Summary
1. The plant community structure of European lowland forests has changed dramatically in the 20th century, leading to biodiversity decline at various spatial scales. However, due to methodological difficulties associated with simultaneous changes in species diversity and composition, ecological processes behind the changes are still poorly understood.
2. We analysed temporal changes in forest plant community after the mid-20th century abandonment of coppicing in a typical Central European forest, which had been managed as coppice for centuries. We used 122 semi-permanent plots first surveyed in the 1950s shortly after the last coppicing and again in the 2000s after half a century of natural succession. We used a novel Temporal Nestedness Analysis to disentangle the immigration and extinction processes underlying temporal changes in community structure and tested whether species gains and losses were ecologically random.
3. The studied vegetation has shifted from the species-rich assemblages of a relatively open and low-nutrient forest towards the impoverished flora of a closed-canopy forest dominated by a few shade-adapted species. The significant reduction of beta diversity, i.e. compositional heterogeneity among plots, indicated taxonomic homogenization of the forest understorey. Temporal species turnover was only a minor component of the community change and recent assemblages are nested subsets of the former ones. Ecologically non-random extinctions dominated these changes. Light-demanding species with a persistent seed-bank were the most prone to extinction, while species with high specific leaf area substantially increased in frequency.
4. Synthesis and applications. The dominant process after the abandonment of coppicing was the ecologically non-random extinction of light-demanding species leading to an impoverished, temporally nested plant community structure. This development is typical for many abandoned lowland coppice forests and poses a significant threat to forest biodiversity in Europe. If forestry and conservation policies continue to prefer closed-canopy stands, many endangered species are likely to pay their extinction debts. To restore declining or even locally extinct species, canopy opening in abandoned coppices is urgently needed.
Keywords: community structure, eutrophication, forest management, taxonomic homogenization, life-history traits, long-term changes, resurvey, specific leaf area, semi-permanent plots, temporal nestedness analysis
Introduction
Disentangling the mechanisms of temporal change in ecological communities, in particular distinguishing between immigration and extinction effects, represents one of the most challenging issues in applied ecology. On ecological time scales, the composition of species assemblages changes through an array of immigration and extinction events (Olden & Poff 2003; Jackson & Sax 2010). If species immigration results in a major compositional turnover or even the creation of a novel community, ecological restoration can be difficult or impossible (Hobbs, Higgs, & Harris 2009). On the other hand, when the resulting assemblage is an impoverished subset of previously present species, the restoration is likely to succeed (Jackson & Hobbs 2009).
These issues are increasingly important because species assemblages change rapidly under human impact. In temperate Europe, lowland forests have gone through dramatic changes since the mid-20th century (Rackham 2008). This has raised many conservation issues because lowland forests are often species rich and host many globally threatened plant and animal species (Ellenberg 1988, Rackham 2006). Substantial changes in plant diversity and composition were recently documented in a number of lowland forests across north-western and central Europe. While there is no common temporal trend in species richness, species composition shifted towards more shade-adapted and nutrient-demanding plant species in most forests (Verheyen et al. 2012). Atmospheric deposition and high numbers of ungulates have been widely recognized as principal drivers of these changes (Chytrý & Danihelka 1993; Kwiatkowska 1994; Thimonier et al. 1994; Diekmann et al. 1999). Recently, these conclusions have been challenged by the hypothesis that the ultimate driver behind the observed changes is the demise of traditional forest management, in particular its most widespread form – coppicing (Decocq et al. 2005; Van Calster et al. 2007; Hédl, Kopecký, & Komárek 2010).
In contrast to the long periods of uniformly dark conditions in today’s forests, coppicing maintained the periodic recurrence of extremely light conditions at short intervals (usually every 7–20 years (Szabó 2010)). These light pulses were a very characteristic feature of European lowland forests, which were managed as short-rotation coppices for centuries (Rackham 2006; Szabó 2010), or even for millennia (Gardner 2002; Haneca, Van Acker, & Beeckman 2005). Due to this long-term influence, the species rich communities of European lowland forests were co-formed by human management (Ellenberg 1988; Decocq 2004a; Rackham 2006).
However, coppicing virtually disappeared from central and north-western Europe by the second half of the 20th century. This was caused by two main factors: 1) traditional management was gradually replaced by high-forest management from the 19th century onwards; and 2) nature conservation policy considered coppicing an undesirable ‘human intervention’ and banned it in most reserves (Szabó 2010). The demise of coppicing led to substantial changes in forest environments, which in turn resulted in changes both in plant communities (Baeten et al. 2009; Hédl, Kopecký, & Komárek 2010) and in invertebrate populations (Benes et al. 2006; Freese et al. 2006; Konvicka et al. 2008).
The changing nature of European lowland forests has been extensively documented, however, the ecological processes behind it are still poorly understood, mainly due to methodological difficulties associated with simultaneous changes in species diversity and composition. Therefore, we developed a novel analytical approach called Temporal Nestedness Analysis, which allows to disentangle processes behind changes in community structure. We used it to analyse changes in forest plant community after the mid-20th century abandonment of coppicing in Central European biodiversity hotspot - Děvín Wood. This site is a typical example of abandoned coppices; many similar forests can be found throughout Europe. We explicitly focused on three hypotheses: 1) Species composition and diversity changed in response to the abandonment of coppicing. 2) Species turnover was only a minor component of the changes and the recent species assemblages are impoverished subsets of the former ones. 3) Species gains and losses were ecologically non-random.
Materials and methods
Study Site
Děvín (48°52' N, 16°39' E) is a conspicuous limestone crest at 550 m a. s. l. which dominates the flat lowlands in the southeastern corner of the Czech Republic (Fig S1 in Supporting Information). It is located in the meeting zone of the Pannonian, Continental and Alpine biogeographic regions, resulting in species rich assemblages. With 643 species of vascular plants, the site is one of the biodiversity hotspots of central Europe. Climate is sub-continental, relatively warm and dry, with an average annual temperature of 9°C and average precipitation of 550 mm year-1. Carbonate-rich substrates vary from shallow lithosols and rendzinas to loamy-clay luvisols of loess deluvial plateaus. Habitat types range from dry rocky grasslands on south-exposed slopes to ravine forests in north-exposed screes and shady gorges.
The habitats of Děvín have been co-formed by human activities for millennia as continuous human presence can be traced since Neolithic times. Most of Děvín is covered by Děvín Wood, which has had a remarkably stable size of 200-260 ha for at least seven centuries. Management has also been characterised by long-term stability: from the 14th to the 20th century, the Děvín Wood was managed as coppice with varying numbers of standard trees. The coppice cycle was 7 years in 1384 AD and gradually grew to 40 years in the 1940s (Szabó 2010). In 1946 the Wood was included in a non-intervention nature reserve, however, forestry operations continued in some parts. As a result, the present structure of the Wood is dominated by abandoned coppices. It also contains high-forests comprised of singled-out coppice stools, and has some plantations of the alien species Pinus nigra and Quercus cerris.
The plant communities of Děvín Wood are dominated by Pannonian oak-hornbeam forests (habitat type 91G0 according to NATURA 2000), ravine forests Tilio-Acerion (type 9180), and Peri-Alpidic basiphilous thermophilous oak forests (type 91H0). Tree dominants include broadleaved lime Tilia platyphyllos, sessile oak Quercus petraea agg., European ash Fraxinus excelsior, European hornbeam Carpinus betulus, and pubescent oak (Quercus pubescens). European beech Fagus sylvatica and native conifers are completely absent from the site.
Data Collection
Between 1953 and 1964 (hereafter ‘the 1950s’), J. Horák sampled Děvín Wood in order to describe its forest vegetation (Horák 1967). The plots were placed subjectively and covered the whole vegetation variability of the wood (average plot density was 0.65 plots per hectare). In each plot, Horák recorded a complete list of vascular plant species, estimated their cover-abundance and assigned them to several vertically defined layers.
In 2002–2003 (hereafter ‘the 2000s’), one of the authors (RH) repeated Horák’s records using the original methodology. Plots were relocated using Horák’s original map scaled 1:10,000 with marked positions of plots and information about slope, aspect and local topography retrieved from Horák’s original field sheets. Based on our field experience, we estimated the relocation error to 10 –20 m for most plots, with occasional maximum error up to 50 m in topographically homogeneous parts. Each plot was re-sampled in the same season as the original record and plot sizes were identical to the originals (200–500 m2 for most plots).
Data Analysis
In this study, we used 244 paired samples recorded from June to September. After merging species with uncertain determination (see Table S1), the dataset comprised 371 understorey species, defined as specimens lower than 1 m. All statistical analyses were performed with R, version 2.14.1 (R Development Core Team 2011) on incidence matrix with species presence–absence data. Specific methods were performed with R packages vegan (Oksanen et al. 2012, functions metaMDS, betadisper), indicspecies (De Cáceres & Legendre 2009, function signassoc) and party (Hothorn, Hornik & Zeileis 2006, function ctree).
Species Diversity and Composition
To analyse temporal changes in species diversity, we tested if the number of all vascular plant species and the number of forest plant species (see Appendix S1) significantly differ between sampling periods using Wilcoxon signed-rank tests.
To test for temporal changes in compositional heterogeneity (beta-diversity), we used the analysis of multivariate homogeneity of group dispersions (PERMDISP, Anderson et al. 2006). As a measure of compositional dissimilarity, we used the Simpson index for presence–absence data because it is independent from richness differences between samples (Koleff, Gaston, & Lennon 2003). We performed the test with 999 permutations restricted within temporally-paired samples.
To compare the vegetation patterns between the 1950s and the 2000s, we performed non-metric multidimensional scaling (NMDS). For NMDS, we used the same dissimilarity matrix as in PERMDISP. The two-dimensional configuration with the lowest stress after 1000 random starts was centred and rotated by principal components analysis in order to maximize variance along the first ordination axis.
To interpret the main compositional gradients extracted by NMDS, we first calculated unweighted means of Ellenberg indicator values (EIV) of the species present within each sample (Ellenberg et al. 1992). Then, we fitted mean EIVs into NMDS space using generalized additive models (Wood 2006). In accordance with the expected environmental changes, we used EIVs for reaction, nutrients, light and temperature. Furthermore, we tested the change in mean Ellenberg indicator values between the surveys with a permutation test that accounts for non-independence between indicator values and vegetation composition (Zelený & Schaffers 2012).
To identify species that have significantly increased or decreased in frequency, we used permutation tests to investigate whether the proportion of plots occupied by each species changed over time. We used 999 permutations and adjusted the P-values through Šidák’s correction for multiple testing (De Cáceres & Legendre 2009).
Temporal Nestedness
Temporal nestedness expresses the extent to which the recent assemblages are impoverished subsets of older assemblages. It is therefore a temporal analogue of spatial nestedness, which expresses the extent to which the poorer species assemblages are nested subsets of richer assemblages (Ulrich, Almeida-Neto, & Gotelli 2009). The important difference is that temporal nestedness is calculated between subsequent samples from the same plots, while spatial nestedness is calculated among all plots in a dataset, usually sorted in order to maximize nestedness (Elmendorf & Harrison 2009; Ulrich, Almeida-Neto, & Gotelli 2009).
To detect which process dominated the observed vegetation changes, we developed a novel Temporal Nestedness Analysis (TNA), which allows differentiation between immigration and extinction processes. TNA is based on a comparison of the observed temporal nestedness between subsequent samples from the same plots and the nestedness of the same samples whose temporal sequence was randomly reshuffled.
Specifically, we proceeded through the following steps. First, we calculated the nestedness measure based on overlap and decreasing fill for sites (NODFsites, Almeida-Neto et al. 2008) between each pair of the temporally sorted old and recent plot samples. Then, we averaged these pairwise NODFsites indexes into temporal nestedness (TN) of the whole dataset. Finally, we compared the observed TN to the distribution of 999 TN values generated by random reshuffling of the survey period between temporally paired samples (see annotated R script in Appendix S2). We reported the difference between the observed TN and the TN values of randomly reshuffled assemblages by standardized effect size (SES), which measures the number of standard deviations that the observed TN value differs from the mean TN value of the simulated assemblages.
Ecology of Species Loss and Gain
To test whether the species gains and losses were ecologically random, we explored how they were related to species life-history traits. First, we selected 18 ecologically relevant species life-history traits from the LEDA (Kleyer et al. 2008) and BiolFlor (Klotz, Kühn & Durka 2002) databases. We included species traits related to competitive ability (e.g. CSR strategy, canopy height), environmental requirements (e.g. specific leaf area, leaf anatomy) and dispersal (e.g. seed weight, vegetative propagation, full overview in Table S2).
Then we tested whether these traits were useful for partitioning species into homogenous groups with respect to changes in species frequency between the surveys. For partitioning, we used classification and regression trees, because these account for non-linear hierarchical relationships, treat categorical, ordinal and quantitative data simultaneously, and deal with missing values (De’ath & Fabricius 2000). In each split of the tree all species traits are tested and the trait which best discriminates between declined and increased species is selected. The procedure goes on until no trait significantly discriminates between species. We used conditional inference trees as these have several crucial advantages over other approaches (e.g. CART) - the statistical testing of each split through permutation, no need for problematic pruning of over-fitted trees and no selection bias towards variables with many possible splits or missing values (Hothorn, Hornik & Zeileis 2006).
Results
Species Diversity and Composition
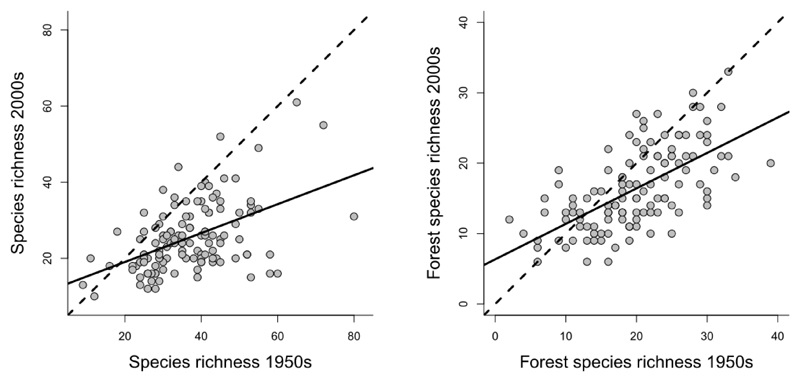
During the second half of 20th century, the forest vegetation of the Děvín Wood became significantly impoverished in vascular plant species (Fig. 1). While the first survey captured 356 species, only 228 species were recorded in the recent survey. The observed decline of gamma diversity was accompanied by a significant reduction in alpha diversity (Fig. 1). Individual plots lost more than one quarter (on average 27.3 %) of their former species richness and this decline was highly significant (Wilcoxon test, V = 6922, P < 0.001). The richness of forest plant species also declined from 110 species found in the 1950s to 89 in the 2000s. At the plot level, it declined significantly (V = 5254, P < 0.001) and the temporal pattern was similar to that of all vascular plant species (Fig. 1). Compositional heterogeneity (beta diversity) significantly declined between sampling periods (PERMDISP, F = 6.77, P = 0.001) indicating taxonomic homogenization of the forest understorey.
Fig. 1.
Relationship between species richness observed in 244 paired samples surveyed in the 1950s and the 2000s. The left diagram shows the relationship for all vascular plant species, the right diagram only for forest plant species (note different scales). The dashed line represents the null hypothesis of no change in species richness between surveys and the solid line is a linear relationship fitted to the data.
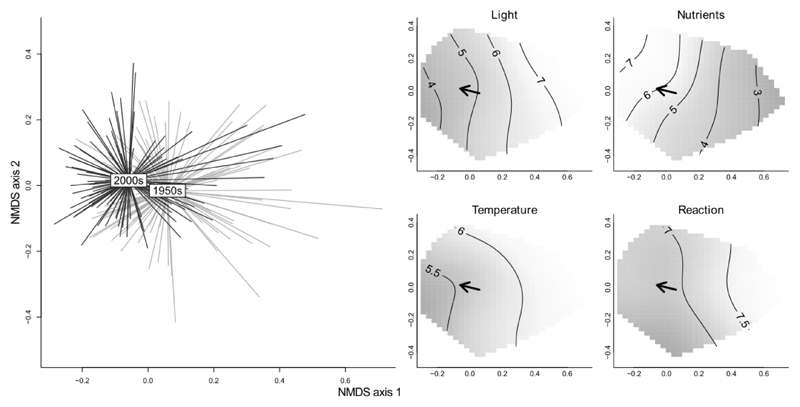
Species composition has shifted from the flora of a relatively open, nutrient-poor forest towards mesic, nutrient-rich forest community (Fig. 2). While mean Ellenberg indicator values for light (EIV-Lold = 5.3, EIV-Lnew = 4.9, P = 0.06) and nitrogen (EIV-Nold = 5.3, EIV-Nnew = 5.6, P = 0.10) changed substantially, indicator values for temperature (EIV-Told = 5.7, EIV-Tnew = 5.7, P = 0.33) and soil reaction (EIV-Rold = 7.0, EIV-Rnew = 7.0, P = 0.54) did not change between the surveys.
Fig. 2.
Non-metric multidimensional scaling of temporal change in the understory species composition. Left: Compositional turnover between 244 paired samples. Each spider connects individual samples with the average score for the 1950s (grey) and the 2000s surveys (black). The species composition in the 1950s was more heterogeneous than in the 2000s indicating taxonomic homogenization of the forest understorey. Right: Smoothed surfaces of mean Ellenberg indicator values fitted into the NMDS space. The arrows show the direction of the compositional change connecting the centroids of the sampling periods. The species composition shifted toward flora of shade adapted and nutrient demanding species, while the species indication of temperature and soil reaction did not change.
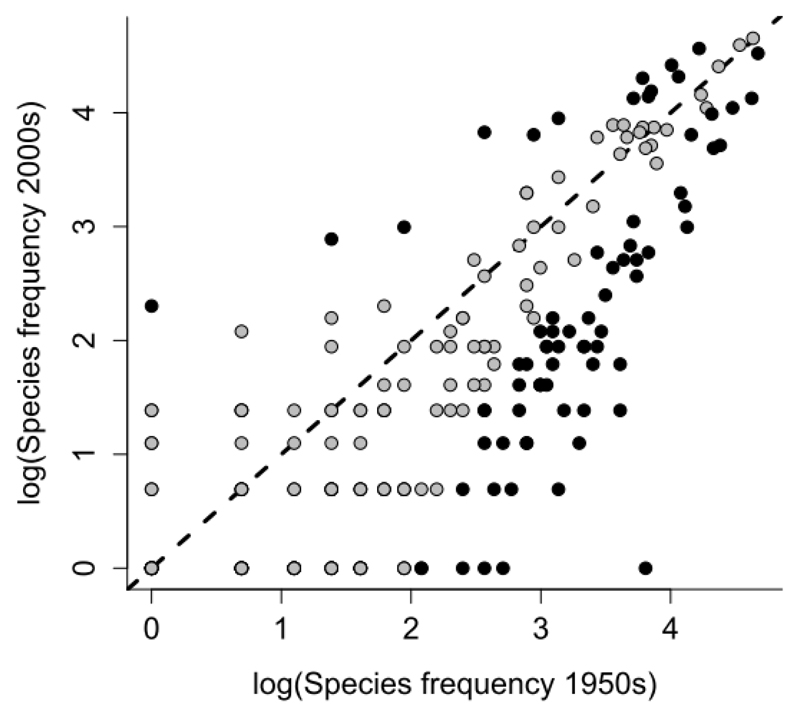
The community-wide shift was based on substantial changes at the species level. From the pool of 371 species encountered during the surveys, almost a quarter (24.5 %) significantly changed their frequencies between the surveys (Fig.3). While only 15 species occurred significantly more frequently in the 2000s survey, 76 declined or even became locally extinct (for individual species, see Table S3).
Fig. 3.
Relationship between species frequency in the 1950s and the 2000s. The dashed line separates species, which increased (above the line) or decreased (below the line). The black symbol marks species whose frequency changed significantly. Note that many species declined, while just a few increased.
Temporal Nestedness
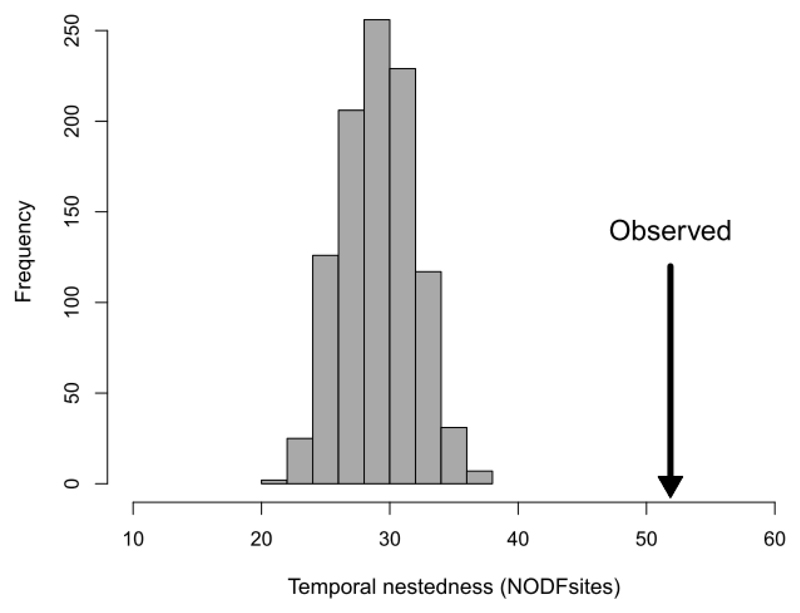
We found that species turnover was only a minor component of the community changes, because species assemblages from the 2000s formed temporally nested subsets of those from the 1950s (SES = 10.98, P = 0.001). None of the randomly generated assemblages exhibited the high degree of temporal nestedness observed in the real dataset (Fig. 4). Assemblages from the 2000s therefore represented highly impoverished subsets of the assemblages present in the 1950s and the prevailing mechanism underlying the community change was the plot-level extinction.
Fig. 4.
Comparison of the observed temporal nestedness and distribution of 999 nestedness values of species assemblages generated by random reshuffling of the survey period between paired samples. To observe such temporal nestedness by chance is highly improbable, therefore, species turnover was only a minor component of the community change and present assemblages are mainly impoverished subsets of the former ones.
Ecology of Species Loss and Gain
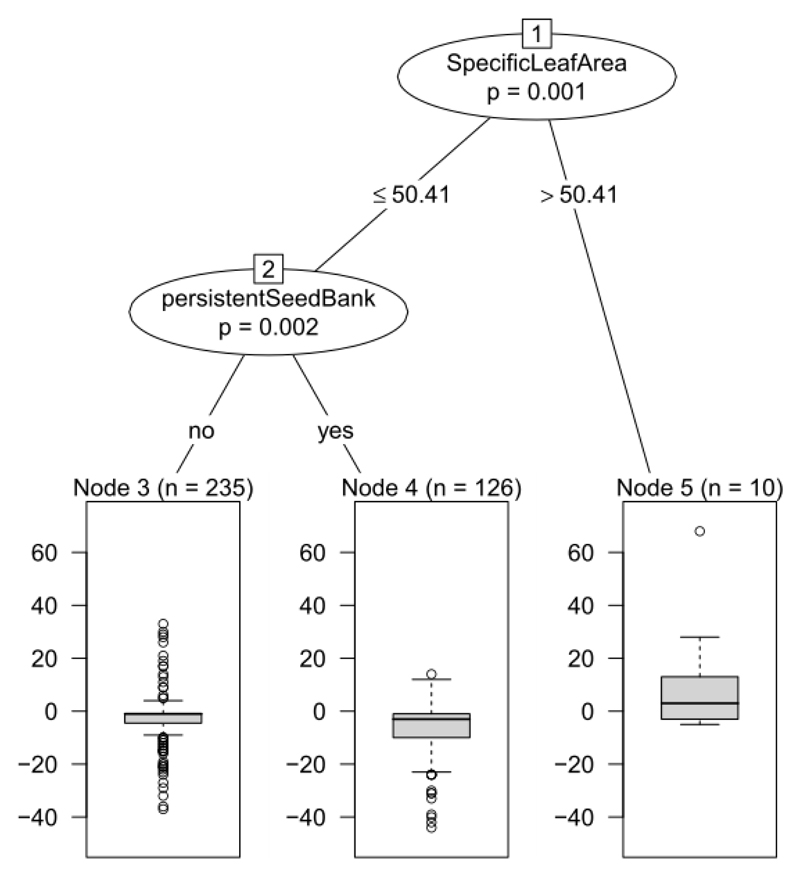
Species gains and losses were not ecologically random because specific leaf area and the ability to form a persistent seed bank significantly discriminate between increased and decreased species (Fig. 5). The species most prone to local extinction were light-demanding species with low specific leaf area. This group was further divided according the ability of species to form a persistent seed bank (Fig. 5). Species with a persistent seed bank were more susceptible to local extinction than species without persistent seed bank. Shade-tolerant species with relatively high specific leaf area significantly increased between the sampling periods (Fig. 5). Other life-history traits did not discriminate significantly between increased and decreased species.
Fig. 5.
Life-history traits predicting species gains and losses displayed by conditional inference tree. In each split of the tree all species traits are tested and the trait which best discriminate between declined and increased species is selected. The procedure goes on until no trait significantly discriminate between species. The response variable (summarized as box-plots) is the difference in the number of plots occupied by each species in the 2000s and the 1950s. Values below zero indicate declining species and values above zero indicate expanding species. Each split of the tree is described by the life-history trait used at the split, the permutation-based significance of the split (P-value) and the trait values at which the split occurs. The number of species (n) is given at each terminal node.
Discussion
Driving Forces of Community Changes
The studied vegetation has shifted from the species-rich assemblages of a relatively open and low-nutrient forest towards the impoverished flora of a closed-canopy forest dominated by shade-adapted species. A similar scenario was observed in nearby Milovice Wood, whose management history has been almost identical (Hédl, Kopecký, & Komárek 2010). Both sites belonged to the same owners, who utilized the forests as short-rotation coppices continuously from at least 14th to the early 20th century (Szabó 2010). Moreover, the changes observed at both sites are in line with other studies conducted across European lowland forests which were previously managed as coppices (Van Calster et al. 2007; Corney et al. 2008; Baeten et al. 2009). Management changes and nitrogen deposition are the most commonly identified drivers of this pan-European trend (Verheyen et al. 2012).
We argue that the demise of coppicing in the mid-20th century was the ultimate driver behind the community changes observed in Děvín Wood. The species that suffered the most significant losses are plants typical of open woodlands characterized by low specific leaf area. The prolonged period of canopy closure is therefore the most probable cause of their decline (Dahlgren et al. 2006). The increased frequency of nutrient demanding species was the consequence of forest succession rather than effect of nitrogen deposition, because the abandonment of coppicing has led to biomass accumulation and alteration of soil nutrient status (Hölscher, Schade, & Leuschner 2001). The increasing proportion of trees requiring high levels of soil nutrients and having base nutrient-rich litter (mainly Fraxinus excelsior, Ulmus glabra and Acer platanoides) further contributed to soil eutrophication, leading to a positive feedback between soil chemistry and changes in tree species composition (Hofmeister, Mihaljevic, & Hosek 2004; Hédl, Kopecký, & Komárek 2010; Verheyen et al. 2012).
During the coppicing cycle, plant species richness first increases and then, after several years, slowly declines to levels before the last coppicing (Ash & Barkham 1976; Mason & Macdonald 2002). However, stand-scale survival of light demanding species and their co-existence with shade-tolerant species is ensured by the shifting mosaic of differently aged patches created by coppicing (Decocq et al. 2004a). After the abandonment of coppicing, lowland forests became structurally homogenized and light demanding species gradually disappeared from the local species pool. The significant taxonomic homogenization detected in our study was most likely caused by the structural homogenization of the forest. In the long run, this unification of vegetation structure and plant species composition can be detrimental to other organisms requiring a fine-scale mosaic of specific habitat patches (Freese et al. 2006; Spitzer et al. 2008).
Biodiversity Threats
Compositional changes observed in abandoned coppices are driven by the population dynamics of species, which react to changing environmental conditions. Unfortunately, these natural changes have significant consequences for biodiversity conservation. The most sensitive indicators of biodiversity change are species with a high population turnover, which react to changing conditions relatively quickly. Butterflies are organisms which possess these characteristics, with the additional benefit of having a well-known ecology and distribution (Beneš et al. 2002). Nation-wide extinctions of butterflies confined to traditionally managed woodlands have already been reported (Van Swaay & Warren 1999; Beneš et al. 2002) and the last remaining populations of other endangered species survive in the few fragments of former coppices (Freese et al. 2006; Konvička et al. 2008). As butterflies are among the most intensively studied organisms, their decline may indicate declines of other, less known species groups (Thomas et al. 2004).
Furthermore, the documented extinctions can be vanguards of future developments, because of extinction debt. Extinction debt occurs when a population persists at a formerly suitable site where the conditions have meanwhile become unfavourable, the population growth rate is negative, and the population is doomed to extinction (Tilman et al. 1994). Long-lived organisms are especially prone to extinction debt because they are able to persist at a site even without successful reproduction (Kuussaari et al. 2009). Recent extinctions of woodland butterflies may be the first signs of future extinctions of other species with slower population turnover, such as forest plants (Krauss et al. 2010). If forestry and management policy continues to prefer closed-canopy forests, many species characteristic for European lowland forests will have to pay their debts and will become extinct.
Management Restoration
Restoration of traditional woodland management has been suggested in order to ensure the long-term survival of light demanding forest species (Benes et al. 2006, Freese et al. 2006, Spitzer et al. 2008). Based on our results, we argue that opening the forest canopy is the first step required for successful restoration as the species most declined were light demanding and formed persistent soil seed banks. Canopy opening could increase the populations of such species on sites where they still survive, or initiate their establishment from the seed bank on sites where they became locally extinct (Decocq et al. 2004b; Van Calster et al. 2008a).
In the lowland forest of Białowieża (Poland), the removal of strongly shading Carpinus betulus led to the successful restoration of a species rich community with characteristic light demanding plant species (Kwiatkowska & Wyszomirski 1990). Similarly, Van Calster et al. (2008b) reported increased performance of several forest species after experimental canopy opening in a Belgian lowland forest. Increased irradiance on the forest floor could also increase the diversity of other organisms living in lowland forests, such as butterflies (Benes et al. 2006), ants (Dolek et al. 2008), xylophagous beetles (Vodka, Konvicka, & Cizek 2009) and epigeic invertebrates (Spitzer et al. 2008).
However, as frequently pointed out by conservation practitioners, a possible negative effect of canopy opening in abandoned coppices is the expansion of ruderal and exotic species. These are frequently nutrient-demanding and would benefit from the faster decomposition of accumulated organic matter after canopy opening. In addition to the internal source of nutrients in accumulated biomass, large amounts of nitrogen have accumulated through atmospheric deposition. Verheyen et al. (2012) suggested that the effect of nitrogen accumulation is not yet apparent in lowland forests because of canopy shading. The authors called this situation the “nitrogen time bomb” and warned against future canopy opening, which could suddenly release all accumulated nitrogen. The N-bomb “explosion” is certainly possible, but we argue that its effect would be reduced by soil phosphorus, which became the limiting resource in many terrestrial ecosystems (Vitousek et al. 2010; Peñuelas et al. 2012), including European lowland forests (Hofmeister et al. 2002; Axmanová et al. 2011). In contrast to nitrogen, soil phosphorus is related to substrate weathering rather than atmospheric deposition and its turnover is much slower (Vitousek et al. 2010; Peñuelas et al. 2012). Limited phosphorus availability is therefore likely to block any effects of nitrogen eventually released by canopy opening.
However, to our knowledge, no papers have been published on long-term responses of plant communities to restoration of coppicing. We therefore encourage ecologists to focus on this applied issue and experimentally explore the consequences of canopy opening in abandoned coppices. This kind of research is urgently needed because of the fast and often irreversible changes in endangered biota of the European lowland forests.
Conclusion
In this paper, we applied a novel Temporal Nestedness Analysis, which successfully disentangled the effects of species immigration and extinction on temporal changes in abandoned coppices. We found that the dominant process after the abandonment of coppicing was the ecologically non-random extinction of light-demanding species. This process has led to impoverished assemblages, which constitute compositionally nested subsets of the former ones. Moreover, compositional heterogeneity among plots significantly decreased and the present vegetation is taxonomically more homogenous. This development is typical for many lowland forests previously managed as coppices and poses a significant threat to forest biodiversity in Europe. If management policy continues to prefer closed-canopy forests, many species characteristic for European lowland forests will become extinct. To restore declining or locally extinct species, canopy opening in abandoned coppices is urgently needed.
Supplementary Material
R script used for calculation of Temporal Nestedness Analysis.
Map of Děvín Wood with temporal nestedness of individual plots.
Acknowledgements
We thank J. Horák for historical vegetation samples, J. Danihelka for field support, M. Chudomelová for the help with species life-history traits and M. Chytrý for providing the dataset for forest species delimitation. We also thank the reviewers for useful comments.
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC Grant agreement n° 278065. Additional funding was provided by grant IAA600050812 and long-term research project RVO 67985939, both from the Academy of Sciences of the Czech Republic.
References
- Almeida-Neto M, Guimaraes P, Guimaraes PR, Jr, Loyola RD, Ulrich W. A consistent metric for nestedness analysis in ecological systems: reconciling concept and measurement. Oikos. 2008;117:1227–1239. [Google Scholar]
- Anderson MJ, Ellingsen KE, McArdle BH. Multivariate dispersion as a measure of beta diversity. Ecology letters. 2006;9:683–693. doi: 10.1111/j.1461-0248.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- Ash J, Barkham J. Changes and variability in the field layer of a coppiced woodland in Norfolk, England. Journal of Ecology. 1976;64:697–712. [Google Scholar]
- Axmanová I, Zelený D, Li C-F, Chytrý M. Environmental factors influencing herb layer productivity in Central European oak forests: insights from soil and biomass analyses and a phytometer experiment. Plant and Soil. 2011;342:183–194. [Google Scholar]
- Baeten L, Bauwens B, De Schrijver A, De Keersmaeker L, Van Calster H, Vandekerkhove K, Roelandt B, Beeckman H, Verheyen K. Herb layer changes (1954-2000) related to the conversion of coppice-with-standards forest and soil acidification. Applied Vegetation Science. 2009;12:187–197. [Google Scholar]
- Benes J, Cizek O, Dovala J, Konvicka M. Intensive game keeping, coppicing and butterflies: The story of Milovicky Wood, Czech Republic. Forest Ecology and Management. 2006;237:353–365. [Google Scholar]
- Benes J, Konvicka M, Dvorak J, Fric Z, Havelda Z, Pavlíčko A, Vrabec V, Weidenhoffer Z. Butterflies of the Czech Republic: Distribution and conservation I, II. SOM; Prague: 2002. [Google Scholar]
- Chytrý M, Danihelka J. Long-term changes in the field layer of oak and oak-hornbeam forests under the impact of deer and mouflon. Folia Geobotanica. 1993;28:225–245. [Google Scholar]
- Corney PM, Kirby KJ, Le Duc MG, Smart SM, McAllister Ha, Marrs RH. Changes in the field-layer of Wytham Woods - assessment of the impacts of a range of environmental factors controlling change. Journal of Vegetation Science. 2008;19:287–298. [Google Scholar]
- Dahlgren JP, Eriksson O, Bolmgren K, Strindell M, Ehrlén J. Specific leaf area as a superior predictor of changes in field layer abundance during forest succession. Journal of Vegetation Science. 2006;17:577–582. [Google Scholar]
- De’ath G, Fabricius KE. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. [Google Scholar]
- De Cáceres M, Legendre P. Associations between species and groups of sites: indices and statistical inference. Ecology. 2009;90:3566–3574. doi: 10.1890/08-1823.1. [DOI] [PubMed] [Google Scholar]
- Decocq G, Aubert M, Dupont F, Alard D, Saguez R, Wattez-Franger A, Foucault BD, Delelis-Dusollier A, Bardat J. Plant diversity in a managed temperate deciduous forest: understorey response to two silvicultural systems. Journal of Applied Ecology. 2004a;41:1065–1079. [Google Scholar]
- Decocq G, Valentin B, Toussaint B, Hendoux F, Saguez R, Bardat J. Soil seed bank composition and diversity in a managed temperate deciduous forest. Biodiversity and Conservation. 2004b;13:2485–2509. [Google Scholar]
- Decocq G, Aubert M, Dupont F, Bardat J, Wattez-Franger A, Saguez R, de Foucault B, Alard D, Delelis-Dusollier A. Silviculture-driven vegetation change in a European temperate deciduous forest. Annals of forest science. 2005;62:313–323. [Google Scholar]
- Diekmann M, Brunet J, Rühling Å, Falkengren-Grerup U. Effects of nitrogen deposition: results of a temporal-spatial analysis of deciduous forests in South Sweden. Plant Biology. 1999;1:471–481. [Google Scholar]
- Dolek M, Freese-Hager A, Bussler H, Floren A, Liegl A, Schmidl J. Ants on oaks: effects of forest structure on species composition. Journal of Insect Conservation. 2008;13:367–375. [Google Scholar]
- Ellenberg H. Vegetation Ecology of Central Europe. 4th edn. Cambridge University Press; Cambridge: 1988. [Google Scholar]
- Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulißen D. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica. 1992;18:1–248. [Google Scholar]
- Elmendorf SC, Harrison SP. Temporal variability and nestedness in California grassland species composition. Ecology. 2009;90:1492–1497. doi: 10.1890/08-1677.1. [DOI] [PubMed] [Google Scholar]
- Freese A, Benes J, Bolz R, Cizek O, Dolek M, Geyer A, Gros P, Konvicka M, Liegl A, Stettmer C. Habitat use of the endangered butterfly Euphydryas maturna and forestry in Central Europe. Animal Conservation. 2006;9:388–397. [Google Scholar]
- Gardner AR. Neolithic to Copper Age woodland impacts in northeast Hungary? Evidence from the pollen and sediment chemistry records. The Holocene. 2002;12:541–553. [Google Scholar]
- Haneca K, Van Acker J, Beeckman H. Growth trends reveal the forest structure during Roman and Medieval times in Western Europe: a comparison between archaeological and actual oak ring series (Quercus robur and Quercus petraea) Annals of Forest Science. 2005;62:797–805. [Google Scholar]
- Hédl R, Kopecký M, Komárek J. Half a century of succession in a temperate oakwood: from species-rich community to mesic forest. Diversity and Distributions. 2010;16:267–276. [Google Scholar]
- Hobbs RJ, Higgs E, Harris Ja. Novel ecosystems: implications for conservation and restoration. Trends in Ecology & Evolution. 2009;24:599–605. doi: 10.1016/j.tree.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Hofmeister J, Mihaljevic M, Hosek J, Sádlo J. Eutrophication of deciduous forests in the Bohemian Karst (Czech Republic): the role of nitrogen and phosphorus. Forest Ecology and Management. 2002;169:213–230. [Google Scholar]
- Hofmeister J, Mihaljevic M, Hosek J. The spread of ash (Fraxinus excelsior) in some European oak forests: an effect of nitrogen deposition or successional change? Forest Ecology and Management. 2004;203:35–47. [Google Scholar]
- Hölscher D, Schade E, Leuschner C. Effects of coppicing in temperate deciduous forests on ecosystem nutrient pools and soil fertility. Basic and Applied Ecology. 2001;2:155–164. [Google Scholar]
- Horák J. Forest types of the Pavlov Hills. PhD thesis, Agricultural University; Brno: 1967. [Google Scholar]
- Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. Journal of Computational and Graphical Statistics. 2006;15:651–674. [Google Scholar]
- Jackson ST, Hobbs RJ. Ecological restoration in the light of ecological history. Science. 2009;325:567–9. doi: 10.1126/science.1172977. [DOI] [PubMed] [Google Scholar]
- Jackson ST, Sax DF. Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends in Ecology & Evolution. 2010;25:153–60. doi: 10.1016/j.tree.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Kleyer M, Bekker RM, Knevel IC, Bakker JP, Thompson K, Sonnenschein M, Poschlod P, van Groenendael JM, Klimeš L, Klimešová J, Klotz S, et al. The LEDA Traitbase: a database of life-history traits of the Northwest European flora. Journal of Ecology. 2008;96:1266–1274. [Google Scholar]
- Koleff P, Gaston KJ, Lennon JJ. Measuring beta diversity for presence-absence data. Journal of Animal Ecology. 2003;72:367–382. [Google Scholar]
- Konvicka M, Novak J, Benes J, Fric Z, Bradley J, Keil P, Hrcek J, Chobot K, Marhoul P. The last population of the Woodland Brown butterfly (Lopinga achine) in the Czech Republic: habitat use, demography and site management. Journal of Insect Conservation. 2008;12:549–560. [Google Scholar]
- Krauss J, Bommarco R, Guardiola M, Heikkinen RK, Helm A, Kuussaari M, Lindborg R, Ockinger E, Pärtel M, Pino J, Pöyry J, et al. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecology letters. 2010;13:597–605. doi: 10.1111/j.1461-0248.2010.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuussaari M, Bommarco R, Heikkinen RK, Helm A, Krauss J, Lindborg R, Ockinger E, Pärtel M, Pino J, Rodà F, Stefanescu C, et al. Extinction debt: a challenge for biodiversity conservation. Trends in Ecology & Evolution. 2009;24:564–571. doi: 10.1016/j.tree.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Klotz S, Kühn I, Durka W. BIOLFLOR – Eine Datenbank mit biologisch-ökologischen Merkmalen zur Flora von Deutschland. Bundesamt für Naturschutz; Bad Godesberg; DE: 2002. [Google Scholar]
- Kwiatkowska AJ. Changes in the species richness, spatial pattern and species frequency associated with the decline of oak forest. Vegetatio. 1994;112:171–180. [Google Scholar]
- Kwiatkowska AJ, Wyszomirski T. Species deletion in Potentillo albae-Quercetum phytocoenoses reversed by the removal of Carpinus betulus. Vegetatio. 1990;87:115–126. [Google Scholar]
- Mason CF, Macdonald SM. Responses of ground flora to coppice management in an English woodland – a study using permanent quadrats. Biodiversity and Conservation. 2002;11:1773–1789. [Google Scholar]
- Olden JD, Poff NL. Toward a mechanistic understanding and prediction of biotic homogenization. The American Naturalist. 2003;162:442–60. doi: 10.1086/378212. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. Vegan: community ecology package. R package version 2.0-3. 2012 http://CRAN.R-project.org/package=vegan.
- Peñuelas J, Sardans J, Rivas-ubach A, Janssens IA. The human-induced imbalance between C, N and P in Earth’s life system. Global Change Biology. 2012;18:3–6. [Google Scholar]
- Rackham O. Woodlands. Collins; London: 2006. [Google Scholar]
- Rackham O. Ancient woodlands: modern threats. The New Phytologist. 2008;180:571–586. doi: 10.1111/j.1469-8137.2008.02579.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. ISBN 3-900051-07-0 http://www.R-project.org. [Google Scholar]
- Spitzer L, Konvicka M, Benes J, Tropek R, Tuf I, Tufova J. Does closure of traditionally managed open woodlands threaten epigeic invertebrates? Effects of coppicing and high deer densities. Biological Conservation. 2008;141:827–837. [Google Scholar]
- Szabó P. Driving forces of stability and change in woodland structure: A case-study from the Czech lowlands. Forest Ecology and Management. 2010;259:650–656. [Google Scholar]
- Thimonier A, Dupouey JL, Bost F, Becker M. Simultaneous eutrophication and acidification of a forest ecosystem in North-East France. The New Phytologist. 1994;126:533–539. doi: 10.1111/j.1469-8137.1994.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Telfer MG, Roy DB, Preston CD, Greenwood JJD, Asher J, Fox R, Clarke RT, Lawton JH. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science. 2004;303:1879–1881. doi: 10.1126/science.1095046. [DOI] [PubMed] [Google Scholar]
- Tilman D, May RM, Lehman CL, Nowak MA. Habitat destruction and the extinction debt. Nature. 1994;371:65–66. [Google Scholar]
- Ulrich W, Almeida-Neto M, Gotelli NJ. A consumer’s guide to nestedness analysis. Oikos. 2009;118:3–17. [Google Scholar]
- Van Calster H, Baeten L, Deschrijver A, Dekeersmaeker L, Rogister J, Verheyen K, Hermy M. Management driven changes (1967–2005) in soil acidity and the understorey plant community following conversion of a coppice-with-standards forest. Forest Ecology and Management. 2007;241:258–271. [Google Scholar]
- Van Calster H, Chevalier R, Wyngene B, Archaux F, Verheyen K, Hermy M. Long-term seed bank dynamics in a temperate forest under conversion from coppice-with-standards to high forest management. Applied Vegetation Science. 2008a;11:251–260. [Google Scholar]
- Van Calster H, Endels P, Antonio K, Verheyen K, Hermy M. Coppice management effects on experimentally established populations of three herbaceous layer woodland species. Biological Conservation. 2008b;141:2641–2652. [Google Scholar]
- Van Swaay CAM, Warren MS. Nature and Environment, no. 99. Council of Europe; Strasbourg: 1999. Red data book of European butterflies (Rhopalocera) [Google Scholar]
- Verheyen K, Baeten L, De Frenne P, Bernhardt-Römermann M, Brunet J, Cornelis J, Decocq G, Dierschke H, Eriksson O, Hédl R, Heinken T, et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. Journal of Ecology. 2012;100:352–365. [Google Scholar]
- Vitousek PM, Porder S, Houlton BZ, Chadwick Oa. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecological Applications. 2010;20:5–15. doi: 10.1890/08-0127.1. [DOI] [PubMed] [Google Scholar]
- Vodka S, Konvicka M, Cizek L. Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: implications for forest history and management. Journal of Insect Conservation. 2009;13:553–562. [Google Scholar]
- Wood SN. Generalized Additive Models: An Introduction with R. Chapman & Hall/CRC; Boca Raton, FL: 2006. [Google Scholar]
- Zelený D, Schaffers AP. Too good to be true: pitfalls of using mean Ellenberg indicator values in vegetation analyses. Journal of Vegetation Science. 2012;23:419–431. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
R script used for calculation of Temporal Nestedness Analysis.
Map of Děvín Wood with temporal nestedness of individual plots.