ABSTRACT
Epithelial cells are polarised within the plane of the epithelium, forming oriented structures that have a coordinated and consistent polarity (planar cell polarity, PCP). In Drosophila, at least two separate molecular systems generate and interpret intercellular polarity signals: Dachsous/Fat, and the ‘core’ or Starry night/Frizzled system. Here, we study the prickle gene and its protein products Prickle and Spiny leg. Much research on PCP has focused on the asymmetric localisation of core proteins in the cell and as a result prickle was placed in the heart of the Starry night/Frizzled system. We investigate whether this view is correct and how the prickle gene relates to the two systems. We find that prickle can affect, separately, both systems; however, neither Prickle nor Spiny leg are essential components of the Dachsous/Fat or the Starry night/Frizzled system, nor do they act as a functional link between the two systems.
KEY WORDS: Drosophila, Planar cell polarity, Abdomen, Dachsous/Fat, Prickle, Starry night/Frizzled
Summary: Drosophila prickle can affect, separately, both the Ds/Ft and the Stan/Fz PCP systems; however, Pk and Sple are not essential for either and do not act as a functional link between the two systems.
INTRODUCTION
Planar cell polarity (PCP) refers to a property that all, or most, epithelial cells have – they are coordinately oriented in the plane of the epithelial sheet and, sometimes, they demonstrate this by forming oriented structures. These oriented structures can be cell organelles such as cilia, or multicellular organs such as mammalian hairs (Tree et al., 2002a; Wang and Nathans, 2007; Goodrich and Strutt, 2011; Butler and Wallingford, 2017). Drosophila has been used to identify most of the genes involved in PCP and has proved the most amenable of all animals for elucidating its mechanisms. Most studies have investigated where PCP gene products are localised in the cell and how these localisations relate to the transmission of polarity from cell to cell. Here, we use genetic methods to investigate the function of prickle (pk), a PCP gene.
The pk gene produces two homologous transcripts that encode the Pk and Sple proteins; both proteins contain protein-protein binding LIM domains, they differ in the N terminus (Gubb et al., 1999) and have sequence elements conserved to vertebrates. In vertebrates, syndromes caused by pk mutations have been identified but it is not clear if they reveal genuine PCP functions of the pk genes (Tissir and Goffinet, 2013). In flies, in the absence of the pk gene, the polarities of bristles and hairs are altered and/or disordered over large areas of the cuticle. In the abdomen, when the pk isoform is overexpressed everywhere, polarity of the anterior (A) compartment is almost entirely reversed, whereas the posterior (P) compartment is normal. By contrast, when the sple isoform is overexpressed everywhere, polarity of the P compartment is completely reversed and the A compartment is normal. These findings led to the hypothesis that the pk gene functions in the wild type to turn around or ‘rectify’ polarity locally so that hair orientation is made consistent in both compartments (Lawrence et al., 2004). These results support the hypothesis that Pk and Sple have similar functions in PCP, the local outcome depending on the distribution of both proteins and varied regional responses to them (Gubb and Garcia-Bellido, 1982). For example, in the wild type, of the two proteins, Pk is found to be the most effective agent in the wing, in the P compartment of the abdomen and the posterior part of the thorax, whereas Sple is thought to predominate in the A compartment of the abdomen and anterior region of the thorax (Gubb et al., 1999; Ayukawa et al., 2014; Merkel et al., 2014; Ambegaonkar and Irvine, 2015).
One set of proteins constitutes the ‘core system’ of PCP. Because of the central importance of Starry night (Stan) and Frizzled (Fz) in the function of this system, we rename it the ‘Stan/Fz system’ (Lawrence et al., 2004). Proteins classified as members of the Stan/Fz system include Stan, Fz, Van Gogh (Vang), Dishevelled (Dsh), Diego (Dgo) and Pk/Sple (for a review, see Goodrich and Strutt, 2011; Adler, 2012; Butler and Wallingford, 2017). Some of these proteins are asymmetrically distributed: in the wing, Pk is enriched on or near the proximal membrane of each cell (Tree et al., 2002b), while Fz is localised distally (Strutt, 2001). The localisation of these proteins is mutually dependent; when one protein is removed, the others become evenly distributed around the cell periphery (reviewed by Strutt and Strutt, 2009). The current view is that Dsh, Pk, Vang and Fz interact with each other to amplify their asymmetric localisation within the cell and thereby consolidate its polarity. Groups of proteins associate in separate complexes on different sides of the wing cell and are associated with each other across the cell membrane, proximally or distally, as a response to an upstream signal (reviewed by Klein and Mlodzik, 2005).
Some argue that PCP is produced by three tiers of gene activity (Tree et al., 2002a, b; Yang et al., 2002; Klein and Mlodzik, 2005; Strutt and Strutt, 2005, 2007; Axelrod, 2009) in which asymmetrical distribution of the protocadherins Dachsous (Ds) and Fat (Ft) provides an initial cue to orient the Stan/Fz system, which amplifies the signal to polarise effector functions. Recently, it has been posited that the pk gene intervenes between the polarising information specified by the direction of the gradients of Ds/Ft activity and its interpretation by the Stan/Fz system (Hogan et al., 2011; Ayukawa et al., 2014; Olofsson et al., 2014; Ambegaonkar and Irvine, 2015). These articles also support earlier conclusions that Pk and Sple act discordantly on polarity output in different tissues and have improved the evidence that changes in the levels of Pk and/or Sple can turn around the orientation of polarised structures (Ayukawa et al., 2014). Moreover, they present arguments that Sple is the main component of a molecular link between the Ds/Ft and Stan/Fz systems.
But, some earlier findings are inconsistent with the hypothesis that Pk/Sple acts as a link. (1) The two systems can work independently of each other, at least in the abdomen (Casal et al., 2006), meaning that neither the Ds/Ft system nor the Stan/Fz system need each other to polarise cells. If so, there would be no need for a general link between the two systems. (2) Genetic experiments argue that, functionally, Pk and Sple are not required for polarity signalling from cell to cell, a central aspect of PCP. Adler et al. (2000) found that a weak allele of pk did not block or inhibit but actually increased those local changes in cell polarity that are induced by clones mutant for other Stan/Fz system genes. Adler's finding was confirmed when Lawrence et al. (2004) showed that complete loss of Pk and Sple increases polarisation by the Stan/Fz system genes; they proposed that the key molecules in the Stan/Fz system do not include Pk but are Stan, Fz and Vang. This conclusion was later supported by Strutt and Strutt (2007) who presented further evidence that Dsh, Pk and Dgo are not needed for the propagation of polarity from cell to cell.
Here, we undertake genetic experiments aimed at clarifying the function of Pk/Sple in the wild-type abdomen, particularly in relation to the two systems. We conclude that Pk and Sple are not essential components of either system nor are they essential components of a link between the two systems. We add to evidence that the Ds/Ft system acts independently of the Stan/Fz system. Surprisingly, we provide data that Pk and Sple can interact with the output of the Ds/Ft system to reverse the hair orientation (even in the absence of a functioning Stan/Fz system). Pk and Sple also, separately, affect the output of the Stan/Fz system; they alter how far the polarising signal can spread.
RESULTS
Explanation of terms and methods
In this study, we make genetically marked clones of cells of different genotypes to investigate how the two different genetic systems, the Ds/Ft system and the Stan/Fz system, define cell polarity in the A and P compartments of the adult abdomen. We assay function of the Ds/Ft system by the ability of ‘sending cells’ in clones that, for example, overexpress ft, to change the polarity of ‘receiving cells’. Such responses that are induced in cells nearby the clone are defined as ‘non-autonomous’. As a result, hairs and bristles around the clones may point ‘inwards’ or ’outwards’, that is, in or away from the clone. For the Ds/Ft system, Ds, Ft and Dachs (D) are each essential; however, removal of only Ds or Ft does break the system but, in addition, causes misdistribution of the D protein in each cell (Ambegaonkar et al., 2012; Pan et al., 2013), leading to an adventitious phenotype of whorly polarity (Ambegaonkar et al., 2012; Lawrence and Casal, 2013). Therefore, the cleanest way to break the Ds/Ft system completely and persuasively is to remove D as well as Ds or Ft. To break the Stan/Fz system we remove Stan; stan– cells cannot send or receive signals, for example stan– receiving cells cannot respond to cells that overexpress fz (Genotype 1) even when those sending cells also express stan (Lawrence et al., 2004; Casal et al., 2006). Using these functional assays we investigate whether and how Pk and Sple cooperate with the Ds/Ft and the Stan/Fz systems.
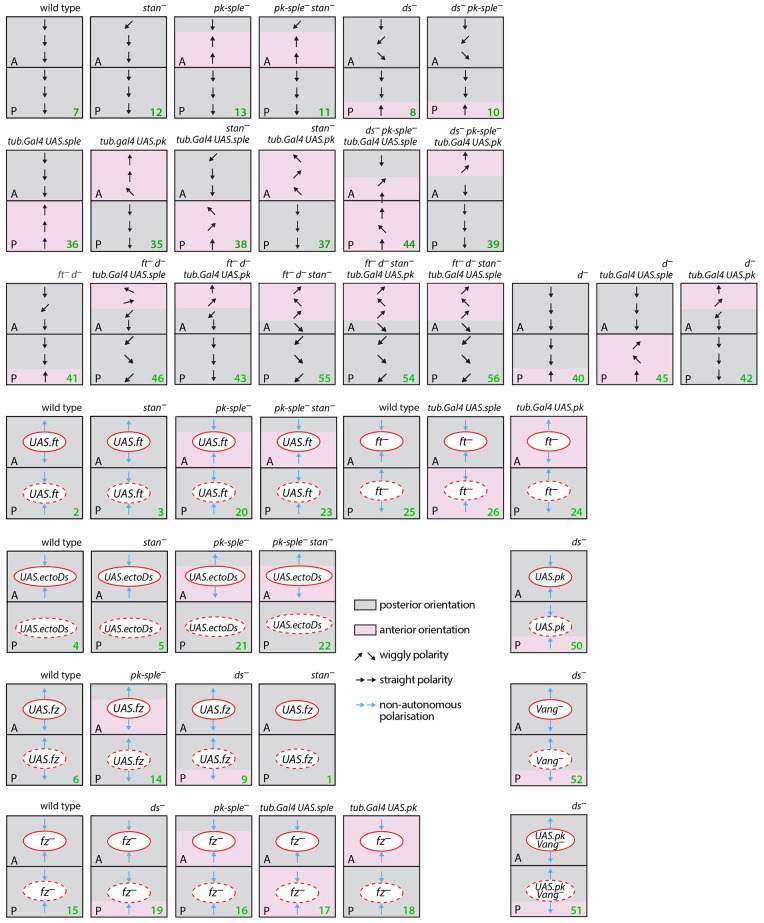
Fig. 1 acts as a summary of, and a guide to, all the experiments and results.
Fig. 1.
A guide to all the experiments. A summary of the results showing the polarities of hairs in the two abdominal compartments plus the effects of clones on polarity. UAS indicates overexpression of the said gene in the clones, tub.Gal4 UAS.x indicates generalised expression of x. Compartments are separated by the horizontal line, anterior compartments (A) above and posterior (P) below. Grey areas define areas where the hairs point predominately posteriorly as in the wild type; pink indicates areas where polarity is mainly reversed, i.e. pointing anteriorly. Polarity is also indicated by the arrows. Genotypes are indicated in this and following figures by green numbers (See Experimental genotypes in the Materials and Methods section). In this and all the following figures, clones in the A compartment are outlined with a continuous red line, and those clones in the P compartment with a dashed red line.
Do the Ds/Ft and Stan/Fz systems act independently in both A and P compartments?
It has been proposed that upstream polarity information specified by the Ds/Ft system is interpreted by the Stan/Fz system (Yang et al., 2002; Ma et al., 2003; Goodrich and Strutt, 2011; reviewed by Butler and Wallingford, 2017). Experiments in the adult abdomen showed that the non-autonomous effects on neighbouring cells by clones, for example, lacking ft, depended on the compartment. ft– clones in the A compartment made the surrounding cells point inwards towards the clone, whereas the same clones in the P compartment caused the surrounding cells to point outwards. We therefore argued that the gradient of Ds activity might have opposite slopes in the A and the P compartments (Casal et al., 2002). But, if that were true, because the hairs in both compartments point the same way in the wild type, hair polarity cannot be a direct readout of the gradient slope of the Ds/Ft system. Experimental evidence provided a solution to this conundrum: perhaps Pk or Sple rectify the reading of a gradient in either the A or the P compartment so that all hairs point in the same direction (Lawrence et al., 2004). But, later experiments argued that the Stan/Fz system and the Ds/Ft system can act independently of each other (Casal et al., 2006; Lawrence et al., 2007; and more recently Brittle et al., 2012), implying that rectification due to Pk and/or Sple does not alter a direct input from the Ds/Ft system into the Stan/Fz system but avoids dissonance between their independent inputs into PCP.
Clones affecting the Ds/Ft system function when the Stan/Fz system is broken
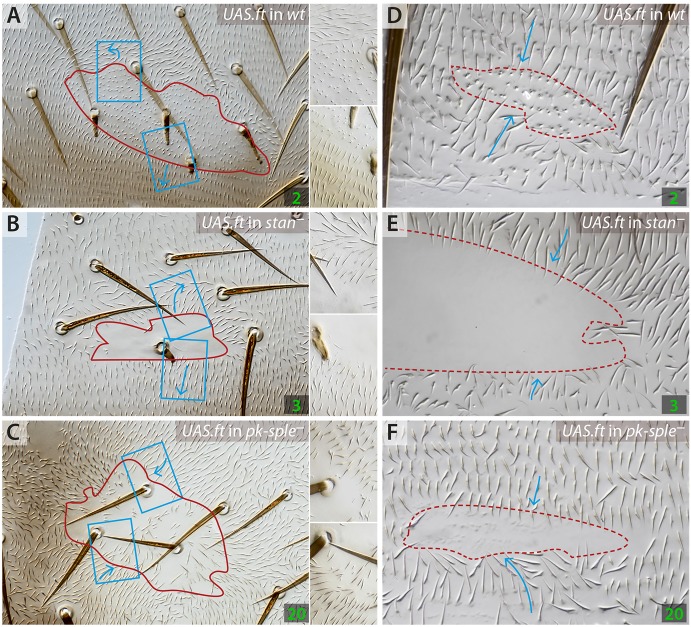
Previously, we showed that clones affecting the Ds/Ft system could polarise cells non-autonomously and do so very well in the absence of a functioning Stan/Fz system. These studies were limited to the A compartments. Here, we show that, in both the A and the P compartments, clones overexpressing ft polarise both wild-type cells (Genotype 2) and cells in which the Stan/Fz system is broken – we have used flies lacking stan, (Genotype 3) or, in the case of A clones, both stan and fz (Casal et al., 2006). In both cases, the receiving cells tend to point hairs outwards from the clone in the A compartments (Casal et al., 2006) and inwards in the P compartments (Figs 2 and 3). Consistent with these results, clones overexpressing the extracellular domain of Ds also polarise both wild-type cells (Genotype 4) and cells in which the Stan/Fz system is broken (stan–, Genotype 5) inwards in the anterior portion of A compartments (Casal et al., 2002, 2006). These clones are ineffective in the posterior parts of A compartments and in P compartments (Fig. S3), probably because the activity of Ds is normally high in these regions (Casal et al., 2002).
Fig. 2.
Clones that overexpress ft in various backgrounds. The receiving cells point outwards in the A compartments (A,B), inwards in P compartments (D,E) of stan– and wild-type cells. The response of pk-sple– cells is inwards in both the A and P compartments (C,F). In this and the following figures, blue boxes delimit the areas detailed at higher magnification (a blue dashed line indicates that the area is part of the P compartment), and blue arrows indicate orientation of hairs. For images of clones expressing fz in the same backgrounds, see Fig. S2.
Fig. 3.
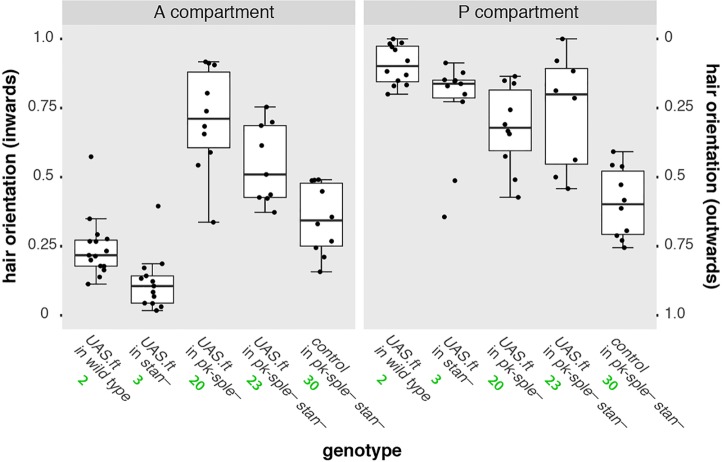
Effects of the ft-overexpressing clones in A and P compartments (cf. Fig. 2). The orientations of hairs immediately adjacent to each clone are counted and displayed in box plots; each dot represents the data from one clone. The responses range from all pointing inwards (top of the graph) to all pointing outwards (bottom). Breaking the Stan/Fz system (stan–) did not much affect any outcome (see Fig. S3 for statistical analysis), confirming that the Ft/Ds system does not act through the Stan/Fz system. However, removing pk and sple changed the sign of response in the A compartment.
Clones affecting the Stan/Fz system function when the Ds/Ft system is broken
We can also look at the independence of the two systems by interfering with the Stan/Fz system in clones in a background in which the Ds/Ft system is broken. Clones that overexpress fz, in either the A or P compartments, normally turn the polarity of receiving cells to point outwards from the clone in A (Casal et al., 2006) and also in P (Genotype 6, Fig. S2). They do the same in ds– flies but with a longer range (Genotype 9; Adler et al., 1998; Ma et al., 2003; Casal et al., 2006; Fig. S1).
These and previous experiments have established that the two systems can act independently in both compartments of the abdomen; we now ask are Pk and Sple essential components of either the Ds/Ft or the Stan/Fz systems?
How do pk and sple interact with each of the two systems?
Evidence from epistasis
ds– and pk-sple– flies differ in phenotype in the dorsal abdomen: the most useful difference is seen in the P compartment, where, in ds– flies, hairs in the anterior region of the P compartment are in whorls (probably due to the misdistribution of Dachs) but in the posterior region of the P compartment the hairs point directly anteriorward. By contrast, in pk-sple– flies, the entire P compartment has normal polarity (Lawrence et al., 2004). If Ds/Ft were to provide an upstream cue that is interpreted by the Stan/Fz system via Pk/Sple, one would expect the double mutant ds– pk-sple– to have a phenotype more similar to the single pk-sple– mutant than to the ds– mutant phenotype. However, we find that, in the abdomen, ds– pk-sple– flies (Genotype 10) are little different from ds– flies (Genotype 8, Fig. 1). It follows that the ds mutation is epistatic to a mutation that removes both pk and sple functions. Turning to interaction between the Stan/Fz system and Pk/Sple. When pk-sple– stan– flies are compared with each single mutant, they differ from both, having an additive phenotype (Genotype 11, Genotype 12, and Genotype 13; Fig. 1), implying that the two genes act independently. Taken together, these findings might suggest that the pk gene acts entirely through the Ds/Ft system. However, other results argue that Pk and Sple can also act independently of the Ds/Ft system (see below), and suggest instead that Pk and Sple act separately but differently on each of the two systems.
The Stan/Fz system functions well, both in cells that lack pk and sple and in cells that have pk or sple overexpressed
It has been proposed that Pk/Sple act as a link between Ds/Ft and the Stan/Fz system, rectifying the polarity output to ensure that the A and the P compartments have the same polarity. Here, we test this link by making clones that alter the Stan/Fz system in pk-sple– mutant flies or in flies with ubiquitous overexpression of pk or sple.
In the abdomen of pk-sple– flies (Genotype 13), polarity of most of the A compartment is reversed, but the P compartment is normal. Clones of cells that overexpress fz (Genotype 14 or, alternatively, lack fz, Genotype 16) in such pk-sple– flies polarise receiving cells ‘strongly’ (i.e. with consistent effects and over several rows of cells) in both A and P compartments; in both compartments the clones affect mutant receiving cells with the same sign as in wild-type receiving cells – that is, outwards from the clones that overexpress fz and inwards towards clones that lack fz – independently of the prevailing polarity of the receiving cells (Fig. S2). Thus, the Stan/Fz system does not need Pk or Sple to send polarity signals or to repolarise receiving cells (cf. Lawrence et al., 2004).
In flies in which either sple or pk are overexpressed, large areas of each abdominal segment show abnormal polarity. Nevertheless, fz– clones made in these flies polarise receiving cells of both compartments inwards – as they do in wild-type flies – independently of the prevailing polarity of those receiving cells (Genotype 17, Genotype 18; Fig. 4). All these results are mutually consistent: they show that polarity changes induced by the Stan/Fz system do not require products of the pk gene, showing that Pk and Sple cannot be essential components of the Stan/Fz system in the wild type.
Fig. 4.

Behaviour of fz– and ft– clones in flies overexpressing isoforms of the pk gene. fz– clones behave normally, polarising receiving cells inwards in both A and P either in tub.Gal4 UAS.pk or tub.Gal4 UAS.sple flies, independently of the polarity of their surroundings (A,B). The effects of ft– clones, but only in territories with reversed polarity, are the opposite of normal: in the wild type these effects are inwards in A, outwards in P whereas in tub.Gal4 UAS.pk the cells close to the anterior clones point outwards (C) and in tub.Gal4 UAS.sple the cells nearby the posterior clones point inwards (D). See Fig. S5 for analysis of maximum range of effects of fz– clones.
However, the fz– clones do not behave exactly as they would in a wild-type background: absence or excess of Pk and Sple change the amount of polarisation caused by clones with altered amounts of Fz. In A compartments of the abdomen, clones of cells that lack fz alter polarity of surrounding wild-type cells. The number of rows of receiving cells affected (the range) varies with the amount of Pk and/or Sple protein: in pk-sple– flies the range of polarisation around the fz– clones or clones that contain excess fz (Genotype 16, Genotype 14; figure 4 in Lawrence et al., 2004) is increased, resembling the increase in range observed when fz– clones are induced in ds– flies (Genotype 19). Raising the level of the Pk isoform ubiquitously does not alter the range of polarisation surrounding fz– clones (Genotype 18), whereas when Sple levels are raised (Genotype 17), this range of polarisation is reduced (Fig. S5). In the P compartments, we detected no effects on the range of repolarisation surrounding fz– clones; either in pk-sple– flies or when the levels of either Pk or Sple were increased (Figs S2 and S5). These results add to evidence that the Stan/Fz system can function independently of Pk and Sple.
The Ds/Ft system functions well, both in cells that lack pk and sple and in cells that have pk or sple overexpressed
If Pk/Sple act as an essential link between Ds/Ft and the Stan/Fz system one may expect that clones with an altered Ds/Ft system not to have any effect on hair polarity in flies lacking both Pk and Sple. However, the results were striking and did not support the link hypothesis.
In pk-sple– flies, clones of cells overexpressing ft repolarise receiving cells strongly, even if they lack Pk and Sple (Genotype 20). However, it surprised us that in the largely reversed A compartment of the pk-sple– abdomen, the hairs around the clones point inwards (the opposite sign induced by such clones in the wild type) and also inwards in the P compartment (the same sign as in wild type, Fig. 2). Clones overexpressing ds in pk-sple– flies (Genotype 21) act comparably: the hairs around such clones point outwards in A (the opposite sign induced by such clones in the wild type) and outwards, but weakly, in the P compartment (the same sign as in wild type, see Fig. S3). Thus, in clones of both genotypes, in the A compartments, the sign of the effect is the opposite to when such clones are made in the wild type (Genotype 2 and Genotype 4). Nevertheless, in both these genotypes, in the P compartments, the sign of the polarising effect is the same as wild type. Quantification of overexpressing ft and ds clones confirms these results and also shows that these clones (in the A compartment) affect the polarity of both wild-type (Genotype 2 and Genotype 4) and stan– receiving cells (Genotype 3 and Genotype 5) to the same extent. They also affect pk-sple– stan+ (Genotype 20 and Genotype 21) and pk-sple– stan– (Genotype 22 and Genotype 23) receiving cells with the same strength (Fig. 3 and Fig. S3). These results show that Pk, Sple and Stan are not required for polarity signalling by the Ds/Ft system, although Pk and Sple can change the sign of the response, suggesting that the formation of Ds-Ft bridges works well even the absence of Pk/Sple and/or the Stan/Fz system. Any effect seen has to be downstream of these bridges. They also show that Pk and Sple do not act as an essential link between the Ds/Ft system and the Stan/Fz system, because if they were such a link, removal of Pk and Sple would block effects on polarity caused by overexpressing ft.
Results supporting these conclusions came from clones that altered the Ds/Ft system in flies in which polarity was altered by excess Pk or Sple. Clones that lack ft made in flies in which pk is generally overexpressed (Genotype 24) behave as follows: where the polarity of much of the surrounding background is reversed from normal, with the hairs pointing forwards (ie in the A compartment), ft– clones act with the opposite sign to that in the wild type (Genotype 25) and hairs around the clone tend to point outwards (Fig. 4). In the P compartment, where overexpression of pk produces no change to polarity, the ft– clones behave as they do in the wild type, that is the hairs point outwards from the clone (Fig. 4). Clones that lack ft made in flies in which sple is generally overexpressed (Genotype 26) behave as follows: in the A compartment, which has normal polarity, these clones affect these receiving cells as they do wild-type cells: hairs around the clone point inwards (Fig. 4). In the P compartment, where the polarity of the surrounding background is reversed from normal with the hairs pointing forwards, the ft– clones now polarise receiving cells with the opposite sign to that in the wild type, that is the hairs point inwards into the clone (Fig. 4).
In the A compartment of the abdomen, clones that lack ds have effects of the opposite sign to ft– clones in both classes of experiments above (see two previous paragraphs), as would be expected. However, ds– clones have little or no effect in the P compartment in all genotypes tested (data not shown; Genotype 27, Genotype 28 and Genotype 29).
These results show that the Ds/Ft system can function independently of Pk and Sple but that Pk and Sple can modulate the sign of its output. This dramatic effect could, in principle, be due to Pk and/or Sple affecting the patterns of expression of ds, and/or fj, and thereby changing the orientation of the Ds/Ft system gradients. To test this, we studied the expression of enhancer traps for ds and fj loci in pk-sple– flies and saw no departure from the wild-type patterns (Genotype 31, Genotype 32, Genotype 33 and Genotype 34; Fig. S6). It follows that Pk and Sple determine whether polarised structures in the cell, the hairs and bristles, point up or down the gradients of Ds and Fj.
Pk and Sple alter polarity even when the Stan/Fz system is broken
Were Pk/Sple to act only upstream of the Stan/Fz system one would not expect overexpression of pk or sple to have an effect on the polarity of stan–flies. However, uniform overexpression of pk causes large changes of polarity in the abdomen of wild-type flies (Genotype 35) and flies with a broken Stan/Fz system (Genotype 37) in the A compartment, without affecting the P compartment (Fig. 5), whereas generalised overexpression of sple also affects the polarity of both wild type (Genotype 36) and stan– flies, altering the polarity of the P compartment of the abdomen, without much affecting the A compartment (Genotype 38; Fig. 6).
Fig. 5.
Effects of overexpressing pk on polarity of cells in which either the Stan/Fz system (stan–) or the Ds/Ft system is broken (ft– d–), or both are broken (ft– d– stan–). Phenotypes of A compartments (A-C,E,G,H,J) and P compartments (D,F,I,K). In the A compartments, generalised overexpression of pk changes the polarity of the anterior region of wild-type, stan– and ft– d– cells (A,G,H). In the P compartments, the region that normally points anteriorly in ft– d– points posteriorly (as in the wild type) when pk is overexpressed (I). Ubiquitous Pk appears to have no effect on ft– d– stan– in either A or P compartments (compare E,F,J,K). Compare Fig. S4 for expression of pk in d– flies.
Fig. 6.
Effects of overexpressing sple on polarity of cells in which either the Stan/Fz system (stan–) or the Ds/Ft system is broken (ft– d–). Overexpression of sple in stan– and the wild type reverses all or most of the P compartment to point forwards (A,B,F) but overexpression of sple in a ft– d– background produces a P compartment of normal polarity (H) and even the rear of the P region, which points forward in ft– d– (D) is now ‘rescued’ to normal polarity. Overexpression of sple in ft– d– flies also alters the polarity at the front of the A compartment (C,G) turning the hairs laterally, whereas overexpressing pk turns the hairs to point anteriorly (Fig. 5). Ubiquitous Sple appears to have no effect on ft– d– stan– in either A or P compartments (compare E and I). Fig. S4 for expression of sple in d– flies.
Pk and Sple affect PCP even when the Ds/Ft system is broken
If Pk and Sple acted exclusively on the Ds/Ft system, one would not expect Pk and Sple proteins to affect PCP if the Ds/Ft system were broken. However, we find that ubiquitous overexpression of pk alters polarity of the A compartment (and part of the P compartment) of ds– pk-sple– (Genotype 39; Fig. 1), d– (Genotype 42; Fig. S4) and ft– d– flies (Genotype 43; Fig. 5). Similarly, general overexpression of sple affects the polarity of the P compartment of the abdomen of ds– pk-sple– (Genotype 44; Fig. 1), d– (Genotype 45; Fig. S4) and ft– d– flies (Genotype 46; Fig. 6).
In d– flies, the A and P compartments are largely normal but a section of the P compartment is reversed, as in ds– (or ft–) flies. When ubiquitous Pk is added to d– or ft– d– flies, the anterior part of the A compartment is altered to point forwards and the reversed rear section of the P compartment is ‘rescued’ so that it points backwards, as in the wild type. Thus, Pk affects both the A and the P compartment in these flies. However, unlike Pk, ubiquitous Sple affects d– and ft– d– flies differentially: in a d– background there is no change to the A compartment, but the whole P compartment is largely reversed. But, in a ft– d– background the anterior region of the A compartment points laterally and, as noted by Sharp and Axelrod (2016), the P compartment is rescued, having a normal orientation. Thus, Pk and Sple have similar effects on ft– d– but very different effects on d– flies. It follows from these findings that Ft has outputs that are independent of D and that these outputs are altered by Sple but not by Pk. Note that both Sple and Pk can rescue the reversed polarity in the P compartment in a completely broken Ds/Ft system (ft– d–) perhaps through their effects on the Stan/Fz system or, maybe, through any other contributors to PCP (Figs 5 and 6 and Fig. S4).
We find that clones of cells overexpressing sple (Genotype 47; Lawrence et al., 2004) or pk (Genotype 48; data not shown), have small non-autonomous effects in the wild type and, more so, in ds– flies (Genotype 49 and Genotype 50) where they polarise receiving cells to point strongly inwards (Fig. 7). Perhaps these clones act via the Stan/Fz system? It is pertinent that both wing and abdominal cells that overexpress the pk gene accumulate Vang uniformly on the cell membrane (Bastock et al., 2003; Olofsson et al., 2014). If this were to happen in our experiments, then the clone could behave as if it were overexpressing Vang and should polarise surrounding cells inwards, as observed; this effect should be stronger in ds– than in ds+ cells, also as observed. To test this hypothesis further, we made Vang– clones that overexpressed pk (Genotype 51), as well as control Vang– clones (Genotype 52), in ds– flies. Both these types of clones behaved like Vang– clones in wild-type flies (Genotype 53), and could not be distinguished from each other, i.e. they polarise ds– receiving cells strongly outwards (Fig. 7), confirming the hypothesis that cells overexpressing pk polarise cells because they accumulate Vang, a Stan/Fz system protein. Thus, overexpressing Pk interferes with the Stan/Fz system. These results show that Pk and Sple do have functions that are independent of the Ds/Ft system.
Fig. 7.
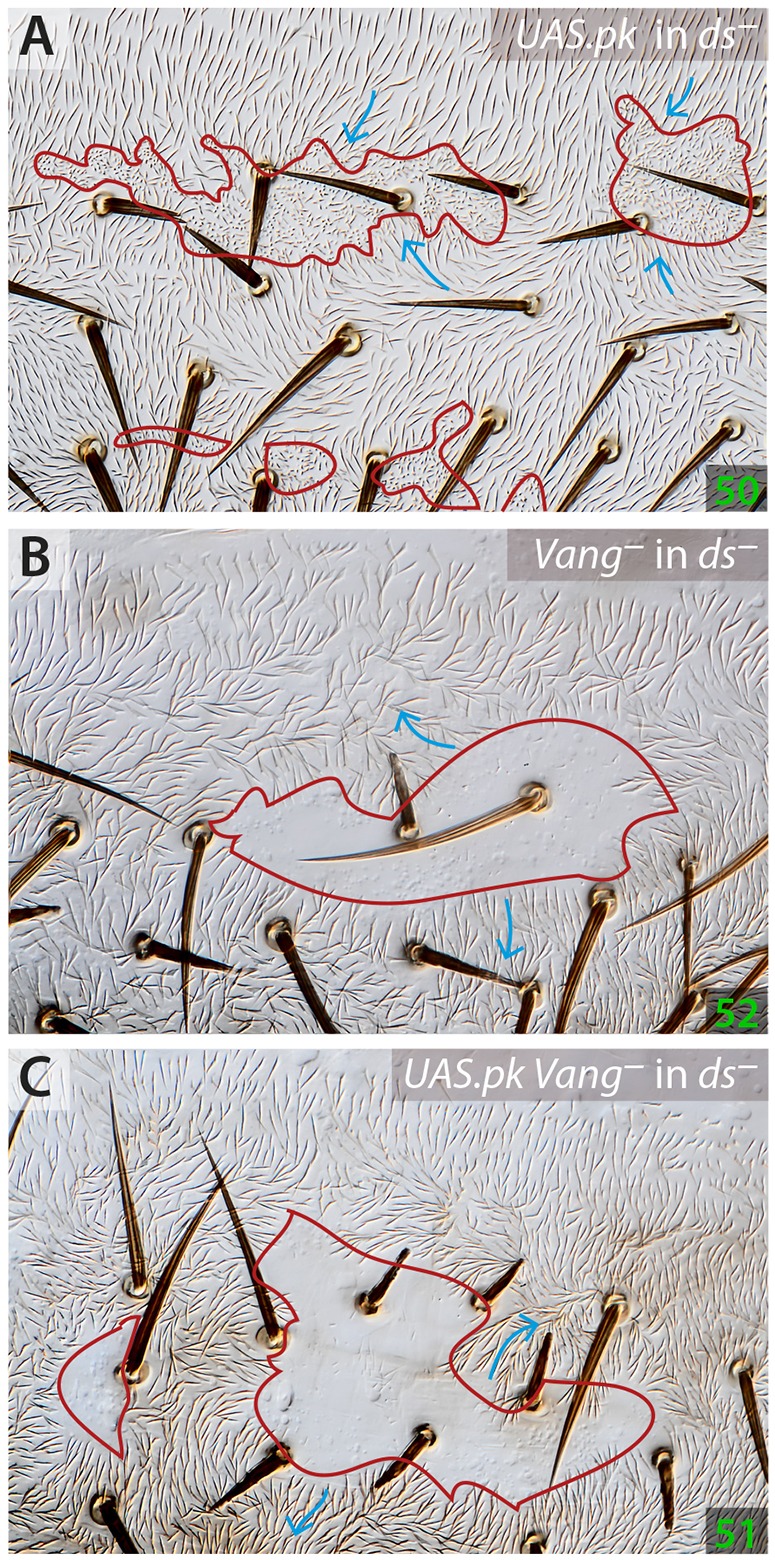
Effects of pk-expressing clones in flies in which the Ds/Ft system is broken. Clones that overexpress pk polarise ds– cells strongly inwards (A). Clones lacking Vang (B) as well as clones that, lacking Vang, also overexpress pk (C), polarise ds– receiving cells strongly outwards.
Can Pk function independently of both systems of PCP?
We have presented evidence that the Pk and Sple modulate, in different ways, both the Ds/Ft and the Stan/Fz systems. We wondered if Pk and Sple proteins could have outputs independently of these two systems. To test this, we overexpressed pk and sple in a genetic background in which both systems are broken: ft– d– stan–. We found, using blind screening, that we could not distinguish the phenotypes of these flies with and without the overexpression of pk (Genotype 54 and Genotype 55; Fig. 5), or sple (Genotype 56; Fig. 6) arguing that the polarity effects of an excess of Pk or Sple are due to them acting through the two systems and by no other route.
DISCUSSION
Our aim is to understand the contribution of Pk and Sple to building PCP in the wild-type fly. The main results and conclusions are listed below.
The Ds/Ft system and the Stan/Fz system act independently and are not linked via Sple and/or Pk
ft-overexpressing clones reorient wild-type receiving cells, outwards in the A compartment (Casal et al., 2006) and inwards in the P (this paper). These clones have the same effects on cells in which the Stan/Fz system of PCP is broken (for example, in stan– flies; Figs 2 and 3 and Fig. S3). It follows that this reorientation of polarity cannot be due to any intracellular interaction between Stan and any component of the Ds/Ft system within the sending cells. However, it could be argued that extra Ft in the sending cell, attracting Ds in the receiving cell, would, non-autonomously, influence some residual capability of the Stan/Fz system in the receiving stan3/stanE59 cells to respond and propagate polarity to neighbouring cells. Yet, even clones that overexpress both fz and stan (i.e. cells that have a fully functional Stan/Fz system) fail to repolarise stan3/stanE59 cells (Casal et al., 2006). Thus, the propagation of a polarity change observed around cells that overexpress ft cannot be due to any non-autonomous effect on the Stan/Fz system. These results show that, at least for both compartments of the abdomen, the Ds/Ft system acts independently of the Stan/Fz system (Casal et al., 2006; Lawrence et al., 2007; Lawrence, 2011).
Here, we make stanE59 clones (stanE59 introduces a premature stop codon in the ectodomain; Usui et al., 1999) that overexpress ft or ds in pk-sple– stan3/ pk-sple– stanE59 flies, and show that these clones repolarise the receiving cells. This polarisation cannot depend on Pk and Sple intervening, inside the cells of the clone, between the Ds/Ft and the Stan/Fz systems because the sending cells lack the stan and pk genes completely; in addition, the receiving cells lack the pk gene and any functional Stan (see previous paragraph). A finding that conflicts with current models in which the pk gene products are proposed to link the two systems of PCP (Hogan et al., 2011; Ayukawa et al., 2014; Merkel et al., 2014; Olofsson et al., 2014; Ambegaonkar and Irvine, 2015).
Another argument is relevant here: clones affecting the Ds/Ft system have outputs of different sign in the A and P compartments (Casal et al., 2002). If this divergent polarisation were to act through and depend on the Stan/Fz system via a molecular link of Pk and/or Sple, then we would expect the polarising output from Stan/Fz system clones (e.g. from fz– clones) to be also of different sign in the two compartments and to be dependent on that link (cf. figure 7 in Ayukawa et al., 2014). However, this is not the case (Fig. S2E,F).
Pk/Sple act independently of the Stan/Fz system
Loss of the pk gene or overexpression of the Pk isoform reverses polarity of most of the A compartment, having strong effects even in flies with a broken Stan/Fz system (stan–). Similarly, overexpressing Sple reverses polarity in the P compartment in stan– flies; it follows that Pk and Sple can act independently of the Stan/Fz system. This does not fit easily with the current view that Pk functions as an essential component of the Stan/Fz system; for example, the lack of requirement for the pk gene contrasts with a requirement for the other key Stan/Fz system genes (i.e. Fz, Stan or Vang) in the receiving cells (Taylor et al., 1998; Lawrence et al., 2004; Strutt and Strutt, 2007; Strutt and Warrington, 2008; Struhl et al., 2012).
The Stan/Fz system proteins Stan, Fz, Vang and Pk are all preferentially localised to specific regions of the cell membrane and this is considered to be important for their functions in PCP. Nevertheless, pk-sple– receiving cells, in which Stan, Fz and Vang no longer appear to be localised (reviewed by Strutt, 2009), can respond at least as well to sending cells that overexpress fz, as wild-type receiving cells (Adler et al., 2000; Lawrence et al., 2004). This dilemma could be resolved if the observed asymmetry were not directly related to function as has been assumed and were more a consequence than a cause of polarity (Lawrence et al., 2004).
Pk and Sple modulate the Ds/Ft system, determining the polarity of its output
Sending cells that overexpress ds or ft, or lack ds or ft, change the polarity of receiving cells, even in the absence of Pk and Sple; it follows that these proteins cannot be necessary for the Ds/Ft system to function and propagate polarity from cell to cell. However, the sign of this change depends on whether the receiving cells contain, lack or overexpress products of the pk gene. These results show that Pk and Sple can alter the sign of polarisation that is produced by the Ds/Ft system. But, how do Pk and Sple have their various effects on polarity? It appears that the sign of polarisation depends on the relative amounts of Pk and Sple in a particular region of the fly (Gubb et al., 1999; Ayukawa et al., 2014). One model is that the Ds/Ft proteins might act through Pk and Sple to bias the orientation of microtubules and thus PCP, because oriented microtubules could transport Stan/Fz system components preferentially to one side of the cell (Shimada et al., 2006; Harumoto et al., 2010; Matis et al., 2014; Olofsson et al., 2014). But, correlation between microtubule orientation, Pk/Sple function, and PCP is inconsistent or lacking, clearly so in the distal half of the wing and the P compartment of the adult abdomen (Harumoto et al., 2010; Sharp and Axelrod, 2016), leading to doubts about the validity of the hypothesis (Ambegaonkar and Irvine, 2015). Also, this model is now contradicted by our results, which show that abnormal amounts of Ds, Ft, Sple or Pk can all affect PCP even when the Stan/Fz system is broken.
A diagram suggesting how the pk gene might fit into the organisation of PCP is given in Fig. 8.
Fig. 8.
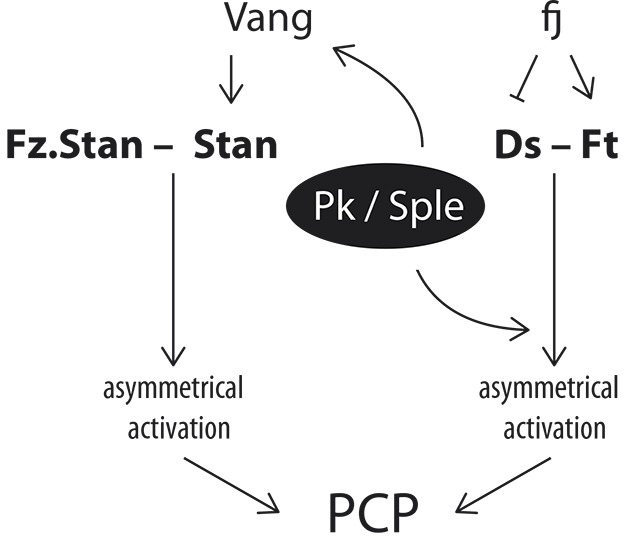
Pk and Sple functions in the context of PCP. PCP depends on molecular bridges between cells: for the Stan/Fz system the key bridge consists of a complex of Stan and Fz in one cell and Stan in the other; Vang promotes function of the Stan pillar of this bridge (Struhl et al., 2012). For the Ds/Ft system, Ds in one cell is linked to Ft in another, the activity of both is modulated by Fj (reviewed by Butler and Wallingford, 2017). Pk and/or Sple bind to Vang and promote asymmetrical distribution of Vang and other PCP molecules. Yet in the absence of Pk and Sple, the Stan/Fz system can still receive and send polarity information, implying that it is the asymmetric activation of protein complexes that polarise a cell rather than asymmetric localisation. Pk and Sple alter the sign of the polarity output of the Ds/Ft system, but by an unknown mechanism. Yet, Pk and Sple can alter polarity output even when the Ds/Ft system is broken. The results show that Pk and Sple can act separately on both systems, implying some general function of Pk and Sple in cell polarity. The indispensable elements of the two systems are shown in bold.
The functions of Pk and Sple
It has been suggested that Pk and Sple do fundamentally different things (Ayukawa et al., 2014; Ambegaonkar and Irvine, 2015); however, our findings fit better with the view that the two isoforms have similar molecular functions and the differences between them are due to their expression in different patterns (Gubb et al., 1999). Indeed, in the Results, section ‘Pk and Sple affect PCP even when the Ds/Ft system is broken’, in which we study the behaviour of ft– or ft-expressing clones, we found that removal of Pk and Sple or ubiquitous expression of either can eliminate any differences in responses between the A and the P compartment cells.
It might appear that ectopic Pk can act in only the A compartment and Sple in the P, but it cannot be so simple, for the universal expression of either Pk or Sple can rescue the reversed polarity at the back of the P compartment in ft– d– flies (Figs 5 and 6; Sharp and Axelrod, 2016). Also, when sple is generally overexpressed in ft– d– flies, polarity of the anterior region of the A compartment is considerably altered (Fig. 6). Looking at the P compartment, the action of Pk appears to be independent of an intact Ds/Ft system, but the effects of Sple in the P compartment depend on whether the background genotype is d– or ft– d– (Fig. 6 and Fig. S4). Part of this difference could be due to direct interaction between Sple and Ds but not between Pk and Ds (Ambegaonkar and Irvine, 2015). However, Ayukawa et al. (2014) find that both Sple and Pk bind to each other and to D (but not to Ds), suggesting the difference may have other causes.
But why are the Pk and Sple proteins asymmetrically localised in the cell? Part of the answer could be that Pk and Sple work with and/or bind to components of the Ds/Ft system, which are themselves asymmetrically localised (Ayukawa et al., 2014; Ambegaonkar and Irvine, 2015). But this cannot be all of the answer as Pk is not properly localised in stan– cells (Tree et al., 2002b), in which Ds and Ft are, presumably, normally localised.
How can we understand the effect of Pk and Sple on the Stan/Fz system, particularly on range? In the A compartment, a high level of Sple reduces polarity changes induced by fz– clones, whereas the loss of the pk gene increases their range. One explanation could depend on Sple and Pk (or the lack of these proteins) acting on the Ds/Ft system; if they made the polarity induced by Ds/Ft in the cells more (or less) robust it would make it more difficult (or easier) for clones affecting the Stan/Fz system to alter PCP. Another explanation could relate to some direct effect of Pk (and Sple) on Vang (Bastock et al., 2003), which fits our observations with clones overexpressing Pk (Fig. 7). The function of Vang in the Stan/Fz system is somewhat unclear; like Pk, Vang is present in larger than stoichiometric amounts in relation to the two molecules that form the intercellular bridge, Stan and Fz (Strutt et al., 2016), yet affects bridge function (Struhl et al., 2012). The abdominal phenotypes of Vang– and pk-sple– are somewhat similar, both having areas of reversed polarity (Lawrence et al., 2004), suggesting a commonality of function. Indeed, there is a recent model proposing that Pk acts on the stability of Fz intracellularly (via Dsh) and in the adjacent cell (via Vang); the former effect may involve endocytosis (Warrington et al., 2017). Our experiments argue that the function of Pk is not limited to the Stan/Fz system but includes, independently, the Ds/Ft system. In any case, we have no explanation for the lack of apparent effects of Pk and Sple on the range of fz– clones in the P compartment.
What could be the purpose of such complexity? In Drosophila the consistent orientation of the wing hairs may have led to an oversimplified and idealised picture. Elsewhere, the presentation of PCP is more complex: consider the mixed orientation of rows of hairs and denticles on the Drosophila larva, differing dorsally and ventrally, or, in mammals, the startlingly diverse orientation of stereocilia in the vestibular system, or the complex patterns of hair orientation on the skin. Two separate genetic systems that generate polarity by reading the slopes of morphogen gradients, plus Pk and Sple to modulate output in different parts of the body, could generate much of this flexibility in PCP.
Conclusion
We have found for the abdomen that Pk and Sple are not essential for the polarising effects of either the Ds/Ft or the Stan/Fz systems. We have shown that they do not function as a link between the two systems. Instead, Pk and Sple appear to modulate the polarity outputs of both the Ds/Ft system and the Stan/Fz system with the most conspicuous effects on the former. Both these systems differ in their components but are similar in their logic; both utilise intercellular molecular bridges that become distributed asymmetrically within each cell. Pk and Sple could help produce this asymmetry, perhaps via a generic function in cell biology the mechanism of which, although still undescribed, may be related to those feedback mechanisms that amplify small asymmetries. Our genetic experiments on the abdomen point to conclusions that differ from the prevailing view that the pk gene mediates between the Ds/Ft and the Stan/Fz systems. We do not know if our conclusions apply to other organs in the fly, but if we adopt the hypothesis that they do, they suggest that current views of the wild-type functions of the pk gene should be reconsidered.
MATERIALS AND METHODS
Mutations and transgenes
The FlyBase (Gramates et al., 2017) entries for relevant mutations and transgenes are the following: tub.Gal4, Scer\GAL4alphaTub84B.PL; tub.Gal80, Scer\GAL80alphaTub84B.P; UAS.ectoDs, dsecto.Scer\UAS; UAS.ft, ftScer\UAS.cMa; UAS.fz, fzScer\UAS.cSa; UAS.pk, pkScer\UAS.cGa; UAS.sple, pksple.Scer\UAS; ckUAH21; dGC13; dsUA071 and ds2D60b; fjp1; ft8 and ftG-rv; fz15; pkpk-sple-13; pwn1; sha1; stan3 and stanE59; trc1.
Experimental genotypes
Experimental genotypes are listed in Table 1.
Table 1.
Experimental genotypes used in this study
Clone induction and microscopy
Clones were induced by heat shocking third instar larvae for 1 h at 34°C. Adult abdominal cuticles were studied as previously published (e.g. Lawrence et al., 2004; Casal et al., 2006).
Quantification
Individual hairs along the entire perimeter of each clone (about 60-100 hairs per clone) were each scored as pointing largely into, outwards or parallel to the clone. Parallel hairs, which averaged 8% of the hairs, were counted; half was added equally to the inwards and outwards sets. The average orientation is then found for each clone (between 10 and 20 clones per genotype).
For range measurements, for each clone (n=20) the maximum extent in cell rows of the induced polarity changes was measured. The observer was blinded to genotype; he chose clones located in the middle of the A compartment and the middle or rear of the hairy region of the P compartment; small clones were avoided. Statistical analysis and graphics were performed in R using standard packages (R Core Team, 2016) and the reshape and ggplot packages (Wickham, 2007, 2009).
Supplementary Material
Acknowledgements
We thank the Department of Zoology, Malcolm Burrows for encouragement, and Gary Struhl and David Strutt for stocks and constructive criticisms.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.C.; Methodology: J.C.; Validation: J.C., P.A.L.; Formal analysis: J.C., P.A.L.; Investigation: J.C., B.I.-J.; Writing - original draft: P.A.L.; Writing - review & editing: J.C., P.A.L.; Visualization: J.C., B.I.-J.; Supervision: J.C.; Project administration: P.A.L.; Funding acquisition: P.A.L.
Funding
This research was funded by the Wellcome Trust (WT096645MA and WT107060MA). Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.168112.supplemental
References
- Adler P. N. (2012). The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr. Top. Dev. Biol. 101, 1-31. 10.1016/B978-0-12-394592-1.00001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler P. N., Charlton J. and Liu J. (1998). Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development 125, 959-968. [DOI] [PubMed] [Google Scholar]
- Adler P. N., Taylor J. and Charlton J. (2000). The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech. Dev. 96, 197-207. 10.1016/S0925-4773(00)00392-0 [DOI] [PubMed] [Google Scholar]
- Ambegaonkar A. A. and Irvine K. D. (2015). Coordination of planar cell polarity pathways through Spiny-legs. Elife 4, e09946 10.7554/eLife.09946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambegaonkar A. A., Pan G., Mani M., Feng Y. and Irvine K. D. (2012). Propagation of Dachsous-fat planar cell polarity. Curr. Biol. 22, 1302-1308. 10.1016/j.cub.2012.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J. D. (2009). Progress and challenges in understanding planar cell polarity signaling. Semin. Cell Dev. Biol. 20, 964-971. 10.1016/j.semcdb.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Ayukawa T., Akiyama M., Mummery-Widmer J. L., Stoeger T., Sasaki J., Knoblich J. A., Senoo H., Sasaki T. and Yamazaki M. (2014). Dachsous-dependent asymmetric localization of spiny-legs determines planar cell polarity orientation in Drosophila. Cell Rep. 8, 610-621. 10.1016/j.celrep.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Bastock R., Strutt H. and Strutt D. (2003). Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development 130, 3007-3014. 10.1242/dev.00526 [DOI] [PubMed] [Google Scholar]
- Brittle A. L., Thomas C. and Strutt D. (2012). Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Curr. Biol. 22, 907-914. 10.1016/j.cub.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. T. and Wallingford J. B. (2017). Planar cell polarity in development and disease. Nat. Rev. Mol. Cell Biol. 18, 375-388. 10.1038/nrm.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J., Struhl G. and Lawrence P. A. (2002). Developmental compartments and planar polarity in Drosophila. Curr. Biol. 12, 1189-1198. 10.1016/S0960-9822(02)00974-0 [DOI] [PubMed] [Google Scholar]
- Casal J., Lawrence P. A. and Struhl G. (2006). Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development 133, 4561-4572. 10.1242/dev.02641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich L. V. and Strutt D. (2011). Principles of planar polarity in animal development. Development 138, 1877-1892. 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates L. S., Marygold S. J., dos Santos G., Urbano J.-M., Antonazzo G., Matthews B., Rey A., Tabone C., Crosby M., Emmert D. et al. (2017). FlyBase at 25: looking to the future. Nucleic Acids Res. 45, D663-D671. 10.1093/nar/gkw1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D. and Garcia-Bellido A. (1982). A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morph. 68, 37-57. [PubMed] [Google Scholar]
- Gubb D., Green C., Huen D., Coulson D., Johnson G., Tree D., Collier S. and Roote J. (1999). The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 13, 2315-2327. 10.1101/gad.13.17.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harumoto T., Ito M., Shimada Y., Kobayashi T. J., Ueda H. R., Lu B. and Uemura T. (2010). Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev. Cell 19, 389-401. 10.1016/j.devcel.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan J., Valentine M., Cox C., Doyle K. and Collier S. (2011). Two frizzled planar cell polarity signals in the Drosophila wing are differentially organized by the fat/dachsous pathway. PLoS Genet. 7, e1001305 10.1371/journal.pgen.1001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. J. and Mlodzik M. (2005). Planar cell polarization: an emerging model points in the right direction. Ann. Rev. Cell Dev. Biol. 23, 23 10.1146/annurev.cellbio.21.012704.132806 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A. (2011). Planar cell polarity fashioning solutions. Fly 5, 126-128. 10.4161/fly.5.2.14396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A. and Casal J. (2013). The mechanisms of planar cell polarity, growth and the Hippo pathway: some known unknowns. Dev. Biol. 377, 1-8. 10.1016/j.ydbio.2013.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Casal J. and Struhl G. (2004). Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development 131, 4651-4664. 10.1242/dev.01351 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G. and Casal J. (2007). Planar cell polarity: one or two pathways? Nat. Rev. Genet. 8, 555-563. 10.1038/nrg2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Yang C. H., McNeill H., Simon M. A. and Axelrod J. D. (2003). Fidelity in planar cell polarity signalling. Nature 421, 543-547. 10.1038/nature01366 [DOI] [PubMed] [Google Scholar]
- Matis M., Russler-Germain D. A., Hu Q., Tomlin C. J. and Axelrod J. D. (2014). Microtubules provide directional information for core PCP function. Elife 3, e02893 10.7554/eLife.02893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel M., Sagner A., Gruber F. S., Etournay R., Blasse C., Myers E., Eaton S. and Julicher F. (2014). The balance of prickle/spiny-legs isoforms controls the amount of coupling between core and fat PCP systems. Curr. Biol. 24, 2111-2123. 10.1016/j.cub.2014.08.005 [DOI] [PubMed] [Google Scholar]
- Olofsson J., Sharp K. A., Matis M., Cho B. and Axelrod J. D. (2014). Prickle/spiny-legs isoforms control the polarity of the apical microtubule network in planar cell polarity. Development 141, 2866-2874. 10.1242/dev.105932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G., Feng Y., Ambegaonkar A. A., Sun G., Huff M., Rauskolb C. and Irvine K. D. (2013). Signal transduction by the Fat cytoplasmic domain. Development 140, 831-842. 10.1242/dev.088534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Sharp K. A. and Axelrod J. D. (2016). Prickle isoforms control the direction of tissue polarity by microtubule independent and dependent mechanisms. Biol. Open 5, 229-236. 10.1242/bio.016162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y., Yonemura S., Ohkura H., Strutt D. and Uemura T. (2006). Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev. Cell 10, 209-222. 10.1016/j.devcel.2005.11.016 [DOI] [PubMed] [Google Scholar]
- Struhl G., Casal J. and Lawrence P. A. (2012). Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development 139, 3665-3674. 10.1242/dev.083550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D. I. (2001). Asymmetric localization of Frizzled and the establishment of cell polarity in the Drosophila wing. Mol. Cell 7, 367-375. 10.1016/S1097-2765(01)00184-8 [DOI] [PubMed] [Google Scholar]
- Strutt D. (2009). Gradients and the specification of planar polarity in the insect cuticle. Cold Spring Harb. Perspect. Biol. 1, a000489 10.1101/cshperspect.a000489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H. and Strutt D. (2005). Long-range coordination of planar polarity in Drosophila. BioEssays 27, 1218-1227. 10.1002/bies.20318 [DOI] [PubMed] [Google Scholar]
- Strutt D. and Strutt H. (2007). Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev. Biol. 302, 181-194. 10.1016/j.ydbio.2006.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H. and Strutt D. (2009). Asymmetric localisation of planar polarity proteins: mechanisms and consequences. Semin. Cell Dev. Biol. 20, 957-963. 10.1016/j.semcdb.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Strutt D. and Warrington S. J. (2008). Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development 135, 3103-3111. 10.1242/dev.025205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H., Gamage J. and Strutt D. (2016). Robust asymmetric localization of planar polarity proteins is associated with organization into signalosome-like domains of variable stoichiometry. Cell Rep. 17, 2660-2671. 10.1016/j.celrep.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Abramova N., Charlton J. and Adler P. N. (1998). Van Gogh: a new Drosophila tissue polarity gene. Genetics 150, 199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F. and Goffinet A. M. (2013). Shaping the nervous system: role of the core planar cell polarity genes. Nat. Rev. Neurosci. 14, 525-535. 10.1038/nrn3525 [DOI] [PubMed] [Google Scholar]
- Tree D. R. P., Ma D. and Axelrod J. D. (2002a). A three-tiered mechanism for regulation of planar cell polarity. Semin. Cell Dev. Biol. 13, 217-224. 10.1016/S1084-9521(02)00042-3 [DOI] [PubMed] [Google Scholar]
- Tree D. R. P., Shulman J. M., Rousset R., Scott M. P., Gubb D. and Axelrod J. D. (2002b). Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109, 371-381. 10.1016/S0092-8674(02)00715-8 [DOI] [PubMed] [Google Scholar]
- Usui T., Shima Y., Shimada Y., Hirano S., Burgess R. W., Schwarz T. L., Takeichi M. and Uemura T. (1999). Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98, 585-595. 10.1016/S0092-8674(00)80046-X [DOI] [PubMed] [Google Scholar]
- Wang Y. and Nathans J. (2007). Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134, 647-658. 10.1242/dev.02772 [DOI] [PubMed] [Google Scholar]
- Warrington S. J., Strutt H., Fisher K. H. and Strutt D. (2017). A dual function for prickle in regulating Frizzled stability during feedback-dependent amplification of planar polarity. Curr. Biol. 27, 2784-2797.e3. 10.1016/j.cub.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2007). Reshaping data with the reshape package. J. Stat. Softw. 21, 1-20. 10.18637/jss.v021.i12 [DOI] [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- Yang C., Axelrod J. D. and Simon M. A. (2002). Regulation of Frizzled by Fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108, 675-688. 10.1016/S0092-8674(02)00658-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.