Abstract
Global change alters the composition and functioning of ecosystems by creating novel environmental conditions and thereby selecting for specific traits of organisms. Thus, trait-based approaches are promising tools to more mechanistically understand compositional and functional shifts in ecological communities as well as the dependency of response and effect traits upon global change. Such approaches have been particularly successful for the study of plant communities in terrestrial ecosystems. However, given the intimate linkages between aboveground and belowground compartments as well as the significance of plants as integrating organisms across those compartments, the role of plant traits in affecting soils communities has been understudied. This special issue contains empirical studies and reviews of plant trait effects on soil organisms and functions. Based on those contributions, we discuss here plasticity in trait expression, the context-dependency of plant trait effects, time lags in soil biotic responses to trait expression, and limitations of measured plant traits. We conclude that plant trait-based approaches are an important tool to advance soil ecological research, but also identify critical limitations and next steps
Keywords: Aboveground-belowground interactions, chemical ecology, climate change, global change, microbial ecology, novel environments, plant-microbe interactions, soil biodiversity, soil food web
Introduction
The quest for overarching principles in ecology has advanced trait-based approaches and unprecedented collaborative efforts (e.g., Kattge et al., 2011; Pey et al., 2014; Iversen et al., 2017) due to their predictive capacity across ecological scales (Shipley et al., 2016). Plant traits have been successfully utilized to predict the distribution of biodiversity (Díaz et al., 2016) as well as the functioning of ecosystems (Kunstler et al., 2016). Thus, plant trait-based approaches have been proposed to also develop a better mechanistic understanding of the composition and functioning of soil communities (Laliberté, 2016; Eisenhauer et al., 2017), as well as means to translate effects on soil communities to the ecosystem scale (Powell et al., 2013). In fact, such an improved understanding of aboveground-belowground linkages may allow soil ecologists to derive predictions from plant community characteristics (Eisenhauer et al., 2017). For instance, Liu et al. (2017; this issue) show in this issue that traits related to tree growth explain tree identity and diversity effects on soil respiration in young tree stands. Despite such promising applications, few studies have been conducted to link plant traits to soil communities and functions (Steinauer et al., 2017; this issue).
In a recent review paper, Laliberté (2016) identified six main belowground frontiers in plant trait ecology that are also reflected by contributions to this special issue:
-
(1)
redefining fine roots as trait data typically is collected on different root orders, which complicates across-study comparisons, syntheses, and thus generalizations (Steinauer et al., 2017; this issue);
-
(2)
quantifying trait dimensionality as it is unclear whether aboveground and belowground traits are coordinated (Ferlian et al., 2017; this issue; Liu et al., 2017; this issue; Powell et al., 2017; this issue);
-
(3)
integrating mycorrhizal fungi due to their ubiquity and functional importance (Powell et al., 2017; this issue; Donn et al., 2017; this issue);
-
(4)
broadening the suite of traits because few belowground traits are typically measured (Tsunoda and van Dam, 2017; this issue);
-
(5)
determining trait-environment linkages due to the limited knowledge regarding trait variation across environmental contexts (Patoine et al., 2017; this issue; Wang et al., 2017; this issue); and
-
(6)
understanding ecosystem-level consequences due to the likely significant effect of root (Steinauer et al., 2017; this issue; Wang et al., 2017; this issue) and mycorrhizal traits (Powell et al., 2017; this issue) for determining ecosystem functions like microbial respiration and carbon storage.
In this special issue, we present recent soil ecological case studies using plant traits and their diversity to predict soil functions as well as studies that introduce heretofore rarely considered plant traits in certain contexts, such as plant secondary metabolites in association with rhizosphere ecology (Tsunoda and van Dam, 2017; this issue) and mycorrhizal associations in relation to leaf flammability (Powell et al., 2017; this issue). Taken together, these studies support the notion that plant traits can advance the development of overarching theories in soil ecology (Bardgett and van der Putten, 2014). Furthermore, there is the increasing awareness that a stronger emphasis on belowground plant traits is required (Bardgett et al., 2014; Laliberté, 2016; Eisenhauer et al., 2017). However, several studies also exemplify the limitations of traditional trait-based approaches by showing the context-dependency of plant trait effects as well as the plasticity of traits in response to their abiotic and biotic environment. Future research should consider and embrace this plasticity and explore the role of soil organisms in co-determining the expression of plant traits.
1. Plasticity in trait expression
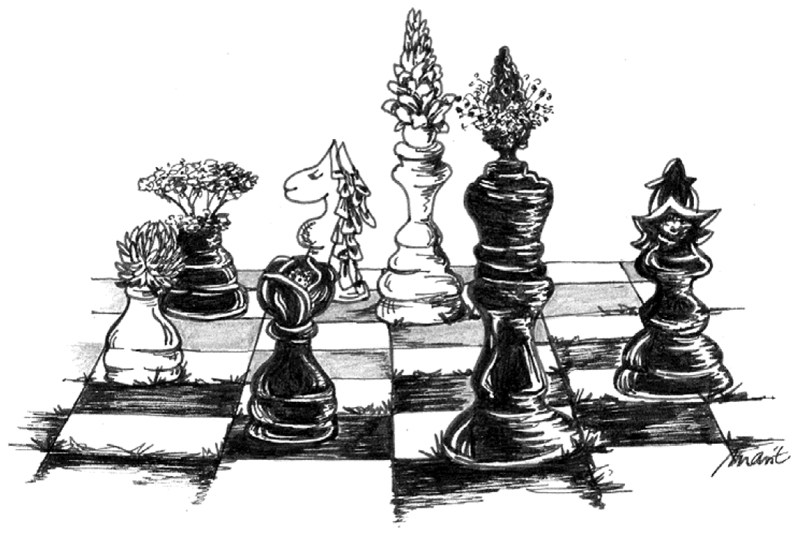
Plant traits are assumed to predict how plants compete and capture resources (e.g., Kunstler et al., 2016; Laliberté, 2016), with subsequent consequences for many ecosystem functions. This has resulted in analytical approaches and experiments to predict how ecosystems function (e.g., Ebeling et al., 2014). However, the extensive use of traits in ecological studies over the last few decades to predict community functions has revealed that morphological plant traits are plastic and respond to various environmental factors (Laliberté, 2016). As a consequence, some researchers called traits moving targets as plants may adjust their resource use strategies depending on the strategies of their competition partners (Fig. 1). Indeed, studies in the Jena Experiment (Roscher et al., 2004) have shown that plant species growing in plant communities of differing diversity vary in the expression of functional traits (e.g., Roscher et al., 2011; Gubsch et al., 2011; Lipowsky et al., 2015).
Figure 1.
Schematic illustration of trait plasticity in plants. Plants express traits in plastic ways (alter their strategy) depending on the abiotic and biotic environment, calling for detailed trait measurements in different contexts. Pawns in the game being placed at the wrong positions (colors) indicate that plant traits might shift in unexpected ways, i.e., they may not play according to predicted rules. Drawing by Marit Bodenstein.
In the present issue, Steinauer et al. (2017; this issue) used a grassland field experiment that manipulated the functional dissimilarity of plant communities based on six traits related to aboveground and belowground resource use as well as plant phenology (Ebeling et al., 2014) to study soil microbial respiration and biomass. They found that plant species richness and trait diversity effects on soil microbial properties were non-significant over the course of the five-year experiment. However, soil basal respiration and biomass were higher in plant communities with smaller leaves and both denser and shallower root systems than in plant communities with taller plants and sparse root systems four to five years after experimental set-up (Steinauer et al., 2017; this issue). Structural equation modeling revealed many correlations among plant traits and that rooting depth was the most important driver of soil microbial functions, stressing the significance of root traits (Bardgett et al., 2014; Laliberté, 2016). Despite the targeted experimental design, the explanatory power of the statistical models was low, indicating that slow soil responses to plant community treatments may take several years to materialize and/or that plant traits are plastic and measurements in plant monocultures have limited capacity to predict trait expression in polyculture. Thus, future studies on drivers of belowground communities and functions should consider root traits as well as their plasticity in different biotic and abiotic contexts (Steinauer et al., 2017; this issue).
2. Context-dependency of plant trait effects
Trait-based ecology strives to identify general principles (e.g., Díaz et al., 2016; Kunstler et al., 2016); however, as explained above, traits can be plastic, and trait effects on soil communities can depend on the environmental context. Contributions to this special issue support this context-dependency. Previous studies reported correlations between the stoichiometry of soil microorganisms and stoichiometry of different plant tissues (e.g., Fanin et al., 2013), although the generality of those relationships is unclear. Here, Ferlian et al. (2017; this issue) investigated associations of C and N concentrations between leaf, root, and soil as well as their ratios and soil microbial biomass C and activity (microbial basal respiration and specific respiratory quotient) across 32 young native angiosperm tree species at two locations in central Germany. At both sites, soil C concentrations rather than N concentrations determined significant effects of soil C:N ratio on soil microbial properties, indicating soil stoichiometry to represent a consistent determinant of soil microbial biomass and activity. However, soil microbial properties were not affected by the stoichiometry of plant tissues in the investigated young trees (Ferlian et al., 2017; this issue), which could be due to the relatively young age of the investigated trees or the dominance of soil abiotic effects in certain environments.
Plant trait effects on soil functions can also depend on the biotic context, such as exemplified by the studies by Patoine et al. (2017; this issue) and Wang et al. (2017; this issue). Patoine et al. (2017; this issue) performed a microcosm study to investigate the influence of litter functional traits and diversity as well as two major groups of soil macro-detritivores (earthworms and isopods) on the litter mass loss of tree leaf mixtures. The effect of functional diversity of the litter material was highest in the presence of both macro-detritivore groups (Patoine et al., 2017; this issue), supporting the notion that litter diversity effects are most pronounced in the presence of different detritivore species (Hättenschwiler and Gasser, 2005; Vos et al., 2011). Notably, these complex interactions among litter traits, diversity, and the macro-detritivore community changed over the course of the experiment. This suggests that the temporal dynamics of litter trait diversity effects and their interaction with detritivore diversity are key to advancing our understanding of litter mass loss in nature.
Moreover, plant trait expression can be significantly affected by soil organisms; for instance, several studies have shown that detritivores can alter nutrient concentrations in plant tissue (e.g., Partsch et al., 2006; Eisenhauer and Scheu, 2008) as well as plant secondary metabolites (e.g., Endlweber et al., 2011). Wang et al. (2017; this issue) examined decomposition of roots and litter collected from two plant species, Eriophorum vaginatum (Cyperaceae) and Betula nana (Betulaceae), each dominant plant species in tundra ecosystems and, for both species, the context in which decomposition was measured affected the outcome: slowly decomposing tissue (dead leaves from both species, dead roots from E. vaginatum, live roots from B. nana) decomposed more rapidly in communities dominated by conspecifics. Such ‘home-field advantage’ effects have been observed previously but are generally highly variable and have mainly targeted leaf litter (Veen et al., 2015). This study provides an example of how changing vegetation and plant traits may interact to influence ecosystem carbon storage mediated by soil biota.
3. Time lags in soil biotic responses to trait expression
The temporal nature of how plants influence ecosystem properties has increasingly become a focus of ecological studies, with observed changes in time being a recent focus in soil ecology. For instance, biodiversity–ecosystem function relationships may be as sensitive to diversity-through-time as they are to diversity-in-space, as evidenced in rotation systems where more diverse crop rotations lead to enhanced microbial diversity and positive effects on ecosystem functioning (e.g., Eisenhauer, 2016; Venter et al., 2016). Similarly, the strength of biodiversity–ecosystem function relationships may increase through time, as observed during two long-term grassland biodiversity experiments where species redundancy became less apparent in later years (Reich et al., 2012). In Steinauer et al. (2017; this issue), the relationships between plant traits and soil biotic responses only became apparent four years after the establishment of the field experiment. This has important consequences for the design of experiments in soil ecology, particularly when it comes to studies where the mechanisms being tested relate to resource acquisition. As pointed out by Steinauer et al. (2017; this issue), the accumulation of plant-derived resources in soil, in the form of organic matter, requires a period of time before differences among treatments can be observed. Similarly, it may take time to establish the soil communities that become integrated with those plant traits and are involved in the ecosystem processes being measured (Eisenhauer et al., 2012). Neutel et al. (2007) observed a gradual increase in complexity of soil food webs when comparing grasslands and various stages of succession, with food webs in those grasslands at very early stages (resembling the start of experiments such as that described in Steinauer et al., 2017; this issue) exhibiting low levels of stability. While technically challenging, our ecological understanding of soil would benefit greatly from studies that are capable of decoupling the time lags associated with the accumulation of effects linked to root traits and with the development of soil communities in new ecosystems. We thus argue that the set-up and maintenance of long-term studies is particularly relevant for assessments of plant traits effects on soil communities and functions as well as to capture soil feedback effects on plant trait expression.
4. Measured traits as poor proxies for ecological processes
For many studies, the focal plant traits are often chosen because they can easily be measured or because the measurements already exist in a database. These traits are proxies, assumed to represent an ecological or biological process that indicates how the plant interacts with its biotic or abiotic environment, but this assumption is not always tested. For example, Liu et al. (2017; this issue) measured two traits indicative of production (tree biomass) and decomposability (litter mass loss) to try to identify the mechanisms underlying tree diversity effects on soil respiration. They were able to explain almost half of the variation in soil respiration, with tree biomass being the only significant predictor – this raises questions about whether decomposability is actually less important or whether measurements of traits more finely associated with litter quality and decomposer interactions with litter (e.g., Fanin and Bertrand 2016) might have explained some variation. Similarly, while the low levels of variation in Steinauer et al. (2017; this issue) may be explained by trait plasticity or by time lags in their effects, they may also represent poor proxies for the mechanisms by which the growing plants are influencing soil ecology. Plant resource-acquisition traits are likely to be a step removed from the microbial functions studied by Steinauer et al. (2017; this issue) (microbial biomass, basal respiration), which will be more tightly linked to the resources to microbes themselves (as observed by Ferlian et al., 2017; this issue). Similarly, the biomass measures in Liu et al. (2017; this issue) themselves may only be indirectly related to the resource-acquisition traits that are likely involved in the observed positive effects of tree diversity on soil respiration (Eisenhauer and Reich, 2012; Pollierer et al., 2007). These studies indicate that future work is needed to further our ecological understanding of these aboveground-belowground interactions as well as the adequate plant traits that mediate these interactions.
In some cases, such as during interactions between roots and root-associated microbes, identifying the correct plant traits to measure may be very important with regard to our ecological understanding of these interactions (Tsunoda and van Dam, 2017; this issue). For instance, several studies have demonstrated that ecosystem-level variation in arbuscular mycorrhizal (AM) fungal communities can be explained by environmental factors, such as vegetation type and disturbance (e.g., Davison et al., 2015; Hart et al., 2016), with root traits proposed as one driver of differences in mycorrization among host species (Vályi et al., 2014). To address this, the study by Donn et al. (2017; this issue) examined, in a single host species, the effects on AM fungal communities of root types differing substantially in morphology. They did observe that AM fungal communities were shaped by root type, but only to a very small extent compared with the trait differences observed among host plants. These results may be due to the tested root morphological traits being less important than other aspects of host biology, but they are consistent with other recent observations that variation among AM fungal communities can be much higher than expected (Caruso et al., 2011; Powell and Bennett, 2016), suggesting that more attention needs to be paid to stochastic aspects of these interactions (Werner and Kiers, 2015). Identification of root traits that help explaining this stochasticity is a major challenge that soil ecologists face, but variation in root turnover may be a promising candidate (Powell and Bennett, 2016).
Conclusions
The papers in this special issue support the notion that plant trait-based approaches are an important tool to advance soil ecological research (Eisenhauer et al., 2017). However, exploring the plasticity in plant trait expression, the context-dependency of plant trait effects, time lags in soil biotic responses to trait expression, and limitations of measured plant traits are critical research frontiers. Because of the intimate interplay between plant traits and soil organisms, we should not only aim at improving the assessment of plant traits and long-term soil responses, but also study the effects of soil biota on plant trait expression and the temporal dynamics of such above-belowground linkages.
Acknowledgements
We thank Nicole van Dam, Olga Ferlian and Anja Pasemann for comments on an earlier version of this paper. Nico Eisenhauer gratefully acknowledges funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; Ei 862/2, Ei 862/3-2; FOR 1451) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no 677232). Further support came from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Research Foundation (FZT 118). Jeff Powell acknowledges funding from the Australian Research Council.
References
- Bardgett RD, van der Putten WH. Belowground biodiversity and ecosystem functioning. Nature. 2014;515:505–511. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- Bardgett RD, Mommer L, De Vries FT. Going underground: root traits as drivers of ecosystem processes. Trends in Ecology & Evolution. 2014;29:692–699. doi: 10.1016/j.tree.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Caruso T, Hempel S, Powell JR, Barto EK, Rillig MC. Compositional divergence and convergence in arbuscular mycorrhizal fungal communities. Ecology. 2011;93:1115–1124. doi: 10.1890/11-1030.1. [DOI] [PubMed] [Google Scholar]
- Davison J, Moora M, Öpik M, Adholeya A, Ainsaar L, Bâ A, Burla S, Diedhiou AG, Hiiesalu I, Jairus T, Johnson NC, et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science. 2015;349:970–973. doi: 10.1126/science.aab1161. [DOI] [PubMed] [Google Scholar]
- Díaz S, Kattge J, Cornelissen JH, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice IC, Garnier E, et al. The global spectrum of plant form and function. Nature. 2016;529:167–177. doi: 10.1038/nature16489. [DOI] [PubMed] [Google Scholar]
- Donn S, Kawasaki A, Delroy B, Chochois V, Watt M, Powell JR. Root type is not an important driver of mycorrhizal colonisation in Brachypodium distachyon. Pedobiologia. 2017 [Google Scholar]
- Ebeling A, Pompe S, Baade J, Eisenhauer N, Hillebrand H, Proulx R, Roscher C, Schmid B, Wirth C, Weisser WW. A trait-based experimental approach to understand the mechanisms underlying biodiversity–ecosystem functioning relationships. Basic and Applied Ecology. 2014;15:229–240. [Google Scholar]
- Eisenhauer N. Plant diversity effects on soil microorganisms: spatial and temporal heterogeneity of plant inputs increase soil biodiversity. Pedobiologia. 2016;59:175–177. [Google Scholar]
- Eisenhauer N, Scheu S. Earthworms as drivers of the competition between grasses and legumes. Soil Biology and Biochemistry. 2008;40:2650–2659. [Google Scholar]
- Eisenhauer N, Reich PB. Above- and below-ground plant inputs both fuel soil food webs. Soil Biology and Biochemistry. 2012;45:156–160. [Google Scholar]
- Eisenhauer N, Antunes PM, Bennett AE, Birkhofer K, Bissett A, Bowker MA, Caruso T, Chen B, Coleman DC, de Boer W, de Ruiter P, et al. Priorities for research in soil ecology. Pedobiologia. 2017;63:1–7. doi: 10.1016/j.pedobi.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer N, Reich PB, Scheu S. Increasing plant diversity effects on productivity with time due to delayed soil biota effects on plants. Basic and Applied Ecology. 2012;13:571–578. [Google Scholar]
- Endlweber K, Krome K, Welzl G, Schäffner AR, Scheu S. Decomposer animals induce differential expression of defence and auxin-responsive genes in plants. Soil Biology and Biochemistry. 2011;43:1130–1138. [Google Scholar]
- Fanin N, Fromin N, Buatois B, Hättenschwiler S. An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter–microbe system. Ecology Letters. 2013;16:764–772. doi: 10.1111/ele.12108. [DOI] [PubMed] [Google Scholar]
- Fanin N, Bertrand I. Aboveground litter quality is a better predictor than belowground microbial communities when estimating carbon mineralization along a land-use gradient. Soil Biology and Biochemistry. 2016;94:48–60. [Google Scholar]
- Ferlian O, Wirth C, Eisenhauer N. Leaf and root C-to-N ratios are poor predictors of soil microbial biomass C and respiration across 32 tree species. Pedobiologia. 2017 doi: 10.1016/j.pedobi.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubsch M, Buchmann N, Schmid B, Schulze E-D, Lipowsky A, Roscher C. Differential effects of plant diversity on functional trait variation of grass species. Annals of Botany. 2011;107:157–169. doi: 10.1093/aob/mcq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MM, Zaitsoff PD, van der Heyde M, Pither J. Testing life history and trait-based predictions of AM fungal community assembly. Pedobiologia. 2016;59:203–213. [Google Scholar]
- Hättenschwiler S, Gasser P. Soil animals alter plant litter diversity effects on decomposition. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1519–1524. doi: 10.1073/pnas.0404977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen CM, McCormack ML, Powell AS, Blackwood CB, Freschet GT, Kattge J, Roumet C, Stover DB, Soudzilovskaia NA, Valverde-Barrantes OJ, van Bodegom PM, et al. A global Fine-Root Ecology Database to address below-ground challenges in plant ecology. New Phytologist. 2017;215:15–26. doi: 10.1111/nph.14486. [DOI] [PubMed] [Google Scholar]
- Kattge J, Diaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC. TRY–a global database of plant traits. Global Change Biology. 2011;17:2905–2935. [Google Scholar]
- Kunstler G, Falster D, Coomes DA, Hui F, Kooyman RM, Laughlin DC, Poorter L, Vanderwel M, Vieilledent G, Wright SJ, Aiba M, et al. Plant functional traits have globally consistent effects on competition. Nature. 2016;529:204–207. doi: 10.1038/nature16476. [DOI] [PubMed] [Google Scholar]
- Laliberté E. Below-ground frontiers in trait-based plant ecology. New Phytologist. 2016;213:1597–1603. doi: 10.1111/nph.14247. [DOI] [PubMed] [Google Scholar]
- Lipowsky A, Roscher C, Schumacher J, Michalski SG, Gubsch M, Buchmann N, Schulze E-D, Schmid B. Plasticity of functional traits of forb species in response to biodiversity. Perspectives in Plant Ecology. Evolution and Systematics. 2015;17:66–77. [Google Scholar]
- Liu M, Xia H, Fu S, Eisenhauer N. Tree diversity regulates soil respiration through accelerated tree growth in a mesocosm experiment. Pedobiologia. 2017 doi: 10.1016/j.pedobi.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutel A-M, Heesterbeek JAP, van de Koppel J, Hoenderboom G, Vos A, Kaldeway C, Berendse F, de Ruiter PC. Reconciling complexity with stability in naturally assembling food webs. Nature. 2007;449:599–602. doi: 10.1038/nature06154. [DOI] [PubMed] [Google Scholar]
- Partsch S, Milcu A, Scheu S. Decomposers (Lumbricidae, Collembola) affect plant performance in model grasslands of different diversity. Ecology. 2006;87:2548–2558. doi: 10.1890/0012-9658(2006)87[2548:dlcapp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Patoine G, Thakur MP, Friese J, Nock C, Hönig L, Haase J, Scherer-Lorenzen M, Eisenhauer N. Plant litter functional diversity effects on litter mass loss depend on the macro-detritivore community. Pedobiologia. 2017 doi: 10.1016/j.pedobi.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pey B, Laporte MA, Nahmani J, Auclerc A, Capowiez Y, Caro G, Cluzeau D, Cortet J, Decaëns T, Dubs F, Joimel S, et al. A thesaurus for soil invertebrate trait-based approaches. PloS One. 2014;9 doi: 10.1371/journal.pone.0108985. e108985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollierer MM, Langel R, Körner C, Maraun M, Scheu S. The underestimated importance of belowground carbon input for forest soil animal food webs. Ecology Letters. 2007;10:729–736. doi: 10.1111/j.1461-0248.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- Powell JR, Anderson IC, Rillig MC. A new tool of the trade: plant-trait based approaches in microbial ecology. Plant and Soil. 2013;365:35–40. [Google Scholar]
- Powell JR, Bennett AE. Unpredictable assembly of arbuscular mycorrhizal fungal communities. Pedobiologia. 2016;59:11–15. [Google Scholar]
- Powell JR, Riley RC, Cornwell W. Relationships between mycorrhizal type and leaf flammability in the Australian flora. Pedobiologia. 2017 [Google Scholar]
- Roscher C, Schumacher J, Baade J, Wilcke W, Gleixner G, Weisser WW, Schmid B, Schulze ED. The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic and Applied Ecology. 2004;5:107–121. [Google Scholar]
- Shipley B, De Bello F, Cornelissen JHC, Laliberté E, Laughlin DC, Reich PB. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia. 2016;180:923–931. doi: 10.1007/s00442-016-3549-x. [DOI] [PubMed] [Google Scholar]
- Reich PB, Tilman D, Isbell F, Mueller K, Hobbie SE, Flynn DFB, Eisenhauer N. Impacts of Biodiversity Loss Escalate Through Time as Redundancy Fades. Science. 2012;336:589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- Roscher C, Schmid B, Buchmann N, Weigelt A, Schulze E-D. Legume species differ in the responses of their functional traits to plant diversity. Oecologia. 2011;165:437–452. doi: 10.1007/s00442-010-1735-9. [DOI] [PubMed] [Google Scholar]
- Steinauer K, Fischer FM, Roscher C, Scheu S, Eisenhauer N. Spatial plant resource acquisition traits explain plant community effects on soil microbial properties. Pedobiologia. 2017 [Google Scholar]
- Tsunoda T, van Dam NM. Root chemical traits and their roles in belowground biotic interactions. Pedobiologia. 2017 [Google Scholar]
- Vályi K, Rillig MC, Hempel S. Land-use intensity and host plant identity interactively shape communities of arbuscular mycorrhizal fungi in roots of grassland plants. New Phytologist. 2015;205:1577–1586. doi: 10.1111/nph.13236. [DOI] [PubMed] [Google Scholar]
- Veen GF (Ciska), Freschet GT, Ordonez A, Wardle DA. Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos. 2015;124:187–195. [Google Scholar]
- Venter ZS, Jacobs K, Hawkins H-J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia. 2016;59:215–223. [Google Scholar]
- Vos VC, van Ruijven J, Berg MP, Peeters ET, Berendse F. Macro-detritivore identity drives leaf litter diversity effects. Oikos. 2011;120:1092–1098. [Google Scholar]
- Wang P, van Ruijven J, Heijmans M, Berendse F, Maksimov A, Maximov T, Mommer L. Short-term root and leaf decomposition of two dominant plant species in a Siberian tundra. Pedobiologia. 2017 [Google Scholar]
- Werner GDA, Kiers ET. Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytologist. 2015;205:1515–1524. doi: 10.1111/nph.13092. [DOI] [PubMed] [Google Scholar]