Abstract
Background
The aim of the study was to examine dietary patterns and risk of adverse pregnancy outcomes among mothers with inflammatory bowel disease (IBD) in the Norwegian Mother and Child Cohort Study (MoBa).
Method
MoBa enrolled participants from all over Norway between 1999 and 2008, and the study comprised 83,988 mothers, of whom there were 183 mothers with Crohn’s disease (CD) and 240 with ulcerative colitis (UC). An additional questionnaire was submitted to mothers with IBD in 2013. We extracted three exploratory dietary patterns: a “Prudent,” a “Western,” and a “Traditional” pattern. We explored the relationship between dietary patterns and IBD and dietary patterns and adverse pregnancy outcomes: small for gestational age (SGA) and preterm delivery (PTD).
Results
IBD mothers had a significantly lower adherence to the Traditional dietary pattern [mean score -0.10 (95% CI: - 0.2 - - 0.01)] than non-IBD mothers. In IBD mothers, middle and high adherence to the Traditional dietary pattern was associated with lower risk of SGA [OR tertile 2 vs. tertile 1: 0.44 (95% CI: 0.20 - 0.97) and OR tertile 3 vs. tertile 1: 0.23 (95% CI: 0.08–0.61)] than in IBD and non-IBD mothers with low adherence. In the IBD-subset analyses, similar results were demonstrated for UC mothers [OR tertile 2 vs. tertile 1: 0.21 (95% CI: 0.05 – 0.80) and OR tertile 3 vs. tertile 1: 0.16 (95% CI: 0.04 – 0.60)].
Conclusion
In IBD mothers, higher adherence to a Traditional dietary pattern, characterized by high consumption of lean fish, fish products, potatoes, rice porridge, cooked vegetables, and gravy, was associated with lower risk of SGA.
Keywords: The Norwegian Mother and Child Cohort Study, diet, inflammatory bowel disease, pregnancy outcomes, nutrition
Inflammatory bowel disease (IBD), Crohn’s disease (CD), and ulcerative colitis (UC) represent chronic complex disorders of the gastrointestinal tract.1 IBD is most prevalent in North America and Northern Europe, suggesting a north-south gradient in incident rates.2-4 This geographical variation suggests environmental and lifestyle-dependent factors as important modifying factors of the disease. The incidence rate of the disease is increasing in the developing world, indicating westernization as a potential risk factor. Although somewhat inconclusive, research on dietary patterns and risk of IBD have shown an association between a western diet with a high proportion of saturated fat and sugar-containing foods and beverages, and IBD.5–7 The results indicate a protective effect of diets rich in fiber, fruits, and vegetables.
IBD is early onset and usually diagnosed in late adolescence and early adulthood, coinciding with the peak reproductive years of women.1 A large body of evidence suggests that pregnant women with IBD have an overall increased risk of adverse pregnancy outcomes.8–13 The most consistent adverse outcomes described are preterm delivery (PTD) (<week 37), cesarean section, low birth weight (<2500 grams) (LBW), and small for gestational age (SGA).14
In pregnancy, the fetus receives all required nutrients through the placenta, solely dependent on the transfer of nutrients from the mother. Maternal dietary habits and placental function thus may influence the fetal development and pregnancy outcome, as well as the long-term health of the child, through the concept of fetal programming.15–17
Patients with IBD are at risk of nutrition deficiencies due to an increased loss and impaired absorption of nutrients from the intestine. Diarrhea, small bowel inflammation, and bowel resections are all contributing factors to malnutrition in IBD.1,18 Prevalent deficiencies include protein-, calcium-, vitamin D-, folic acid-, iron-, vitamin B12-, and zinc deficiencies.18,19
Previous studies have investigated the relationship between dietary patterns and pregnancy outcomes in healthy women.20–28 Although somewhat inconsistent, the studies found that diets characterized by a high consumption of vegetable oils, fruits, vegetables, whole grains, and fish were protective of adverse pregnancy outcomes including PTD and SGA.21, 22, 24
The role of dietary patterns in pregnant women with IBD has, to the best of our knowledge, not yet been investigated. The aim of this study was to investigate the relationship between IBD and dietary patterns, and the impact of dietary patterns on pregnancy outcomes in women with IBD.
MATERIALS AND METHODS
Ethical Considerations
MoBa is approved by The Regional Committee for Medical Research Ethics (REK). The current study is a part of a project administered by Akershus University hospital, and was approved by REK (2011/1317) and the owners of the registries and databases, in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants before the study.29
Population and Study Design
Participants were recruited from the Norwegian Mother and Child Cohort Study (MoBa).29 MoBa is a population-based, prospective cohort conducted by the Norwegian Institute of Public Health. Participants were recruited from all over Norway by postal invitation from 1999-2008.29 The women consented to participation in 41% of the pregnancies.30 The cohort now includes 114,500 children, 95,200 mothers, and 75,200 fathers. The current study is based on version 7 of the quality-assured data files released for research in 2012.
Follow-up is conducted through questionnaires and by linkage to national health registries.29, 31 A total of three questionnaires were sent out during the pregnancy period. The present study includes data from the two first questionnaires: Q1 and Q2. Q1 was submitted at gestational week 15 and covered the medical history of the mother before and during pregnancy, including lifestyle habits and various environmental exposures. Q2 was a food frequency questionnaire (FFQ) submitted at gestational week 22, sent out from 2002 and onwards. Pregnancy and birth records from the Medical Birth Registry of Norway (MBRN) are linked to the MoBa database.31 To be eligible for inclusion in the present study, participants had to be registered in MBRN with a singleton, live birth and to have answered Q1 and Q2 (2002 version). In addition, we excluded women with an invalid energy intake in the FFQ, ie,<4.5MJ and >20MJ.32 A total of n = 83,988 participants fulfilled these criteria. Of the 739 mothers who claimed to suffer from IBD reported in questionnaire (Q1), 655 mothers were available for the present study.
We mailed out an additional questionnaire in 2013 including questions regarding sub-classification of IBD, surgery, and disease activity during pregnancy.
Of the responders, 136 mothers suffered from CD and 192 suffered from UC. Of the 327 non-responders, 79 were recorded as CD and 95 women were recorded as UC by the Norwegian Patient Registry, which comprises 502 IBD women; 215 women with CD, and 287 women with UC. After excluding multiple births and extreme energy intake, 360 pregnancies, 156 CD, and 204 UC, fulfilled the criteria for further analyses reflecting the association between dietary patterns and pregnancy outcomes in women with IBD. The final numbers of non-IBD SGA pregnancies were 60,960 and 69,979 for PTD pregnancies.
Dietary Information
Dietary information was collected in the FFQ sent out to the participants from 2002 onwards.29 The FFQ was a semi-quantitative questionnaire, designed to obtain information on dietary habits and use of dietary supplements during the first four to five months of pregnancy.
The FFQ was developed especially for the MoBa study, and has been validated in a subsample of the cohort participants.33 This study found that the questionnaire produces realistic estimates of food and nutrient intakes and is a valid tool to rank pregnant women according to high and low intakes of energy, nutrients, and foods.
Extraction of Dietary Patterns
We extracted exploratory dietary patterns using principal component factor analysis (PCA) with orthogonal (varimax) rotation to obtain optimal interpretability of the extracted components (dietary patterns). Factor analysis is a way of identifying variables that correlate and form a pattern across the sample population.34
A total of 98 nonoverlapping food and beverage variables (g/day) were used as input variables in the analysis. Suitability of the data before the analysis was tested using correlation matrix, Bartlett’s Test of Sphericity (P< 0.001) and Kaiser- Meyer-Olkin test (KMO = 0.640).
Coefficients with absolute value above 0.25 were considered relevant for interpretability of the patterns. We used scree-plot and interpretability of the factors to determine the number of components to retain. Three distinct dietary patterns were extracted from the PCA, explaining 10.97% of the total variance in food consumption for all women (Table 1). Their eigenvalues were 5.1, 3.3, and 2.6, respectively.
Table 1:
Structures of the Three Rotated Components Identified by PCA in 83,988 Singleton Births, from MoBa 2002–2008.
| Dietary Pattern | Food | Loading Coefficienta | Cummulative Variance Explained. % |
|---|---|---|---|
| “Prudent” | Green salads | 0.602 | 5.116 |
| Raw vegetables | 0.571 | ||
| Onions, leek, garlic | 0.546 | ||
| Cooked vegetables | 0.518 | ||
| Cooking oils | 0.516 | ||
| Tomato, cucumber | 0.466 | ||
| Mushroom | 0.456 | ||
| Green beans | 0.435 | ||
| Vegetables as main course | 0.429 | ||
| Olive oil | 0.427 | ||
| Fruit, traditional | 0.398 | ||
| Fruit, exotic | 0.368 | ||
| Rice, millet, coscous | 0.343 | ||
| Almonds, nuts | 0.316 | ||
| Dried fruits | 0.313 | ||
| Green-, herbal-, rose hip tea | 0.306 | ||
| Water, bottled or tap | 0.302 | ||
| Breakfast cereal, porridge | 0.297 | ||
| Corn | 0.288 | ||
| Poultry | 0.285 | ||
| Berries | 0.270 | ||
| Tuna fish | 0.265 | ||
| Processed meat products (burgers, hotdogs) | -0.458 | ||
| Casseroles, meat | -0.308 | ||
| White bread | -0.271 | ||
| “Western” | Salty snacks (crisps, popcorn, peanuts) | 0.471 | 8.452 |
| Potatoes, fried (French Fries) | 0.421 | ||
| Chocolates | 0.419 | ||
| Tomato ketchup | 0.390 | ||
| Caramels, pastilles, candies | 0.385 | ||
| Cakes | 0.379 | ||
| Dairy desserts | 0.375 | ||
| Gravy | 0.373 | ||
| White bread | 0.355 | ||
| Mayonnaise, mayonnaise salads | 0.340 | ||
| Processed meat products | 0.335 | ||
| Sugared sodas | 0.319 | ||
| Buns | 0.294 | ||
| Corn | 0.281 | ||
| Potetatoes, cooked or mashed | 0.265 | ||
| Mustard | 0.254 | ||
| Waffles, pancakes | 0.252 | ||
| “Traditional” | Processed fish products | 0.573 | 10.973 |
| Potatoes, cooked or mashed | 0.555 | ||
| Lean fish | 0.496 | ||
| Gravy | 0.334 | ||
| Rice porridge | 0.325 | ||
| Cooked vegetables | 0.271 | ||
| Poultry | -0.329 | ||
| Pizza, tacos | -0.310 | ||
| Casseroles, meat | -0.291 |
aLoading coefficients denote correlation between the input variables (food group) and component (dietary pattern). Only loading coefficients with an absolute value > 0.25 shown in the table.
Food items are denoted with a positive or negative coefficient within the dietary pattern, so-called factor loadings. Food items may correlate with several dietary patterns, and dietary patterns are not mutually exclusive. Participants are assigned factors scores for all patterns based on their consumption of the given food items (from the FFQ) multiplied with the factor loadings for each item in the given dietary pattern. The factor scores, which were approximately normally distributed and with mean zero, reflect the adherence of an individual to each of the patterns. Factor scores were divided into tertiles indicating low, medium, or high intake of food items associated with the score.
Pregnancy Outcomes
Pregnancy outcomes are defined as the dichotomous variables PTD (< 37 weeks of gestation) and SGA defined as birth weight below the 10th percentile of the mean Norwegian weight curve.35
Expected date of birth is estimated (in MoBa) based on ultrasound scan, or the recorded date of last menstrual period, if ultrasound scan is missing.29 The information regarding birth outcomes was obtained from MBRN.31
Covariates
We adjusted for a set of potential confounders: maternal prepregnant body mass index (BMI) as a continuous variable; age divided into ≤ 34 and > 34 years; educational status divided into high school or less, 3 years of college/university, or master degree or higher; total energy intake in quartiles: <1870, 1871–2224, 2225–2657, and > 2657 kcal; and smoking during pregnancy as a dichotomous variable. Maternal diabetic condition, recorded as a dichotomous variable, includes diabetes I and II as well as gestational diabetes. Maternal hypertension was defined as systolic blood pressure ≥ 140 mm Hg, or diastolic blood pressure ≥ 90 mm Hg. These variables are all known as important predictors on pregnancy outcomes.
Disease Activity
Mothers with IBD were asked to report disease activity during the month before pregnancy and during pregnancy. Disease activity was defined as fulfilment of at least one of the following consequences of flares; change of medication, IBD-related surgery, or IBD-related hospital admissions.
Statistical Analysis
One-way ANOVA and independent sample t tests were performed to analyze the distribution of dietary patterns in relation to characteristics and pregnancy outcomes.
Logistic regression analysis was used to model the relationship between the exposure variables and the outcome variables. The models were adjusted for potential confounding by maternal BMI, age, educational status, total energy intake, and smoking during pregnancy. All dietary patterns were entered into the same model, controlling for each other. Diabetes mellitus and chronic hypertension were excluded from the analyses due to low prevalence in the mothers with IBD.
We fitted two sets of logistic regression models. In the first set, we included both mothers with IBD and non-IBD mothers. The relationship between dietary pattern and IBD and between dietary pattern and pregnancy outcomes in women with IBD was estimated by entering dietary patterns as three distinct interaction terms with the IBD variable. Non-IBD women and women with IBD adhering to the lowest third in the dietary pattern weres used as reference category. The parameters reported show excess risk of outcome for women with IBD in the second and third tertiles compared to the women in the reference category.
In the second set of logistic regression models, we included only mothers with IBD. We estimated the relationship between dietary pattern and pregnancy outcome in women with IBD. In these analyses, all three dietary patterns were included in the same model as single variables divided into tertiles. The lowest tertiles of dietary patterns were used as the reference categories. Disease activity was added as confounder in separate models.
Risks of the stated adverse pregnancy outcomes are presented in odds ratios (OR) with corresponding p-values and confidence intervals (CI). The statistical analyses were performed using SPSS version 23. Statistical significance was considered for P values < 0.05.
RESULTS
Dietary Patterns
The first dietary pattern explained 5.1% of the variance and was labeled “Prudent.” This pattern had positive factor loadings for fruit, berries, vegetables (cooked and raw), beans, olive oil, rice, corn, poultry, nuts, tuna fish, cereal, and water as beverage. The Prudent dietary pattern had negative factor loadings for processed meats (hamburgers, hot dogs), casseroles, and white bread. The second dietary pattern explained 3.3% of the variance was labeled “Western” and had high factor loadings for foods and beverages rich in sugar (such as sodas, waffles, pancakes, cakes, candy, dairy desserts, and white bread) and fat (such as chocolate, dairy desserts, gravy, salty snacks, processed meats, mayonnaise, and fried potatoes). The third pattern explained 2.6% of the variance and was labeled “Traditional” and had high factor loadings for fish (lean) and fish products, gravy, potatoes, rice porridge, and cooked vegetables, and negatively factor loadings for poultry, casseroles, pizza, and tacos (Table 1).
The distribution of IBD, CD, and UC in relation to maternal characteristics, dietary patterns, and pregnancy outcomes are presented in Table 2.
Table 2:
Distribution of 423 Singleton Births with Maternal IBD by Maternal Characteristics, from MoBa 2002–2008
| IBD (n = 423) | CD (n= 183) | UC (= 240) | Non-IBD (n= 83.565) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Age (yrs) | ||||
| ≥ 34 | 341 (80.6) | 151 (82.5) | 190 (79.2) | 69230 (82.3) |
| >34 | 82 (19.4) | 32 (17.5) | 50 (20.8) | 14758 (17.7) |
| Missing | 0 | 0 | 0 | 0 |
| Body mass index | ||||
| <25 | 297 (70.2) | 130 (71.0) | 167 (69.6) | 55372 (66.2) |
| ≥25 | 115 (27.2) | 48 (26.2) | 67 (27.9) | 25626 (30.7) |
| Missing | 11 (2.6) | 5 (2.7) | 6 (2.5) | 2578 (3.1) |
| Diabetes mellitus | ||||
| No | 419 (99.1) | 181 (98.9) | 237 (98.8) | 82312 (98.5) |
| Yes | 4 (0.9) | 2 (1.1) | 3 (1.2) | 1263 (1.5) |
| Missing | 0 | 0 | 0 | 0 |
| Education (completed level) | ||||
| High school or less | 130 (30.7) | 63 (34.4) | 67 (27.9) | 26869 (32.2) |
| 3 years of college/university | 183 (43.3) | 75 (41.0) | 108 (45.0) | 32780 (39.2) |
| Master degree or higher | 83 (19.6) | 31 (16.9) | 52 (21.7) | 19118 (22.9) |
| Missing | 27 (6.4) | 14 (7.7) | 13 (5.4) | 4825 (5.7) |
| Energy (kcal) | ||||
| ≤ 1876 | 111 (26.2) | 52 (28.4) | 59 (24.6) | 20903 (25.0) |
| 1877–2223 | 94 (22.2) | 32 (17.5) | 62 (25.8) | 20904 (25.0) |
| 2224–2647 | 111 (26.2) | 49 (26.8) | 62 (25.8) | 20870 (25.0) |
| ≥2648 | 107 (25.3) | 50 (27.3) | 57 (23.8) | 20888 (25.0) |
| Missing | 0 | 0 | 0 | 0 |
| Chronic hypertension | ||||
| No | 422 (99.8) | 183 (100.0) | 239 (99.6) | 83143 (99.5) |
| Yes | 1 (0.2) | 0 | 1 (0.4) | 422 (0.5) |
| Missing | 0 | 0 | 0 | 0 |
| Smoking during pregnancy | ||||
| No | 360 (85.1) | 151 (82.5) | 209 (87.1) | 69623 (83.3) |
| Yes | 35 (8.3) | 22 (12.0) | 13 (5.4) | 6135 (7.3) |
| Missing | 28 (6.6) | 10 | 18 (7.5) | 7835 (9.4) |
| Disease activity | ||||
| No | 270 (86.3) | 120 (91.3) | 150 (82.5) | N/A |
| Yes | 58 (13.7) | 16 (8.7) | 42 (17.5) | N/A |
| Missing | 96 | 47 | 48 | |
| Prudent dietary pattern | ||||
| Low | 140 (33.0) | 58 (31.7) | 82 (34.2) | 27854 (33.3) |
| Middle | 168 (39.7) | 73 (39.9) | 95 (39.6) | 27825 (33.3) |
| High | 115 (27.2) | 52 (28.4) | 63 (26.3) | 27886 (33.4) |
| Missing | 0 | 0 | 0 | 0 |
| Western dietary pattern | ||||
| Low | 129 (30.5) | 54 (29.5) | 75 (31.3) | 27864 (33.3) |
| Middle | 124 (29.3) | 46 (25.1) | 78 (32.5) | 27869 (33.4) |
| High | 170 (40.2) | 83 (45.4) | 87 (36.3) | 27832 (33.3) |
| Missing | 0 | 0 | 0 | |
| Traditional dietary pattern | ||||
| Low | 154 (36.4) | 72 (39.3) | 82 (34.2) | 27839 (33.3) |
| Middle | 147 (34.8) | 62 (33.9) | 85 (35.4) | 27846 (33.3) |
| High | 122 (28.8) | 49 (26.8) | 73 (30.4) | 28880 (33.4) |
| Missing | 0 | 0 | 0 | 0 |
| Preterm delivery | ||||
| >week 37 | 396 (93.6) | 169 (92.3) | 227 (94.6) | 79502 (95.1) |
| <week 37 | 27 (6.4) | 14 (7.7) | 13 (5.4) | 4156 (5.0) |
| Missing | 0 | 0 | 0 | 330 (0.4) |
| Small for gestational age | ||||
| Above 10th percentile | 384 (90.8) | 165 (90.2) | 219 (91.2) | 77614 (92.9) |
| Below 10th percentile | 39 (9.2) | 18 (9.8) | 21 (8.8) | 5456 (6.5) |
| Missing | 0 | 0 | 0 | 495 (0.6) |
Mean factor scores for dietary patterns in relation to characteristics for IBD, CD, and UC are presented in Table 3. The mean factor score was negative for almost all characteristics in the Prudent and Traditional dietary pattern in women with IBD. The mean factor score in the complete dataset is zero. This indicates that women with IBD have, in general, lower adherence to the Prudent dietary pattern and the Traditional dietary pattern, and higher adherence to the Western dietary pattern than controls [mean factor score: 0.14 (95% CI: 0.04 – 0.25)], and this is consistent across all characteristics (Table 3). Women with IBD had a significant lower adherence to the Traditional dietary pattern than non-IBD women [mean factor score: -0.10 (95% CI: -0.2 - - 0.01)] (Table 3).
Table 3:
Mean Factor Scores in Relation to Maternal Characteristics in 423 Births, from MoBa 2002–2008a
| IBD (n = 423) | CD (n= 183) | UC (n = 240) | Non-IBD (n = 83.565) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prudent | Western | Traditional | Prudent | Western | Traditional | Prudent | Western | Traditional | Prudent | Western | Traditional | |
| All women (mean (CI)) | -0.06 (-0.16 - 0.04) |
0.14 (0.04 - 0.25) |
-0.10
(-0.2 - -0.01) |
-0.01 (-0.16 - 0.13) | 0.27 (0.1 - 0.44) | -0.14(-0.28 - 0) | -0.09 (-0.23 – 0.03) |
0.05 (-0.07 - 0.17) |
-0.07 (-0.19 - 0.05) |
0.00 (-0.01 - 0.01) |
0.00 (-0.01 - 0.01) |
0.00 (-0.01 - 0.01) |
| Age (yrs) | ||||||||||||
| ≤34 | -0.06 | 0.14 | -0.19 | 0.01 | 0.32 | -0.19 | -0.12 | -0.01 | -0.18 | -0.05 | 0.02 | -0.04 |
| >35 | -0.06 | 0.18 | 0.24 | -0.13 | 0.02 | 0.08 | -0.01 | 0.28 | 0.34 | 0.24 | -0.11 | 0.21 |
| Body mass index | ||||||||||||
| <24.9 | -0.05 | 0.11 | -0.05 | 0.02 | 0.25 | -0.06 | -0.10 | 0.00 | -0.04 | 0.07 | -0.05 | 0.03 |
| ≥25 | -0.16 | 0.23 | -0.28 | -0.26 | 0.43 | -0.44 | -0.09 | 0.08 | -0.16 | -0.16 | 0.10 | -0.07 |
| Diabetes mellitus | ||||||||||||
| No | -0.06 | 0.15 | -0.10 | -0.01 | 0.27 | -0.14 | -0.09 | 0.05 | -0.06 | 0.00 | 0.00 | 0.00 |
| Yes | -0.59 | -0.11 | -0.78 | -1.16 | 0.33 | 0.24 | -0.41 | -0.26 | -1.11 | 0.00 | 0.03 | -0.07 |
| Education (completed level) | ||||||||||||
| High school or less | -0.33 | 0.47 | -0.02 | -0.32 | 0.62 | -0.05 | -0.34 | 0.34 | -0.003 | -0.26 | 0.27 | 0.06 |
| 3 years of college/ university | -0.05 | 0.08 | -0.04 | 0.02 | 0.10 | -0.10 | -0.10 | 0.06 | -0.002 | -0.02 | -0.05 | 0.00 |
| Master degree or higher | 0.32 | -0.21 | -0.33 | 0.48 | 0.12 | -0.54 | 0.23 | -0.41 | -0.18 | 0.38 | -0.29 | -0.07 |
| Energy (kcal) | ||||||||||||
| ≤ 1876 | -0.33 | -0.56 | -0.42 | -0.35 | -0.51 | -0.39 | -0.31 | -0.59 | -0.44 | -0.27 | -0.64 | -0.47 |
| 1877–2223 | -0.20 | -0.16 | -0.33 | -0.17 | -0.16 | -0.50 | -0.22 | -0.17 | -0.23 | -0.10 | -0.26 | -0.15 |
| 2224–2647 | 0.004 | 0.21 | -0.07 | 0.04 | 0.21 | -0.20 | -0.03 | 0.22 | -0.05 | 0.02 | 0.08 | 0.11 |
| ≥2648 | 0.27 | 1.07 | 0.40 | 0.38 | 1.42 | 0.32 | 0.20 | 0.76 | 0.54 | 0.33 | 0.81 | 0.54 |
| Chronic hypertension | ||||||||||||
| No | -0.08 | 0.14 | -0.11 | -0.01 | 0.27 | -0.14 | -0.12 | 0.04 | -0.08 | 0.00 | 0.00 | 0.00 |
| Yes | 6.64 | 2.00 | 1.37 | N/A | N/A | N/A | 6.64 | 2.00 | 1.37 | -0.04 | 0.08 | -0.04 |
| Smoking during pregnancy | ||||||||||||
| No | -0.03 | 0.11 | -0.13 | 0.04 | 0.27 | -0.17 | -0.07 | 0.00 | -0.09 | 0.05 | -0.05 | -0.03 |
| Yes | -0.10 | 0.68 | -0.12 | -0.28 | 0.56 | -0.11 | 0.21 | 0.88 | -0.13 | -0.37 | 0.57 | 0.09 |
| Disease activity | ||||||||||||
| No | -0.06 | 0.13 | 0.08 | 0.01 | 0.25 | 0.16 | 0.12 | 0.03 | 0.01 | N/A | N/A | N/A |
| Yes | 0.04 | 0.21 | 0.23 | 0.25 | 0.45 | 0.11 | 0.03 | 0.12 | 0.36 | N/A | N/A | N/A |
aDietary patterns are extracted through PCA.
bDifferences between categories were tested using Independent samples ttest
cDifferences between categories were tested using One-way ANOVA, with Dunnett’s posthoc analysis, comparing the different levels to the lowest level. Results were considered statistical significant for P<0.00.
Dietary Patterns in Relation to Pregnancy Outcomes
We list the results from the adjusted logistic regression analysis in Table 4, where we included IBD, CD, and UC and dietary patterns as interaction terms.
TABLE 4:
Results from Regression Analysis of all Eligible Women in the Moba Cohort 2002–2008 (n = 70.320 or 70.339, Dependent on Pregnancy Outcomes).
| SGA | PTD | |||||
|---|---|---|---|---|---|---|
| Pregnancies (total= 70.320) |
Pregnancy Outcome in IBD |
OR (95% KI)a | Pregnancies (total = 70.339) |
Pregnancy Outcome in IBD |
OR (95% KI)1 | |
| IBD*Dietary pattern | ||||||
| Prudent | ||||||
| Low (controls + IBD) | 70078 | 17 | 1 (ref.) | 70097 | 9 | 1 (ref.) |
| Middle (IBD) | 145 | 10 | 2.30 (0.92 - 5.73) | 145 | 9 | 1.16 (0.39 - 3.44) |
| High (IBD) | 97 | 8 | 0.98 (0.37 - 2.65) | 97 | 6 | 1.00 (0.34 - 2.96) |
| Western | ||||||
| Low (controls + IBD) | 70068 | 9 | 1 (ref.) | 70087 | 7 | 1 (ref.) |
| Middle (IBD) | 107 | 8 | 0.63 (0.28 - 1.51) | 107 | 4 | 0.78 (0.30 - 2.06) |
| High (IBD) | 145 | 18 | 0.52 (0.22 - 1.28) | 145 | 13 | 0.42 (0.13 - 1.35) |
| Traditional | ||||||
| Low (controls + IBD) | 70094 | 13 | 1 (ref.) | 70113 | 12 | 1 (ref.) |
| Middle (IBD) | 126 | 6 | 0.44 (0.20 - 0.97) | 126 | 7 | 1.75 (0.59 - 5.21) |
| High (IBD) | 100 | 16 | 0.23 (0.08 - 0.61) | 100 | 5 | 1.04 (0.32 - 3.43) |
| CD*Dietary pattern | ||||||
| Prudent | ||||||
| Low (controls + IBD) | 70205 | 7 | 1 (ref.) | 70232 | 4 | 1 (ref.) |
| Middle (IBD) | 65 | 6 | 2.05 (0.46 - 9.02) | 65 | 6 | 1.35 (0.23 - 7.80) |
| High (IBD) | 42 | 3 | 1.46 (0.33 - 6.53) | 42 | 2 | 1.85 (0.35 - 9.86) |
| Western | ||||||
| Low (controls + IBD) | 702050 | 2 | 1 (ref.) | 70224 | 2 | 1 (ref.) |
| Middle (IBD) | 39 | 3 | 0.25 (0.05 - 1.22) | 39 | 3 | 0.54 (0.11 - 2.78) |
| High (IBD) | 76 | 11 | 0.45 (0.11 - 1.79) | 76 | 7 | 0.91 (0.22 - 3.80) |
| Traditional | ||||||
| Low (controls + IBD) | 706230 | 8 | 1 (ref.) | 70249 | 6 | 1 (ref.) |
| Middle (IBD) | 50 | 2 | 0.62 (0.19 – 2.04) | 50 | 4 | 1.60 (0.30 - 8.55) |
| High (IBD) | 40 | 6 | 0.21 (0.04 - 1.12) | 40 | 2 | 1.45 (0.25 - 8.48) |
| UC*Dietary Pattern | ||||||
| Prudent | ||||||
| Low (controls + IBD) | 70185 | 10 | 1 (ref.) | 70204 | 5 | 1 (ref.) |
| Middle (IBD) | 80 | 4 | 2.28 (0.70 - 7.48) | 80 | 3 | 1.27 (0.31 - 5.29) |
| High (IBD) | 55 | 5 | 0.63 (0.16 - 2.51) | 55 | 4 | 0.59 (0.12 - 2.85) |
| Western | ||||||
| Low (controls + IBD) | 70183 | 7 | 1 (ref.) | 70202 | 1 (ref.) | |
| Middle (IBD) | 68 | 5 | 1.11 (0.35 – 3.51) | 68 | 3 | 1.04 (0.29 – 3.71) |
| High (IBD) | 69 | 7 | 0.63 (0.18 – 2.19) | 69 | 5 | 0.17 (0.022 – 1.46) |
| Traditional | ||||||
| Low (controls + IBD) | 705184 | 5 | 1 (ref.) | 70559 | 6 | 1 (ref.) |
| Middle (IBD) | 76 | 4 | 0.34 (0.11 - 1.07) | 76 | 3 | 1.92 (0.44 - 8.30) |
| High (IBD) | 60 | 10 | 0.23 (0.07 - 0.80) | 60 | 3 | 0.71 (0.14 - 3.75) |
aAdjusted for maternal age, BMI, educational level, total energy intake and smoking during pregnancy. The table shows the excess risk of birth outcomes of the three dietary patterns. Reference category is non-IBD women and IBD women adherent to the lowest tertile of the dietary patterns. The values associated with the middle tertiles of the dietary pattern show the risk of adverse pregnancy outcomes in women with IBD adherent to the middle dietary pattern compared to non-IBD women and women with IBD in the lowest tertile (reference). Parallel for IBD women in the highest dietary patterns. The table shows the excess risk of birth outcomes of the three dietary patterns. Reference category is non-IBD women and IBD women adherent to the lowest tertile of the dietary patterns. The values associated with the middle tertiles of the dietary pattern show the risk of adverse pregnancy outcomes in women with IBD adherent to the middle dietary pattern compared to non-IBD women and women with IBD in the lowest tertile (reference). Parallel for IBD women in the highest dietary patterns.
There were no significant differences in mean factor score for the different dietary patterns between IBD mothers with and without SGA or PTD (data not shown).
Women with IBD in the middle and highest third of the Traditional dietary pattern had a significant lower risk of giving birth to babies with SGA compared to controls and women with IBD in the lower third of the dietary pattern, for the same pregnancy outcome [OR for tertile 2 vs. tertile 1: 0.44 (95% CI: 0.20 – 0.97) and OR for tertile 3 vs. tertile 1: 0.23 (95% CI: 0.08–0.61)] (Table 4). This significant protective effect was found only in the UC subgroup [OR for tertile 3 vs. tertile 1: 0.23 (95% CI: 0.07 – 0.80)] (Table 4). These findings suggest a protective effect of the Traditional dietary pattern, reducing the odds (excess risk) of SGA with 56% and 77% among IBD women and UC women, respectively. We did not find any significant associations between dietary patterns and PTD among IBD mothers
In Table 5, we list the results from the logistic regression analyses within the IBD subgroup. A significant protective effect of the Traditional dietary pattern was found only in the UC subgroup [OR for tertile 2 vs. tertile 1: 0.21 (0.05–0.80) and OR for tertile 3 vs. tertile 1: 0.16 (0.04–0.60)] (Table 5).
Table 5:
Risk of Adverse Pregnancy Outcomes of Three Dietary Patterns
| SGA | PTD | ||||
|---|---|---|---|---|---|
| Pregnancies (total= 360) |
Pregnancy Outcome |
OR (95% KI)a | Pregnancy Outcome |
OR (95% KI)a | |
| IBD | |||||
| Prudent | |||||
| Low | 118 | 17 | 1 (ref.) | 9 | 1 (ref.) |
| Medium | 145 | 10 | 1.90 (0.70 - 5.10) | 9 | 1.66 (0.49 – 5.60) |
| High | 97 | 8 | 0.90 (0.30 - 2.61) | 6 | 1.39 (0.41 - 4.08) |
| Western | |||||
| Low | 108 | 9 | 1 (ref.) | 7 | 1 (ref.) |
| Medium | 107 | 8 | 0.52 (0.18 - 1.54) | 4 | 1.83 (0.53 – 5.92) |
| High | 145 | 18 | 0.40 (0.14 - 1.15) | 13 | 1.05 (0.31 - 3.63) |
| Traditional | |||||
| Low | 135 | 13 | 1 (ref.) | 12 | 1 (ref.) |
| Medium | 125 | 6 | 0.43 (0.18 - 1.10) | 7 | 1.24 (0.35 - 4.43) |
| High | 100 | 16 | 0.20 (0.07 - 0.60) | 5 | 0.56 (0.15 – 6.32) |
| SGA | PTD | ||||
|
Pregnancies
(total= 156) |
Pregnancy
Outcome |
Model OR (95% KI)a |
Pregnancy
Outcome |
Model OR (95% KI)a | |
| CD | |||||
| Prudent | |||||
| Low | 49 | 7 | 1 (ref.) | 4 | 1 (ref.) |
| Medium | 65 | 6 | 2.55 (0.49 - 13.23) | 6 | 4.53 (0.48 - 42.89) |
| High | 42 | 3 | 1.71 (0.33 – 8.79) | 2 | 5.66 (0.72 - 44.21) |
| Western | |||||
| Low | 41 | 2 | 1 (ref.) | 2 | 1 (ref.) |
| Medium | 39 | 3 | 0.42 (0.06 - 3.09) | 3 | 6.01 (0.44 - 81.31) |
| High | 76 | 11 | 0.73 (0.14 - 3.96) | 7 | 1.48 (0.21 - 10.59) |
| Traditional | |||||
| Low | 67 | 8 | 1 (ref.) | 6 | 1 (ref.) |
| Medium | 50 | 2 | 1.00 (0.24 – 4.09) | 4 | 3.57 (0.48 - 26.4) |
| High | 40 | 5 | 0.26 (0.05 - 1.47) | 2 | 2.26 (0.30 –17.17) |
| SGA | PTD | ||||
|
Pregnancies
(total= 204) |
Pregnancy
Outcome |
Model OR (95% KI)a |
Pregnancy
Outcome |
Model OR (95% KI)a | |
| UC | |||||
| Prudent | |||||
| Low | 69 | 10 | 1 (ref.) | 5 | 1 (ref.) |
| Medium | 80 | 4 | 1.48 (0.40 - 5.50) | 3 | 0.72 (0.14 – 3.78) |
| High | 55 | 5 | 0.46 (0.11 - 2.00) | 4 | 0.45 (0.08 - 2.61) |
| Western | |||||
| Low | 67 | 7 | 1 (ref.) | 5 | 1 (ref.) |
| Medium | 68 | 5 | 0.69 (0.17 - 2.89) | 3 | 0.92 (0.18 – 4.77) |
| High | 69 | 7 | 0.34 (0.08 - 1.44) | 5 | 0.12 (0.01 - 1.29) |
| Traditional | |||||
| Low | 68 | 5 | 1 (ref.) | 6 | 1 (ref.) |
| Medium | 76 | 4 | 0.21 (0.05 - 0.80) | 3 | 0.91 (0.18 - 4.57) |
| High | 60 | 10 | 0.16 (0.04 - 0.60) | 3 | 0.28 (0.04 - 1.83) |
*Adjusted for maternal age, BMI, educational level, total energy intake and smoking during pregnancy.
Results from regression analysis of IBD women in the Moba cohort 2002–2008 (N=360). The table shows the risk of pregnancy outcomes of the three dietary patterns. Reference category is IBD women adherent to the lowest tertile of the dietary patterns. The values of the odd ratios associated with the middle tertile of the dietary patterns shows the risk of adverse pregnancy outcomes in women with IBD compared women to IBD in the lowest tertile (reference). Parallel for women in the highest tertile.
Disease Activity Before and During Pregnancy
Of the responders to our mail-out questionnaire, 132 of 136 CD and 177 of 192 UC mothers completed questions about disease activity (88%). Flares were reported twice as often among UC (47/177, 26.6%) compared to CD mothers (18/132, 13.6%) (crude OR = 1.95, 95% CI: 1.19–3.19, p = 0.006). Of the 65 IBD mothers (65/309, 21%) who reported flares during pregnancy, 57 reported change in medication (57/65). Furthermore, 16 mothers with flares reported IBD-related hospital admission and 5 mothers IBD-related surgery.
Disease Activity and Pregnancy Outcomes
Disease activity was neither associated with PTD nor with SGA among IBD mothers (adjusted OR = 0.64, 95 % CI: 0.14 –3.01 and OR = 0.87, 95% CI: 0.28 –2.72, respectively). Disease activity was not significant in any of the fitted models and was therefore excluded from the results presented in Table 5.
DISCUSSION
The main finding in this study was that a high adherence to the Traditional dietary pattern was associated with lower likelihood of SGA in IBD mothers than in non-IBD mothers and IBD mothers with low scores on the Traditional pattern. This effect was found to be significant only in UC mothers.
Searching for predictors of adverse pregnancy outcomes in IBD has been an important task for the follow-up of pregnant IBD women in clinical care. Although disease activity has been demonstrated as a strong predictor of adverse pregnancy outcomes in IBD, several investigations have revealed that having IBD is a risk factor of its own.8–13, 36, 37 Knowing that IBD patients in general are prone to malnutrition, we hypothesized that dietary patterns during pregnancy influence the risk of adverse pregnancy outcomes in women with IBD.38, 39
To the best of our knowledge, associations between dietary patterns and pregnancy outcomes in women with IBD have not been investigated in previous research. However, a number of studies have examined the relationship between dietary patterns and pregnancy outcomes in the general population.20–22, 24, 28 The results from these studies indicate a protective effect of a dietary pattern characterized by high consumption of food items such as fruits and vegetables, olive oil, unrefined grains, and fish (a prudent or Mediterranean diet) on preterm delivery and birth weight.21, 24, 25, 27, 28 A previous study in MoBa found that higher adherence to a prudent, as well as a traditional dietary pattern, was associated with reduced risk of PTD.21 However, in the present study no associations between the dietary patterns and PTD were indicated in IBD mothers.
A study within the Danish National Birth Cohort extracted two exploratory dietary patterns and used the scores to assign participants into mutually exclusive groups reflecting a predominant prudent diet, a predominant Western diet, and a mixed diet group. The results indicated that women in the Western diet group had increased risk of SGA.24
Diet has been suggested to play a role in the etiology of IBD and may act as a modifier of disease activity.5–7, 40 A Western diet has been associated with changes in the microflora and the intestinal barrier and,thereby, causing intestinal inflammation. Diet rich in fibers from fruits, vegetables, and unrefined cereals may modulate the microflora, resulting in higher concentration of short chain fatty acids that has been shown to be protective against intestinal inflammation.41 We found higher mean factor scores in the Western dietary pattern and lower scores in the Traditional pattern in IBD mothers than in non-IBD mothers (Table 3). However, high scores in the Western dietary pattern did not influence the risk of SGA and PTD.
An earlier MoBa-study by Hillesund et al. revealed a link between high adherence to the New Nordic Diet (NND), which shares similarities with the Traditional diet, and SGA in the general population. High adherence to NND, as compared with low, was associated with reduced odds of SGA.42 The present study showed similar effects of Traditional diet among IBD mothers, high adherence was associated with reduced odds of SGA.
Patients with IBD are vulnerable to nutrition deficiencies due to an increased loss, drug-nutrient interaction, and decreased absorption from the ileum.1, 18 Furthermore, exclusion of certain foods or food groups from the diet has been suggested as a main cause of malnutrition among IBD patients.40, 43–45 In the present study, women with IBD had a significantly lower intake of the Traditional diet than non-IBD women. Deficiencies and alterations in diet may be of great importance in pregnancy, with nutrition being an important predictor for adverse pregnancy outcomes and future life course in the offspring.15, 20–22, 24, 28 In addition, all nutrient requirements are increased in pregnancy.46 This suggests several mechanisms for biological effects of dietary patterns on pregnancy.
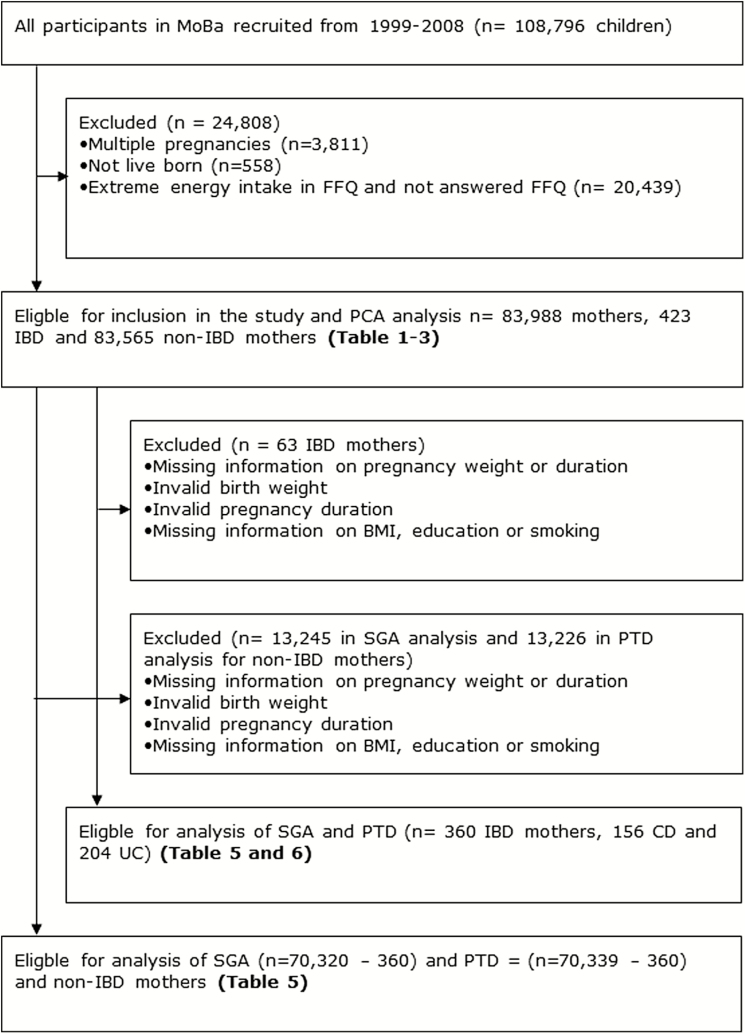
FIGURE 1.
Flow chart showing selection of participants from The Norwegian Mother and Child Cohort Study (MoBa).30
The Traditional dietary pattern was found to lower the risk of SGA significantly in IBD women compared to controls, indicating an interaction between IBD and the Traditional dietary pattern. It is possible that the Traditional pattern correlates with nutrients especially important in IBD and in fetal development. Such nutrients may be protein, calcium, zinc, or folic acid, in which IBD patients have been found to have deficiencies.18 In the study by Englund-Ögge et al., potassium, magnesium, protein, and dietary fiber were found to correlate with the scores on a traditional dietary pattern similar to the one in our study 21. The protective effect of the Traditional dietary pattern was found in the UC group only. This is somewhat surprising in a nutrition perspective, as nutrition deficiencies are more prevalent in CD patients given the location of the disease.47, 48
Active disease in pregnancy has been found to be a potential risk factor for adverse pregnancy outcomes in maternal IBD.49–51 Disease activity is an important determinant of the nutritional status of the mother, as active disease may impair absorption and lead to an increased loss of nutrients through diarrhea.18 The requirement of nutrients may be increased in active state of the disease.
Disease activity may influence weight, energy intake, and diet of the mother.52, 53 We adjusted for BMI and total energy intake. An inadequate transfer of nutrients from the placenta may impair growth and development in the fetus. This proposes disease activity both as a confounder and a potential mediator between diet and pregnancy outcomes. In our analyses, we did not find any significant effects from disease activity on pregnancy outcomes.
Many IBD patients report specific dietary preferences related to symptom relief.40, 43–45 Foods rich in dietary fiber, such as fruits and vegetables are frequently reported as worsening symptoms.40 Foods rich in fiber may be hard to digest during active inflammation. Dairy products are also frequently excluded during active disease due to a transient lactose intolerance.44, 54 The Traditional dietary pattern has low factor loadings for berries, raw fruits and vegetables, and dairy products. It is possible that during active disease, more women will adhere to the Traditional dietary pattern. In this scenario, disease activity may act as a confounder, or the Traditional diet may act as an effect modifier between disease activity and pregnancy outcome. Nevertheless, in our study, disease activity did not confound the association between dietary patterns and birth outcomes.
Strengths and Weaknesses
The MoBa study is a nationwide study including women with a broad range of characteristics.29 The strengths of the present study are the large sample size and the number of women with IBD. In addition, this study separates CD and UC. Nevertheless, IBD is a rare disease, and relatively few births were registered with the maternal condition; only 360 were included in the analyses. Relatively small numbers of subjects can potentially produce large confidence intervals for the effect measures. However, the total number of women with IBD in the present study is in accordance with the prevalence rates in the general population.55, 56
This study is of an observational design and we cannot establish causality. Our results reflect associations between dietary pattern and adverse pregnancy outcomes in IBD mothers. In addition, the results cannot easily be pertained to IBD women outside Scandinavia, who may adhere to different dietary patterns and have different genetics (influencing the disease, and diet/nutrient-disease-interactions). The use of questionnaires may impair the validity of the study. However, the prospective cohort design minimizes potential differential misclassification errors of diet, because dietary habits were measured before pregnancy outcomes. Differential misclassification of pregnancy outcome in relation to the exposure is very unlikely given the fact that outcome information is attained from MBRN, which has no direct linkage to the FFQ.31
The FFQ covers the first four to five months of pregnancy. Although the adherence to dietary patterns are shown to be stable throughout pregnancy 57, it is still possible that we did not obtain the actual dietary habits in the last part of the pregnancy. Furthermore, recalling, averaging, and reporting food intakes are rather complex cognitive tasks. This is even more challenging when the questionnaire asks for intake since the beginning of pregnancy, which is a time period when most women experience nausea and changes in taste, smell, and appetite.32
Dietary patterns are considered a valuable method when studying associations between diet and health outcomes in pregnant populations (58). Analysis of dietary patterns may give a wider perspective of the importance of nutrition in pregnancy in women with IBD, and may provide more relevant and applicable information than investigation of single nutrients or food groups alone. Nutrients and foods are not consumed independently of each other, and may interact in different ways. The three dietary patterns extracted from the factor analysis only accounted for 10.98% of the total variance in food intakes in MoBa. However, the variance explained by the dietary patterns is similar to that in previous studies.20, 21, 57
There are considerable limitations with the study design concerning disease activity, which cannot be corrected. First, the information regarding disease activity was collected retrospectively in 2013 through a survey that introduced a significant time lag of 5 to 13 years between pregnancy and the survey. Second, disease activity was self-reported. The mothers with IBD were asked to report disease activity before and during pregnancy by three options: change in medication, IBD-related surgery, or hospital admission. These options are proxies for disease activity53, 59, 60, usually used in retrospective studies and register studies and donot represent objective markers. These proxies donot include mild and moderate disease activity. We decided to define disease activity as the fulfillment of one of these three options, because we believe that these events during pregnancy would be easier to recall compared to grading level of disease from mild to severe disease.
The retrospective nature of the data collection on disease activity, along with the time lag between pregnancy and questionnaire, are associated with recall bias. The data on disease activity therefore must be treated with caution. Furthermore, prepregnancy factors including medications the IBD mothers were on, as well as disease activity, could have influenced the results including food choices, regardless of the presented data on disease activity.
Conclusion
This study revealed, for the first time to our knowledge, that women with IBD had a lower adherence to a prudent and traditional dietary pattern, and a higher adherence to a western dietary pattern, than non-IBD controls. Furthermore, we found that middle and high adherence to a traditional dietary pattern, characterized by a consumption of lean fish and fish products, potatoes, rice porridge, cooked vegetables, and gravy, was associated with a lower risk of SGA in women with UC compared to non-IBD mothers and IBD mothers with low adherence. Disease activity did not influence these associations.
Our study has an observational design and includes a relative small number of IBD mothers, and the results should therefore be considered as preliminary data.
However, our findings indicate that dietary quality may be of importance for the follow-up of the pregnant IBD patient, and may serve as a basis for further research.
Further research is required to explore the differences between the dietary patterns on a macro- and micro nutrient level.
ACKNOWLEDGEMENTS
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1). We are grateful to all the participating families in Norway who take part in this on-going cohort study.
REFERENCES
- 1. Abraham C, Cho JH. Mechanisms of disease: Inflammatory Bowel Disease. N Engl J Med. 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loftus EV. Clinical Epidemiology of Inflammatory Bowel Disease: Incidence, Prevalence, and Environmental Influences. Gastroenterology. 2004;126:1504–17. [DOI] [PubMed] [Google Scholar]
- 3. Ng SC, Bernstein CN, Vatn MH et al. . Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–49. [DOI] [PubMed] [Google Scholar]
- 4. Schultz M, Butt AG. Is the north to south gradient in inflammatory bowel disease a global phenomenon?Expert Rev. Gastroenterol Hepatol. 2012;6:445–7. [DOI] [PubMed] [Google Scholar]
- 5. Spooren CEGM, Pierik MJ, Zeegers MP et al. . Review article: the association of diet with onset and relapse in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:1172–87. [DOI] [PubMed] [Google Scholar]
- 6. Racine A, Carbonnel F, Chan SM et al. . Dietary patterns and risk of inflammatory bowel disease in Europe: Results from the EPIC Study. Inflamm BowelDis. 2016;22:345–54. [DOI] [PubMed] [Google Scholar]
- 7. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–73. [DOI] [PubMed] [Google Scholar]
- 8. Kornfeld D, Cnattingius S, Ekbom A. Pregnancy outcomes in women with inflammatory bowel disease - a population-based cohort study. Am J Obstet Gynecol. 1997;177:942–6. [DOI] [PubMed] [Google Scholar]
- 9. Mahadevan U, Sandborn WJ, Hakimian S et al. . Pregnancy outcomes in women with inflammatory bowel disease: a large community-based study from Northern California. Gastroenterology. 2007;133:1106–12. [DOI] [PubMed] [Google Scholar]
- 10. Bengtson MB, Solberg IC, Aamodt G et al. . Relationships between inflammatory bowel disease and perinatal factors: both maternal and paternal disease are related to preterm birth of offspring. Inflamm Bowel Dis. 2010;16:847–55. [DOI] [PubMed] [Google Scholar]
- 11. Norgard B, Fonager K, Sørensen HT et al. . Birth Outcomes of Women With Ulcerative Colitis: A Nationwide Danish Cohort Study. Am J Gastroenterol. 2000;95:3165–71. [DOI] [PubMed] [Google Scholar]
- 12. Fonager K, Sørensen HT, Olsen J et al. . Pregnancy Outcome for Women With Crohn’s Disease: A Follow-up Study Based on Linkage Between National Registries. Am J Gastroenterol. 1998;93:2426–31. [DOI] [PubMed] [Google Scholar]
- 13. Cornish J, Tan E, Teare J et al. . A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2006;56:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng SW, Mahadevan U. Management of inflammatory bowel disease in pregnancy. Expert Rev Clin Immunol. 2013;9:161–74. [DOI] [PubMed] [Google Scholar]
- 15. Barker DJP, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2:105–12. [DOI] [PubMed] [Google Scholar]
- 16. Godfrey KM, Barker DJP. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:13448–528. [DOI] [PubMed] [Google Scholar]
- 17. Geraghty AA, Lindsay KL, Alberdi G et al. . Nutrition during pregnancy impacts offspring’s epigenetic status - evidence from human and animal studies. Nutrition and Metabolic Insights. 2015;8:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goh J, O’Morain CA. Review article: nutrition and adult inflammatory bowel disease. Aliment Pharmacol Ther. 2003;17:307–20. [DOI] [PubMed] [Google Scholar]
- 19. Gassull MA, Cabré E. Nutrition in inflammatory bowel disease. Curr Opin XClin NutrMet Care. 2001;4:561–9. [DOI] [PubMed] [Google Scholar]
- 20. Brantsæter AL, Haugen M, Samuelsen SO et al. . A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant norwegian women. J Nutr. 2009;139:1162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Englund-Ögge L, Brantsæter AL, Sengpiel V et al. . Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ. 2014;348 ;g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haugen M, Meltzer HM, Brantsæter AL et al. . Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet Gynecol Scand. 2008;87:319–24. [DOI] [PubMed] [Google Scholar]
- 23. Martin CL, Sotres-Alvarez D, Siega-Riz AM. Maternal Dietary Patterns during the Second Trimester Are Associated with Preterm Birth. J Nutr. 2015;145:1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB et al. . Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr. 2008;62:463–470. [DOI] [PubMed] [Google Scholar]
- 25. Akbari Z, Mansourian M, Kelishadi R. Relationship of the intake of different food groups by pregnant mothers with the birthweights and gestational age: need for public and individual eductational programs. J Edu Health Promot[Internet]. 2015; 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saunders L, Guldner L, Costet N et al. . Effect of a Mediterranean Diet during Pregnancy on Fetal Growth and Preterm Delivery: Results From a French Caribbean Mother-Child Cohort Study (TIMOUN). Paediatr Perinat Epidemiol. 2014;28:235–244. [DOI] [PubMed] [Google Scholar]
- 27. Khoury J, Henriksen T, Christophersen B et al. . Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am J Obstet Gynecol. 2005;193:1292–301. [DOI] [PubMed] [Google Scholar]
- 28. Colón-Ramos U, Racette SB, Ganiban J et al. . Association between Dietary Patterns during Pregnancy and Birth Size Measures in a Diverse Population in Southern US. Nutrients. 2015;7:1318–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magnus P, Nystad W, Stoltenberg C et al. . MoBa Study G: Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006:1146–50. [DOI] [PubMed] [Google Scholar]
- 30. Magnus P, Birke C, Vejrup K et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa)2016. 2016;45(2):382–8. [DOI] [PubMed]
- 31. Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–9. [PubMed] [Google Scholar]
- 32. Meltzer HM, Brantsæter AL, Ydersbond TA et al. . Methodological challenges when monitoring the diet of pregnant women in a large study: experiences from the Norwegian Mother and Child Cohort Study (MoBa). Maternal & Child Nutrition. 2008;4:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brantsæter AL, Haugen M, Alexander J et al. . Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Maternal & Child Nutrition. 2008;4:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Newby PK, Tucker KL. Empircally derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- 35. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79:440–9. [PubMed] [Google Scholar]
- 36. Bush MC, Patel S, Lapinski RH et al. . Perinatal outcomes in inflammatory bowel disease. J Matern Fetal Med. 2004;15:237–41. [DOI] [PubMed] [Google Scholar]
- 37. Morales M, Berney T, Jenny A et al. . Crohn’s disease as a risk factor for the outcome of pregnancy. Hepatogastroenterology 2000;47:1595–8. [PubMed] [Google Scholar]
- 38. Riordan AM, Hunter JO, Cowan RE et al. . Treatment of active Crohn’s disease by exclusion diet: East Anglian multicentre controlled trial. Lancet. 1993;342:1131–4. [DOI] [PubMed] [Google Scholar]
- 39. Lucendo AJ, De Rezende LV. Importance of nutrition in inflammatory bowel disease. WJG. 2009;15:2081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen AB, Lee D, Long M et al. . Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013;58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruemmele FM. Role of Diet in Inflammatory Bowel disease. Ann Nutr Metab. 2016;68:33–41. [DOI] [PubMed] [Google Scholar]
- 42. Hillesund ER, Bere E, Haugen M et al. . Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth - a study performed in the Norwegian Mother and Child Cohort Study (MoBa). PHN. 2014;17:1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zallot C, Quilliot D, Chevaux JB et al. . Dietary Beliefs and Behavior Among Inflammatory Bowel Disease Patients. Inflamm Bowel Dis. 2013;19:66–72. [DOI] [PubMed] [Google Scholar]
- 44. Lopes MB, Rocha R, Lyra AC et al. . Restriction of dairy products; a reality in inflammatory bowel disease patients. Nutrición Hospitalaria. 2013;29:575–81. [DOI] [PubMed] [Google Scholar]
- 45. Hwang C, Ross V, Mahadevan U. Popular Exclusionary Diets for Inflammatory Bowel Disease: The Search for a Dietary Culprit. Inflamm.Bowel Dis. 2014;20:732–41. [DOI] [PubMed] [Google Scholar]
- 46. Williamson C, Wyness L. Nutritional requirements in pregnancy and use of dietary supplements. Journal of Primary Care & Community Health. 2013;86:44–7. [PubMed] [Google Scholar]
- 47. Filippi J, Al-Jaouni R, Wiroth JB et al. . Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis. 2006;12:185–91. [DOI] [PubMed] [Google Scholar]
- 48. Sousa Guerreiro C, Cravo M, Costa AR et al. . A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: a case-control study. Am J Gastroenterol. 2007;102:2551–6. [DOI] [PubMed] [Google Scholar]
- 49. Bar-Gil Shitrit A, Grisaru-Granovsky S, Ben Ya’acov A et al. Management of Inflammatory Bowel Disease During Pregnancy:2016. 2016;61(8):2194–204. [DOI] [PubMed]
- 50. Hanan IM. Inflammatory bowel disease in the pregnant woman. Compr Ther. 1993;19:91–5. [PubMed] [Google Scholar]
- 51. Schulze H, Esters P, Dignass A. Review article: the management of Crohn′s disease and ulcerative colitis during pregnancy and lactation. Aliment Pharmacol Ther. 2014;40:991–1008. [DOI] [PubMed] [Google Scholar]
- 52. Bengtson MB, Aamodt G, Mahadevan U et al. . Inadequate Gestational Weight Gain, the Hidden Link Between Maternal IBD and Adverse Pregnancy Outcomes: Results from the Norwegian Mother and Child Cohort Study. Inflammatory bowel diseases. 2017;23(7):1225–33. [DOI] [PubMed] [Google Scholar]
- 53. Oron G, Yogec Y, Shcolnick S. Inflammatory bowel disease: risk factors for adverse pregnancy outcome and the impact of maternal weight gain. J J Matern Fetal Med. 2012;25:2256–60. [DOI] [PubMed] [Google Scholar]
- 54. Mishkin S. Dairy sensitivity, lactose malabsorption, and e limination diets in inflammatory bowel disease. Am J Clin Nutr. 1997;65:564–7. [DOI] [PubMed] [Google Scholar]
- 55. Moum B, Vatn MH, Ekbom A et al. . Insidence of Crohn’s disease in four counties in Southeastern Norway, 1990–93. A prospective population-based study. Scand J Gastroenterol. 1996;31:355–61. [DOI] [PubMed] [Google Scholar]
- 56. Moum B, Vatn MH, Ekbom A et al. . Insidence of ulcerative colitis and indeterminate colitis in four countries of southeastern Norway, 1990–93. A prospective population-based study. Scand J Gastroenterol. 1996;31:362–6. [DOI] [PubMed] [Google Scholar]
- 57. Cúco G, Fernández-Ballart J, Sala J et al. . Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur J Clin Nutr. 2006;60:364–71. [DOI] [PubMed] [Google Scholar]
- 58. Northstone K, Emmet PM, Rogers I. Dietary patterns in pregnancy and associations with nutrient intakes. Br J Nutr. 2007;99:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stephansson O, Larsson H, Pedersen L et al. . Crohn’s disease is a risk factor for preterm birth. CGH. 2010;8:509–15. [DOI] [PubMed] [Google Scholar]
- 60. Stephansson O, Larsson H, Pedersen L. Congenital abnormalities and other birth outcomes in children born to women with ulcerative colitis in Denmark and Sweden. Inflammy Bowel Dis. 2011;17:795–801. [DOI] [PubMed] [Google Scholar]