Abstract
One of the best characterized mouse models of the inflammatory bowel diseases (IBD; Crohn’s disease, ulcerative colitis) is the CD4+CD45RBhigh T cell transfer model of chronic colitis. Following our relocation to Texas Tech University Health Sciences Center (TTUHSC), we observed a dramatic reduction in the incidence of moderate-to-severe colitis from a 16-year historical average of 90% at Louisiana State University Health Sciences Center (LSUHSC) to <30% at TTUHSC. We hypothesized that differences in the commensal microbiota at the 2 institutions may account for the differences in susceptibility to T cell-induced colitis. Using bioinformatic analyses of 16S rRNA amplicon sequence data, we quantified and compared the major microbial populations in feces from healthy and colitic mice housed at the 2 institutions. We found that the bacterial composition differed greatly between mice housed at LSUHSC vs TTUHSC. We identified several genera strongly associated with, and signficantly overrepresented in high responding RAG-/- mice housed at LSUHSC. In addition, we found that colonization of healthy TTUHSC RAG-/- mice with feces obtained from healthy or colitic RAG-/- mice housed at LSUHSC transferred susceptibility to T cell-induced colitis such that the recipients developed chronic colitis with incidence and severity similar to mice generated at LSUHSC. Finally, we found that the treatment of mice with preexisting colitis with antibiotics remarkably attenuated disease. Taken together, our data demonstrate that specific microbial communities determine disease susceptibility and that manipulation of the intestinal microbiota alters the induction and/or perpetuation of chronic colitis.
Keywords: inflammatory bowel diseases, Crohn’s disease, ulcerative colitis, microbiota, mouse models
INTRODUCTION
Animal models of the inflammatory bowel diseases [(IBD); Crohn’s disease (CD), ulcerative colitis (UC)] have been invaluable in advancing our understanding of the immunopathologic mechanisms responsible for chronic intestinal inflammation.1, 2 Following more than 2 decades of investigations using a variety of genetically-engineered, spontaneous, and immune-manipulated mouse models of IBD, investigators have concluded that chronic intestinal inflammation results from a dysregulated immune response to intestinal microbiota in genetically susceptible mice.3 The apparent interaction among genetics, the immune system, and intestinal microbiota is not surprising given the results from genome-wide association studies of patients with IBD.4 More than 160 different polymorphisms/susceptibility loci have been identified in patients with CD or UC.4 In CD, several susceptibility loci are known to be important for recognition and/or elimination of intracellular bacteria including nucleotide-binding oligomerization domain 2 (NOD2), autophagy-related protein 16-1 (ATG16L1), and immunity-related GTPase M (IRGM).4, 5 These genetic risk factors together with polymorphisms related to epithelial barrier function and IL-10, IL-17, and IL-23 signaling suggest that CD may develop because of defects in response against microorganisms.4, 5 Indeed, the low concordance rates for CD (<40%) or UC (<20%) in genetically identical twins strongly suggests that certain environmental factors (eg, intestinal microbiota) are important in the induction and perpetuation of chronic intestinal inflammation in genetically susceptible individuals.6, 7 Investigators have found in both mouse models of IBD and in human CD and UC, major alterations in luminal and mucosa-associated microbial communities resulting in a situation called dysbiosis, strongly suggesting that intestinal bacteria play a major role in driving IBD in genetically susceptible mice or humans.8–10 Despite the intense interest in the role that dysbiosis may play in the immuno-pathogenesis of chronic intestinal inflammation, it is currently not clear whether dysbiosis is a cause or consequence of chronic tissue inflammation.
One of the best characterized mouse models of IBD is the CD45RBhigh T cell transfer model of chronic colitis.11, 12 Adoptive transfer of naive CD4+CD45RBhigh T cells from healthy wild-type (WT) mice into recombinase activating gene-1 or -2-deficient (RAG-1-/- or RAG-2-/-) recipients generates large numbers of disease-producing Th1 and Th17 effector cells with little or no production of regulatory T cells (Tregs).11 Once generated, these colitogenic effector cells home to the colon and other tissues where they induce chronic and unrelenting colitis within 6–8 weeks post T cell transfer.11, 12 Due to the conversion of the naive T cells to disease-producing effector cells in the absence of Tregs, colitis will develop in most T cell-deficient recipients (eg, SCID, RAG-1-/-, RAG-2-/-, athymic nude, TCRβ-/-x δ-/-) following T cell transfer.3, 11, 13 In addition, it is well known that intestinal bacteria are required for induction of disease in this and several other mouse models of IBD.14, 15 The T cell transfer model has also proven to be particularly useful in understanding the critical role that Tregs play in suppressing the activation of naive T cells by commensal intestinal bacteria.11, 16 A non-exhaustive search of PubMed identifies more than 280 studies that have appeared over the past 2 decades using this mouse model of IBD. Unlike other mouse models of chronic intestinal inflammation that develop spontaneous and, in some cases, highly variable disease, the vast majority of published studies using the T cell transfer model consistently report similar incidence and severity of colitis. Surprisingly, there has been no metagenomic analysis of the colonic microbiome before and following induction of colitis in this model.
Following our relocation to Texas Tech University Health Sciences Center (TTUHSC), we witnessed a surprising change in the phenotype of our T cell transfer model of IBD. We observed a significant reduction in our 16-year historical incidence of moderate-to-severe colitis of ~90% at Louisiana State University Health Sciences Center (LSUHSC)11 to a much more variable disease with an incidence of moderate-to-severe disease of ~30% at TTUHSC. This change in phenotype occurred despite the fact that animal vendor, housing conditions and T cell preparations were virtually identical at both institutions. While alterations in mouse model phenotype following changes in animal husbandry, institution, and/or animal source have been appreciated for a number of years, it has only been relatively recently that investigators have begun to systematically define how and why these types of changes affect disease phenotype in mouse models of autoimmune and chronic inflammatory diseases.15, 17–19 For example, it is well known that the onset and severity of chronic colitis that develops spontaneously in IL-10-/- mice may be quite variable depending upon the strain, background, and host microbial composition.20, 21 More recent studies by Yang and coworkers reported that when identical C57BL/6J IL-10-/- mice were housed under specific pathogen-free conditions (SPF) at 2 different animal facilities within the same city, 1 group of mice responded to Helicobacter hepaticus infection with robust typhlocolitis, whereas the other group failed to develop significant gut inflammation.22 They concluded that differences in the microbiota were a major contributor to the susceptibility of developing colonic inflammation. A similar set of observations have been reported by another group using mice rendered deficient in the flagellin receptor toll-like receptor 5 (TLR5-/-).23, 24 These investigators found that TLR5-/- mice develop a spontaneous and highly variable colitis with only 10% of the mutant mice exhibiting overt colitis. Furthermore, they demonstrated that the few mice that did develop chronic colitis displayed a dysbiosis that was characterized by enrichment of Proteobacteria when compared to their noncolitic TLR5-/- littermates. The vast majority of published studies using the T cell transfer model have reported much less variation with respect to incidence and severity of colonic inflammation than other spontaneous models of IBD. However, there are published studies demonstrating that adoptive transfer of CD45RBhigh T cells into lymphopenic recipients either fails to induce colitis or induces only mild-to-moderate colonic inflammation that can be enhanced by infection with certain pathobionts such as Helicobacter hepaticus.13, 25–30
The change in phenotype coupled with the lack of any detailed microbial data in this model prompted us to quantify and compare the colonic microbiota in healthy and colitic mice obtained from TTUHSC and LSUHSC and determine whether manipulation of colonic microbiota may alter the incidence, severity, and/or perpetuation of chronic colitis at our current institution.
MATERIALS AND METHODS
Animals and Housing
All animal experiments were conducted according to the protocols approved by the TTUHSC Institutional Animal Care and Use committee . Male C57Bl/6J recombinase activating gene-1 deficient (RAG-1-/-) mice and wild-type C57Bl/6J (WT) mice were obtained from the Jackson Laboratories (Bar Harbor, ME) at 6 weeks of age and were housed for 2 weeks in the animal care facilities associated with TTUHSC and LSUHSC in standard microisolator cages (see below) at 5 mice per cage in standard microisolator conditions. Freshly collected feces from mice housed at LSUHSC were provided by our collaborator (Dr. Dmitry V. Ostanin). Both facilities maintain animals in an environment that is free of specified microbial agents that could interfere with the research carried out at the institutions and maintained on a 6:00 am-6:00 pm light cycle. Both animal facilities maintain sentinel testing programs that screen mice semiannually for mouse hepatitis virus, mouse parvovirus (MPV1, MPV2, MPV3), minute virus of mice, mouse norovirus, Theiler’s murine encephalomyelitis virus, mouse rotavirus, Sendai virus, Mycoplasma pulmonis, pneumonia virus of mice, reovirus 3, Lymphocytic choriomeningitis virus, Ectromelia virus, and ectoparasites. All animals in our study were found to be free of the microorganisms mentioned above for both institutions. All animals housed at TTUHSC were maintained in microisolator housing in standard size cages (12 x 6.25 inches) with a Reemay filter (spunbonded polyester nonwovens, Farmingdale, NJ) ventilated on a Tecniplast vent rack (Varese, Italy) connected to the Tecniplast Smart Flow air circulation system. Mice housed at LSUHSC were maintained in an identical manner except that an Allentown air circulation system was used. The rooms and each individual cage at both institutions were subjected to positive pressure relative to the outside environment to prevent microbial contamination. All cages at both institutions were sterilized and furnished with wood chip bedding (7090 Sani-Chips, Harlan® Laboratories Inc., Indianapolis, IN) and cotton material for nest construction. Animals maintained at TTUHSC were provided irradiated Prolab Isopro RMH 3000 (LabDiet, St. Louis, MO) rodent chow and nonacidified tap water ad libitum, whereas mice housed at LSUHSC were given Teklad (Envigo) 7012 LM-485 rodent chow (Envigo, City) and nonacidified tap water ad libitum (Table S1).
Induction of Chronic Colitis
Because mice from the same cage share their microbiota via coprophagy, we reasoned that multiple rounds of mixed cohousing would minimize these potential “cage effects” for our in vivo experiments. Thus, mice were subjected to a total of 4 rounds of mixed cohousing before their randomization into the different treatment groups. Briefly, mice were housed 4 to a cage upon their arrival from the vendor. Following 2–3 days of housing, mice underwent an additional 3 rounds of mixed cohousing over the course of 7–10 days such that all mice would possess a similar microbiota. Randomization of mice into the different treatment groups was accomplished using the Stat Trek random number generator (http://stattrek.com/statistics/random-number-generator.aspx). This PC-based calculator uses a statistical algorithm to produce random numbers for each mouse in the study.
Chronic colitis was induced in RAG-/- recipients via adoptive transfer of naive (CD4+CD45RBhigh) T cells using our well-established protocol.11 Briefly, spleens were removed from healthy male C57Bl/6J WT mice immediately upon killing and placed in FACS buffer [PBS containing 4% fetal calf serum (FCS) (Atlanta Biologicals)] on ice. Spleens were gently ground between 2 frosted slides into a cell suspension and passed through a 70 μm cell strainer (Falcon, Corning, NY) using a 10 mL syringe with a 26G x ¾ needle (BD, Franklin Lakes, NJ), CD4 cells were enriched by negative selection using Dynabeads Untouched Mouse CD4 Cell kit (Life Technologies AS, Norway) according to manufacturer’s instructions. CD4 cells were then stained with allophycocyanin (APC) rat antimouse CD4 and phycoerythrin (PE) rat antimouse CD45RB antibodies (BD Pharminigen Franklin Lakes, NJ). CD4+ T cells were sorted using the BD FACSJAZZ cell sorter for the brightest 40% (CD45RBhigh cells) and dimmest 15% (CD45RBlow cells). Male C57BL/6J RAG-/- recipients were then injected (ip, 0.5 mls) with 5 x 105 CD4+CD45RBlow T cells to be used as controls and with CD4+CD45RBhigh T cells to induce chronic colitis. For some studies, CD4+CD45RBhigh→ RAG-/- mice were generated at LSUHSC and shipped to TTUHSC at 2 weeks following T cell transfer where they were housed for an additional 6 weeks.
Fecal Microbial Transplant
In addition to the conventional model of T cell induced chronic colitis, we also wished to assess the effects of colonizing RAG-/- mice housed at TTUHSC with feces obtained from healthy RAG-/- mice or from CD4+CD45RBhigh →RAG-/- mice with active colitis (6–8 weeks post T cell transfer) that were generated at LSUHSC. Colonization of RAG-/- mice housed at TTUHSC with feces obtained from LSUHSC mice was accomplished by Fecal Microbial Tranplant (FMT) using a minor modification of the method described by Markle et al.31 Briefly, pellets were weighed, transferred into a sterile container, suspended in sterile room temperature water, and gently dispersed using the PowerGen 125 homogenizer (Fisher Scientific; Waltman, MA) to create a homogeneous suspension. Suspensions were then placed on ice. One 250 microliter (250μl) aliquot of fecal suspension was delivered to each mouse via gastric gavage using sterile 20G X 1.5” single use feeding needles (Cadence Science; Stauton, VA). Gastric gavage with fecal suspension was repeated 24 hours later such that each mouse received a total of 2.0 mg feces/g body weight via the 2 transfers.
Antibiotic Adminstration and/or Fecal Microbial Transplantation
Chronic colitis was induced as described above. Briefly, RAG-/- mice were colonized with feces obtained from colitic mice housed at LSUHSC followed by adoptive tranfer of 5 × 105 CD4+CD45RBhigh T cells. At 4 weeks post T cell transfer, mice were randomized into 4 groups (n = 8 mice per group) that received no treatment (control group), an antibiotic cocktail (ABX), FMT, or both ABX and FMT (ABX+FMT). Previous studies have demonstrated that >80% of the mice develop chronic colitis at 4 weeks post T cell transfer.32 Mice in the ABX group received (po, ad libitum) a cocktail of 1.125 g of Aspartame (Asp-Phe- methyl ester; Sigma-Aldrich), 0.15g of vancomycin (Sigma-Aldrich), and 0.3 g of neomycin (Sigma-Aldrich) in 300 ml of sterile water according to the method of Shen et al.33 Briefly, mice received the ABX cocktail for 60 hours at which time the ABX cocktail was replaced with drinking water containing 10% polyethelyne glycol (PEG) that was adminstered (po, ad libitum) to fasting mice for an additional 12 hours. Following PEG treatment, mice were again placed on ABX-containing drinking water and allowed free access to food for an additional 7 weeks. The FMT group received 5 daily gavages followed by 1 weekly gavage until the end of the experiment with feces from a healthy WT mouse. All FMT treatments were performed using fecal pellets obtained from 1 healthy WT mouse that was the same sex and age as treatment groups. The ABX+FMT group received the same ABX protocol as described above followed by the 5 daily and 1 weekly FMT protocol (same as FMT only group).
Fecal and Tissue Collection and Preparation
For mice housed at TTUHSC, colonic fecal pellets were removed from the colons of each mouse immediately following killing using aseptic technique. Briefly, colons were excised, opened longitudinally, and fecal pellets removed and quick frozen in liquid nitrogen. All fecal samples were stored at -80°C. Colonic tissue was saved for histopathological evaluation and tissue leukocyte determinations (see below). For mice housed at LSUHSC, fresh fecal pellets were obtained from healthy RAG-/- mice or from CD4+CD45RBhigh→ RAG-/- mice with active colitis (6–8 weeks post T cell transfer). Because of the nature of these interinstitutional studies using mice from ongoing studies at LSUHSC, collection of individual fecal samples from each animal was not possible. Thus, fresh fecal pellets from either healthy or colitic RAG-/- mice housed at LSUHSC were collected from approximately 10–12 mice from each group and placed into 1 of 2 sterile collection tubes for each group. The fecal pellets were immediatedly frozen in liquid nitrogen and stored at -80°C for shipment (on dry ice) to TTUHSC for further analyses. Microbial DNA was isolated from ~100 mg of fecal pellets from each mouse generated at TTUHSC and from 5 random samples of fecal pellets (~100 mg each) from the pooled healthy or colitic RAG-/- mice collected at LSUHSC. The MoBio PowerSoilDNA Isolation Kit (Carlsbad, CA) and FastPrep 24 bead beater (MP Biomedicals. Santa Ana, California) were used to extract microbial DNA from each fecal sample. Each sample was subjected to 3 rounds of bead beating for 60 seconds each at 4.0 m/s (resting one minute between rounds) in the PowerBead Tubes following the manufacturer’s guidelines.
Bacterial 16S rRNA Sequencing
Fecal DNA was sequenced using an Illumina Miseq desktop analyzer (Illumina), San Diego, CA. We used dual index paired-end sequencing strategy and prepared the samples for high throughput sequencing using a 2-step PCR approach according to the protocol supplied by Illumina (Illumina 16S Metagenomic Sequencing Library Preparation). The variable region V3 and V4 of bacterial 16S rRNA gene was amplified using universal bacterial primer set 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) that contained Illumina adaptors.34 The PCR products were then verified on 1.5% agarose gel to test the integrity. Index PCR was performed using Nextera XT index kit v2 (Illumina) in which a unique index for each sample was attached to the Illumina sequencing adapter on each end. Following PCR, the amplicon products were cleaned using AMPure XP beads (Beckman Coulter Inc.) following the manufacturer’s instructions and eluted in 50 μL of 10 mM Tris buffer (pH 8.5). The cleanup products were then quantified in triplicates on Qubit 3.0 fluorometer using dsDNA HS Assay kit (Invitrogen, Carlsbad, CA). DNA concentration in nM was calculated for each sample based on the average of triplicates of concentration (ng/μL) and an average library size of 630 bp. All the libraries were then normalized by diluting to 4 nM using 10 mM Tris (at pH 8.5). Pooling of libraries was performed by aliquoting 5 μL each of 4 nM diluted library. The library pool with unique indices was run on the Agilent 2200 tape station (Agilent Technologies, Santa Clara, CA) using D1000 Screen Tape (Agilent Technologies, Santa Clara, CA) following the manufacturer’s instructions to get the final size of the library pool. The final library concentration was determined by fluorometric quantification using Qubit (Invitrogen). Sequencing was performed using the MiSeq Reagent Kit v3 (600-cycle) (Illumina) and the cartridge and reagents were handled according to the manufacturer’s instructions. The library was denatured using 0.2 N NaOH and diluted to 9.0 pM using prechilled HT1 buffer solution. Similarly, PhiX control libraries were denatured and diluted to 9.0 pM. To get a spike-in of ~10% for low diversity libraries, 60 μL of 9.0 pM PhiX library and 540 μL of 9.0 pM library were mixed. To ensure efficient template loading, the combined sample was heat denatured at 96°C for 2 minutes followed by chilling on ice water bath for 5 minutes and loaded into the MiSeq reagent cartridge. A mean cluster density of 961 K /mm2 was obtained for the library and 85% of clusters passed the quality filter. The samples were demultiplexed and FASTQ files were generated utilizing MiSeq reporter software (Illumina).
Bioinformatics Analyses
The 16S rRNA amplicon sequence data was analyzed using the Bioconductor35 workflow described by Callahan et al.36 All functions described in this section are part of the DADA2 package37 unless mentioned otherwise. Briefly, reads derived from paired end libraries were quality trimmed and dereplicated, using the fastqPairedFilter, derepFastq functions. The dada function was then used to infer sequence variants for each member of the pairs and the mergePairs function was used to merge forward and reverse reads of the inferred variants. Putative chimeric sequences were filtered from the dataset using removeBimeraDenovo function and taxonomic identities were assigned to the inferred sequence variants using the assign Taxonomy function, which uses the RDP-classifier.38 Because the mouse microbiome can contain a large proportion of previously uncharacterized taxa [(eg, families like ‘S24-7’ (Bacteroidales)], we created a custom 16S rRNA database based on sequences of strains characterized as part of the Mouse Intestinal Bacterial Collection (miBC)39 and all available type strains stored in the Ribosomal Database Project (accessed August 2016).
Quantitative insights into microbial ecology (QIIME, version 1.8.0) pipeline was used to calculate the species richness and diversity indices (Shannon, phylogenetic, and Chao1) to measure alpha diversity within the sample. Rarefactions were created for all diversity measures and richness assessments by 10 repeated iterations at a sub-sampling depth of 10 to 90,010 sequences. All the alpha diversity indices and richness estimates were normalized to a sampling depth of 1000 to correct the differences between samples as a result of sampling depth. Pairwise distances between microbial communities based on phylogenic relatedness of whole communities were calculated using UniFrac method (beta diversity between samples).40 Indicator species analysis was performed to determine the indicative species of each group of samples using ‘indicspecies function41 in R (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2016). Statistical analyses were performed in R (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2016) using the PhyloSeq package,42 that uses ggplot2 (H. Wickham. 2009. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag NY) to plot graphs. Additionally, the R MSA package43 was used for sequence alignment and the Phangorn package44 was used for phylogenetic inference. Differential abundance was tested using DeSeq2.45 R scripts, the custom database and rds files containing the operational taxonomic units Operational taxonomic unit (OTU) tables are available on GitHub (https://github.com/hcdenbakker/DADA2MouseMicrobiome) and sequence data deposited at the NCBI Sequence Read Archive.
For taxonomic and indicator species analyses described in Table S2, Figure 9, and Supplemental Figure 5, 16S rRNA sequencing reads in fastq format were obtained by unzipping the fastq.gz files by gunzip command on linux server. Sequencing reads in fastq format were obtained by unzipping the fastq.gz files by gunzip command on linux server. Processing of the demultiplexed samples was performed using the QIIME pipeline developed at CBG. Assembling of the paired end reads of each sample was done using PEAR software. This was followed by quality trimming the assembled sequences to remove poor quality reads using split_libraries_fastq.py script with default parameters that included: truncation of any reads that has 3 consecutive low-quality base calls and trimming of sequence to last high quality score position that was defined by q equals to 3. Sequences that were not at least 75% of the expected amplicon length together with ambiguous base calls were removed from the data pool. The 16S rRNA gene sequences were clustered based on 97% similarity of the reads and OTUs were recognized against the subset Greengenes database (http://greengenes.lbl.gov) using UCLUST algorithm. Alignment of the sequences on PyNast aligner46 and allocating representative sequence from each OTU to taxonomy against Greengenes 16S rRNA reference sequences (gg_97_otus_aug_13) was done. Removal of singleton from data pool was performed before taxonomy was assigned and further analyzed. TTU high computational resource, Hrothgar, was used to accomplish this computational data analysis.40 Indicator species analysis was performed to determine the indicative species of each group of samples using ‘indicspecies’ function41 in R (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2016).
FIGURE 9.
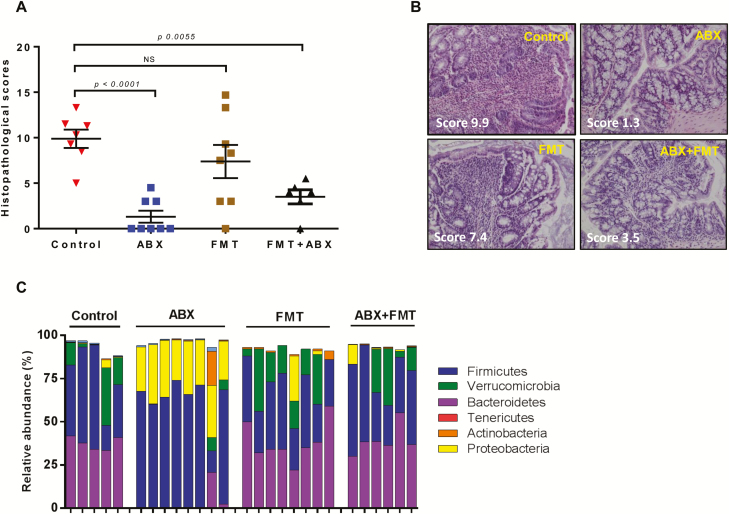
Therapeutic effects of antibiotic treatment and/or fecal microbiota transplant in mice with preexisting colitis. Treatment with antibiotics (ABX; neomycin and vancomycin; N = 7), fecal microbial transplant (FMT; N = 8) or both ABX and FMT (N = 5) began 4 weeks following T cell transfer as described in the Methods section. Seven mice were not treated and served as the control group. A, Blinded histopathology scores of colons for all treatment groups at 11 weeks post T cell transfer. Data are expressed as the mean±SEM. B,. Representative micrographs from each group. Note the extensive inflammatory infiltrate (blue circles) and goblet cell dropout in the control (untreated) and FMT groups. Scores in each micrograph (in white) represent the mean histopathology scores for that group. C, Relative abundance of the 5 major phyla in feces obtained from mice subjected to ABX and/or FMT treatment. Note the large expansion of Proteobacteria and major reductions of Bacteroidetes and Verrucomicrobia in mice treated with the ABX cocktail.
Quantification of Total Bacterial Load
Total bacterial load in fecal samples was quantified using real-time PCR with domain-specific primers F340 and R514.47 Briefly, using Lactobacillus rhamnosus (ATCC 7469) genomic DNA as a reference organism, a 5-point standard curve was generated using iTaq Universal Supermixes (Bio-Rad, Hercules, CA) on an Applied Biosystems cycler (Foster City, CA) with the 7500 cycler program: 1 cycle at 95°C for 5minutes, followed by 40 cycles of 94°C for 15seconds and 63°C for 45seconds.48
Macroscopic and Histopathologic Scoring
At 8 weeks following transfer of naive T cells or when mice lost >15% of their original body weight, RAG−/− mice were killed and their colons were removed, cleaned of fecal material, and scored for macroscopic evidence of colitis using our previously published scoring criteria.11 Normal appearing colons were assigned a score of 0, mild bowel wall thickening in the absence of visible hyperemia was assigned a score of 1, moderate bowel wall thickening and hyperemia was given a score of 2, severe bowel wall thickening with rigidity and marked hyperemia was assigned a score of 3, and severe bowel wall thickening with rigidity, hyperemia, and colonic adhesions was given a score of 4. Colonic length and weight also were measured to calculate weight-to-length ratios that provide a quantitative index of inflammation.11 In addition to macroscopic inflammation, representative sections of the proximal, mid, and distal colon were fixed overnight in 10% PBS-formalin, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E). The degree of inflammation in cross sections of the colon was assessed by an experienced pathologist (Dr. Yava Jones-Hall) blinded to treatment allocation, as previously described.49 The severity of the leukocyte infiltrate in the mucosa was assessed as none, mild, moderate, or severe with scores of 0–3, respectively.The distribution of leukocytes was denoted as present in the lamina propria only, extending to the submucosa, and extending to the serosa with scores of 1–3, respectively. The distribution of erosion/ulceration was assessed as none, focal, multifocal, or diffuse with scores of 0–3, respectively. Necrosis was assessed as none, mild, moderate, or severe with scores of 0–3, respectively. Goblet cell loss was assessed as none, focal, multifocal, or diffuse with scores of 0–3, respectively. The number of crypt abscesses was quantified per 10 high power fields and categorized as 1–2, 3–4, or 4+ (scores of 0–3, respectively). A score of 0 was assigned for each criterion not noted. Total disease score ranges from 0 to a maximum of 18 points based upon summation of each assigned criterion. In addition, 10 high power fields were evaluated and the infiltration of PMNs or mononuclear cells was assigned scores that ranged from 0–3 corresponding to no, mild, moderate, or severe infiltration, respectively. All PMN and mononuclear scores were normalized to the overall severity of inflammation in the tissue.
Statistical Analysis of Animal Studies
For all animal studies, results are expressed in ±SEM. Statistical significance between 2 groups was assesed by using a 2-tailed student’s unpaired t test. For multiple comparisons, analysis of variance (ANOVA) was done followed by Šidák post hoc test to compare different groups.
RESULTS
Differential Susceptibility to T cell-induced Colitis of RAG-/- mice Housed at 2 Different Insitutions
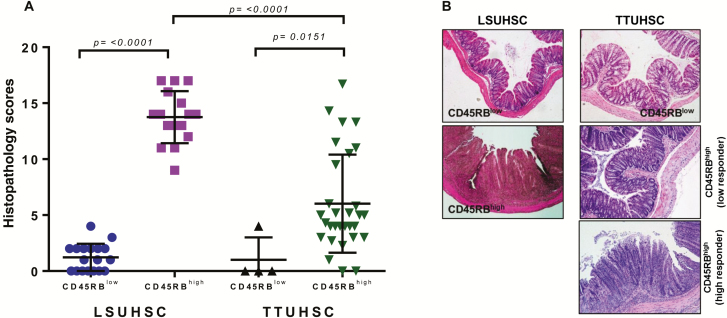
Over the past 2 decades, a number of different laboratories from around the world have used the CD45RBhigh T cell transfer model of IBD to investigate the immuno-pathological and immunoregulatory mechanisms responsible for induction and supression of chronic gut inflammation driven by the intestinal microbiota.11–14 Indeed, we have a 16-year history of using this model of IBD at our former institution (LSUHSC) that consistently produced moderate-to-severe colitis in 85%–90% of the reconsituted recipients.11 Following our relocation to TTUHSC, we observed surprising yet consistent differences in the incidence and severity of disease. Figure 1A illustrates the individual variability in the development of chronic colitis in mice generated at TTUHSC when compared to data generated by our collaborators at LSUHSC. We found that mice housed at TTUHSC developed chronic colitis that appeared to segregate into 2 distinct groups with only 27% (8 of 30 mice) developing moderate-to-severe disease (blinded histopathological scores >7; termed high responders) while 73% (22 of 30 mice) developed mild or no colitis (blinded histopathological scores <7; termed low responders) (Figs. 1A, B). In contrast, the incidence and severity of disease in mice housed at LSUHSC was found to be much greater with 15 of 16 mice exhibiting severe disease (blinded histopathological scores >10) (Figs. 1A, B). No colitis developed in either group of mice reconstituted with CD45RBlow T cells.
FIGURE. 1.
Induction of chronic colitis in mice housed at 2 different institutions. A, Blinded histopathology scores of colons from mice at 6–8 weeks following transfer of either CD4+CD45RBlow or CD4+CD45RBhigh T cells into RAG-/- mice housed at LSUHSC or TTUHSC. A total of 18 and 16 mice were used for the CD4+CD45RBlow or CD4+CD45RBhigh T cell groups at LSUHSC, respectively whereas 4 and 30 mice were used for the CD4+CD45RBlow or CD4+CD45RBhigh T cell groups at TTUHSC, respectively. B, Representative micrographs of colons from mice reconstituted with CD4+CD45RBlow or CD4+CD45RBhigh T cells housed at the two institutions. Data are presented as the mean±SEM for the animals indicated.
Interestingly, if RAG-/- mice were reconstituted with CD45RBhigh T cells at LSUHSC, transported to TTUHSC at 2 weeks following T cell transfer, and then housed at the TTUHSC animal facility for an additional 6 weeks, 80% of the mice developed moderate-to-severe colitis (Fig. S1). These data appeared to suggest that the environment (ie, microbiota) in mice housed at LSUHSC is much more conducive to induction of robust disease. These data, coupled with the incomplete penetrance of robust disease in mice generated at TTUHSC, prompted us to quantify the intestinal microbial composition of healthy RAG-/- mice housed at the 2 institutions.
Microbial Composition of Healthy RAG-/- mice Housed at LSUHSC vs TTUHSC
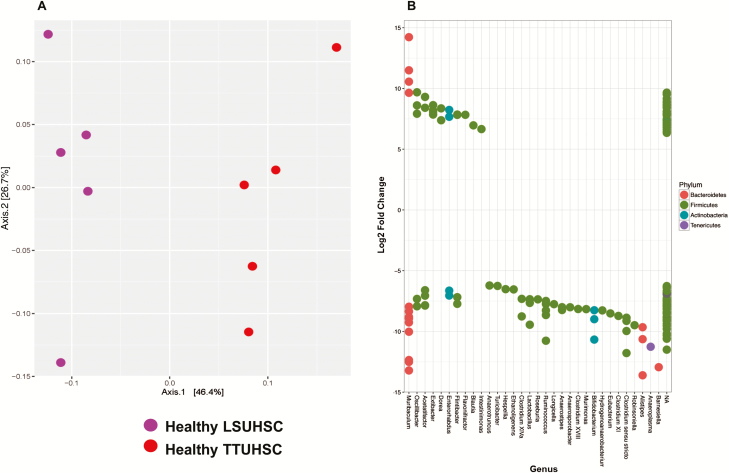
In order to ascertain how the housing environment may affect the composition of colonic microbiota in mice housed at the 2 animal facilities, we analyzed the fecal microbiota of healthy RAG-/- mice housed in ventilated microisolator cages at LSUHSC and TTUHSC. Taxonomic analyses showed similar relative abundances of the 5 major phyla in both groups of mice with no significant differences between groups (Figure S2A). OTU-based measures of community richness and diversity (Observed species, Chao1, and Shannon) showed that samples from LSUHSC (with the exception of 2 outliers) were less heterogeneous in OTU richness than those obtained from TTUHSC mice (Fig. S2B). Although not statistically significant, Chao1 and Shannon indices suggested a trend for higher OTU diversity for the LSUHSC samples when eveness of abundance of OTUs were taken into account (Fig. S2B). These data indicate that the microbial community in TTUHSC samples may be dominated by fewer but highly abundant taxa. In contrast, Principal Coordinate Analysis (PCoA) of mouse fecal microbiota that takes into consideration OTU composition and relative abundance of these OTUs within a community (weighted UniFrac), revealed a clear separation of the 2 groups indicating significant differences between the OTU composition and relative abundance of the microbiota obtained from mice housed at LSUHSC vs TTUHSC (Fig. 2A). Similarly, DESeq2 analysis revealed significant differences (< 0.01 multiple comparison adjusted p-value) within and between groups at the genus level, including differences in strain and/or species that may otherwise be missed when looking at higher taxonomic classifications. For example, we observed multiple OTU expression within 7 of the 22 genera significantly and differentially expressed in feces obtained from LSUHSC vs TTUHSC mice suggesting the presence of different strains and/or species within these genera (Fig. 2B). The vast majority of differentially expressed OTUs in feces from LSUHSC mice were associated with Firmicutes with fewer genera assigned to Bacteroidetes, Actinobacteria, and Tenericutes (Fig. 2B). Some of the most overrepresented genera associated with LSUHSC mice (>10 log2 fold change) were Ruminococcus, Bifidobacterium, Clostrium sensu stricto, Alistipes, Anaeroplasma, and Barnesiella. In addition, DESeq2 analyses of microbiota of TTUHSC mice revealed only 5 genera that were differentially and significantly overrepresented albeit at lower relative abundances in TTUHSC vs LSUHSC mice including Extibacter, Dorea, Flavonifactor, Blautia, and Intestimonas (Fig. 2B). For reference, a log2 fold change of 10 represents a change of 210 or a 1024 change in OTU expression.
FIGURE 2.
Principal coordinate and DESeq analyses of fecal microbiota from healthy RAG -/- mice housed at LSUHSC or TTUHSC. A, Principal coordinate analysis using weighted UniFrac of fecal microbiota for healthy RAG-/- mice housed at the 2 institutions. Weighted UniFrac takes into consideration the OTU composition and relative abundance of the OTUs within a community. Each axis shows the percentage of variation that is present in the dataset, Axis1 being the largest representation of variability. LSUHSC and TTUHSC microbiota samples are shown as purple and red circles, respectively. Note the clear separation of the 2 groups indicating significant differences in the bacterial populations between the 2 institutions. B, Differential overexpression of bacterial OTUs (genera) in feces obtained from healthy RAG-/- mice housed at LSUHSC or TTUHSC. Using DESeq2 analysis, values >0 represent OTUs (genera) that are significantly overexpressed in healthy RAG-/- mice housed at TTUHSC, whereas values <0 represent genera that are significantly overrepresented in healthy mice housed at LSUHSC. Multiple dots aligning within 1 genera represent different strains and/or species.
Microbial Composition of RAG-/- mice Following Induction of Chronic Colitis at LSUHSC vs TTUHSC
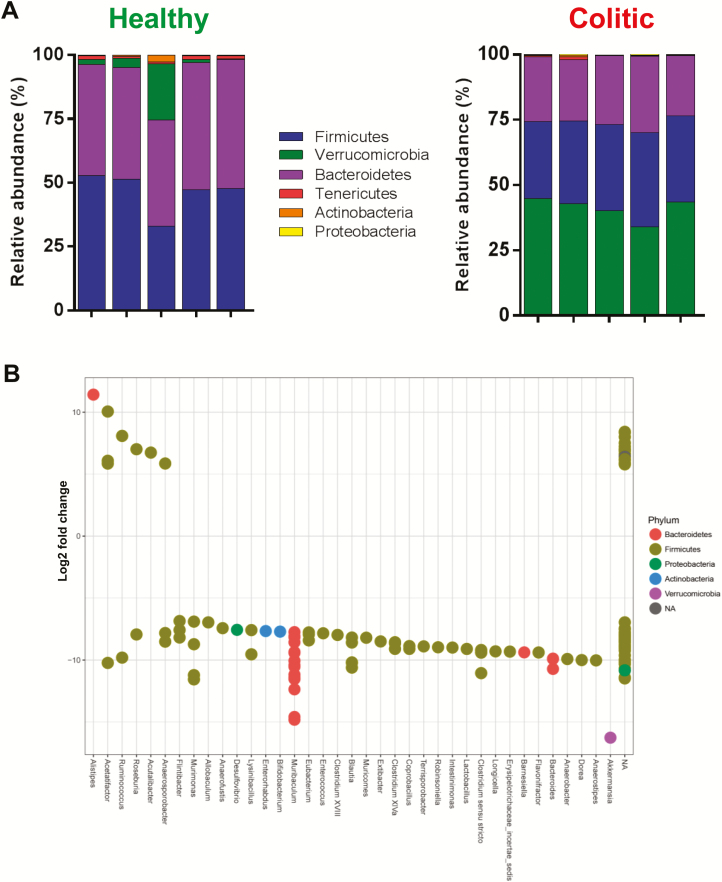
To identify microbial communities that may be differentially represented in colitic mice housed at the 2 institutions, we quantified and compared the 16S rRNA amplicon sequence data obtained from healthy vs colitic mice housed at the 2 institutions. As we noted above, 93% of the mice generated and housed at LSUHSC developed severe colitis (histopathological scores >10). Thus, we first wished to quantify and compare the fecal bacterial communities obtained from healthy vs colitic mice housed at LSUHSC. We observed an expansion of the phlya Verrucomicrobia with a concurrent contraction of Firmicutes and Bacteriodetes in colitic mice when compared to healthy RAG-/- animals housed at LSUHSC (Fig. 3A). In contrast to other animal and human studies, we did not observe significant expansion of Proteobacteria. Using DeSeq2 analyis to asertain whether specific genera were differentially represented in the 2 groups, we found numerous genera whose relative abundance were significantly overrepresented in the mice with active colitis when compare to healthy RAG-/- mice. Of the 31 genera that were differentially and significantly overrepresented in the colitic vs healthy group, Murimonas, Muribaculum, Blautia, Clostridium sensu stricto, Bacteroides, and Akkermansia were the 6 most abundant genera in the colitic group (>10 log2 fold change) (Fig. 3B). Of note, 9 of the 31 OTUs that were differentially overexpressed in LSUHSC coltic vs healthy mice contained multiple OTUs within each genera suggesting different species and/or strains. Interestingly, we observed only 1 genus in the phyla Proteobacter (Desulfovibrio) that was differentially overexpressed in the colitic group albeit at a lower relative abundance than the 6, most prevlent genera (Fig. 3B). In fact, the large majority of the differentially expressed genera in colitic LSUHSC mice were associated with the phyla Firmicutes.
FIGURE 3.
Relative abundance and differential overexpression of fecal bacterial communities in healthy and colitic RAG -/- mice housed at LSUHSC. A, Relative abundance of the6 major phyla in 5 individual fecal samples each from healthy and colitic mice housed at LSUHSC. B, Differential overexpression of OTUs (genera) in feces from healthy and colitic mice housed at LSUHSC. Using DESeq2 analysis, values >0 represent OTUs (genera) that are significantly overexpressed in healthy RAG-/- mice, whereas values <0 represent OTUs (genera) that are significantly overexpressed in colitic RAG-/- mice. Again, multiple dots aligning within 1 genera represent different strains and/or species.
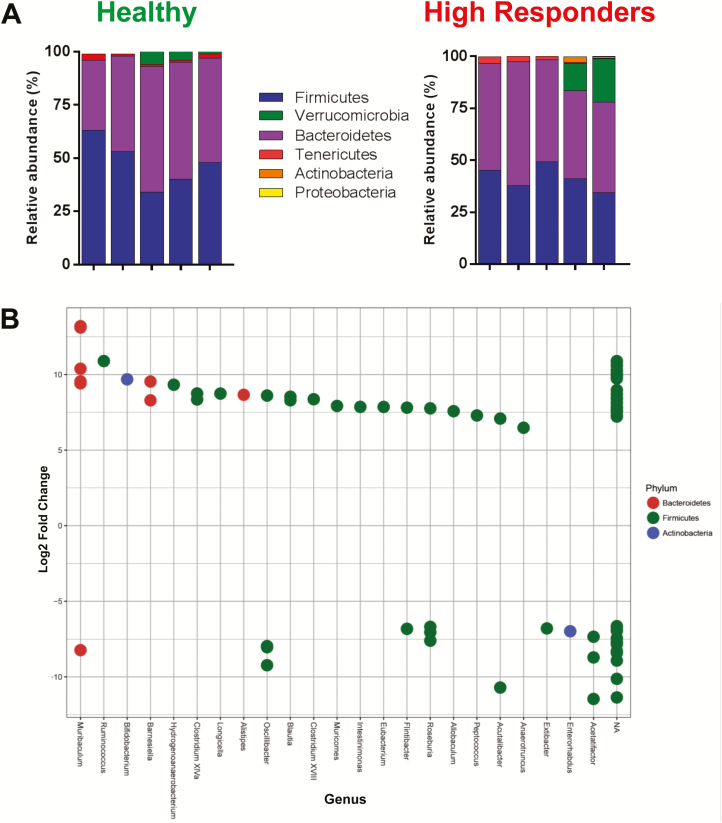
Using the same approach, we next wished to quantify and compare the fecal microbial communities in the TTUHSC colitic mice (ie, high responders) with those of healthy RAG-/- mice housed at TTUHSC. We observed no significant differences between the 5 major phyla of high responders vs healthy RAG-/- mice (Fig. 4A). Again, we failed to observe the presence of Proteobacteria in the colitic mice. DeSeq2 analysis did reveal significant increases in expression of 15 OTUs (genera) from high responders with Ruminococcus, Bifidobacterium, Barnesiella, Hydrogenoanaeobacterium, Clostrium XIVa, Longicella, and Alistipes representing the 7 most abudant genera in the high responder group (>7.5 log2 fold change) (Fig. 4B). As we observed previously, the large majority of the differentially expressed genera in the high responders were members of the phyla Firmicutes.
FIGURE 4.
Fecal bacterial communities in healthy and colitic mice (high responders) housed at TTUHSC. A, Relative abundance of the 6 major phyla in feces from healthy (N = 5) and colitic RAG-/- mice (high responders; N = 5) housed at TTUHSC. B, Differential overexpression of OTUs (genera) in feces from healthy and colitic (high responders) mice housed at TTUHSC. Using DESeq2 analysis, values >0 represent OTUs (genera) that are significantly overexpressed in colitic RAG-/- mice (high responders), whereas values <0 represent OTUs (genera) that are significantly overexpressed in healthy RAG-/- mice. Multiple dots aligning within 1 genera represent different strains and/or species.
We next wished to compare the fecal bacterial communities obtained from TTUHSC high vs low responders. We found that α diversity largely overlaped suggesting no major differences in phylogenetic composition and taxon abundance between these 2 groups (not shown). Despite the lack of clear differences in α- and β- diversity, we observed a significant increase in the relative abundance of Bacteriodetes (49.1% vs 26.1%; P < 0.01) in high vs low responders, respectively; whereas, the relative abundance of Verrucomicrobia in the low responder group was found to be significantly higher vs the high responder group (40.3% vs 6.8%; P < 0.01) (Fig. S3A). When we tested for the presence of differentially expressed OTUs between the 2 groups, we observed 8 different genera from 2 different phyla (ie, Firmicutes and Bacteroidetes) that were significantly overrepresented in the high responder group including Longicella, Blautia, Roseburia, Dorea, Acutalibacter, Hydrogenoanaerobacterium, Bifidobacterium, and Erysipelotrichaceae incertae sedis; only Akkermansia and Oscilibacter were overrepresented in the low responder group (Fig. S3B).
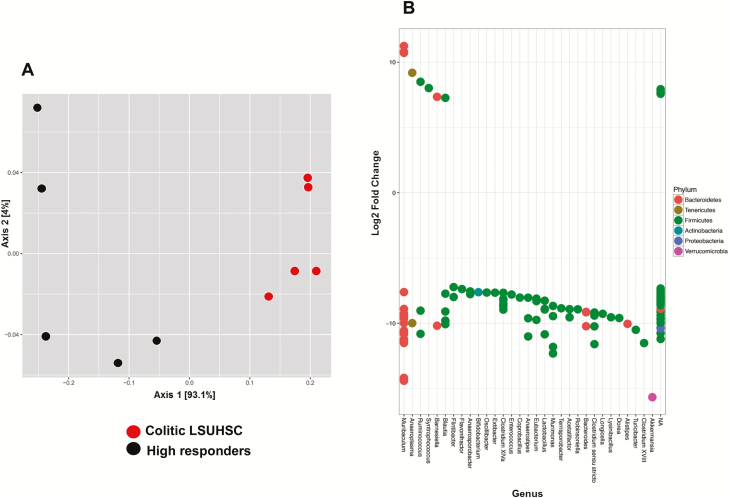
Given the histopatholgic similarly between LSUHSC colitic mice and the TTUHSC high responders, we wished to ascertain the similarities and differences in the colonic microbial communities in these 2 groups. Principal coordinate analysis using weighted unifrac revealed a clear separation of both groups demonstrating a significant difference in their phylogenetic composition and taxon abundance (Fig. 5A). We found that whereas both groups of mice had similar abundances of Bacteriodetes, Firmicutes, Tenericutes, and Actinobacteria, the relative abundance of Verrucomicrobia was significantly increased in colitic LSUHSC mice vs TTUHSC high responders (Figs. S3A, 3A). Furthermore, we observed significant differences in the expression of OTUs between the 2 groups. For example, colitic mice generated at LSUHSC contained at least 25 different genera that were differentially and significantly overexpressed when compared to TTUHSC high responders (Fig. 5B). The vast majority of these overrepresented genera were associated with Firmicutes with many fewer genera assigned to Bacteriodetes and Verrricumicrobia. Some of the most overrepresented genera associated with the colitic LSUHSC group (>10 log2 fold change) were Anaerostipes, Lactobacillus, Murimonas, Bacteroides, Clostridium sensu stricto, Turicibacter, Clostridium XVIII, and Akkermansia (Fig. 5B). Syntrophococcus was the only genera that was significantly overrespresented in TTUHSC-high responders. In addition, we observed multiple OTU expression within 12 of the 31 genera, again suggesting the presence of different strains and/or species within these genera.
FIGURE 5.
Principal coordinate and DESeq analyses of fecal microbiota from colitic RAG -/- mice housed at LSUHSC or TTUHSC. A, Principal coordinate analysis using weighted UniFrac of fecal microbiota from colitic mice housed at the 2 institutions. LSUHSC and TTUHSC (high responder) samples (N = 5) are shown as red and black circles, respectively. Note the clear separation of the 2 groups indicating significant differences in microbial populations between the 2 institutions (beta diversity). B, Differential overexpression of OTUs (genera) in feces from colitic mice housed at TTUHSC or LSUHSC. Values >0 represent OTUs (genera) that are significantly overexpressed in TTUHSC high responders, whereas values <0 represent OTUs (genera) that are significantly overexpressed in colitic mice housed at LSUHSC.
Transmission of Chronic Colitis via Fecal Microbial Transplantation
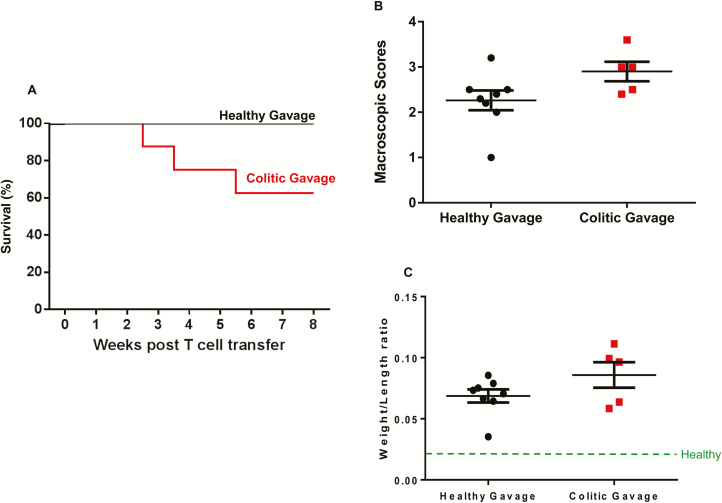
Data presented in the preceding section clearly identified major differences in the colonic bacterial communities of mice housed at LSUHSC vs TTUHSC. Therefore, we next wished to determine whether colonization of RAG-/- mice housed at TTUHSC with feces obtained from healthy or colitic RAG-/- mice housed at LSUHSC would alter the onset and/or severity of chronic colitis induced by the adoptive transfer of naive T cells. Mice that received colitic RAG-/- feces exhibited early clinical signs of disease including loose stools with occasional occult blood, kyphosis, piloerection and inactivity starting at 3 weeks post T cell transfer. Indeed, 3 of the 8 mice in this group died unexpectedly within the first 3 weeks following T cell transfer (Fig. 6A). In contrast, mice that received fecal gavage from healthy RAG-/- mice began to develop clincial signs of disease beginning 4–5 weeks post T cell transfer with all 8 mice in the healthy gavage group surviving the entire 8-week observation period (Fig. 6A). Upon killing, most of the RAG-/- mice that were colonized with feces from healthy donors (5 of 8 mice) exhibited macroscopic signs of moderate-to-severe colitis including bowel wall thickening, rigidity, mild hyperemia and increased colon weight/length ratios (Figs. 6B, C). In contrast, all 5 of the surviving mice that received colitic gavage exhibited macroscopic evidence of moderate-to-severe disease such as bowel wall thickening, rigidity, colonic hypermia and increased colon weight/length ratios (Fig. 6B, C). Although the mean macroscopic scores and weight/length ratios for mice that received colitic gavage tended to be higher than those of mice that received healthy fecal gavage, the differences were not significantly different.
FIGURE 6.
Chronic colitis develops in RAG -/- mice colonized with healthy or colitic feces prior to T cell transfer. A total of 16 RAG-/- mice housed at TTUHSC were colonized (via gavage) with a suspension of feces obtained from either healthy or colitic RAG-/- mice housed at LSUHSC before T cell transfer (N = 8 for each group; see methods section). A, Survival of mice gavaged with healthy (N = 8 to start; all survived) or colitic (N = 8 to start; 3 died) feces. B, Macroscopic colon scores at 8 weeks post T cell transfer. Macroscopic scores were based upon a 0–4 scoring system for gut thickness, rigidity, hyperemia, and shortening (a score of 0 represents a healthy colon). C, Colonic weight-to-length ratios at 8 weeks post T cell transfer. A weight/length ratio of a healthy colon is indicated by green dashed line (~0.020). Data in Figures B and C were derived from the surviving 8 and 5 mice in the healthy and colitic gavage groups, respectively and are presented as the mean±SEM.
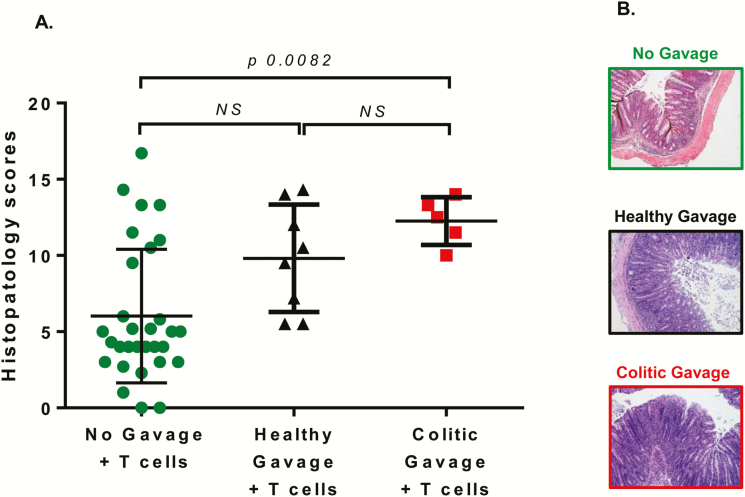
Blinded histopathological analyses of these 2 groups of mice revealed that 71% (6 of 8 mice) that received healthy fecal gavage and 100% (5 of 5 mice) of the surviving mice that received colitic fecal gavage had moderate-to-severe disease (scores >7; Fig. 7A, B). Mean histopathological scores for healthy vs colitic gavage mice were 9.8 ± 1.3 and 12.6 ± 0.7, respectively. This contrasts greatly with data presented in Figure 1 showing that only 27% (8 of 30 mice) of TTUHSC RAG-/- mice that received T cells (but no fecal gavage) exhibited moderate-to-severe disease. Blinded histopathological analysis of colons from mice gavaged with either healthy or colitic feces exhibited transmural inflammation with similar numbers of leukocyte infiltration (T cells, PMNs, macrophages) into the mucosa, submucosa, and muscle layer. Goblet cell dropout, epithelial erosions, and crypt abscesses were observed to a greater extent in the mice gavaged with colitic feces.
FIGURE 7.
Blinded histopathology scores of colons from mice colonized with healthy or colitic feces before T cell transfer. A total of 16 RAG-/- mice housed at TTUHSC were colonized (via gavage) with a suspension of feces obtained from either healthy or colitic RAG-/- mice housed at LSUHSC prior to T cell transfer. All 8 mice in the healthy gavage group survived, whereas only 5 animals in the colitic gavage group survived the 8-week observation period. A, Blinded histopathology scores were determined at 6–8 weeks following T transfer. B, Representative micrographs of colons from mice in the different groups. Data from Figure 1A showing T cell-engrafted RAG-/- mice housed at TTUHSC (No gavage + T cell group; N = 30) is included for comparison. Data are presented as the mean±SEM.
The similar degree of colonic inflammation in the 2 groups prompted us to quantify and compare the fecal microbiome in RAG-/- mice that were colonized with microbiota derived from healthy or colitic feces before engraftment with T cells. Alpha and beta diversity analyses failed to identify any significant differences between the 2 groups (data not shown). Furthermore, we did not observe any significant differences in the relative abundance of the major phyla between the healthy vs colitic gavage mice, nor did we observe significant differences in the total bacterial load between the 2 groups (Figures S4A). However, DESeq 2 analysis revealed major differences within and between certain genera in the 2 different groups. We observed increased expression of different OTUs within the genera Muribaculum, Clostrium XIVa, Eubacterium, Ruminococcus, Blautia, Murimonas, Anaerostipes, and Bacteroides in colitic gavage vs healthy gavage groups (Fig. S4B). In addition, we identified several genera that were significantly and differentially overrepresented in the colitic gavage microbiome with Anearoplasma, Ruminococcus, Murimonas, Anaerostipes, Bacteroides, Turicibacter, and Dorea representing the most abundant genera (>10 log2 fold change) in this group (Fig. S4B). We did not observe any genera that were uniquely overrepresented in the healthy gavage group. As with all other comparisons, most of the differentially overrepresented genera in either group were members of the phyla Firmicutes. We noticed a trend for a smaller bacterial load in mice that received colitic vs healthy gavage, however these data were not statistically significant (Fig. S4C)
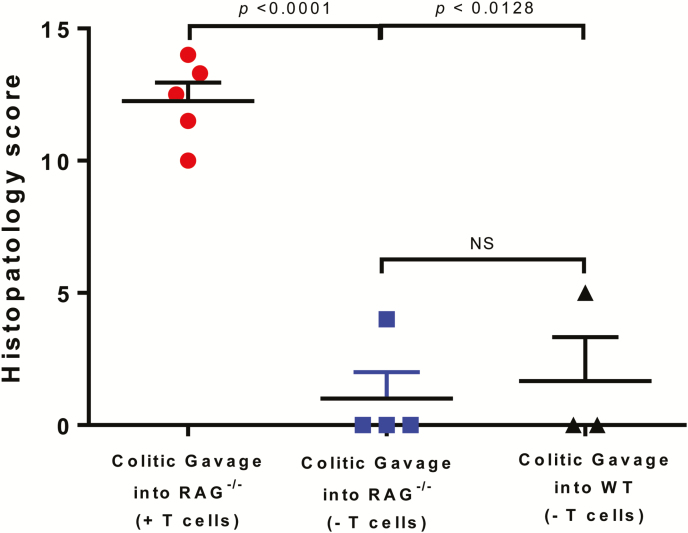
To determine whether the increase mortality in the colitic gavage mice was due to the introduction of pathogenic bacteria present in feces obtained from colitic LSUHSC mice, we colonized healthy WT or RAG-/- mice housed at TTUHSC with colitic feces obtained from LSUHSC. In the absence of T cell transfer, neither group of mice developed any macroscopic or histopathological evidence of colitis at 8–10 weeks postfecal transfer suggeting that colitic feces do not contain pathogens that induce colitis (Fig. 8).
FIGURE 8.
Chronic colitis fails to develop in RAG -/- or wild-type mice colonized with colitic feces. RAG-/- (N = 4) and wild-type (WT; N = 3) mice housed at TTUHSC were colonized with colitic feces (from LSUHSC) but were not engrafted with T cells. Data from Figure 7A showing histopathology scores for RAG-/- mice colonized with colitic feces and engrafted with T cells (N = 5) is included for comparison. Data are presented as the mean±SEM.
Therapeutic Efficacy of Antibiotic Administration and/or Fecal Microbial Transplantation to Treat Preexisting Colitis
It is well known that commensal microbiota is required for induction of chronic colitis in a number of mouse models of chronic colitis. Indeed, a number of studies have demonstrated that antibiotic treatment or germ-free conditions dramatically attenuates or eliminates the development of colonic inflammation in several mouse models of IBD, including the T cell transfer model of chronic colitis.14, 15, 50, 51 Furthermore, FMT has been identified as a promising therapeutic for diseases where the intestinal microbiota play a role in the induction and/or perpetuation of intestinal inflammation. Nieuwdorp and coworkers demonstrated that the transplant of fecal microbiota obtained from healthy donors into patients with recurrent Clostridium difficile-induced colitis essentially cures these individuals with a rate of >90% compared to a rate of <35% with vancomycin. These data suggest that reestablishing a “healthy” microbiota may be a more potent and longer lasting treatment for inflammatory diseases such as IBD.52 No studies have been reported evaluating the therapeutic efficacy of antibiotic administration and/or FMT to treat preexisting colitis in our mouse model of IBD. Therefore, we initiated a series of studies to evaluate the therapeutic effects of ABX administration, FMT administration, or both treatments in mice with established disease. Colitis was induced in mice via gastric gavage with feces obtained from colitic RAG-/- mice housed at LSUHSC followed by adoptive tranfer of naive T cells. All treatments started at 4 weeks post T cell transfer based on our previous studies demonstrating that >80% of the mice develop active colitis at 4 weeks following T cell transfer.32
We found that ABX and ABX+FMT, but not FMT alone were effective at reducing the increases in colon weight/length ratios in untreated mice (Fig. S5A). Blinded histopathological analysis revealed moderate-to-severe disease in the control and FMT groups, whereas colonic inflammation in mice treated with ABX or ABX+FMT was significantly attenuated (Fig. 9A). Colons from the control group showed focal, moderate-to-severe PMN, and mononuclear leukocyte infiltration extending into the serosa, whereas colons from the FMT group revealed focal PMN and mononuclear infiltrate that extended to the submucosa. Epithelial cell necrosis, crypt absesses, and globlet cell drop out appeared more prominent in the control group (Fig. 9B). Treatment with ABX dramatically and significantly reduced the severity of colitis when compared to the control group (Fig. 9A, B). Microscopic examination of colons from the ABX group revealed little or no infiltration of PMNs with only 2 animals exhibiting mild infiltration of monocytes that was restricted to the lamina propria. In addition, these mice did not show necrosis, crypt absesses, globlet cell loss, or erosions. Blinded histopathological analysis of colons from mice in the ABX+FMT group showed little or no infiltration of PMNs but moderate infiltration of monocytes distributed into the lamina propria (Fig. 9B). Similar to the ABX-treated group, colons from ABX+FMT mice did not show necrosis, crypt absesses, globlet cell loss, or erosion (Fig. 9B). Surprisingly, FMT treatment failed to attenuate chronic colitis when compared to their untreated controls.
We next wished to determine how the different treatement groups affected the major bacterial populations in each animal. Whereas total bacterial load was reduced by 100 fold in response to ABX treatment (data not shown), this difference was not significant. We did observe, however, a dramatic increase in relative abundance of Proteobacteria with a concomminent disappearance in Bacteroidetes in the ABX- treated group (Fig. 9C). We also observed a modest but not significant expansion of the phlya Verrucomicrobia in the control, FMT, and ABX+FMT groups (Fig. 9C). Principal coordinate analysis revealed that the ABX-treated group was the only group that was significantly different from the control group (Fig. S5B). Thus, we wished to determine which bacterial species were unique to the ABX-treated group when compared to the control group by performing the indicator species analysis (ISA). ISA identifies bacterial OTUs that are significantly associated with one group when compared to the other by taking into consideration fidelity (exclusivity) and relative abundance of the organism.41 When we compared both groups, we identified several indicator species that were significantly (P < 0.05) and uniquely associated with the control and ABX-treated groups. Not surprisingly, many of the genera uniquely overrepresented in the ABX treatment group were members of the phyla Proteobacteria, whereas those uniquely overrepresented in the control group belonged to the phyla Firmicutes (Table S2).
DISCUSSION
Mouse models of IBD have been used extensively for more than 25 years to gain a better understanding of the immunopathogenesis of IBD and to identify and evaluate new therapeutic strategies that may be used to treat individuals with these chronic inflammatory disorders. Unfortunately, the penetrance/incidence and severity of disease in several different mouse models of IBD may be quite variable, presenting investigators with significant challenges in experimental design due to the inability to control the onset and uniformity of disease. This is not surprising given the growing body of evidence demonstrating that the intestinal microbiota within the same strain of mouse may vary greatly depending upon the animal vendor, the vendor location, and even the room where the animals are housed within the same facility.53–55 Given our experience with differences in disease incidence and severity at 2 different institutions, coupled with the lack of detailed microbiome analyses in the T cell transfer model of IBD, we quantified and compared the major microbial populations in feces obtained from healthy and colitic mice housed at the 2 institutions. We identified several genera associated with the colonic microbiota obtained from the highly susceptible RAG-/- mice housed at LSUHSC that were signficantly overrepresented compared to that obtained from the more resistant TTUHSC RAG-/- mice. In addition, we found that severe disease developed in RAG-/- mice at TTUHSC provided that T cell transfer occurred and animals were housed at the LSUHSC animal facility for an initial 2-week period before transfer to TTUHSC. Furthermore, we observed that colonization of TTUHSC RAG-/- mice with fecal microbiota from healthy LSUHSC RAG-/- mice could transfer disease susceptibility such that the incidence and severity of colonic inflammation approximated that observed in the highly susceptible LSUHSC mice following T cell transfer. Finally, we found that ABX but not FMT treatment significantly attenuated preexisting disease. We discuss our novel findings of this study in the context of disease pathogenesis.
Although several studies have characterized the intestinal microbiota in different mouse models of IBD with varying susceptibilities to spontaneous colitis, no studies have reported alterations in the colonic microbiome in the T cell transfer model of chronic colitis. In the current study, we observed significant increases in abundance of a number of specific genera within the colonic microbiota of LSUHSC vs TTUHSC mice. We found a total of 22 different genera that were significantly overrepresented in the highly susceptible LSUHSC RAG-/- mice with Ruminococcus, Bifidobacterium, Clostria sensu stricto, Alistipes, Anaeroplasma, and Barbesiella representing the genera with the highest abundance in this group (Fig. 2B). Although we did not observe significant differences in the overall abundance of Muribaculum between the 2 groups, DESeq2 analysis revealed significantly more strains and/or species within this genus in feces collected from healthy LSUHSC RAG-/- vs TTUHSC RAG-/- mice (8 vs 4 strains/species, respectively; Fig. 2B). It is not now apparent which of the different genera are required for induction of colitis. There are, however, a few reports demonstrating that certain mucolytic bacteria from the genus Ruminococcus (eg, R. gnavus and R. torques) are increased in the noninflamed and inflamed mucosa of patients with IBD.56 We are currently attempting to identify the specific species that are overrepresented in the highly susceptible LSUHSC animals.
In addition to the unique genera that colonize healthy RAG-/- mice from LSUHSC, we observed 4 genera that were significantly overrepresented in the TTUHSC mice when compared to LSUHSC RAG-/- animals. These genera include Extibacter, Dorea, Flavonifractor, Blautia, and Intestimonas (Fig. 2B). Very little is known about the relationship between these genera and susceptibility to gut inflammation; however, Blautia has recently been identified as a major butyrate (and/or acetate) producer.57 In fact, Blautia spp. are among the most abundant bacteria within the gastrointestinal tract comprising between 2.5% and 16% of the total microbiota.58 In addition, Blautia has been suggested to possess anti-inflammatory activity as it has been shown to be associated with reduced risk of graft-versus-host-disease, as well as improved outcomes in colorectal cancer, inflammatory pouchitis, and liver cirrhosis.59 It is intriguing to speculate that future studies may reveal the immunosuppressive/protective properties of specific species within these genera that may be useful in suppressing intestinal inflammation.
The reasons for the differences in composition of the commensal microbiota between the two groups of RAG-/- mice have not been clearly defined at the present time. It is well documented that a number of factors may affect the composition of the intestinal microbiota in rodents including diet, genetics/strain, age, sex, pH of the drinking water, and stress.55, 60, 61 Given that all except for 1 of these variables and housing conditions are very similar, if not identical at the two institutions, we believe that differences in diet may have had a major effect on bacterial composition and consequent induction of chronic gut inflammation. Indeed, retrospective examination of the rodent chow composition used at the 2 institutions reveals a few major differences (Table S1). One difference is the addition of porcine animal fat that is preserved with butylated hydroxyanisol (BHA) in the TTUHSC chow. This lipid-soluble compound is well known to be a potent antioxidant and free radical scavenger. It is used widely in the human and animal food industry to reduce lipid peroxidation limiting the rancidification of polyunsaturated fatty acids. It is quite possible that addition of this xenobiotic in combination with porcine fat alters the intestinal microbiome from one that promotes T cell activation and expansion of disease-producing effector cells (eg, Th1 and Th17 cells) to a commensal community that is unfavorable for induction of disease. An equally intriguing hypothesis is that BHA/porcine fat does not affect the initial T cell responses to commensal bacterial antigens but suppresses the subsequent inflammatory cascade including inhibition of T cell and/or myeloid cell trafficking to the gut and suppression of inflammatory cytokine production by T cells, monocytes, and granulocytes (ie, PMNs). It is well known that antioxidants, including BHA, possess potent anti-inflammatory activity in vitro and in vivo.62, 63 Another major difference between the 2 diets is the addition of fish meal to the TTUHSC chow (Table S1). However, it is not clear how addition of this protein source might affect microbial communities and/or the inflammatory process.
Another novel observation made in the current study was that robust disease does in fact develop in RAG-/- mice housed at TTUHSC, provided that T cell transfer occurs and animals are housed at the LSUHSC animal facility for an initial 2-week period before transfer to TTUHSC (Fig. S1). These studies suggest that once the initial immunological interactions occur between naive CD4+ T cells and “appropriate” microbial antigens, progression of chronic disease will continue over the ensuing 6 weeks irrespective of the animal care facility. How this occurs is only a matter of speculation; however, one could envision a scenario in which low-grade, intestinal inflammation is induced during the first 2 weeks following T cell transfer at LSUHSC thereby creating a dysbiotic microbiota.5, 64 This subclinical, inflammation-induced dysbiosis would continue unabated over the ensuing weeks resulting in the expansion of pathobionts that are ultimately responsible for the induction of chronic colitis.64 Inflammation-induced dysbiosis not only would initiate disease in our lymphopenic RAG-/- recipients, but also it would perpetuate colitis irrespective of the animal care facility. If this scenario is correct, then one would predict that colonization of healthy RAG-/- mice housed at TTUHSC with feces obtained from RAG-/- donors housed at LSUHSC would render these mice more susceptible to induction of chronic colitis. In fact, that is exactly what we observed (Figs. 6, 7). If, as Schaubeck et al suggest using the TNFΔARE mouse model of chronic ileitis, that inflammation-induced dysbiosis may “cause transmission of disease to the susceptible host”,65 then we would predict that colonization of TTUHSC RAG-/- mice with feces obtained from colitic LSUHSC donors would produce robust disease following T cell transfer. These data would be consistent with the concept that time-dependent, inflammation-induced dysbiosis is a rate-determining step in induction of disease.
One of the most consistent observations reported in patients with IBD and in some (but not all) mouse models of IBD is the expansion of Proteobacteria with a concommitant reduction in Firmicutes.64, 66 Therefore, we were surprised to find no significant expansion of Proteobacteria or its associated families (eg, Enterobacteriaceae, Helicobacteraceae, etc) in colitic mice generated at LSUHSC or at TTUHSC (ie, high responders) (Figs. 3-5, S3). On the contrary, we observed that the large majority of the top 5–7 most overrepresented genera in colitic mice generated at the 2 institutions were members of Firmicutes with substantially fewer genera associated with Bacteroidetes and Verricumicrobia (Figs. 3-5 S3). In addition, we observed that the composition of the genera in the different groups of colitic mice were, in most cases, quite different with little overlap. At first glance, these data appear to be surprising since colitic mice at LSUHSC and TTUHSC (high responders) were generated using the same protocol. In fact, this is not the case. These novel and somewhat provocative data suggest that depending upon the initial microbial composition of the healthy mice, the components of the dysbiotic microbiota in colitic mice may be very different. Thus, the ability to identify specific genera and/or species may be much more difficult than originally thought. For example, we did observe a large and significant overabundance of 2 genera found within the phyla Bacteroidetes (eg, Muribaculum and Bacteroides) in colitic LSUHSC mice (Fig. 3). It has been reported that a member of Bacteriodes (eg Bacteroides thetaiotaomicron) is capable of digesting mucin and inducing chronic colitis in antibiotic-treated, genetically-susceptible mice.67 However, these authors also noted that whereas Enterobacteriaeae were >100 fold enriched in spontaneous colitis in these same mice, monoassociation of antibiotic-treated mice with these facultative anaerobes did not induce chronic colitis in genetically susceptible animals.67 These data are similar to those reported by Perez-Munoz et al who found that development of spontaneous colitis in their genetically-engineered mouse model of IBD was associated with large and significant increases in abundance of Lactobacillus and Clostridum species.68 However, monoassociation of their germ-free mice with either species failed to induce disease, whereas colonization with Bacteroides thetaiotaomicron induced robust colitis. Complicating the situation even more, Garret et al reported that although the relative abundance of 2 members of Enterobacteriaceae were increased and correlated well with the development of spontaneous colitis in their genetically-susceptible mice, they could only induce disease in germ-free mice when Enterobacteriaceae were administered together with commensal bacteria.69
Another novel observation we made in the current study was that the microbiota within the colons of LSUHSC colitic mice contain a large and significant expansion of the genera Akkermansia (Fig. 3). The only known species of this genera is the mucin-degrading bacterium Akkermansia muciniphilia. This bacterium has been shown to be increased in experimental and human IBD.70–72 Paradoxically, we also observed large and significant increases in abundance of this same bacterium in the feces of mice that develop little or no colitis at TTUHSC (low responders; Fig. S3). Indeed, others have reported the protective effects of Akkermansia muciniphilia in mice and humans.73 Although it is not clear how and why this bacterium can be overrepresented in feces of mice with active and quiescent disease, we hypothesize that different strains of Akkermansia muciniphilia may affect disease outcome. It is also possible that Akkermansia muciniphilia alone plays no role in disease pathogenesis and/or protection or it affects disease phenotype only in the presence of other bacterial communities. In addition, it is quite possible that the disease outcome differences may be related to the loss of low-abundance organisms. Although certain organisms could be present in a relatively low abundance, they could be metabolically very active, and their metabolites could be detrimental or beneficial to the host and other microbial comunities.
As mentioned previously, it is well known that commensal bacteria are required for induction of chronic intestinal inflammation in several different mouse models of IBD. Virtually all studies implicating the intestinal microbiota in the pathogenesis of experimental IBD have used either prophylactic ABX treatment or germ-free mice. To our knowledge, no studies have been performed evaluating the therapeutic efficacy of ABX treatment in a more clinically relevant situation where mice have preexisting colitis. Data obtained in the current study demonstrate, for the first time, that administration of broad spectrum ABX to mice with established disease remarkably attenuates colonic inflammation (Fig. 9). In addition, while ABX-treated mice appeared to have many fewer bacteria than controls, we observed a significant expansion of Proteobacteria and loss of Bacteroidetes, when compared to untreated controls (Fig. 9). At first glance, these data appear to be counterintuitive as many human and some animal studies suggest that expansion of certain bacteria within Proteobacteria are strongly associated with disease. Although the reasons for this apparent disconnect are not known, it may be that 1 or more of the overrepresented genera following ABX treatment are protective or antiinflammatory in nature [eg, Lactobacillus (phyla Firmicutes); Table 1]. Alternatively, the surviving members of Proteobacteria are not disease-producing pathobionts or simply cannot induce chronic colitis at their reduced numbers following ABX treatment. In addition, it may be that ABX treatment may itself have some sort of immunomodulatory effect independent of their antibiotic activity. Nevertheless, ABX treatment can be effective in attenuating distal bowel disease; it is currently used as an adjunctive therapy.74
Table 1.
Indicator Species Analysis of Control vs Antibiotic Treatment Groupa
| Untreated Control Group | Antibiotic-Treated Group | ||||||
|---|---|---|---|---|---|---|---|
| Phyla | Family | Genera | P value | Phyla | Family | Genera | P value |
| Firmicutes | Dehalobacteriaceae | Dehalobacterium | 0.001 | Proteobacteria | Oxalobacteraceae | Cupriavidus | 0.001 |
| Firmicutes | Ruminococcaceae | Anaerotruncus | 0.001 | Proteobacteria | Brucellaceae | Ochrobactrum | 0.0031 |
| Firmicutes | Lachnospiraceae | Blautia | 0.002 | Firmicutes | Lactobacillaceae | Lactobacillus | 0.0037 |
| Tenericutes | Anaeroplasmataceae | Anaeroplasma | 0.0028 | Proteobacteria | Xanthomonadaceae | Stenotrophomonas | 0.0091 |
| Firmicutes | Lachnospiraceae | Roseburia | 0.0066 | Actinobacteria | Streptomycetaceae | Streptomyces | 0.0128 |
| Actinobacteria | Coriobacteriaceae | Adlercreutzia | 0.0071 | Bacteroidetes | Chitinophagaceae | Chitinophaga | 0.017 |
| Firmicutes | Lachnospiraceae | Dorea | 0.0073 | Actinobacteria | Promicromonosporaceae | Xylanimicrobium | 0.0182 |
| Firmicutes | Lachnospiraceae | Anaerostipes | 0.008 | Proteobacteria | Rhodospirillaceae | Inquilinus | 0.0184 |
| Firmicutes | Erysipelotrichaceae | Coprobacillus | 0.0081 | Actinobacteria | Microbacteriaceae | Salinibacterium | 0.021 |
| Firmicutes | Turibacteraceae | Turicibacter | 0.0086 | Actinobacteria | Rubrobacteraceae | Rubrobacter | 0.0346 |
Indicator species analysis determines bacterial OTUs that are significantly associated (P < 0.05) based on fidelity (exclusivity) and relative abundance of the organism in each comparison
Another objective of the current study was to assess the therapeutic efficacy of FMT in mice with preexisting disease. As mentioned previously, a great deal of excitement has been generated following the report by Nieuwdorp et al in the New England Journal of Medicine demonstrating that FMT essentially cured (>90% efficacy) recurrent Clostridium difficile-induced colitis.52 We found that FMT administration alone did not attenuate colonic inflammation even when administered multiple times following the onset of disease (Fig. 9). In some respects, these data are not terribly surprising given recent clinical reports demonstrating little or only modest effects of FMT in patients with active IBD.75 Although we observed that the combination of ABX+FMT significantly reduced colonic inflammation, protection was similar to ABX treatment alone suggesting that broad-spectrum antibiotics are much more effective than FMT in treating established disease (Fig. 9).
CONCLUSIONS
When taken together, this study of the colonic microbial composition of mice with differential susceptibility to T cell- induced colitis should allow for the generation of testable hypotheses to ascertain which bacteria may be responsible for promoting inflammation or suppressing disease. Although we were able to identify relatively few candidate genera that were differentially overrepresented in highly susceptible LSUHSC mice vs those in the resistant TTUHSC mice, we recognize the limitations of our study. Data obtained in our current study and those obtained in numerous others by different laboratories are limited by the inability to definitively define the causive species and/or strains of bacteria in mouse models of chronic disease. To a large degree, this is due to limitations inherent in the methods used for the analyses of intestinal microbial communities. The bioinformatics platforms used by the large majority of investigators for 16S rRNA amplicon gene and metagenomics/transcriptome analyses are currently less than optimal. For example, most of the commonly used 16S rRNA analyses are incapable of providing phylogenetic data lower than the genus level.76, 77 In addition, databases for analyzing metagenomic and transcriptomic analyses are incomplete, thereby, hindering our ability to gain insight into microbial structure and function.76, 77 Nevertheless, we have taken a major step forward in defining the role of intestinal bacteria in the pathogenesis of chronic gut inflammation.
Supplementary Material
ACKNOWLEGEMENTS
Work reported in this study was supported by grants from the DOD (W81XWH-11-1-0666; MBG); the NIH (R01-DK091269; MBG) and the Presidents’ Collaborative Research Initiative at Texas Tech University Health Sciences Center.
Conflicts of Interest: None of the authors have any conflicts of interest to report.
Supported by: funding from a grant from the Presidents’ Collaborative Research Initiative supported the work of Hendrik den Bakker, PhD and Kameswara Rao Kottapalli, PhD reported in this manuscript. The work reported in this manuscript by Matthew Grisham, PhD was supported by grants from the DOD (W81XWH-11-1-0666), the NIH (R01-DK091269) and the Presidents’ Collaborative Research Initiative.
REFERENCES
- 1. Koboziev I, Karlsson F, Zhang S, et al. Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm Bowel Dis. 2011;17:1229–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valatas V, Bamias G, Kolios G. Experimental colitis models: insights into the pathogenesis of inflammatory bowel disease and translational issues. Eur J Pharmacol. 2015;759:253–264. [DOI] [PubMed] [Google Scholar]
- 3. Powrie F, Leach MW. Genetic and spontaneous models of inflammatory bowel disease in rodents: evidence for abnormalities in mucosal immune regulation. Ther Immunol. 1995;2:115–123. [PubMed] [Google Scholar]
- 4. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium (IIBDGC). Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buttó LF, Schaubeck M, Haller D. Mechanisms of microbe-host interaction in crohn’s disease: dysbiosis vs. Pathobiont selection. Front Immunol. 2015;6:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57:1185–1191. [DOI] [PubMed] [Google Scholar]
- 7. Ventham NT, Kennedy NA, Nimmo ER, et al. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–1728. [DOI] [PubMed] [Google Scholar]
- 9. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset crohn’s disease. Cell Host Microbe. 2014;15:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manichanh C, Borruel N, Casellas F, et al. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. [DOI] [PubMed] [Google Scholar]
- 11. Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–G146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powrie F, Leach MW, Mauze S, et al. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. [DOI] [PubMed] [Google Scholar]
- 13. Kanai T, Kawamura T, Dohi T, et al. TH1/TH2-mediated colitis induced by adoptive transfer of CD4+CD45RBHIGH T lymphocytes into nude mice. Inflamm Bowel Dis. 2006;12:89–99. [DOI] [PubMed] [Google Scholar]
- 14. Aranda R, Sydora BC, McAllister PL, et al. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBHIGH T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 15. Stepankova R, Powrie F, Kofronova O, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in Scid mice reconstituted with CD45RBHIGH Cd4+ T cells. Inflamm Bowel Dis. 2007;13:1202–1211. [DOI] [PubMed] [Google Scholar]
- 16. Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. [DOI] [PubMed] [Google Scholar]
- 17. Kriegel MA, Sefik E, Hill JA, et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2011;108:11548–11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathis D, Benoist C. The influence of the microbiota on type-1 diabetes: on the threshold of a leap forward in our understanding. Immunol Rev. 2012;245:239–249. [DOI] [PubMed] [Google Scholar]
- 19. Ericsson AC, Franklin CL. Manipulating the gut microbiota: methods and challenges. Ilar J. 2015;56:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bristol IJ, Farmer MA, Cong Y, et al. Heritable susceptibility for colitis in mice induced by IL-10 deficiency. Inflamm Bowel Dis. 2000;6:290–302. [DOI] [PubMed] [Google Scholar]
- 21. Mähler M, Leiter EH. Genetic and environmental context determines the course of colitis developing in IL-10-deficient mice. Inflamm Bowel Dis. 2002;8:347–355. [DOI] [PubMed] [Google Scholar]
- 22. Yang I, Eibach D, Kops F, et al. Intestinal microbiota composition of interleukin-10 deficient C57BL/6J mice and susceptibility to helicobacter hepaticus-induced colitis. Plos One. 2013;8:e70783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carvalho FA, Koren O, Goodrich JK, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12:139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cahill RJ, Foltz CJ, Fox JG, et al. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fox JG, Ge Z, Whary MT, et al. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laroux FS, Norris HH, Houghton J, et al. Regulation of chronic colitis in athymic nu/nu (nude) mice. Int Immunol. 2004;16:77–89. [DOI] [PubMed] [Google Scholar]
- 28. Leon F, Contractor N, Fuss I, et al. Antibodies to complement receptor 3 treat established inflammation in murine models of colitis and a novel model of psoriasiform dermatitis. J Immunol. 2006;177:6974–6982. [DOI] [PubMed] [Google Scholar]
- 29. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. [DOI] [PubMed] [Google Scholar]
- 30. Valatas V, He J, Rivollier A, et al. Host-dependent control of early regulatory and effector T-cell differentiation underlies the genetic susceptibility of RAG2-deficient mouse strains to transfer colitis. Mucosal Immunol. 2013;6:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. [DOI] [PubMed] [Google Scholar]
- 32. Karlsson F, Martinez NE, Gray L, et al. Therapeutic evaluation of ex vivo-generated versus natural regulatory T-cells in a mouse model of chronic gut inflammation. Inflamm Bowel Dis. 2013;19:2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen TC, Albenberg L, Bittinger K, et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015;125:2841–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huber W, Carey VJ, Gentleman R, et al. Orchestrating high-throughput genomic analysis with bioconductor. Nat Methods. 2015;12:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Callahan BJ, Sankaran K, Fukuyama JA, et al. Bioconductor workflow for microbiome data analysis: from raw reads to community analyses. F1000Res. 2016;5:1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods. 2016;13:581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cole JR, Wang Q, Fish JA, et al. Ribosomal database project: data and tools for high throughput rrna analysis. Nucleic Acids Res. 2014;42:D633–D642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lagkouvardos I, Pukall R, Abt B, et al. The mouse intestinal bacterial collection (mibc) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol. 2016;1:16131. [DOI] [PubMed] [Google Scholar]
- 40. Lozupone C, Knight R. Unifrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Cáceres M, Legendre P. Associations between species and groups of sites: indices and statistical inference. Ecology. 2009;90:3566–3574. [DOI] [PubMed] [Google Scholar]
- 42. McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. Plos One. 2013;8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bodenhofer U, Bonatesta E, Horejš-Kainrath C, et al. Msa: an R package for multiple sequence alignment. Bioinformatics. 2015;31:3997–3999. [DOI] [PubMed] [Google Scholar]
- 44. Schliep KP. Phangorn: phylogenetic analysis in R. Bioinformatics. 2011;27:592–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with deseq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wlodarska M, Kostic AD, Xavier RJ. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe. 2015;17:577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang H, Sparks JB, Karyala SV, et al. Host adaptive immunity alters gut microbiota. Isme J. 2015;9:770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones-Hall YL, Kozik A, Nakatsu C. Ablation of tumor necrosis factor is associated with decreased inflammation and alterations of the microbiota in a mouse model of inflammatory bowel disease. Plos One. 2015;10:e0119441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rooks MG, Veiga P, Wardwell-Scott LH, et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. Isme J. 2014;8:1403–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singh V, Yeoh BS, Carvalho F, et al. Proneness of TLR5 deficient mice to develop colitis is microbiota dependent. Gut Microbes. 2015;6:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nieuwdorp M. Faecal microbiota transplantation. Br J Surg. 2014;101:887–888. [DOI] [PubMed] [Google Scholar]
- 53. Gill N, Finlay BB. The gut microbiota: challenging immunology. Nat Rev Immunol. 2011;11:636–637. [DOI] [PubMed] [Google Scholar]
- 54. Weldon L, Abolins S, Lenzi L, et al. The gut microbiota of wild mice. Plos One. 2015;10:e0134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ericsson AC, Davis JW, Spollen W, et al. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. Plos One. 2015;10:e0116704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Png CW, Lindén SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–2428. [DOI] [PubMed] [Google Scholar]
- 57. Bui TP, Shetty SA, Lagkouvardos I, et al. Comparative genomics and physiology of the butyrate-producing bacterium intestinimonas butyriciproducens. Environ Microbiol Rep. 2016;8:1024–1037. [DOI] [PubMed] [Google Scholar]
- 58. Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38:996–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jenq RR, Taur Y, Devlin SM, et al. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laukens D, Brinkman BM, Raes J, et al. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2016;40:117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sofi MH, Gudi R, Karumuthil-Melethil S, et al. Ph of drinking water influences the composition of gut microbiome and type 1 diabetes incidence. Diabetes. 2014;63:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reimund JM, Allison AC, Muller CD, et al. Antioxidants inhibit the in vitro production of inflammatory cytokines in crohn’s disease and ulcerative colitis. Eur J Clin Invest. 1998;28:145–150. [DOI] [PubMed] [Google Scholar]
- 63. Stosic-Grujicic SD, Miljkovic DM, Cvetkovic ID, et al. Immunosuppressive and anti-inflammatory action of antioxidants in rat autoimmune diabetes. J Autoimmun. 2004;22:267–276. [DOI] [PubMed] [Google Scholar]
- 64. Buttó LF, Haller D. Dysbiosis in intestinal inflammation: cause or consequence. Int J Med Microbiol. 2016;306:302–309. [DOI] [PubMed] [Google Scholar]
- 65. Schaubeck M, Clavel T, Calasan J, et al. Dysbiotic gut microbiota causes transmissible crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Robinson AM, Gondalia SV, Karpe AV, et al. Fecal microbiota and metabolome in a mouse model of spontaneous chronic colitis: relevance to human inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2767–2787. [DOI] [PubMed] [Google Scholar]
- 67. Bloom SM, Bijanki VN, Nava GM, et al. Commensal bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Perez-Muñoz ME, Bergstrom K, Peng V, et al. Discordance between changes in the gut microbiota and pathogenicity in a mouse model of spontaneous colitis. Gut Microbes. 2014;5:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vigsnaes LK, van den Abbeele P, Sulek K, et al. Microbiotas from UC patients display altered metabolism and reduced ability of LAB to colonize mucus. Sci Rep. 2013;3:1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang Y, Huang D, Chen KY, et al. Fucosylation deficiency in mice leads to colitis and adenocarcinoma. Gastroenterology. 2017;152:193–205.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kang CS, Ban M, Choi EJ, et al. Extracellular vesicles derived from gut microbiota, especially akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. Plos One. 2013;8:e76520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gupta V, Rodrigues R, Nguyen D, et al. Adjuvant use of antibiotics with corticosteroids in inflammatory bowel disease exacerbations requiring hospitalisation: a retrospective cohort study and meta-analysis. Aliment Pharmacol Ther. 2016;43:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–118.e4. [DOI] [PubMed] [Google Scholar]
- 76. Miyoshi J, Chang EB. The gut microbiota and inflammatory bowel diseases. Transl Res. 2017;179:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schloss PD, Westcott SL. Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rrna gene sequence analysis. Appl Environ Microbiol. 2011;77:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.