSD1 inhibition has effects in erythroid cells beyond increased γ-globin expression, such as impaired erythroid maturation [11–13], although these effects have been incompletely characterized. To determine the effects of LSD1 inhibition on erythroid maturation, β-yac ESREs were placed in maturation media and treated with either 1 μmol/L TCP or vehicle control (dimethyl sulfoxide), and their maturation assessed. Significant phenotypic differences were observed between the TCP-treated cells and the vehicle-treated controls. By day 3 of maturation, the vehicle-treated cultures were composed primarily of late-stage erythroid precursors and enucleated cells (Fig. 5A, top panel). In contrast, the TCP-treated cultures were much more heterogeneous in appearance. Although some late-stage erythroid precursors and enucleated cells could be seen, the culture was composed primarily of less mature cells, which were more basophilic with a larger cell and nuclear area (Fig. 5A, bottom panel). The TCP-treated cultures also had a significantly lower rate of benzidine accumulation (Fig. 5B).
Figure 5.
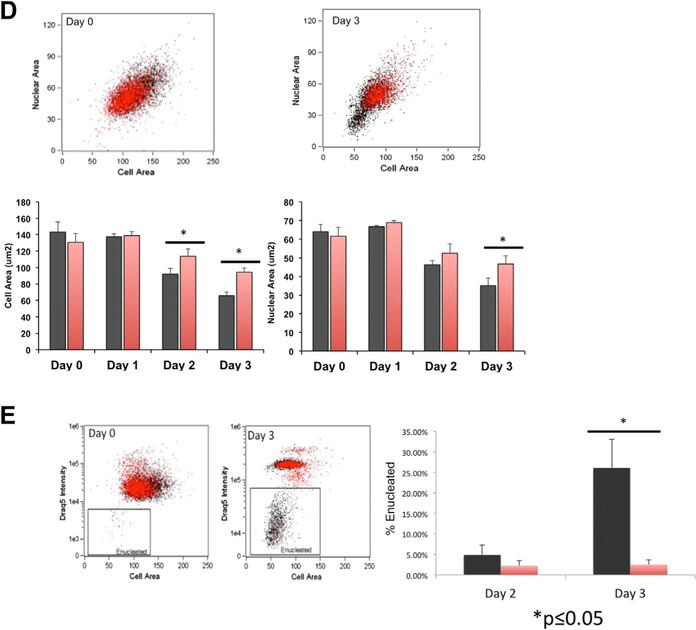
Treatment of β-yac ESREs with an LSD1 inhibitor results in abnormal maturation and impaired enucleation. (A) Photomicrographs from vehicle-treated cultures (upper panel) and TCP-treated cultures (lower panel) on day 3 of maturation. The vehicle-treated culture consists of late-stage erythroid precursors and enucleated cells. The TCP-treated cultures are more heterogeneous, with both late-stage erythroid precursors and enucleated cells, as well as less mature erythroid precursors. (B) Benzidine staining of TCP-treated samples (red bars) and vehicle-treated control (black bars). Data represent mean and SEM of a minimum of three independent experiments. (C) Histogram of Kit expression determined by imaging flow cytometry on β-yac ESREs treated with TCP or vehicle control after 3 days of maturation. The Kit levels are higher in the TCP-treated samples than in the vehicle-treated control. (D) Comparison of cell and nuclear size in vehicle- and TCP-treated cells. Vehicle-treated cells are in black, and TCP-treated cells are in red. Graphs represent mean of three independent experiments. After 3 days of maturation, the TCP-treated cells have both a larger cell and nuclear size. (E) TCP treatment impairs enucleation. Vehicle-treated cells are in black, and TCP-treated cells are in red. The percent of enucleated cells was determined by comparing DRAQ5 intensity to cell area. Graph represents mean and SEM of three independent experiments.
Imaging flow cytometry was used to quantify the differences in maturation observed in vehicle and TCP treated cultures. After 3 days in maturation media, the TCP-treated cells had prolonged Kit expression (Fig. 5C), as well as larger cell and nuclear areas (Fig. 5D), compared with vehicle treated controls. The TCP-treated samples also had a significant deficit in enucleation, determined by comparing DRAQ5 intensity to cell area. On day 3 of maturation, 3% of TCP-treated cells were enucleated, compared with 26% of vehicle-treated cells (Fig. 5E). Image stream analyses were repeated on maturation day 4, to determine whether the maturation of TCP-treated cultures was simply delayed, as opposed to abnormal. On maturation day 4, nuclear size was similar between TCP-and vehicle-treated samples; however, the TCP-treated cells continued to be slightly larger, with continued Kit expression and lower rates of enucleation than vehicle treated control (Supplementary Figure E9, online only, available at www.exphem.org).
Discussion
At the molecular level, erythroid maturation is driven by a complex combination of cis- and transacting factors that act in concert to drive the expression of erythroid-specific genes. The establishment of stage-specific patterns of transcription factor binding and chromatin architecture is necessary both for normal erythroid maturation and for the control of erythroid-specific genes, such as γ-globin. Chromatin modifiers, such as HDACs, DNMTs, and the histone demethylase LSD1, play important roles in the regulation of erythroid maturation and also influence globin gene expression. Inhibitors to these chromatin modifiers represent potential treatments for the β-globinopathies, but it is also important to study how these inhibitors influence erythroid maturation.
Consistent with previous studies [6], treatment of β-yac ESREs with the LSD1 inhibitor TCP leads to a significant increase in γ-globin expression. Interestingly, the magnitude of the γ-globin increase observed in β-yac ESREs, which lack promiscuous γ-globin expression, was somewhat lower than was previously reported in CD34+ derived erythroid cultures [6], which have a significantly higher baseline level of γ-globin expression. Despite the fact that LSD1 inhibitors have been shown to increase γ-globin levels in a variety of model systems [6], their role in the in the treatment of the β-globinopathies remains controversial due to their secondary effects on erythroid maturation [11,13]. In β-yac ESREs, TCP treatment was associated with significant impairments in erythroid maturation, including increased cell and nuclear size, altered cell surface marker expression, and decreased rates of enucleation. Further studies are needed to determine if the benefits associated with increased γ-globin expression will outweigh the deleterious effects of LSD1 inhibition on erythroid maturation.
The study of globin switching has long been hampered by a lack of model systems that accurately model globin switching or erythroid maturation. Several recent reports have attempted to create model systems that could be utilized to study agents that increase γ-globin expression [19,20]. One study attempted to use MEL cells engineered with florescent reporter constructs under the control of the human β-globin locus to identify agents that increase γ-globin expression. When challenged with known inducers of γ-globin expression, these cells were unable to upregulate their γ-globin expression unless they were on a B cell lymphoma/leukemia 11a (BCL11a)-depleted background [19], limiting their utility as a screening tool. Another recent study attempted to use fetal liver cell lines derived from p53 null mice that carry florescent reporters directed by the human β-globin locus [20] to identify agents that increase γ-globin expression. Technical difficulties with autoflorescence hampered the use of the fluorescent reporter, and the p53 null background limits the use of these cells for studies of erythroid maturation and predisposes them to karyotypic abnormalities [37].
β-yac mice are well accepted as an in vivo model of globin switching [17,18]. Despite the limitation that they contain a human transgene on a murine background, studies utilizing β-yac mice have lead to important insights into the regulation of globin switching [38–40]. β-yac ESREs were developed to facilitate studies of erythroid maturation and globin switching that are impractical to perform in a mouse model. β-yac ESREs are nontransformed and double daily in culture, while maintaining the ability to mature and enucleate as a relatively synchronous cohort, generating ample cell numbers for high-quality genomic and functional studies. In addition, β-yac ESREs upregulate their γ-globin expression in response to a variety of inhibitors with different mechanisms of action, without being on a genetically altered background.
β-yac ESREs also have some disadvantages. They share the same limitation as the β-yac mice, with a human trans-gene on a murine background, although this does not appear to limit their utility for identifying modulators of γ-globin expression. In addition, as they expand as proerythroblasts, they cannot be utilized to study developmental globin switching or changes in gene expression that occur before this stage of maturation. Similar to the CD34+ culture system, ESREs are derived from primary cells and are therefore cytokine dependent, requiring SCF, IGF1, and erythropoietin, making them significantly more expensive to maintain than cell lines such as K562 or MEL. Lastly, similar to CD34+ cells or other culture-based models of erythroid maturation, there are inherent differences between these cells and uncultured primary cells. The data presented here confirm our expectation that, although these cells are an excellent representation of erythroid maturation, on a molecular level there are differences between ESREs and primary uncultured cells. Despite their limitations, the data presented support β-yac ESREs as a robust model of erythroid maturation and maturational globin regulation that can be used for multiple functional and genomics applications.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Cooley’s Anemia Foundation (New York, NY, USA) and NIDDK K08 DK090153 (L.A.S.).
Footnotes
Conflict of interest disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.exphem.2014.03.006.
References
- 1.Mai A, Jelicic K, Rotili D, et al. Identification of two new synthetic histone deacetylase inhibitors that modulate globin gene expression in erythroid cells from healthy donors and patients with thalassemia. Mol Pharmacol. 2007;72:1111–1123. [DOI] [PubMed] [Google Scholar]
- 2.Cao H, Stamatoyannopoulos G, Jung M. Induction of human gamma globin gene expression by histone deacetylase inhibitors. Blood. 2004; 103:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Migliaccio AR, Rotili D, Nebbioso A, Atweh G, Mai A. Histone deacetylase inhibitors and hemoglobin F induction in beta-thalassemia. Int J Biochem Cell Biol. 2008;40:2341–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mabaera R, Richardson CA, Johnson K, Hsu M, Fiering S, Lowrey CH. Developmental- and differentiation-specific patterns of human gamma- and beta-globin promoter DNA methylation. Blood. 2007; 110:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley TJ, Anagnou NP, Noguchi CT, et al. DNA methylation and globin gene expression in patients treated with 5-azacytidine. Prog Clin Biol Res. 1983;134:457–474. [PubMed] [Google Scholar]
- 6.Shi L, Cui S, Engel JD, Tanabe O. Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction. Nat Med. 2013;19: 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanabe O, McPhee D, Kobayashi S, et al. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J. 2007;26(9):2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSimone J, Koshy M, Dorn L, et al. Maintenance of elevated fetal hemoglobin levels by decitabine during dose interval treatment of sickle cell anemia. Blood. 2002;99:3905–3908. [DOI] [PubMed] [Google Scholar]
- 9.Ley TJ, Nienhuis AW. Induction of hemoglobin F synthesis in patients with beta thalassemia. Annu Rev Med. 1985;36:485–498. [DOI] [PubMed] [Google Scholar]
- 10.Ley TJ, DeSimone J, Anagnou NP, et al. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982;307:1469–1475. [DOI] [PubMed] [Google Scholar]
- 11.Sprüssel A, Schulte JH, Weber S, et al. Lysine-specific demethylase 1 restricts hematopoietic progenitor proliferation and is essential for terminal differentiation. Leuke Leukemia. 2012;26:2039–2051. [DOI] [PubMed] [Google Scholar]
- 12.Saleque S, Kim J, Rooke HM, Orkin SH. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol Cell. 2007;27:562–572. [DOI] [PubMed] [Google Scholar]
- 13.Kerenyi MA, Shao Z, Hsu YJ, et al. Histone demethylase Lsd1 represses hematopoietic stem and progenitor cell signatures during blood cell maturation. Elife. 2013;2:e00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks PA, Rifkind RA. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. [DOI] [PubMed] [Google Scholar]
- 15.England SJ, McGrath KE, Frame JM, Palis J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117:2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Migliaccio AR, Baiocchi M, Durand B, Eddleman K, Migliaccio G, Adamson JW. Stem cell factor and the amplification of progenitor cells from CD34+ cord blood cells. Blood Cells. 1994;20:129–138. discussion 138–139. [PubMed] [Google Scholar]
- 17.Peterson KR, Li QL, Clegg CH, et al. Use of yeast artificial chromosomes (YACs) in studies of mammalian development: production of beta-globin locus YAC mice carrying human globin developmental mutants. Proc Natl Acad Sci U S A. 1995;92:5655–5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson KR, Navas PA, Stamatoyannopoulos G. beta-YAC transgenic mice for studying LCR function. Ann N Y Acad Sci. 1998;850:28–37. [DOI] [PubMed] [Google Scholar]
- 19.Chan KS, Xu J, Wardan H, McColl B, Orkin S, Vadolas J. Generation of a genomic reporter assay system for analysis of gamma- and beta-globin gene regulation. FASEB J. 2012;26:1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulos P, Guti errez L, van der Linden R, et al. A dual reporter mouse model of the human beta-globin locus: applications and limitations. PLoS One. 2012;7:e51272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David M, Dzamba M, Lister D, Ilie L, Brudno M. SHRiMP2: sensitive yet practical SHort Read Mapping. Bioinformatics. 2011;27:1011–1012. [DOI] [PubMed] [Google Scholar]
- 22.Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics. 2011;27:2325–2329. [DOI] [PubMed] [Google Scholar]
- 23.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrath KE, Bushnell TP, Palis J. Multispectral imaging of hematopoietic cells: where flow meets morphology. J Immunol Methods. 2008;336:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palis J Ontogeny of erythropoiesis. Curr Opin Hematol. 2008;15:155–161. [DOI] [PubMed] [Google Scholar]
- 26.Ji P, Murata-Hori M, Lodish HF. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol. 2011;21:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kingsley PD, Greenfest-Allen E, Frame JM, et al. Ontogeny of erythroid gene expression. Blood. 2013;121:e5–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin J, Singh M, Banzon V, et al. Transcriptional activation of the gamma-globin gene in baboons treated with decitabine and in cultured erythroid progenitor cells involves different mechanisms. Exp Hematol. 2009;37:1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akpan I, Banzon V, Ibanez V, Vaitkus K, DeSimone J, Lavelle D. Decitabine increases fetal hemoglobin in Papio anubis by increasing gamma-globin gene transcription. Exp Hematol. 2010;38:989–993. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrine SP, Ginder GD, Faller DV, et al. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med. 1993;328:81–86. [DOI] [PubMed] [Google Scholar]
- 31.Perrine SP, Olivieri NF, Faller DV, Vichinsky EP, Dover GJ, Ginder GD. Butyrate derivatives. New agents for stimulating fetal globin production in the beta-globin disorders. Am J Pediatr Hematol Oncol. 1994;16:67–71. [PubMed] [Google Scholar]
- 32.Weinberg RS, Ji X, Sutton M, et al. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood. 2005;105: 1807–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chada K, Magram J, Costantini F. An embryonic pattern of expression of a human fetal globin gene in transgenic mice. Nature. 1986;319: 685–689. [DOI] [PubMed] [Google Scholar]
- 34.Hill A, Hardies SC, Phillips SJ, Davis MG, Hutchison CA 3rd, Edgell MH. Two mouse early embryonic beta-globin gene sequences. Evolution of the nonadult beta-globins. J Biol Chem. 1984;259:3739–3747. [PubMed] [Google Scholar]
- 35.Shen SH, Slightom JL, Smithies O. A history of the human fetal globin gene duplication. Cell. 1981;26:191–203. [DOI] [PubMed] [Google Scholar]
- 36.McGrath KE, Frame JM, Fromm GJ, et al. A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood. 2011;117:4600–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukasawa K, Wiener F, Vande Woude GF, Mai S. Genomic instability and apoptosis are frequent in p53 deficient young mice. Oncogene. 1997;15:1295–1302. [DOI] [PubMed] [Google Scholar]
- 38.Harju-Baker S, Costa FC, Fedosyuk H, Neades R, Peterson KR. Silencing of Agamma-globin gene expression during adult definitive erythropoiesis mediated by GATA-1-FOG-1-Mi2 complex binding at the −566 GATA site. Mol Cell Biol. 2008;28:3101–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson KR, Fedosyuk H, Zelenchuk L, et al. Transgenic Cre expression mice for generation of erythroid-specific gene alterations. Genesis. 2004;39:1–9. [DOI] [PubMed] [Google Scholar]
- 40.Costa FC, Fedosyuk H, Chazelle AM, Neades RY, Peterson KR. Mi2beta is required for gamma-globin gene silencing: temporal assembly of a GATA-1-FOG-1-Mi2 repressor complex in beta-YAC transgenic mice. PLoS Genet. 2012;8:e1003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.