ABSTRACT
The primary cilium is an antenna-like organelle assembled on most types of quiescent and differentiated mammalian cells. This immotile structure is essential for interpreting extracellular signals that regulate growth, development and homeostasis. As such, ciliary defects produce a spectrum of human diseases, termed ciliopathies, and deregulation of this important organelle also plays key roles during tumor formation and progression. Recent studies have begun to clarify the key mechanisms that regulate ciliary assembly and disassembly in both normal and tumor cells, highlighting new possibilities for therapeutic intervention. Here, we review these exciting new findings, discussing the molecular factors involved in cilium formation and removal, the intrinsic and extrinsic control of cilium assembly and disassembly, and the relevance of these processes to mammalian cell growth and disease.
KEY WORDS: Primary cilia, Ciliopathies, Cilium assembly, Cilium disassembly, Cancer
Summary: This Review discusses the molecular factors involved in cilium formation and removal, the relevance of these processes to development and how recent findings are highlighting new possibilities for therapeutic intervention in disease.
Introduction
Cilia and flagella are evolutionarily conserved hair-like microtubule-based structures that project from cells. These organelles are membrane bound and, although their membrane is contiguous with the plasma membrane, they retain a unique identity, with a compartmentalized structure dedicated to signaling. Ciliated cells can be monociliated or multiciliated. Furthermore, cilia can be categorized as motile or immotile, with the former possessing an ability to beat rhythmically and move extracellular fluids. In higher organisms, motile cilia are found in multiple organs, including the brain, lungs, middle ear and reproductive organs, where they drive fluid flow and/or produce signaling gradients that play diverse and important roles, e.g. in left-right patterning, neurogenesis, mucus clearance, hearing and movement of ova. In lower organisms, such as Chlamydomonas and paramecia, motile cilia or flagella are used for cell motility. Recent studies suggest that certain types of motile cilia also have sensory functions (Jain et al., 2012; Shah et al., 2009). By contrast, immotile cilia, also known as primary cilia, act as physical and chemical sensors and transducers of extracellular cues in a wide variety of cell and tissue types (Fig. 1).
Fig. 1.

Structure of the primary cilium. The overall architecture and key structural elements of the primary cilium are shown. IFT, intraflagellar transport.
The primary cilium is enriched in numerous ion channels, such as PKD1, PKD2, TRPV4 and AC6 (also known as ADCY6), that have been shown to play important roles in mechano-transduction (Pablo et al., 2017; Spasic and Jacobs, 2017). The primary cilium can sense fluid flow and extracellular stress, and can translate these signals to control the left-right specification of organ development, calcium influx in kidney and liver cells, nitric oxide production in endothelial cells and osteogenic differentiation in mesenchymal stem cells. The primary cilium is also enriched in receptors that mediate transduction of Hedgehog (Hh), Wnt, Notch, Hippo, G protein-coupled receptors, receptor tyrosine kinases, mTOR, and TGFβ signals (Elliott and Brugmann, 2018; Wheway et al., 2018). Given the important functions of cilia listed above, it is not surprising that defects in cilium assembly and signaling have been linked to at least 35 diseases, termed ciliopathies, that affect nearly all organ systems (for a review, see Reiter and Leroux, 2017). A detailed understanding of cilium assembly and cilium-associated signaling pathways is therefore crucial to the treatment of ciliopathies.
Progress over the past decade has begun to shed light on the cues that promote the assembly of primary cilia in mammalian cells. Such studies have shown that primary cilia are able to assemble upon cell cycle exit (Fig. 2) triggered by mitogen deprivation or differentiation (Aughsteen, 2001; Choksi et al., 2014; Fu et al., 2014; Marion et al., 2009; Wheatley et al., 1996). For this reason, cilia assembly is often studied in well-established in vitro models, including mouse 3T3 fibroblasts and human retinal pigment epithelial (RPE1) cells (Tucker et al., 1979a,b), wherein ciliation can be efficiently induced through serum withdrawal. However, even in this simplified setting, the intracellular signaling events that promote ciliogenesis remain largely unknown at the molecular level. Nonetheless, in recent years, elegant cell biology and time-lapse microscopy experiments have demonstrated that ciliogenesis occurs sequentially, through a series of interdependent steps involving a number of intrinsic and extrinsic control mechanisms (Fig. 3). In this Review, we provide an overview of the process of ciliogenesis, with an emphasis on recent developments in understanding primary cilium assembly, disassembly and function in mammalian cells, and we include a discussion of the many unanswered questions that should be addressed in future studies.
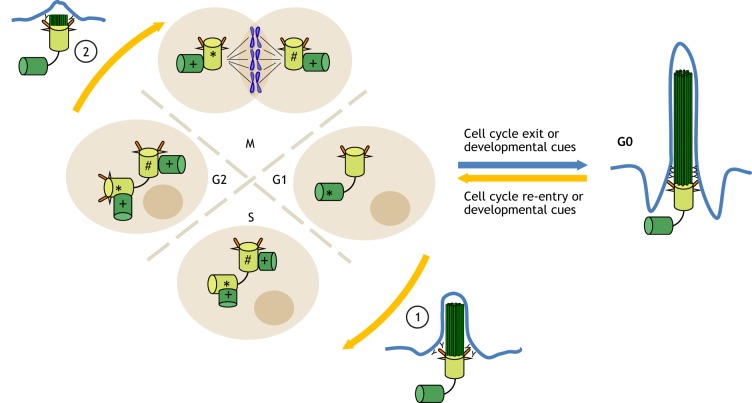
Fig. 2.
A tale of two cycles: cell cycle-linked control of cilium formation and disassembly. A newly formed daughter centriole matures into a MC in two consecutive cell cycles. In the first cell cycle, new daughter centrioles (+) are assembled from an existing (mother) and older (grandmother, #) centriole. In the next cell cycle, the newly formed daughter centriole (*, dark green cylinder) gradually matures into a MC (*, light green cylinder), beginning with the loss of daughter centriole proteins at the G1/S phase and followed by the acquisition of distal appendages and sub-distal appendages in late G2 phase. The primary cilium then assembles when the cell exits the cell cycle (to enter G0) or receives developmental cues (blue arrow). Disassembly of the primary cilium occurs in a biphasic manner (yellow arrows), with the first wave occurring in G1 (1) and a second wave occurring before mitosis (2).
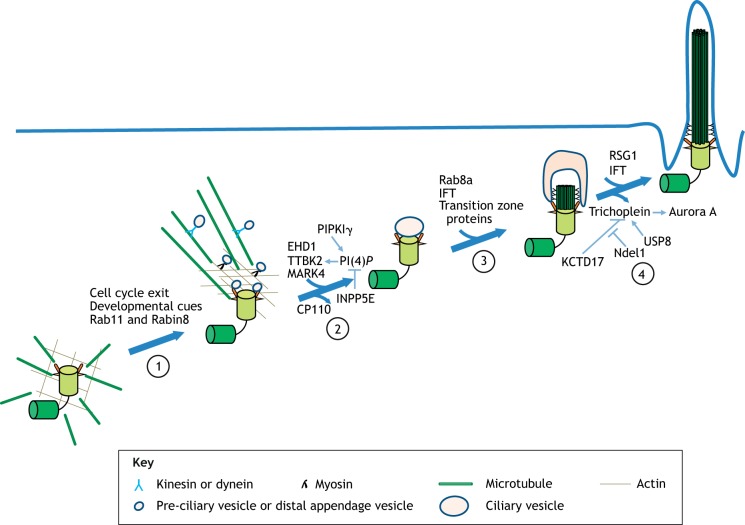
Fig. 3.
The multiple phases and regulation of cilium assembly. The process of cilium assembly involves several successive stages. Cilium assembly is initiated upon cell cycle exit or after receiving developmental signals (1). PCVs are transported via microtubule-actin networks to the distal end of the MC and fuse into a larger CV (2). The process is accompanied by reorganization of the cytoskeleton, which drives the migration of centrioles from the cytoplasm to the cell membrane. CP110 is removed. Next, IFT complexes are continuously recruited to the ciliary base to allow axoneme elongation, while Rab8a is recruited to the MC to facilitate ciliary membrane extension (3). The transition zone is then assembled, and this is followed by axoneme elongation and membrane fusion. Inhibition of the ciliary disassembly pathway also allows outgrowth of the cilium (4). Key proteins that play a regulatory role at each stage of the assembly process are shown. PI(4)P, PtdIns(4)P.
An overview of the cilium assembly process
Ciliogenesis proceeds through two distinct pathways, termed the extracellular and intracellular pathways, depending upon whether cilium growth initiates at the cell surface or within the cytoplasm, respectively. The extracellular pathway is observed in epithelial cells of the kidney or lung, whereas intracellular assembly is observed in fibroblasts and retinal epithelial cells. In the well-studied intracellular pathway, cilium assembly initiates through a series of rapid and well-orchestrated events (Fig. 3), as demonstrated by time-lapse microscopy (Westlake et al., 2011), beginning with maturation of the mother centriole (MC) into a basal body, and culminating in docking of the basal body and growth of the axoneme at the plasma membrane.
First, within 15 min of serum withdrawal, small cytoplasmic vesicles (termed pre-ciliary vesicles, PCVs) that are believed to originate from the Golgi and the recycling endosome, accumulate in the vicinity of MC distal appendages. These vesicles then appear to dock at the centriole, initiating the centriole-to-basal-body transition (Kobayashi et al., 2014; Lu et al., 2015b; Schmidt et al., 2012). After the initial docking of PCVs, the subsequent fusion of vesicles with the basal body produces a cap-like structure, or ciliary vesicle (CV) (Sorokin, 1962). The axoneme is assembled by extension of centriolar microtubules underneath this vesicular cap, and subsequent trafficking of post-Golgi vesicles enlarges the cap in coordination with microtubular growth, resulting in an axoneme compartmentalized by a double membrane. The nascent cilium subsequently docks to the plasma membrane through vesicular fusion with the membranous ciliary sheath, resulting in a projection from the cell surface.
Although the general events underlying ciliogenesis have been uncovered, a number of key questions remain unanswered. Namely, how are vesicles vectorially directed to the centrosome after mitogen deprivation? What are the proteins – on the vesicles and on the MC – that control the docking of PCVs to the centriole? What are the regulators that control the early events of cilium biogenesis, and how is axoneme elongation controlled? As we describe below, a number of recent studies have shed considerable light on these questions, revealing some of the proteins that convert centrioles to basal bodies and, eventually, to primary cilia.
Preparing cells for cilium assembly: mother centriole maturation
Assembly of the primary cilium requires a mature MC that is formed in two consecutive cell cycles from a daughter centriole (Fig. 2). In the first cell cycle, new daughter centrioles are assembled from an existing (mother) and older (grandmother) centriole during the G1-to-S transition. These newborn daughter centrioles are distinguished by the recruitment of daughter centriole proteins such as CEP120, centrobin and NEURL4 (Li et al., 2012; Mahjoub et al., 2010; Zou et al., 2005). In the next cell cycle, the newly formed daughter centriole in each cell gradually matures into a mother centriole, beginning with the loss of daughter centriole proteins at the G1/S phase and followed by the acquisition of distal appendages (DAs) and subdistal appendages (SDAs) in the late G2 phase. Both types of appendages play important roles for the future assembly of cilia: the DAs are essential for vesicle docking and for the recruitment of intraflagellar transport (IFT) machinery during the initiation of ciliogenesis, whereas the SDAs determine cilia positioning through the anchoring of centrioles to the cytoplasmic microtubular network (Sánchez and Dynlacht, 2016).
A number of proteins, including six DA proteins and approximately ten SDA proteins, which are hierarchically recruited to the distal end of the MC have been discovered thus far (Graser et al., 2007; Huang et al., 2017; Joo et al., 2013; Kodani et al., 2013; Kurtulmus et al., 2017 preprint; Mazo et al., 2016; Schmidt et al., 2012; Tanos et al., 2013; Wei et al., 2013). Using super-resolution microscopy, the DA proteins CEP83, CEP89, SCLT1 and CEP164 were shown to form the pinwheel-like spokes of DAs, whereas the interspoke matrix is populated by FBF1 (Yang et al., 2018). The recruitment of DA proteins requires several distal centriolar proteins, including C2CD3, OFD1 and MAPK15 (Kazatskaya et al., 2017; Singla et al., 2010; Ye et al., 2014). The pivotal importance of these distal centriolar proteins was demonstrated by the identification of mutations in the human C2CD3 and OFD1 genes, which produce ciliopathic syndromes with developmental abnormalities (Singla et al., 2010; Thauvin-Robinet et al., 2014). The recruitment of SDA proteins is regulated by a different set of proteins, including trichoplein (also known as TCHP) and CC2D2A (Ibi et al., 2011; Veleri et al., 2014); the ablation of CC2D2A impairs recruitment of the SDA proteins ODF2 and ninein, whereas trichoplein depletion abolishes the recruitment of ninein. Nevertheless, it remains unclear how MC maturation is regulated by this constellation of proteins, and how maturation is linked to the cell cycle and developmental programs. For example, OFD1 and C2CD3 are recruited to newly formed centrioles during centriole duplication, but they are not able to initiate DA assembly until the next cell cycle (Singla et al., 2010; Ye et al., 2014). Indeed, how centriolar asymmetry is generated in the first place – producing one mature MC and therefore only one primary cilium per cell – remains an unsolved mystery in the field.
Vesicle emergence, trafficking, docking and remodeling
After serum starvation, or in response to developmental signals (see below), PCVs are transported to the mature MC along microtubules in a kinesin- and dynein-dependent manner (Fig. 3) (Li et al., 2017; Wu et al., 2018). PCVs can originate from the Golgi or recycling endosomes, and their trafficking appears to depend on cues that trigger ciliogenesis (Sánchez and Dynlacht, 2016), as well as specific proteins such as kinesins, dyneins and myosins.
Golgi-derived PCVs are most likely transported by specific kinesins and dyneins, including KIFC1, which is recruited to the Golgi upon serum starvation (Lee et al., 2017). Upon depletion of KIFC1, ciliary membrane proteins are unable to traffic to the centriole and they accumulate at the Golgi. The dynein DYNC1H1 has also recently been shown to be required for PCV trafficking along microtubules (Wu et al., 2018). After trafficking to the vicinity of centrioles, the docking of PCVs to the DAs relies upon a branched centrosomal actin network and, indeed, growing evidence suggests that the centrosome acts as an actin-organizing center in addition to its established role as a microtubule organizer (Farina et al., 2016). In support of this role, the actin nucleator complex ARP2/3 and the nucleation-promoting complex WASH localize on or near centrioles and promote assembly of a centrosomal actin network. Focal adhesion proteins also localize to centrioles and help to anchor them to the actin cytoskeleton (Antoniades et al., 2014). The actin cytoskeleton remodeling factors LIMK2 and TESK1 have also been visualized in the vicinity of centrioles (Kim et al., 2015a); interestingly, depletion of either of these kinases, which regulate the phosphorylation of cofilin 1, an actin-depolymerizing factor and positive regulator of ciliogenesis, provokes increased ciliation. These findings suggest that a dynamic balance between actin polymerization and depolymerization regulates ciliogenesis.
Recent observations suggest that the transport of PCVs to distal appendages via the actin network is also mediated by the motor protein myosin Va (Wu et al., 2018). Myosin Va+ PCVs can be found near centrioles as early as 30 min after serum starvation, and depletion of myosin Va, as with disruption of the centrosomal actin network, leads to the blockage of PCV docking. This finding suggests that myosin Va is among the earliest known markers of PCV trafficking. It is likely that microtubular and actin networks collaborate during PCV trafficking and docking. Although the details are largely lacking, putative regulators include MACF1A (May-Simera et al., 2016), which helps to organize the centrosomal microtubule-actin network through direct interaction with both microtubules and actin. As with myosin Va depletion, silencing of MACF1A leads to the blockage of PCV docking.
Apart from the Golgi-centriole pathway, components of the multi-subunit endosomal sorting complex required for transport (ESCRT) are essential for sustained docking of PCVs, supporting the involvement of the endosome trafficking pathway in CV growth (Ott et al., 2018). After PCV docking, the membrane-shaping protein EHD1 is recruited to PCVs to facilitate their fusion into a large CV, which caps the entire distal ends of MCs (Fig. 3). Although the cues are not fully understood, a sequence of events, triggered by Rab11 and Rabin8 (also known as RAB3IP), promotes the recruitment of the small GTPase Rab8a to CV to facilitate their extension (Bhattacharyya et al., 2016; Lu et al., 2015b; Wu et al., 2018). Rab8a facilitates the docking of PCVs to DAs by interacting with a group of distal centriolar proteins, including CEP164, Chibby (also known as CBY1), AHI1 and Talpid3 (also known as KIAA0586) (Burke et al., 2014; Hsiao et al., 2009; Kobayashi et al., 2014; Schmidt et al., 2012). Growing evidence also suggests that centriolar satellites, which are electron-dense cytoplasmic granules (Fig. 1), play an important role in PCV trafficking, fusion and extension. In particular, C2CD3 and OFD1, which localize to centriolar satellites as well as to the distal end of centrioles, have been shown to be required for DA assembly (Singla et al., 2010; Ye et al., 2014), whereas an integral centriolar satellite protein, PCM1, interacts with proteins of the BBsome complex and assists in BBsome trafficking, which in turn facilitates activation of Rab11-Rabin8-Rab8a signaling (Nachury et al., 2007; Westlake et al., 2011). PCM1 can also tether the centriolar satellite protein and E3 ligase Mib1, which regulates centrosomal levels of Talpid3, a protein required for Rab8a recruitment (Kobayashi et al., 2014; Wang et al., 2016). Rab8a recruitment, and hence PCV fusion and extension, is also regulated by CEP290, a protein that localizes to both centriolar satellites and the ciliary transition zone (Kim et al., 2008; Tsang et al., 2008). Furthermore, the WD-repeat containing protein WDR8 functions in partnership with SSX2IP and CEP135 in both the assembly of centriolar satellites and PCV docking (Gupta et al., 2015; Klinger et al., 2014; Kurtulmus et al., 2016). It should be noted that centriolar recruitment of C2CD3, OFD1, CEP290 and WDR8 does not depend on their ability to localize to centriolar satellites (Kim et al., 2008; Kurtulmus et al., 2016; Lopes et al., 2011; Ye et al., 2014) and, therefore, additional work is needed to clarify which pool(s) are needed to regulate PCV docking. On the other hand, centriolar satellites are also involved in the organization of microtubular and actin networks through unknown mechanisms, and thus they could also contribute to microtubule-actin-mediated vesicle trafficking (Hori and Toda, 2017). To fully understand the earliest events that trigger ciliogenesis, it will be important to elucidate how vesicle transport is instigated upon receiving the initial cue to ciliate, to dissect how the cytoskeleton and transport between endoplasmic reticulum/endosomes and the centrosome are dynamically remodeled, and to identify the motors involved in each of these pathways.
Centrosome migration and cilium positioning
The morphology and positioning of the cilium vary extensively according to cell type, and the cilium can reside at the cell surface or assume a more submerged position (for a review, see Bernabé-Rubio and Alonso, 2017). For example, in mammals, the primary cilia in epithelial cells of kidney tubules are at the cell surface (Latta et al., 1961), whereas those in smooth muscle cells, fibroblasts and pancreatic β cells are found at a more submerged position (Munger, 1958; Sorokin, 1962). Although the precise mechanisms that dictate cilium positioning remain unclear, some of the factors that regulate centrosome migration during ciliogenesis, and hence the final positioning of the cilium, are beginning to be identified.
During ciliogenesis, ciliary membrane assembly is accompanied by centrosome migration from the center of the cell towards the cell surface (Fig. 3), where the basal body then anchors to the plasma membrane via DAs (Sánchez and Dynlacht, 2016). This process is driven by mechanical forces produced during cytoskeleton remodeling. Indeed, time-lapse microscopy has revealed a dramatic increase in microtubule nucleation and stabilization around the centrosome, coinciding with migration of the centrosome (Pitaval et al., 2017). Microtubules cluster into large bundles between the centrosome and the basal pole of the cell, and point toward the apical pole of the cell, thus pushing the centrosome towards the apical membrane. In addition to the microtubule network, the actin network is also remodeled after serum starvation: the radial symmetry of the network is broken and actin filaments cluster to one side of the cell, resulting in the asymmetrical co-partitioning of microtubules with F-actin (Pitaval et al., 2017).
Beyond studies in tissue culture, the process of centrosome migration during ciliogenesis has been captured in a recent study of Caenorhabditis elegans neuronal development (Li et al., 2017), wherein the centriole is transported along microtubules from the cell body to the tip of the dendrite in a dynein 1-dependent manner. These findings are significant because they suggest that actin and microtubules may cooperatively promote centrosome migration during ciliogenesis. A number of other ciliogenesis effectors have been implicated in centrosome migration, including FLNA, CEP83, CEP164, nesprin 2 (also known as Syne2), meckelin (also known as Tmem67), KIF3A, Pard3 and IFT20 (Adams et al., 2012; Li et al., 2017; Pitaval et al., 2017). As many of the genes encoding these proteins have been implicated in human ciliopathies (Reiter and Leroux, 2017), it will be interesting to investigate the role of centriole maturation, migration and positioning in these pathologies.
Cilia can also assume a more submerged position (Bernabé-Rubio and Alonso, 2017) and a recent study suggests that this positioning is determined by SDAs through their association with the Golgi (Mazo et al., 2016). In particular, it has been shown that the depletion of two SDA proteins, CEP128 and c-NAP1 (also known as CEP250), leads to dissociation of centrosomes from the Golgi, promoting the formation of cilia at the cell surface. It will be interesting to investigate whether, and how, this mechanism might be used in diverse cell types, and whether there are distinct functional outcomes associated with each position (surface versus submerged). Further studies are also needed to clarify the mechanisms that underlie centrosome migration and its relationship with other key events during ciliogenesis, described below.
Crucial control mechanisms in the basal body-to-cilium transition
A crucial mechanism in the decision to ciliate involves the removal of two proteins, CP110 (also known as CCP110) and CEP97, which are the first reported inhibitors of ciliogenesis. In cycling cells, CP110 and CEP97 localize at the distal ends of both mother and daughter centrioles to block inappropriate cilium formation (Spektor et al., 2007). After CV formation, the tau tubulin kinase TTBK2 is recruited by CEP164 to the DA of MCs, where it triggers removal of the CP110-CEP97 inhibitory complex (Fig. 3) (Cajanek and Nigg, 2014; Goetz et al., 2012). It is noteworthy that the exact relationship between CV remodeling and CP110 removal remains to be defined, given that PCV docking and CV formation may not be essential for CP110 removal (Lee et al., 2017; Wu et al., 2018). Intriguingly, and in contrast to studies in cultured cells, it is also worth noting that ciliogenesis fails in CP110 knockout mice, perhaps owing to a failure of SDA assembly and basal body anchoring to the membrane (Yadav et al., 2016). Interestingly, the recruitment of TTBK2 by CEP164 is regulated by phosphatidylinositol 4-phosphate [PtdIns(4)P] levels at the centrosome/ciliary base (Xu et al., 2016); PtdIns(4)P binds to CEP164 and TTBK2 and inhibits their interaction in proliferating cells. The centrosomal pool of PtdIns(4)P is regulated by phosphatidylinositol 5-phosphatase (INPP5E) and the PtdIns(4)P 5-kinase PIPKIγ. Upon serum starvation, INPP5E departs from the centrosome, and centrosomal PIPKIγ promotes TTBK2 recruitment and CP110 removal by depleting PtdIns(4)P at the centrosome. TTBK2 recruitment is also regulated by a centriolar satellite protein, MCRS1, which can directly bind to and recruit TTBK2 to the centriole (Lee et al., 2016b). An siRNA screen for kinases regulating ciliogenesis further identified a second kinase, MARK4, as another catalyst for CP110 removal (Kuhns et al., 2013). Recent studies have also shown that a small GTPase, RSG1, is recruited to MCs by TTBK2 and is required to initiate axoneme elongation (Agbu et al., 2018), which commences after CP110 removal (Fig. 3). Although this protein is important in finalizing the maturation of basal bodies before axoneme elongation, future studies will be required to mechanistically dissect an exact role for RSG1 in this process.
Suppression of cilium disassembly, mediated by the kinase Aurora A (also known as Aurka), is also required for ciliogenesis (Inaba et al., 2016; Inoko et al., 2012; Kasahara et al., 2018, 2014). The SDA assembly regulator and ciliogenesis inhibitor trichoplein localizes at SDAs in cycling cells, where it activates Aurora A to promote cilium disassembly (Inoko et al., 2012) (discussed below). During ciliogenesis, however, KCTD17 acts as a substrate adaptor for Cul3-RING ubiquitin ligases (CRL3s) that polyubiquitylate and degrade trichoplein (Kasahara et al., 2014). Depletion of KCTD17 in RPE1 cells prevents removal of trichoplein and inactivation of Aurora A, resulting in the blockage of ciliogenesis before axoneme elongation. In cycling cells, Ndel1 protects trichoplein from CRL3/KCTD17-mediated degradation (Inaba et al., 2016). EGFR also directly phosphorylates USP8 to promote its deubiquitylase (DUB) activity, which in turn stabilizes trichoplein, thus tying together growth-promoting signals with cilium removal (Kasahara et al., 2018). On the other hand, the MST1/2-SAV1 complex of the Hippo pathway also participates in the dissociation of the Aurora A/HDAC6 cilia-disassembly complex by phosphorylating Aurora A (Kim et al., 2014). In the future, it will be important to investigate how the removal, destruction and/or mis-localization of negative regulators of ciliogenesis are integrated with cell cycle progression, and whether mutations that cripple these proteins are linked to human disease.
Axoneme elongation
The ciliary membrane and microtubules within the axoneme further elongate after the CV-capped basal body fuses with the plasma membrane (for a review, see Sánchez and Dynlacht, 2016). During this process of axoneme elongation, vast amounts of tubulin enter the cilium from the cytoplasm by diffusion and via IFT – the motor-dependent bi-directional cargo transport mechanism used within cilia for their formation, maintenance and function (Harris et al., 2018 preprint; Ishikawa and Marshall, 2017). Perturbation of cytoplasmic soluble tubulin levels or IFT transport can affect axoneme elongation and cilium length. Indeed, elegant studies in Chlamydomonas and mammalian cells suggest that increasing soluble tubulin production leads to longer cilia, whereas stabilization of tubulin leads to cilium shortening (Sharma et al., 2011; Wang et al., 2013b).
IFT requires various anterograde (IFT-B) and retrograde (IFT-A) transport complexes, and recent studies have begun to shed light on how these complexes are recruited to cilia. The recruitment of IFT-B to the ciliary base, for example, is mediated by TTBK2 and DA proteins (Goetz et al., 2012; Tanos et al., 2013). Super-resolution microscopy has also suggested that IFT molecules are concentrated within the matrix of DAs, wherein IFT complexes are formed with the assistance of the BBsome (Wei et al., 2012; Yang et al., 2018). The entry of IFT complexes into the cilium is also partially mediated by another small GTPase, RABL2B, which is recruited to the ciliary base by CEP19, a protein that is tethered at the distal end of the MC by CEP350 and FOP (also known as FGFR1OP). Active RABL2B then binds to the IFT-B complex, promoting entry of the latter into cilia (Kanie et al., 2017; Nishijima et al., 2017), and IFT complexes are subsequently transported via the kinesinII complex, the motor for anterograde movement, to the ciliary tip, where tubulins are integrated into the growing axoneme. The loading and unloading of cargos by kinesinII are regulated by its phosphorylation state. At the ciliary base, FLA8 (a subunit of the kinesinII motor, and a homolog of KIF3B) in the unphosphorylated state allows IFT-B to bind to the kinesinII motor, which conveys it to the cilium tip. At the ciliary tip, CrCDPK1 phosphorylates FLA8 and disrupts the interaction between kinesinII and IFT particles, thereby facilitating cargo unloading (Liang et al., 2014).
Previous studies from several model organisms, primarily Chlamydomonas and C. elegans, have shown that the perturbation of IFT by disruption of IFT genes or motors blocks axonemal elongation and promotes assembly of short cilia. The velocity of IFT transport also contributes to the regulation of cilium length. Increased velocity of anterograde transport is associated with elongated cilia, whereas decreased velocity is linked to short cilia (Besschetnova et al., 2010; Marshall et al., 2005; Marshall and Rosenbaum, 2001). IFT is known to be regulated by a group of protein kinases, including DYF-5, DYF-18, PKG-1, GCK-2 and the DLK-1/p38 MAPK pathway (Burghoorn et al., 2007; Muthaiyan Shanmugam et al., 2018; Phirke et al., 2011; van der Vaart et al., 2015), although the mechanisms are unknown. Cilium length can also be regulated at the IFT cargo loading step. A recent study suggests that the loading of axonemal cargo onto IFT complexes decreases with increasing ciliary length (Pan and Snell, 2014; Wren et al., 2013), suggesting the existence of an uncharacterized feedback mechanism that senses the length of the cilium and thereby regulates cargo loading.
Axoneme elongation is also coordinated with ciliary membrane extension during ciliogenesis. Ciliary membrane growth can surpass the rate of axoneme extension when active Rab8a is overexpressed in mammalian cells or after depletion of IFT genes in trypanosomes (Absalon et al., 2008; Nachury et al., 2007). Abnormal ciliary membrane extension can also promote axoneme elongation and produce longer cilia (Lu et al., 2015a; Nachury et al., 2007). These studies indicate that there may be mechanisms that sense the length of the axoneme and, in turn, control the levels of regulators of membrane extension, including Rab8a and Arl13b. Moreover, the actin network, ectocytosis (Fig. 1) and endocytosis are also involved in the regulation of cilium length, probably by regulating ciliary membrane composition and protein transport or localization (Kaplan et al., 2012; Fu et al., 2016; Scheidel et al., 2018). Once fully assembled and elongated, the cilium retains its unique composition, in part, by virtue of gating mechanisms: at the base of the cilium, a soluble protein barrier and the transition zone function to maintain the protein composition of the cilium (for comprehensive reviews, see Jensen and Leroux, 2017; Nachury, 2018).
Despite these new findings regarding axoneme elongation, cilium length control and ciliary protein trafficking, much remains unknown, and additional studies will be required to determine how axoneme elongation is intricately coordinated with ciliary membrane extension and trafficking of signaling components. Finally, it should be noted that cilium length is also under the control of cilium disassembly pathways, which counteract assembly pathways during the cell cycle (see below).
Cilium disassembly
In contrast with cilium assembly, much less is known about the mechanisms that underlie cilia disassembly/resorption, which has to happen before mitosis. Experiments using cultured mammalian cells suggest that cilia disassemble in a biphasic manner, with the first major ‘wave’ occurring in G1 shortly after mitogenic stimulation of quiescent cells (Fig. 2) and a second wave occurring before mitosis (Pugacheva et al., 2007; Tucker et al., 1979b). Below, we summarize recent studies that have provided insights into cilium disassembly, a process with immense implications in human disease, in particular cancer.
Disassembly of axonemal microtubules
Cilium disassembly requires the destabilization and depolymerization of axonemal microtubules (Figs 2 and 4). The mitotic kinase Aurora A appears to play a key role in promoting the latter process and thereby promotes both waves of cilium disassembly (Pugacheva et al., 2007). Aurora A is activated in response to cell cycle re-entry cues, whereupon it phosphorylates and stimulates the histone deacetylase HDAC6, which de-acetylates and destabilizes tubulins within the axoneme. HDAC6 also de-acetylates cortactin and thus enhances actin polymerization, which also promotes cilium disassembly (Plotnikova et al., 2012; Pugacheva et al., 2007; Ran et al., 2015). The activation of Aurora A is under stringent control by complex signaling pathways that include calcium influx, which induces the binding of Ca2+/calmodulin (CaM) to Aurora A and its partner, NEDD9 (also known as HEF1). This stabilizes the interaction between these proteins and promotes Aurora A activation (Plotnikova et al., 2012). Moreover, PDGFRβ promotes cilium disassembly by activating PLCγ, which causes release of intracellular Ca2+ and activation of CaM and Aurora A (Nielsen et al., 2015). Another histone deacetylase, HDAC2, has recently been shown to play a role in cilium disassembly (Kobayashi et al., 2017). Interestingly, HDAC2 positively regulates Aurora A expression, and its depletion promotes cilium assembly in cancer cells that have lost this organelle. Furthermore, Plk1, a G2/M phase kinase recruited to the pericentriolar matrix before mitotic entry, interacts with and activates HDAC6 to promote ciliary deacetylation and resorption (Lee et al., 2012; Wang et al., 2013a). These studies illustrate how HDACs can regulate cilium disassembly through discrete substrates and mechanisms, highlighting the potential of HDAC inhibition as a therapeutic strategy to reverse cilia loss.
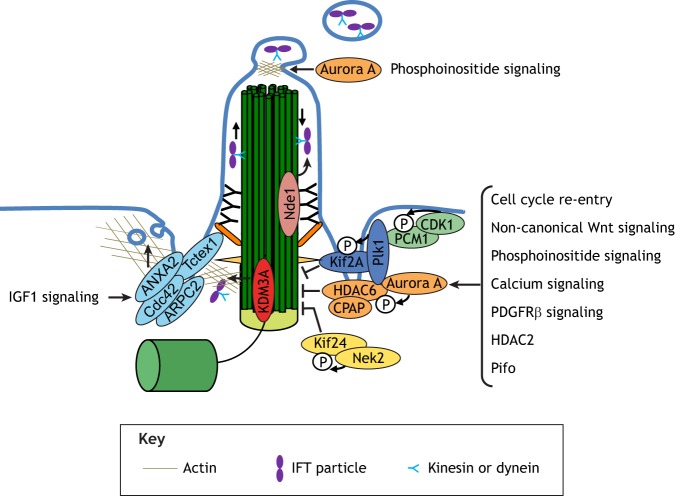
Fig. 4.
The regulation of cilium disassembly. Cell cycle re-entry, accompanied by several signaling pathways and regulatory proteins (including non-canonical Wnt signaling, phosphoinositide signaling, calcium signaling, PDGFRβ signaling, HDAC2 and Pifo), can trigger cilium disassembly via the activation of Aurora A. Aurora A then phosphorylates and stimulates the histone deacetylase HDAC6, which de-acetylates and destabilizes microtubules within the axoneme. During cilium disassembly, Plk1 and Nek2 activate the kinesins Kif2a and Kif24, respectively, which are required for the depolymerization of microtubules. Plk1 is recruited by PCM1 with the help of CDK1. Cilium disassembly also requires the modulation of IFT transport: KDM3A, for example, inhibits entry of the IFT complex into the cilium, whereas Nde1 regulates retrograde IFT transport. Growth signaling also triggers the removal of IFT-B particles through ciliary ectosomes released from the ciliary tip, which is also under the control of Aurora A. Cilium disassembly also requires remodeling of the ciliary pocket, accompanied by the enhancement of clathrin-mediated endocytosis. This process is controlled by Tctex1 and associated proteins (such as ANXA2, Cdc42 and ARPC2) and can be induced by IGF1 signaling. PDGFRβ, platelet-derived growth factor receptor β.
The non-canonical Wnt signaling pathway also participates in Aurora A activation and, thus, ciliary disassembly. Wnt5a treatment induces the phosphorylation of Dvl2 by CK1ε (also known as CSNK1E) and the formation of the Dvl2-Plk1 complex, which stabilizes NEDD9 and promotes Aurora A activation (Lee et al., 2012). Another protein, pitchfork (Pifo), promotes cilium disassembly through activation of Aurora A. Interestingly, heterozygous mutations in Pifo in mice and in humans lead to ciliopathy-related phenotypes, attesting to the biological consequences of perturbing cilium disassembly (Kinzel et al., 2010). Aurora A activity is also regulated by phosphoinositide signaling. Cultured cells grown in three dimensions develop a lumen with cilia and, in this context, the phosphatidylinositol phosphatase SHIP2 was found to bind Aurora A and NEDD9, and to promote their basolateral localization at the expense of their luminal expression associated with cilium resorption (Hamze-Komaiha et al., 2016). Moreover, INPP5E regulates Aurora A protein levels through transcriptional mechanisms that are mediated, at least in part, by AKT activity (Plotnikova et al., 2015). Finally, it was shown that serum stimulation can induce the formation of a cilium disassembly complex (CDC), which consists of Aurora A, CPAP (also known as CENPJ), Nde1 and OFD1, and in which CPAP functions as a scaffold protein that facilitates the recruitment of this complex to the ciliary base (Gabriel et al., 2016). Collectively, these studies illustrate how Aurora A acts as a nexus or focal point for a multitude of signals that impinge on the cilium to promote its disassembly (Fig. 4).
Two kinesins that are able to depolymerize microtubules, Kif2a and Kif24, have also been implicated in the disassembly of primary cilia before mitosis (Fig. 4) (Kim et al., 2015b; Kobayashi et al., 2011; Miyamoto et al., 2015). Kif2a, which is recruited to the SDAs of the MC and to the proximal ends of both centrioles, is activated by Plk1 during G2/M phase (Miyamoto et al., 2015). Activation of this kinesin in the wake of a proliferative signal promotes ciliary microtubule depolymerization and cilium disassembly; in quiescent cells, by contrast, Kif2a is degraded through the APC/C-mediated ubiquitin/proteasome system to facilitate ciliogenesis. Kif24, identified through its association with CP110, also negatively regulates primary cilium assembly (Kobayashi et al., 2011). The ablation of this kinesin promotes aberrant assembly of cilia in growing cells, similar to CP110 loss. The microtubule depolymerizing activity of Kif24 can be enhanced by phosphorylation through Nek2, which is expressed during S and G2 phase (Kim et al., 2015b). Therefore, in a manner analogous to Kif2a, the cell cycle-specific phosphorylation of Kif24 ties the activation of this enzyme to cilium disassembly before mitosis. As this step is distinct from the initiation of cilium disassembly by Aurora A and HDAC6, microtubule depolymerization by Nek2/Kif24 could favor the irreversibility of this process once S phase begins, and thereby safeguard against aberrant assembly of cilia. It is notable that the cell invests a considerable amount of energy in maintaining a deciliated state, and the fact that relatives of Kif24 and Nek2 – as well as Aurora A – play a role in axonemal assembly and disassembly in flagellated and ciliated species (Bradley and Quarmby, 2005; Hilton et al., 2013; Mahjoub et al., 2002; Pan et al., 2004; Piao et al., 2009; Wloga et al., 2006) attests to the conservation and importance of this process. Future studies will be required to determine whether the abrogation of these pathways leads to pathological states, akin to defects in the cilium assembly process that lead to ciliopathies.
Ciliary membrane remodeling during cilium disassembly
Cilium disassembly also requires the remodeling of ciliary membranes (Fig. 4), and several recent studies have identified key mechanisms and proteins that play a role in this process. The ciliary pocket (CiPo) is an actin-rich, periciliary subdomain that surrounds the proximal region of the ciliary axoneme (Fig. 1). It is a dynamic center for endocytosis and, upon cilium resorption, the CiPo membrane undergoes active remodeling, accompanied by enhanced endocytosis (Phua et al., 2017). Importantly, the perturbation of CiPo membrane endocytosis by depletion of clathrin heavy chain or expression of a dominant-negative Rab5 (S34N) mutant specifically blocks ciliary resorption, suggesting that CiPo membrane endocytosis is actively involved in ciliary disassembly/resorption. At the molecular level, remodeling of the CiPo membrane and enhancement of clathrin-mediated endocytosis is dependent on actin polymerization and is regulated by Tctex1 (also known as Dynlt1) (Saito et al., 2017), a protein that has previously been identified as a dynein light chain (Lader et al., 1989). Before S-phase entry, phospho (T94) Tctex1, the functionally active form of Tctex1, is recruited to the transition zone. Tctex1 directly binds to F-actin and interacts with three actin polymerization regulators, ANXA2, ARPC2 and Cdc42 (Saito et al., 2017); perturbing the expression and/or function of any of these three proteins blocks ciliary resorption. The recruitment of phospho (T94) Tctex1 to the transition zone is regulated by the IGF1 signaling pathway, which thus provides a link between mitogenic stimulation and cilium disassembly. Upon IGF binding, ciliary IGF1R translocates to the base of the cilium to activate Gβγ, which competes with the dynein intermediate chain for binding to Tctex1 and thus facilitates the generation of the dynein-free Tctex1 necessary for Thr94 phosphorylation (Li et al., 2011; Saito et al., 2017; Yeh et al., 2013).
Recent studies indicate that the distal ciliary membrane also undergoes active remodeling during cilium disassembly (Fig. 4) (Nager et al., 2017; Phua et al., 2017). In particular, the release of CVs from the distal region of cilia (through ectocytosis; Figs 1 and 4) was periodically observed after growth stimulation, and this was achieved through cilium decapitation mediated by intra-ciliary actin polymerization (Nager et al., 2017; Phua et al., 2017). The exact position of ciliary decapitation is determined by the ciliary distribution of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], which induces actin polymerization in coordination with the actin regulators cofilin 1, fascin and Kras (Phua et al., 2017). Upon growth stimulation, Aurora A drives INPP5E depletion and PI(4,5)P2 re-distribution in the cilium to facilitate actin nucleation at a specific location. As IFT complexes are actively released by vesicles formed through growth-induced ciliary decapitation at the ciliary tip, this mechanism provides another layer of IFT regulation. Notably, proteomic analyses have demonstrated that CV release preferentially removes IFT-B, rather than IFT-A, from primary cilia, and that the removal of IFT-B from primary cilia can limit cilia re-growth and thereby promote cilia disassembly (Phua et al., 2017). As perturbation of ciliary decapitation could abolish cilium disassembly (Nager et al., 2017; Phua et al., 2017), future studies will be required to understand the role of this process in normal and pathological conditions.
Other factors that regulate cilium disassembly
As mentioned above, IFT is important for axonemal extension and length control, and, as such, this process – and the proteins that regulate it – also participates in cilium disassembly (Fig. 4). A number of factors (discussed above) are known to promote the recruitment of IFT complexes to cilia. By contrast, this recruitment process is restricted by local actin networks (Ishikawa and Marshall, 2017). Interestingly, the histone lysine demethylase KDM3A promotes the formation of actin bundles by regulating actin gene expression and by binding to the actin cytoskeleton (Yeyati et al., 2017); in its absence, the actin network is depolymerized, resulting in a delay in cilium resorption and an abnormal distribution of IFT within cilia. Nde1, which localizes at the transition zone, is another negative regulator of cilium length that controls cilium disassembly (Gabriel et al., 2016; Kim et al., 2011; Maskey et al., 2015). Nde1 is highly expressed in mitotic cells but is depleted in G0/G1 cells through CDK5-mediated phosphorylation, which targets the protein for ubiquitylation by the F-box protein Fbxw7, leading to its subsequent destruction. During cell cycle re-entry, CDK5 activity decreases, allowing Nde1 levels to accumulate and promote ciliary resorption. However, it is not known how Nde1 regulates cilium disassembly mechanistically. One Nde1 effector protein, LC8, the dynein light chain (also known as Dynll1), is tethered to Nde1 at the basal body. As LC8 is important for IFT transport and cilium assembly, Nde1 may regulate cilium disassembly in part through perturbation of IFT transport.
Additional mechanisms that induce deciliation have also been described. For example, cilia can be removed by severing mechanisms, as occurs in the green alga Chlamydomonas, in which deciliation can be enforced through the action of katanin, a microtubule-severing enzyme that separates basal bodies from axonemes before mitosis (Lohret et al., 1998; Rasi et al., 2009). In neurons, deciliation or apical abscission can also be accomplished by pinching off the cilium from the centrosome via another actomyosin-dependent process (Das and Storey, 2014). The pervasiveness of this abscission mechanism has not been determined, and it is unknown whether it can be implemented in a cell type- and context-dependent manner to regulate key signaling events under proliferative conditions or during differentiation.
Recent insights into the developmental control of ciliogenesis
It is clear that the correct formation of cilia plays exceptionally important and widespread roles in mammalian development (for a review, see Goetz and Anderson, 2010). By contrast, the dynamic regulation of cilium assembly and disassembly in development has been less thoroughly studied. However, in recent years, new and interesting insights have been reported that now link cilium assembly to diverse developmental states.
In multicellular organisms, the initiation of cilium assembly is regulated by intricate transcriptional programs (reviewed in Choksi et al., 2014; Spassky and Meunier, 2017). Our current knowledge of the transcriptional control of ciliogenesis primarily stems from studies in multiciliated cells, whereas the transcriptional program in monociliated cells has been largely left unexplored. Recently, the evolutionarily conserved RFX family of transcriptional factors was found to regulate cilium assembly in both types of cells by controlling the expression of core ciliary genes, including components of the transition zone, the BBsome and IFT (Choksi et al., 2014). The timely and spatially accurate expression of RFX transcription factors is also determined by key signaling factors, including FGF, as well as the neural transcription factors atonal and noto (Beckers et al., 2007; Cachero et al., 2011; Neugebauer et al., 2009). Ciliogenesis can also be regulated during development through posttranscriptional mechanisms involving microRNAs. For example, miR-129-3p initiates ciliogenesis in diverse tissues by downregulating CP110 and repressing branched F-actin formation (Cao et al., 2012).
In certain tissues, such as blood vessels, the heart, myoblasts and adipocytes (Fu et al., 2014; Goetz et al., 2014; Marion et al., 2009; Mohieldin et al., 2016), primary cilium assembly occurs transiently and is linked to a specific developmental phase, after which the structure disappears, suggesting that a cilium disassembly program may be involved. In these tissues, the transient appearance of primary cilia plays important, but poorly defined, roles during development. A good example here is the endothelial cilia, which are mechanical sensors of fluid flow, that are required for blood vessel maturation and homeostasis (Mohieldin et al., 2016). In zebrafish, endothelial cilia are exclusively present between 24 and 28 h after fertilization, and their disappearance can be ascribed, in part, to cilium disassembly induced by fluid shear stress (Goetz et al., 2014; Mohieldin et al., 2016). However, how shear stress leads to cilium disassembly remains unknown.
In other tissues, the developmental program can shift from a primary ciliated to multiciliated state through inhibition of Notch signaling, as occurs in the mammalian respiratory system and the zebrafish pronephros (Jain et al., 2010; Liu et al., 2007; Spassky and Meunier, 2017). Moreover, during mammalian brain development, the active resorption of primary cilia from progenitor cells is required for maintenance of the progenitor cell pool and for the control of brain size (Li et al., 2011; Yeh et al., 2013). This process is controlled by the cilia repressor Tctex1 and its regulators and effectors, as mentioned above, although it is not yet known how the disappearance of cilia is regulated. Therefore, although much progress has been made, additional studies will be required to understand the precise mechanisms that induce the assembly and disassembly of cilia upon activation of diverse developmental programs.
Defects associated with cilium assembly and disassembly
A range of developmental disorders has been linked to ciliary defects, and manifestations include brain malformations, congenital heart defects and skeletal malformations (for a comprehensive review, see Reiter and Leroux, 2017). Here, we focus on recent studies of ciliopathies and ciliary aberrations that are caused by cell cycle perturbations and, specifically, cancer.
Cilia as tumor suppressors
Loss of cilia has been observed in a multitude of tumors including, but not limited to, pancreatic ductal adenocarcinoma, renal cell carcinoma, thyroid cancer, breast cancer, ovarian cancer, prostate cancer, cholangiocarcinoma, glioblastoma and melanoma (Egeberg et al., 2012; Gradilone et al., 2013; Han et al., 2009; Hassounah et al., 2013; Lee et al., 2016a; Moser et al., 2009; Schraml et al., 2009; Seeley et al., 2009; Yuan et al., 2010). The importance of this cilia loss in tumor initiation, maintenance and progression, as well as in chemotherapeutic resistance, is now beginning to emerge, and it appears to be linked to the key roles played by cilia in various signaling pathways (Fig. 5).
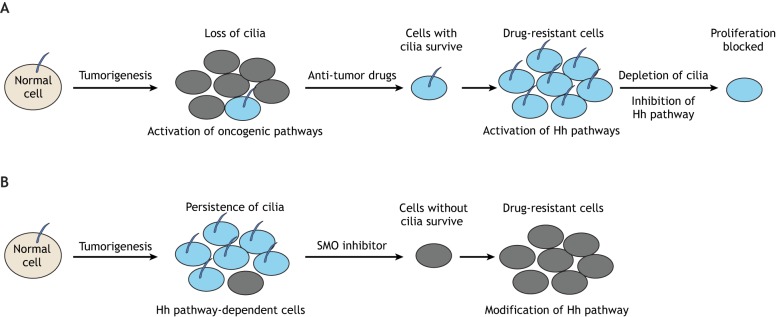
Fig. 5.
The function of primary cilia in cancer. (A) Loss of cilia has been observed in several types of tumors and can lead to aberrant activation of many oncogenic pathways. After treatment with anti-tumor drugs, tumor cells with cilia, and hence aberrant activation of Hh pathway, survive and can further proliferate. Depletion of cilia or inhibition of Hh signal pathway can block the proliferation of these drug-resistant cells. (B) The persistence of cilia can also be observed in a range of tumors, in which they appear to help maintain the oncogenic Hh pathway. After treatment with an SMO inhibitor, tumor cells with cilia die and the surviving cells (without cilia) evolve a modified Hh pathway that confers resistance to SMO inhibition.
Primary cilia are important in Hh signaling (for a review, see Bangs and Anderson, 2017), which plays an essential role in development and the abnormal activation of which is crucial for the development of many cancers (Pak and Segal, 2016). In a breast cancer model, inhibition of ciliogenesis was shown to result in non-canonical activation of Hh signaling, thereby accelerating tumor formation and enhancing the growth of malignant lesions (Hassounah et al., 2017). Moreover, disruption of ciliogenesis promotes pancreatic intraepithelial neoplasia (PanIN) formation during oncogenic Kras (G12D)-driven tumorigenesis (Seeley et al., 2009). Cilia are also known to restrain activation of β catenin-T cell factor (TCF) signaling (Lancaster et al., 2011), and a study using a pancreatic cancer model showed that disruption of ciliogenesis activates the mevalonate (MVA) pathway through β catenin-TCF signaling, which further boosts oncogenic Ras-Erk signaling (Deng et al., 2018). In glioblastoma, mitogenic signaling through lysophosphatidic acid is restricted in normal cells with a primary cilium because of the segregation of lysophosphatidic acid receptor (LPAR1) in the cilium from its downstream G-protein effectors: Gα12 and Gαq (Loskutov et al., 2018). However, during tumorigenesis and upon loss of primary cilia, LPAR1 redistributes to the plasma membrane, allowing Gα12 and Gαq to bind to the receptor, which promotes increased mitogenic signaling and proliferation of tumor cells. These data demonstrate that the absence of cilia observed in tumors could mediate, or repurpose, multiple signaling pathways and promote the formation of tumors.
Notably, a recent study suggested how the loss of cilia promotes tumor survival after chemotherapy (Zhao et al., 2017). Resistance to an inhibitor of smoothened (SMO), an activator of the Hh pathway, is frequently observed in Hh pathway-dependent cancers. In a transposon mutagenesis screen aimed at understanding the mechanism underlying this resistance in medulloblastomas, recurrent mutations in OFD1 – a gene that is defective in ciliopathies – were identified. Following on from this, it was shown that loss of cilia by depletion of OFD1 and other ciliogenesis genes confers resistance to SMO inhibition by achieving a cilium-independent state that is able to transduce low-level Hh signaling and is capable of evolving into a more-aggressive tumor. This study pinpoints an important role for cilia in tumor evolution and drug resistance and, given the pivotal role of cilia in Hh and growth factor signaling, it is likely that many additional mediators of chemo-resistance that localize to centrosomes and cilia will be identified in the future.
Cilia as tumor promoters
In contrast with the above examples, cilia have been shown to persist in a range of tumors (Fu et al., 2014; Yasar et al., 2017) and can be found in medulloblastomas exhibiting activation of Hh or Wnt signaling (Han et al., 2009). Strikingly, tumorigenesis is blocked in a medulloblastoma mouse model driven by constitutively active SMO when primary cilia are genetically ablated (Han et al., 2009). In the same model, INPP5P inactivation increases cilia-localized PI3-kinase/AKT signaling, which promotes cilia loss, thereby reducing both oncogenic Hh signaling and tumor growth (Conduit et al., 2017). Furthermore, inhibiting cilia-dependent oncogenic sonic hedgehog overactivation, through depletion of the ciliary GTPase Arl13b, can also suppress medulloblastoma growth without ablating cilia (Bay et al., 2018). Similarly, the loss of cilia protects mice from tumorigenesis in a basal cell carcinoma model driven by constitutively active SmoM2 (Wong et al., 2009). In this context, the expression of inturned (Intu), a planar cell polarity effector required for cilium assembly that regulates apical actin networks, is aberrantly elevated, whereas disruption of Intu prevents the formation of basal cell carcinoma by suppressing primary cilia formation and Hh signaling (Yang et al., 2017).
In a novel example of ciliary repurposing by tumors, cells were able to switch fates to allow assembly of a primary cilium and activation of Hh signaling (Li et al., 2016): in this example – a choroid plexus (CP) tumor model driven by sustained expression of Notch – monociliated CP tumor cells arise from multiciliated CP epithelial cells following elevated Notch signaling, which suppresses multiciliation in favor of primary ciliogenesis. This leads to enhanced Hh signaling that promotes CP tumor cell proliferation. Together, these data demonstrate that the presence of primary cilia in tumors can mediate Hh signaling and promote the formation of tumors. Furthermore, a recent study suggested that the acquisition of cilia, ciliary tip fragmentation and increased cilium length are frequently observed in tumor cells after anti-neoplastic drug treatment (Jenks et al., 2018), including in EGFR-mutant non-small cell lung carcinoma cells treated with an EGFR inhibitor, in rhabdoid tumors treated with a tyrosine kinase inhibitor, in EML4-ALK-fusion-positive lung cancers after ALK inhibitor treatment and in KRAS mutant lung cancer cells treated with a MEK inhibitor. The aberrant ciliogenesis is accompanied by activation of Hh signaling, and depletion of cilia or inhibition of Hh signaling can thus reduce the viability of drug-resistant cells. This study highlights that cilium assembly pathways can be hijacked by tumor cells to gain resistance to anti-tumor drugs, opening up exciting new avenues for the treatment of drug-resistant tumors.
Conclusions and future directions
In the past few years, significant advances have been made in understanding the events required during cilium assembly. In particular, new DA and SDA components required for centriole maturation have been identified, their localization defined with high-resolution methods, and their assembly pathways and function during vesicle docking and cytoskeleton connection elucidated. Importantly, novel markers of vesicles and regulators of vesicle trafficking, fusion and growth have also been revealed. Future studies will need to uncover the signals that mediate the onset of basal body maturation and cilium assembly, as well as the remodeling events – and specific molecules – at basal bodies that facilitate capture and growth of early ciliary vesicles, and extension of the nascent axoneme. In terms of cilium disassembly, we have a better understanding of the regulation of axonemal microtubule depolymerization and ciliary membrane remodeling, and additional signaling pathways have been found to control cilium disassembly. Given the pivotal role of cilia in development and disease, it will be essential to continue unraveling the mechanisms that link ciliogenesis and cilium disassembly to the cell cycle, mitogen deprivation and developmental cues. Improvements in real-time microscopy and lineage-tracing, as well as in single-cell sequencing technology, will hopefully lead to a better understanding of the developmental control of ciliogenesis in specific lineages and help us to understand the cilium-related mechanisms that govern chemoresistance in specific tumor types. It will also be interesting to understand the co-evolution of cilia structures and ciliary signaling pathways, in particular whether, and how, the ciliogenic program and related ciliary signaling pathways are coordinately switched on and off in a specific cell type or during certain developmental processes.
The role of cilia in tumorigenesis is further complicated by recent studies implicating this organelle in drug resistance. It is unclear how tumor cells manipulate cilium assembly or disassembly pathways for their own survival and how these pathways are harnessed by tumors to promote resistance to chemotherapeutic agents. Understanding these basic issues could contribute to the early detection of cancer and assist with the development of new anti-cancer regimens.
Acknowledgements
We apologize to the many researchers whose work could not be cited owing to space constraints.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by a U.S. Department of Defense fellowship (W81XWH-16-1-0392) to L.W., and a National Institutes of Health grant (9R01GM120776) to B.D.D. Deposited in PMC for release after 12 months.
References
- Absalon S., Blisnick T., Kohl L., Toutirais G., Doré G., Julkowska D., Tavenet A. and Bastin P. (2008). Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol. Biol. Cell 19, 929-944. 10.1091/mbc.e07-08-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M., Simms R. J., Abdelhamed Z., Dawe H. R., Szymanska K., Logan C. V., Wheway G., Pitt E., Gull K., Knowles M. A. et al. (2012). A meckelin-filamin A interaction mediates ciliogenesis. Hum. Mol. Genet. 21, 1272-1286. 10.1093/hmg/ddr557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbu S. O., Liang Y., Liu A. and Anderson K. V. (2018). The small GTPase RSG1 controls a final step in primary cilia initiation. J. Cell Biol. 217, 413-427. 10.1083/jcb.201604048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades I., Stylianou P. and Skourides P. A. (2014). Making the connection: ciliary adhesion complexes anchor basal bodies to the actin cytoskeleton. Dev. Cell 28, 70-80. 10.1016/j.devcel.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Aughsteen A. A. (2001). The ultrastructure of primary cilia in the endocrine and excretory duct cells of the pancreas of mice and rats. Eur. J. Morphol. 39, 277-283. 10.1076/ejom.39.5.277.7380 [DOI] [PubMed] [Google Scholar]
- Bangs F. and Anderson K. V. (2017). Primary cilia and mammalian hedgehog signaling. Cold Spring Harb. Perspect Biol. 9, a028175 10.1101/cshperspect.a028175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay S. N., Long A. B. and Caspary T. (2018). Disruption of the ciliary GTPase Arl13b suppresses Sonic hedgehog overactivation and inhibits medulloblastoma formation. Proc. Natl. Acad. Sci. USA 115, 1570-1575. 10.1073/pnas.1706977115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers A., Alten L., Viebahn C., Andre P. and Gossler A. (2007). The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc. Natl. Acad. Sci. USA 104, 15765-15770. 10.1073/pnas.0704344104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabé-Rubio M. and Alonso M. A. (2017). Routes and machinery of primary cilium biogenesis. Cell. Mol. Life Sci. 74, 4077-4095. 10.1007/s00018-017-2570-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besschetnova T. Y., Kolpakova-Hart E., Guan Y., Zhou J., Olsen B. R. and Shah J. V. (2010). Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr. Biol. 20, 182-187. 10.1016/j.cub.2009.11.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Rainey M. A., Arya P., Dutta S., George M., Storck M. D., McComb R. D., Muirhead D., Todd G. L., Gould K. et al. (2016). Endocytic recycling protein EHD1 regulates primary cilia morphogenesis and SHH signaling during neural tube development. Sci. Rep. 6, 20727 10.1038/srep20727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B. A. and Quarmby L. M. (2005). A NIMA-related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. J. Cell Sci. 118, 3317-3326. 10.1242/jcs.02455 [DOI] [PubMed] [Google Scholar]
- Burghoorn J., Dekkers M. P. J., Rademakers S., de Jong T., Willemsen R. and Jansen G. (2007). Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104, 7157-7162. 10.1073/pnas.0606974104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M. C., Li F.-Q., Cyge B., Arashiro T., Brechbuhl H. M., Chen X., Siller S. S., Weiss M. A., O'Connell C. B., Love D. et al. (2014). Chibby promotes ciliary vesicle formation and basal body docking during airway cell differentiation. J. Cell Biol. 207, 123-137. 10.1083/jcb.201406140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachero S., Simpson T. I., Zur Lage P. I., Ma L., Newton F. G., Holohan E. E., Armstrong J. D. and Jarman A. P. (2011). The gene regulatory cascade linking proneural specification with differentiation in Drosophila sensory neurons. PLoS Biol. 9, e1000568 10.1371/journal.pbio.1000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajanek L. and Nigg E. A. (2014). Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole. Proc. Natl. Acad. Sci. USA 111, E2841-E2850. 10.1073/pnas.1401777111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Shen Y., Zhu L., Xu Y., Zhou Y., Wu Z., Li Y., Yan X. and Zhu X. (2012). miR-129-3p controls cilia assembly by regulating CP110 and actin dynamics. Nat. Cell Biol. 14, 697-706. 10.1038/ncb2512 [DOI] [PubMed] [Google Scholar]
- Choksi S. P., Lauter G., Swoboda P. and Roy S. (2014). Switching on cilia: transcriptional networks regulating ciliogenesis. Development 141, 1427-1441. 10.1242/dev.074666 [DOI] [PubMed] [Google Scholar]
- Conduit S. E., Ramaswamy V., Remke M., Watkins D. N., Wainwright B. J., Taylor M. D., Mitchell C. A. and Dyson J. M. (2017). A compartmentalized phosphoinositide signaling axis at cilia is regulated by INPP5E to maintain cilia and promote Sonic Hedgehog medulloblastoma. Oncogene 36, 5969-5984. 10.1038/onc.2017.208 [DOI] [PubMed] [Google Scholar]
- Das R. M. and Storey K. G. (2014). Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 343, 200-204. 10.1126/science.1247521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y.-Z., Cai Z., Shi S., Jiang H., Shang Y. R., Ma N., Wang J.-J., Guan D.-X., Chen T.-W., Rong Y.-F. et al. (2018). Cilia loss sensitizes cells to transformation by activating the mevalonate pathway. J. Exp. Med. 215, 177-195. 10.1084/jem.20170399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeberg D. L., Lethan M., Manguso R., Schneider L., Awan A., Jorgensen T. S., Byskov A. G., Pedersen L. B. and Christensen S. T. (2012). Primary cilia and aberrant cell signaling in epithelial ovarian cancer. Cilia 1, 15 10.1186/2046-2530-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. H. and Brugmann S. A. (2018). Sending mixed signals: Cilia-dependent signaling during development and disease. Dev. Biol. 10.1016/j.ydbio.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina F., Gaillard J., Guerin C., Coute Y., Sillibourne J., Blanchoin L. and Théry M. (2016). The centrosome is an actin-organizing centre. Nat. Cell Biol. 18, 65-75. 10.1038/ncb3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W., Asp P., Canter B. and Dynlacht B. D. (2014). Primary cilia control hedgehog signaling during muscle differentiation and are deregulated in rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 111, 9151-9156. 10.1073/pnas.1323265111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W., Wang L., Kim S., Li J. and Dynlacht B. D. (2016). Role for the IFT-A complex in selective transport to the primary cilium. Cell Rep 17, 1505-1517. 10.1016/j.celrep.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E., Wason A., Ramani A., Gooi L. M., Keller P., Pozniakovsky A., Poser I., Noack F., Telugu N. S., Calegari F. et al. (2016). CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J. 35, 803-819. 10.15252/embj.201593679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S. C. and Anderson K. V. (2010). The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331-344. 10.1038/nrg2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S. C., Liem K. F. Jr. and Anderson K. V. (2012). The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell 151, 847-858. 10.1016/j.cell.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz J. G., Steed E., Ferreira R. R., Roth S., Ramspacher C., Boselli F., Charvin G., Liebling M., Wyart C., Schwab Y. et al. (2014). Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep 6, 799-808. 10.1016/j.celrep.2014.01.032 [DOI] [PubMed] [Google Scholar]
- Gradilone S. A., Radtke B. N., Bogert P. S., Huang B. Q., Gajdos G. B. and LaRusso N. F. (2013). HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 73, 2259-2270. 10.1158/0008-5472.CAN-12-2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser S., Stierhof Y.-D., Lavoie S. B., Gassner O. S., Lamla S., Le Clech M. and Nigg E. A. (2007). Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179, 321-330. 10.1083/jcb.200707181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G. D., Coyaud E., Goncalves J., Mojarad B. A., Liu Y., Wu Q., Gheiratmand L., Comartin D., Tkach J. M., Cheung S. W. et al. (2015). A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell 163, 1484-1499. 10.1016/j.cell.2015.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamze-Komaiha O., Sarr S., Arlot-Bonnemains Y., Samuel D. and Gassama-Diagne A. (2016). SHIP2 regulates lumen generation, cell division, and ciliogenesis through the control of basolateral to apical lumen localization of aurora A and HEF 1. Cell Rep 17, 2738-2752. 10.1016/j.celrep.2016.11.033 [DOI] [PubMed] [Google Scholar]
- Han Y.-G., Kim H. J., Dlugosz A. A., Ellison D. W., Gilbertson R. J. and Alvarez-Buylla A. (2009). Dual and opposing roles of primary cilia in medulloblastoma development. Nat. Med. 15, 1062-1065. 10.1038/nm.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. A., Van De Weghe J. M., Kubo T., Witman G. B. and Lechtreck K. (2018). Diffusion rather than IFT provides most of the tubulin required for axonemal assembly. bioRxiv. 10.1101/268573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassounah N. B., Nagle R., Saboda K., Roe D. J., Dalkin B. L. and McDermott K. M. (2013). Primary cilia are lost in preinvasive and invasive prostate cancer. PLoS ONE 8, e68521 10.1371/journal.pone.0068521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassounah N. B., Nunez M., Fordyce C., Roe D., Nagle R., Bunch T. and McDermott K. M. (2017). Inhibition of ciliogenesis promotes hedgehog signaling, tumorigenesis, and metastasis in breast cancer. Mol. Cancer Res. 15, 1421-1430. 10.1158/1541-7786.MCR-17-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton L. K., Gunawardane K., Kim J. W., Schwarz M. C. and Quarmby L. M. (2013). The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Curr. Biol. 23, 2208-2214. 10.1016/j.cub.2013.09.038 [DOI] [PubMed] [Google Scholar]
- Hori A. and Toda T. (2017). Regulation of centriolar satellite integrity and its physiology. Cell. Mol. Life Sci. 74, 213-229. 10.1007/s00018-016-2315-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.-C., Tong Z. J., Westfall J. E., Ault J. G., Page-McCaw P. S. and Ferland R. J. (2009). Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Hum. Mol. Genet. 18, 3926-3941. 10.1093/hmg/ddp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Xia Y., Zhang D., Wang S., Bao Y., He R., Teng J. and Chen J. (2017). Hierarchical assembly of centriole subdistal appendages via centrosome binding proteins CCDC120 and CCDC68. Nat. Commun. 8, 15057 10.1038/ncomms15057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi M., Zou P., Inoko A., Shiromizu T., Matsuyama M., Hayashi Y., Enomoto M., Mori D., Hirotsune S., Kiyono T. et al. (2011). Trichoplein controls microtubule anchoring at the centrosome by binding to Odf2 and ninein. J. Cell Sci. 124, 857-864. 10.1242/jcs.075705 [DOI] [PubMed] [Google Scholar]
- Inaba H., Goto H., Kasahara K., Kumamoto K., Yonemura S., Inoko A., Yamano S., Wanibuchi H., He D., Goshima N. et al. (2016). Ndel1 suppresses ciliogenesis in proliferating cells by regulating the trichoplein-Aurora A pathway. J. Cell Biol. 212, 409-423. 10.1083/jcb.201507046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoko A., Matsuyama M., Goto H., Ohmuro-Matsuyama Y., Hayashi Y., Enomoto M., Ibi M., Urano T., Yonemura S., Kiyono T. et al. (2012). Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J. Cell Biol. 197, 391-405. 10.1083/jcb.201106101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H. and Marshall W. F. (2017). Intraflagellar transport and ciliary dynamics. Cold Spring Harb. Perspect Biol. 9 10.1101/cshperspect.a021998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Pan J., Driscoll J. A., Wisner J. W., Huang T., Gunsten S. P., You Y. and Brody S. L. (2010). Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 43, 731-739. 10.1165/rcmb.2009-0328OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Javidan-Nejad C., Alexander-Brett J., Horani A., Cabellon M. C., Walter M. J. and Brody S. L. (2012). Sensory functions of motile cilia and implication for bronchiectasis. Front. Biosci. (Schol Ed) 4, 1088-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks A. D., Vyse S., Wong J. P., Kostaras E., Keller D., Burgoyne T., Shoemark A., Tsalikis A., de la Roche M., Michaelis M. et al. (2018). Primary cilia mediate diverse kinase inhibitor resistance mechanisms in cancer. Cell Rep 23, 3042-3055. 10.1016/j.celrep.2018.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen V. L. and Leroux M. R. (2017). Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr. Opin. Cell Biol. 47, 83-91. 10.1016/j.ceb.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Joo K., Kim C. G., Lee M.-S., Moon H.-Y., Lee S.-H., Kim M. J., Kweon H.-S., Park W.-Y., Kim C.-H., Gleeson J. G. et al. (2013). CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc. Natl. Acad. Sci. USA 110, 5987-5992. 10.1073/pnas.1220927110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanie T., Abbott K. L., Mooney N. A., Plowey E. D., Demeter J. and Jackson P. K. (2017). The CEP19-RABL2 GTPase complex binds IFT-B to initiate intraflagellar transport at the ciliary base. Dev. Cell 42, 22-36 e12. 10.1016/j.devcel.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan O. I., Doroquez D. B., Cevik S., Bowie R. V., Clarke L., Sanders A. A., Kida K., Rappoport J. Z., Sengupta P. and Blacque O. E. (2012). Endocytosis genes facilitate protein and membrane transport in C. elegans sensory cilia. Curr. Biol. 22, 451-460. 10.1016/j.cub.2012.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K., Kawakami Y., Kiyono T., Yonemura S., Kawamura Y., Era S., Matsuzaki F., Goshima N. and Inagaki M. (2014). Ubiquitin-proteasome system controls ciliogenesis at the initial step of axoneme extension. Nat. Commun. 5, 5081 10.1038/ncomms6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K., Aoki H., Kiyono T., Wang S., Kagiwada H., Yuge M., Tanaka T., Nishimura Y., Mizoguchi A., Goshima N. et al. (2018). EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat. Commun. 9, 758 10.1038/s41467-018-03117-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazatskaya A., Kuhns S., Lambacher N. J., Kennedy J. E., Brear A. G., McManus G. J., Sengupta P. and Blacque O. E. (2017). Primary cilium formation and ciliary protein trafficking is regulated by the atypical MAP kinase MAPK15 in Caenorhabditis elegans and human cells. Genetics 207, 1423-1440. 10.1534/genetics.117.300383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Krishnaswami S. R. and Gleeson J. G. (2008). CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum. Mol. Genet. 17, 3796-3805. 10.1093/hmg/ddn277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Zaghloul N. A., Bubenshchikova E., Oh E. C., Rankin S., Katsanis N., Obara T. and Tsiokas L. (2011). Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat. Cell Biol. 13, 351-360. 10.1038/ncb2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Kim M., Lee M.-S., Kim C.-H. and Lim D.-S. (2014). The MST1/2-SAV1 complex of the Hippo pathway promotes ciliogenesis. Nat. Commun. 5, 5370 10.1038/ncomms6370 [DOI] [PubMed] [Google Scholar]
- Kim J., Jo H., Hong H., Kim M. H., Kim J. M., Lee J.-K., Heo W. D. and Kim J. (2015a). Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat. Commun. 6, 6781 10.1038/ncomms7781 [DOI] [PubMed] [Google Scholar]
- Kim S., Lee K., Choi J.-H., Ringstad N. and Dynlacht B. D. (2015b). Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat. Commun. 6, 8087 10.1038/ncomms9087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzel D., Boldt K., Davis E. E., Burtscher I., Trümbach D., Diplas B., Attié-Bitach T., Wurst W., Katsanis N., Ueffing M. et al. (2010). Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev. Cell 19, 66-77. 10.1016/j.devcel.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger M., Wang W., Kuhns S., Bärenz F., Dräger-Meurer S., Pereira G. and Gruss O. J. (2014). The novel centriolar satellite protein SSX2IP targets Cep290 to the ciliary transition zone. Mol. Biol. Cell 25, 495-507. 10.1091/mbc.e13-09-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Tsang W. Y., Li J., Lane W. and Dynlacht B. D. (2011). Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell 145, 914-925. 10.1016/j.cell.2011.04.028 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Kim S., Lin Y.-C., Inoue T. and Dynlacht B. D. (2014). The CP110-interacting proteins Talpid3 and Cep290 play overlapping and distinct roles in cilia assembly. J. Cell Biol. 204, 215-229. 10.1083/jcb.201304153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Nakazono K., Tokuda M., Mashima Y., Dynlacht B. D. and Itoh H. (2017). HDAC2 promotes loss of primary cilia in pancreatic ductal adenocarcinoma. EMBO Rep. 18, 334-343. 10.15252/embr.201541922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodani A., Salome Sirerol-Piquer M., Seol A., Garcia-Verdugo J. M. and Reiter J. F. (2013). Kif3a interacts with Dynactin subunit p150 Glued to organize centriole subdistal appendages. EMBO J. 32, 597-607. 10.1038/emboj.2013.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhns S., Schmidt K. N., Reymann J., Gilbert D. F., Neuner A., Hub B., Carvalho R., Wiedemann P., Zentgraf H., Erfle H. et al. (2013). The microtubule affinity regulating kinase MARK4 promotes axoneme extension during early ciliogenesis. J. Cell Biol. 200, 505-522. 10.1083/jcb.201206013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtulmus B., Wang W., Ruppert T., Neuner A., Cerikan B., Viol L., Dueñas-Sánchez R., Gruss O. J. and Pereira G. (2016). WDR8 is a centriolar satellite and centriole-associated protein that promotes ciliary vesicle docking during ciliogenesis. J. Cell Sci. 129, 621-636. 10.1242/jcs.179713 [DOI] [PubMed] [Google Scholar]
- Kurtulmus B., Yuan C., Schuy J., Neuner A., Hata S., Kalamakis G., Martin-Villalba A. and Pereira G. (2017). Analysis of LRRC45 indicates cooperative functions of distal appendages at early steps of ciliogenesis. bioRxiv. 10.1101/205625 [DOI] [Google Scholar]
- Lader E., Ha H.-S., O'Neill M., Artzt K. and Bennett D. (1989). tctex-1: a candidate gene family for a mouse t complex sterility locus. Cell 58, 969-979. 10.1016/0092-8674(89)90948-3 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A., Schroth J. and Gleeson J. G. (2011). Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat. Cell Biol. 13, 700-707. 10.1038/ncb2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta H., Maunsbach A. B. and Madden S. C. (1961). Cilia in different segments of the rat nephron. J Biophys Biochem Cytol 11, 248-252. 10.1083/jcb.11.1.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Johmura Y., Yu L.-R., Park J. E., Gao Y., Bang J. K., Zhou M., Veenstra T. D., Yeon Kim B. and Lee K. S. (2012). Identification of a novel Wnt5a-CK1varepsilon-Dvl2-Plk1-mediated primary cilia disassembly pathway. EMBO J. 31, 3104-3117. 10.1038/emboj.2012.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Yi S., Kang Y. E., Chang J. Y., Kim J. T., Sul H. J., Kim J. O., Kim J. M., Kim J., Porcelli A. M. et al. (2016a). Defective ciliogenesis in thyroid hurthle cell tumors is associated with increased autophagy. Oncotarget 7, 79117-79130. 10.18632/oncotarget.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Lee M.-S., Choi T.-I., Hong H., Seo J.-Y., Kim C.-H. and Kim J. (2016b). MCRS1 associates with cytoplasmic dynein and mediates pericentrosomal material recruitment. Sci. Rep. 6, 27284 10.1038/srep27284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Joo K., Jung E. J., Hong H., Seo J. and Kim J. (2017). Export of membrane proteins from the Golgi complex to the primary cilium requires the kinesin motor, KIFC1. FASEB J. 32, 957-968. 10.1096/fj.201700563R [DOI] [PubMed] [Google Scholar]
- Li A., Saito M., Chuang J.-Z., Tseng Y.-Y., Dedesma C., Tomizawa K., Kaitsuka T. and Sung C.-H. (2011). Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat. Cell Biol. 13, 402-411. 10.1038/ncb2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kim S., Kobayashi T., Liang F.-X., Korzeniewski N., Duensing S. and Dynlacht B. D. (2012). Neurl4, a novel daughter centriole protein, prevents formation of ectopic microtubule organizing centres. EMBO Rep. 13, 547-553. 10.1038/embor.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Grausam K. B., Wang J., Lun M. P., Ohli J., Lidov H. G. W., Calicchio M. L., Zeng E., Salisbury J. L., Wechsler-Reya R. J. et al. (2016). Sonic Hedgehog promotes proliferation of Notch-dependent monociliated choroid plexus tumour cells. Nat. Cell Biol. 18, 418-430. 10.1038/ncb3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Yi P., Zhu Z., Zhang X., Li W. and Ou G. (2017). Centriole translocation and degeneration during ciliogenesis in Caenorhabditis elegans neurons. EMBO J. 36, 2553-2566. 10.15252/embj.201796883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Pang Y., Wu Q., Hu Z., Han X., Xu Y., Deng H. and Pan J. (2014). FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Dev. Cell 30, 585-597. 10.1016/j.devcel.2014.07.019 [DOI] [PubMed] [Google Scholar]
- Liu Y., Pathak N., Kramer-Zucker A. and Drummond I. A. (2007). Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development 134, 1111-1122. 10.1242/dev.02806 [DOI] [PubMed] [Google Scholar]
- Lohret T. A., McNally F. J. and Quarmby L. M. (1998). A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation. Mol. Biol. Cell 9, 1195-1207. 10.1091/mbc.9.5.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes C. A. M., Prosser S. L., Romio L., Hirst R. A., O'Callaghan C., Woolf A. S. and Fry A. M. (2011). Centriolar satellites are assembly points for proteins implicated in human ciliopathies, including oral-facial-digital syndrome 1. J. Cell Sci. 124, 600-612. 10.1242/jcs.077156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loskutov Y. V., Griffin C. L., Marinak K. M., Bobko A., Margaryan N. V., Geldenhuys W. J., Sarkaria J. N. and Pugacheva E. N. (2018). LPA signaling is regulated through the primary cilium: a novel target in glioblastoma. Oncogene 37, 1457-1471. 10.1038/s41388-017-0049-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Toh M. T., Narasimhan V., Thamilselvam S. K., Choksi S. P. and Roy S. (2015a). A function for the Joubert syndrome protein Arl13b in ciliary membrane extension and ciliary length regulation. Dev. Biol. 397, 225-236. 10.1016/j.ydbio.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Lu Q., Insinna C., Ott C., Stauffer J., Pintado P. A., Rahajeng J., Baxa U., Walia V., Cuenca A., Hwang Y.-S. et al. (2015b). Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat. Cell Biol. 17, 228-240. 10.1038/ncb3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub M. R., Montpetit B., Zhao L., Finst R. J., Goh B., Kim A. C. and Quarmby L. M. (2002). The FA2 gene of Chlamydomonas encodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation. J. Cell Sci. 115, 1759-1768. [DOI] [PubMed] [Google Scholar]
- Mahjoub M. R., Xie Z. and Stearns T. (2010). Cep120 is asymmetrically localized to the daughter centriole and is essential for centriole assembly. J. Cell Biol. 191, 331-346. 10.1083/jcb.201003009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion V., Stoetzel C., Schlicht D., Messaddeq N., Koch M., Flori E., Danse J. M., Mandel J.-L. and Dollfus H. (2009). Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc. Natl. Acad. Sci. USA 106, 1820-1825. 10.1073/pnas.0812518106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. F. and Rosenbaum J. L. (2001). Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 155, 405-414. 10.1083/jcb.200106141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. F., Qin H., Rodrigo Brenni M. and Rosenbaum J. L. (2005). Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol. Biol. Cell 16, 270-278. 10.1091/mbc.e04-07-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskey D., Marlin M. C., Kim S., Kim S., Ong E.-C., Li G. and Tsiokas L. (2015). Cell cycle-dependent ubiquitylation and destruction of NDE1 by CDK5-FBW7 regulates ciliary length. EMBO J. 34, 2424-2440. 10.15252/embj.201490831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Simera H. L., Gumerson J. D., Gao C., Campos M., Cologna S. M., Beyer T., Boldt K., Kaya K. D., Patel N., Kretschmer F. et al. (2016). Loss of MACF1 abolishes ciliogenesis and disrupts apicobasal polarity establishment in the retina. Cell Rep 17, 1399-1413. 10.1016/j.celrep.2016.09.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo G., Soplop N., Wang W.-J., Uryu K. and Tsou M.-F. B. (2016). Spatial control of primary ciliogenesis by subdistal appendages alters sensation-associated properties of cilia. Dev. Cell 39, 424-437. 10.1016/j.devcel.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Hosoba K., Ochiai H., Royba E., Izumi H., Sakuma T., Yamamoto T., Dynlacht B. D. and Matsuura S. (2015). The microtubule-depolymerizing activity of a mitotic kinesin protein KIF2A drives primary cilia disassembly coupled with cell proliferation. Cell Rep. 10.1016/j.celrep.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohieldin A. M., Zubayer H. S., Al Omran A. J., Saternos H. C., Zarban A. A., Nauli S. M. and AbouAlaiwi W. A. (2016). Vascular endothelial primary cilia: mechanosensation and hypertension. Curr Hypertens Rev 12, 57-67. 10.2174/1573402111666150630140615 [DOI] [PubMed] [Google Scholar]
- Moser J. J., Fritzler M. J. and Rattner J. B. (2009). Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer 9, 448 10.1186/1471-2407-9-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger B. L. (1958). A light and electron microscopic study of cellular differentiation in the pancreatic islets of the mouse. Am. J. Anat. 103, 275-311. 10.1002/aja.1001030207 [DOI] [PubMed] [Google Scholar]
- Muthaiyan Shanmugam M., Bhan P., Huang H. Y., Hsieh J., Hua T. E., Wu G. H., Punjabi H., Lee Aplicano V. D., Chen C. W. and Wagner O. I. (2018). Cilium length and intraflagellar transport regulation by kinases PKG-1 and GCK-2 in Caenorhabditis elegans sensory neurons. Mol. Cell. Biol. 38 10.1128/MCB.00612-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M. V. (2018). The molecular machines that traffic signaling receptors into and out of cilia. Curr. Opin. Cell Biol. 51, 124-131. 10.1016/j.ceb.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M. V., Loktev A. V., Zhang Q., Westlake C. J., Peränen J., Merdes A., Slusarski D. C., Scheller R. H., Bazan J. F., Sheffield V. C. et al. (2007). A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201-1213. 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- Nager A. R., Goldstein J. S., Herranz-Perez V., Portran D., Ye F., Garcia-Verdugo J. M. and Nachury M. V. (2017). An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 168, 252-263 e214. 10.1016/j.cell.2016.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer J. M., Amack J. D., Peterson A. G., Bisgrove B. W. and Yost H. J. (2009). FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 458, 651-654. 10.1038/nature07753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B. S., Malinda R. R., Schmid F. M., Pedersen S. F., Christensen S. T. and Pedersen L. B. (2015). PDGFRbeta and oncogenic mutant PDGFRalpha D842V promote disassembly of primary cilia through a PLCgamma- and AURKA-dependent mechanism. J. Cell Sci. 128, 3543-3549. 10.1242/jcs.173559 [DOI] [PubMed] [Google Scholar]
- Nishijima Y., Hagiya Y., Kubo T., Takei R., Katoh Y. and Nakayama K. (2017). RABL2 interacts with the intraflagellar transport-B complex and CEP19 and participates in ciliary assembly. Mol. Biol. Cell 28, 1652-1666. 10.1091/mbc.e17-01-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott C., Nachmias D., Adar S., Jarnik M., Sherman S., Birnbaum R. Y., Lippincott-Schwartz J. and Elia N. (2018). VPS4 is a dynamic component of the centrosome that regulates centrosome localization of gamma-tubulin, centriolar satellite stability and ciliogenesis. Sci. Rep. 8, 3353 10.1038/s41598-018-21491-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablo J. L., DeCaen P. G. and Clapham D. E. (2017). Progress in ciliary ion channel physiology. J. Gen. Physiol. 149, 37-47. 10.1085/jgp.201611696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak E. and Segal R. A. (2016). Hedgehog signal transduction: key players, oncogenic drivers, and cancer therapy. Dev. Cell 38, 333-344. 10.1016/j.devcel.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J. and Snell W. J. (2014). Organelle size: a cilium length signal regulates IFT cargo loading. Curr. Biol. 24, R75-R78. 10.1016/j.cub.2013.11.043 [DOI] [PubMed] [Google Scholar]
- Pan J., Wang Q. and Snell W. J. (2004). An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev. Cell 6, 445-451. 10.1016/S1534-5807(04)00064-4 [DOI] [PubMed] [Google Scholar]
- Phirke P., Efimenko E., Mohan S., Burghoorn J., Crona F., Bakhoum M. W., Trieb M., Schuske K., Jorgensen E. M., Piasecki B. P. et al. (2011). Transcriptional profiling of C. elegans DAF-19 uncovers a ciliary base-associated protein and a CDK/CCRK/LF2p-related kinase required for intraflagellar transport. Dev. Biol. 357, 235-247. 10.1016/j.ydbio.2011.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua S. C., Chiba S., Suzuki M., Su E., Roberson E. C., Pusapati G. V., Setou M., Rohatgi R., Reiter J. F., Ikegami K. et al. (2017). Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168, 264-279 e215. 10.1016/j.cell.2016.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao T., Luo M., Wang L., Guo Y., Li D., Li P., Snell W. J. and Pan J. (2009). A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc. Natl. Acad. Sci. USA 106, 4713-4718. 10.1073/pnas.0808671106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaval A., Senger F., Letort G., Gidrol X., Guyon L., Sillibourne J. and Théry M. (2017). Microtubule stabilization drives 3D centrosome migration to initiate primary ciliogenesis. J. Cell Biol. 216, 3713-3728. 10.1083/jcb.201610039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova O. V., Nikonova A. S., Loskutov Y. V., Kozyulina P. Y., Pugacheva E. N. and Golemis E. A. (2012). Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Mol. Biol. Cell 23, 2658-2670. 10.1091/mbc.e11-12-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova O. V., Seo S., Cottle D. L., Conduit S., Hakim S., Dyson J. M., Mitchell C. A. and Smyth I. M. (2015). INPP5E interacts with AURKA, linking phosphoinositide signaling to primary cilium stability. J. Cell Sci. 128, 364-372. 10.1242/jcs.161323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva E. N., Jablonski S. A., Hartman T. R., Henske E. P. and Golemis E. A. (2007). HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351-1363. 10.1016/j.cell.2007.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran J., Yang Y., Li D., Liu M. and Zhou J. (2015). Deacetylation of alpha-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci. Rep. 5, 12917 10.1038/srep12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi M. Q., Parker J. D., Feldman J. L., Marshall W. F. and Quarmby L. M. (2009). Katanin knockdown supports a role for microtubule severing in release of basal bodies before mitosis in Chlamydomonas. Mol. Biol. Cell 20, 379-388. 10.1091/mbc.e07-10-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter J. F. and Leroux M. R. (2017). Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533-547. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Otsu W., Hsu K. S., Chuang J. Z., Yanagisawa T., Shieh V., Kaitsuka T., Wei F. Y., Tomizawa K. and Sung C. H. (2017). Tctex-1 controls ciliary resorption by regulating branched actin polymerization and endocytosis. EMBO Rep. 18, 1460-1472. 10.15252/embr.201744204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez I. and Dynlacht B. D. (2016). Cilium assembly and disassembly. Nat. Cell Biol. 18, 711-717. 10.1038/ncb3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidel N., Kennedy J. and Blacque O. E. (2018). Endosome maturation factors Rabenosyn-5/VPS45 and caveolin-1 regulate ciliary membrane and polycystin-2 homeostasis. EMBO J. 37, e98248 10.15252/embj.201798248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K. N., Kuhns S., Neuner A., Hub B., Zentgraf H. and Pereira G. (2012). Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J. Cell Biol. 199, 1083-1101. 10.1083/jcb.201202126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml P., Frew I. J., Thoma C. R., Boysen G., Struckmann K., Krek W. and Moch H. (2009). Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Mod. Pathol. 22, 31-36. 10.1038/modpathol.2008.132 [DOI] [PubMed] [Google Scholar]
- Seeley E. S., Carriere C., Goetze T., Longnecker D. S. and Korc M. (2009). Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 69, 422-430. 10.1158/0008-5472.CAN-08-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. S., Ben-Shahar Y., Moninger T. O., Kline J. N. and Welsh M. J. (2009). Motile cilia of human airway epithelia are chemosensory. Science 325, 1131-1134. 10.1126/science.1173869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Kosan Z. A., Stallworth J. E., Berbari N. F. and Yoder B. K. (2011). Soluble levels of cytosolic tubulin regulate ciliary length control. Mol. Biol. Cell 22, 806-816. 10.1091/mbc.e10-03-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V., Romaguera-Ros M., Garcia-Verdugo J. M. and Reiter J. F. (2010). Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell 18, 410-424. 10.1016/j.devcel.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. (1962). Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363-377. 10.1083/jcb.15.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic M. and Jacobs C. R. (2017). Primary cilia: cell and molecular mechanosensors directing whole tissue function. Semin. Cell Dev. Biol. 71, 42-52. 10.1016/j.semcdb.2017.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N. and Meunier A. (2017). The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 18, 423-436. 10.1038/nrm.2017.21 [DOI] [PubMed] [Google Scholar]
- Spektor A., Tsang W. Y., Khoo D. and Dynlacht B. D. (2007). Cep97 and CP110 suppress a cilia assembly program. Cell 130, 678-690. 10.1016/j.cell.2007.06.027 [DOI] [PubMed] [Google Scholar]
- Tanos B. E., Yang H.-J., Soni R., Wang W.-J., Macaluso F. P., Asara J. M. and Tsou M.-F. B. (2013). Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 27, 163-168. 10.1101/gad.207043.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin-Robinet C., Lee J. S., Lopez E., Herranz-Pérez V., Shida T., Franco B., Jego L., Ye F., Pasquier L., Loget P. et al. (2014). The oral-facial-digital syndrome gene C2CD3 encodes a positive regulator of centriole elongation. Nat. Genet. 46, 905-911. 10.1038/ng.3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang W. Y., Bossard C., Khanna H., Peränen J., Swaroop A., Malhotra V. and Dynlacht B. D. (2008). CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev. Cell 15, 187-197. 10.1016/j.devcel.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. W., Pardee A. B. and Fujiwara K. (1979a). Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell 17, 527-535. 10.1016/0092-8674(79)90261-7 [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Scher C. D. and Stiles C. D. (1979b). Centriole deciliation associated with the early response of 3T3 cells to growth factors but not to SV40. Cell 18, 1065-1072. 10.1016/0092-8674(79)90219-8 [DOI] [PubMed] [Google Scholar]
- Van der Vaart A., Rademakers S. and Jansen G. (2015). DLK-1/p38 MAP kinase signaling controls cilium length by regulating RAB-5 mediated endocytosis in Caenorhabditis elegans. PLoS Genet. 11, e1005733 10.1371/journal.pgen.1005733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleri S., Manjunath S. H., Fariss R. N., May-Simera H., Brooks M., Foskett T. A., Gao C., Longo T. A., Liu P., Nagashima K. et al. (2014). Ciliopathy-associated gene Cc2d2a promotes assembly of subdistal appendages on the mother centriole during cilia biogenesis. Nat. Commun. 5, 4207 10.1038/ncomms5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Chen Q., Zhang X., Zhang B., Zhuo X., Liu J., Jiang Q. and Zhang C. (2013a). PCM1 recruits Plk1 to the pericentriolar matrix to promote primary cilia disassembly before mitotic entry. J. Cell Sci. 126, 1355-1365. 10.1242/jcs.114918 [DOI] [PubMed] [Google Scholar]
- Wang L., Piao T., Cao M., Qin T., Huang L., Deng H., Mao T. and Pan J. (2013b). Flagellar regeneration requires cytoplasmic microtubule depolymerization and kinesin-13. J. Cell Sci. 126, 1531-1540. 10.1242/jcs.124255 [DOI] [PubMed] [Google Scholar]
- Wang L., Lee K., Malonis R., Sanchez I. and Dynlacht B. D. (2016). Tethering of an E3 ligase by PCM1 regulates the abundance of centrosomal KIAA0586/Talpid3 and promotes ciliogenesis. Elife 5, e12950 10.7554/eLife.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Zhang Y., Li Y., Zhang Q., Ling K. and Hu J. (2012). The BBSome controls IFT assembly and turnaround in cilia. Nat. Cell Biol. 14, 950-957. 10.1038/ncb2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Xu Q., Zhang Y., Li Y., Zhang Q., Hu Z., Harris P. C., Torres V. E., Ling K. and Hu J. (2013). Transition fibre protein FBF1 is required for the ciliary entry of assembled intraflagellar transport complexes. Nat. Commun. 4, 2750 10.1038/ncomms3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake C. J., Baye L. M., Nachury M. V., Wright K. J., Ervin K. E., Phu L., Chalouni C., Beck J. S., Kirkpatrick D. S., Slusarski D. C. et al. (2011). Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc. Natl. Acad. Sci. USA 108, 2759-2764. 10.1073/pnas.1018823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley D. N., Wang A. M. and Strugnell G. E. (1996). Expression of primary cilia in mammalian cells. Cell Biol. Int. 20, 73-81. 10.1006/cbir.1996.0011 [DOI] [PubMed] [Google Scholar]
- Wheway G., Nazlamova L. and Hancock J. T. (2018). Signaling through the primary cilium. Front. Cell Dev. Biol. 6, 8 10.3389/fcell.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D., Camba A., Rogowski K., Manning G., Jerka-Dziadosz M. and Gaertig J. (2006). Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol. Biol. Cell 17, 2799-2810. 10.1091/mbc.e05-05-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. Y., Seol A. D., So P.-L., Ermilov A. N., Bichakjian C. K., Epstein E. H. Jr., Dlugosz A. A. and Reiter J. F. (2009). Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat. Med. 15, 1055-1061. 10.1038/nm.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren K. N., Craft J. M., Tritschler D., Schauer A., Patel D. K., Smith E. F., Porter M. E., Kner P. and Lechtreck K. F. (2013). A differential cargo-loading model of ciliary length regulation by IFT. Curr. Biol. 23, 2463-2471. 10.1016/j.cub.2013.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-T., Chen H.-Y. and Tang T. K. (2018). Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nat. Cell Biol. 20, 175-185. 10.1038/s41556-017-0018-7 [DOI] [PubMed] [Google Scholar]
- Xu Q., Zhang Y., Wei Q., Huang Y., Hu J. and Ling K. (2016). Phosphatidylinositol phosphate kinase PIPKIgamma and phosphatase INPP5E coordinate initiation of ciliogenesis. Nat. Commun. 7, 10777 10.1038/ncomms10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S. P., Sharma N. K., Liu C., Dong L., Li T. and Swaroop A. (2016). Centrosomal protein CP110 controls maturation of the mother centriole during cilia biogenesis. Development 143, 1491-1501. 10.1242/dev.130120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Leung E. L.-H., Liu C., Li L., Eguether T., Jun Yao X.-J., Jones E. C., Norris D. A., Liu A., Clark R. A. et al. (2017). INTU is essential for oncogenic Hh signaling through regulating primary cilia formation in basal cell carcinoma. Oncogene 36, 4997-5005. 10.1038/onc.2017.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. T., Chong W. M., Wang W.-J., Mazo G., Tanos B., Chen Z., Tran T. M. N., Chen Y.-D., Weng R. R., Huang C.-E. et al. (2018). Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat. Commun. 9, 2023 10.1038/s41467-018-04469-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar B., Linton K., Slater C. and Byers R. (2017). Primary cilia are increased in number and demonstrate structural abnormalities in human cancer. J. Clin. Pathol. 70, 571-574. 10.1136/jclinpath-2016-204103 [DOI] [PubMed] [Google Scholar]
- Ye X., Zeng H., Ning G., Reiter J. F. and Liu A. (2014). C2cd3 is critical for centriolar distal appendage assembly and ciliary vesicle docking in mammals. Proc. Natl. Acad. Sci. USA 111, 2164-2169. 10.1073/pnas.1318737111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C., Li A., Chuang J.-Z., Saito M., Cáceres A. and Sung C.-H. (2013). IGF-1 activates a cilium-localized noncanonical Gbetagamma signaling pathway that regulates cell-cycle progression. Dev. Cell 26, 358-368. 10.1016/j.devcel.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeyati P. L., Schiller R., Mali G., Kasioulis I., Kawamura A., Adams I. R., Playfoot C., Gilbert N., van Heyningen V., Wills J. et al. (2017). KDM3A coordinates actin dynamics with intraflagellar transport to regulate cilia stability. J. Cell Biol. 216, 999-1013. 10.1083/jcb.201607032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K., Frolova N., Xie Y., Wang D., Cook L., Kwon Y.-J., Steg A. D., Serra R. and Frost A. R. (2010). Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J. Histochem. Cytochem. 58, 857-870. 10.1369/jhc.2010.955856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Pak E., Ornell K. J., Pazyra-Murphy M. F., MacKenzie E. L., Chadwick E. J., Ponomaryov T., Kelleher J. F. and Segal R. A. (2017). A transposon screen identifies loss of primary cilia as a mechanism of resistance to SMO inhibitors. Cancer Discov. 7, 1436-1449. 10.1158/2159-8290.CD-17-0281 [DOI] [PubMed] [Google Scholar]
- Zou C., Li J., Bai Y., Gunning W. T., Wazer D. E., Band V. and Gao Q. (2005). Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J. Cell Biol. 171, 437-445. 10.1083/jcb.200506185 [DOI] [PMC free article] [PubMed] [Google Scholar]