ABSTRACT
The soluble transhydrogenase (UdhA) is one of two transhydrogenases that play a role in maintaining the balance between NAD(H) pools and NADP(H) pools in Escherichia coli. Although UdhA has been extensively used in metabolic engineering and biocatalysis for cofactor regeneration, its role in acid resistance has not been reported. Here we used DNA microarray to explore the impact of UdhA on transcript levels. We demonstrated that during growth on acetate, the expression of genes involved in the respiratory chain and Gad acid resistance system was inhibited in the udhA-knockout strain. The deletion of udhA significantly repressed the expression of six genes (gadA, gadB, gadC, gadE, hdeA and hdeB) which are involved in Gad acid resistance and resulted in low survival of the bacterium at a low pH of 4.9. Moreover, UdhA was essential for NADH production which is important for the adaptive growth of E. coli on acetate, while NADH concentration in the udhA-knockout strain was quite low and supplemental NADH significantly increased the expression of acid resistance genes and survival of the udhA-knockout strain. These results demonstrated that UdhA is an important source of NADH of E. coli growth on acetate and affects Gad acid resistance system under acetate stress.
KEY WORDS: Acid resistance, Escherichia coli, NADH, Transhydrogenase, UdhA
Summary: UdhA function stated in this study helps us to understand the physiological roles of UdhA affecting NADH production and Gad acid resistance system in E.coli in acetate environment.
INTRODUCTION
To pass through the stomach and survive in the intestine, enteric pathogens have evolved a number of strategies for adaptation to extremely acidic environments. For instance, Escherichia coli O157:H7, a particularly virulent form of E. coli, can shift from the nurturing pH 7 environment of a hamburger to the harsh pH 2 milieu of the stomach within moments (Foster, 2004). This organism is a highly acid-resistant food-borne pathogen (Foster, 2004; Price et al., 2004). Comparison studies have revealed that enterohemorrhagic E. coli O157:H7 (pathogenic) and commensal E. coli (non-pathogenic) possess similar acid resistance (AR) systems, conferring upon them equally outstanding capabilities to overcome acidic barriers (Foster, 1991, 2004; Price et al., 2004).
Four acid resistance systems have been identified in E. coli (Foster, 2004; Stincone et al., 2011; Sun et al., 2011). The first system (AR1), which is poorly understood, is active in the absence of amino acids (Lin et al., 1996), requires the sigma factor RpoS (Castanie-Cornet et al., 1999; Price et al., 2000) and the catabolite repressor protein CRP (Castanie-Cornet and Foster, 2001), and consumes ATP (Sun et al., 2011). The other three systems are dependent on the external supply of amino acids and are composed of dedicated pairs of amino acid decarboxylases and antiporters (Foster, 2004). The second system (AR2), which is the most effective glutamate-dependent system, involves two glutamate decarboxylase isozymes (GadA and GadB) and a putative glutamate/γ-amino butyric acid antiporter (GadC) (Castanie-Cornet et al., 1999). The third system (AR3), which is an arginine-dependent system, requires arginine decarboxylase (AdiA) and an arginine/agmatine antiporter (AdiC) (Castanie-Cornet et al., 1999; Gong et al., 2003; Iyer et al., 2003). The fourth system (AR4), which is a lysine-dependent, but much less efficient system, relies on lysine decarboxylase (CadA) and a lysine/cadaverin antiporter (CadB) (Iyer et al., 2003; Meng and Bennett, 1992; Vazquez-Juarez et al., 2008). The ARs hold up an ‘umbrella’ that protects E. coli under a variety of different acid stress situations. Moreover, it has been reported that many regulator proteins are directly or indirectly involved in ARs, such as CysB, EvgA/EvgS, GadE, GadX, GadW, HU, SspA and YdeO (Bi et al., 2009; Foster, 2004; Hansen et al., 2005; Lochowska et al., 2004; Masuda and Church, 2002; Sayed et al., 2007; Tramonti et al., 2006, 2002), and that ATP is required for survival under extremely acidic conditions (Sun et al., 2011).
The model organism E. coli, a typical Gram-negative bacterium, has been used to represent the group of enteric pathogens (such as Salmonella and Campylobacter). Although the genomes of various E. coli strains have been sequenced since 1997 (Blattner et al., 1997; Latif et al., 2014; Monk et al., 2013; Yoon et al., 2012), the physiological roles of many genes are still unclear, such as a soluble pyridine nucleotide transhydrogenase (STH). STH is only found in Gram-negative enteric bacteria, Actinomycetes and some other bacteria (Boonstra et al., 2000a). The soluble transhydrogenase, encoded by udhA in E. coli, is an energy-independent flavoprotein and forms remarkably large polymers (Boonstra et al., 2000a). Several experiments suggest that UdhA plays a role in reoxidizing excess NADPH into NADP and transferring the hydrogen (H) electron to NADH (Boonstra et al., 2000b; Canonaco et al., 2001; Sauer et al., 2004; Voordouw et al., 1983). Also, STH has been extensively employed for applications in metabolic engineering and biocatalysis. For example, STH from Pseudomonas fluorescens has been used in a cell-free system for efficient coenzyme cycling, resulting in high yields of hydromorphone, a semisynthetic opiate (Boonstra et al., 2000b). However, despite these technological advances, the functions of UdhA in vivo remain obscure.
In this study, DNA microarrays and mutant strains were used to explore the physiological roles of UdhA in E. coli. Our developing model implies that UdhA is an important source of NADH for the adaptive growth of E. coli on acetate and affects Gad acid resistance system of E. coli under acetate stress.
RESULTS
Growth rates of wild-type and mutant E. coli
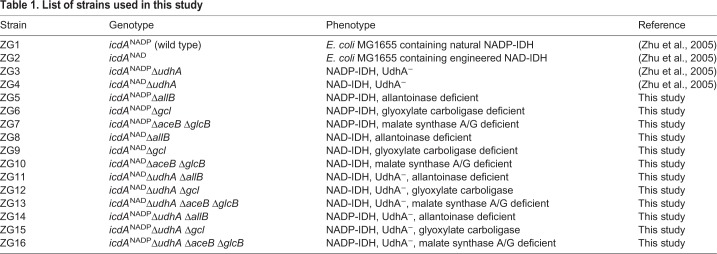
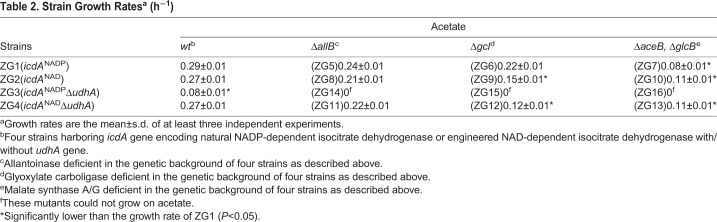
The growth rates of ZG2 (icdANAD, E. coli containing an engineered NAD+-dependent isocitrate dehydrogenase), ZG3 (icdANADPΔudhA, udhA-knockout E. coli containing NADP+-dependent isocitrate dehydrogenase) and ZG4 (icdANADΔudhA, udhA-knockout E. coli containing an engineered NAD+-dependent isocitrate dehydrogenase) were similar to that of the wild-type ZG1 (icdANADP, wild-type E. coli containing NADP+-dependent isocitrate dehydrogenase) when glucose was the sole source of carbon and energy (Zhao et al., 2008). The growth rate of ZG3 on acetate was significantly lower than that of ZG1. The growth rate of ZG4 was recovered when the coenzyme specificity of isocitrate dehydrogenase (IDH) was changed from NADP-dependency to NAD-dependency (Table 1 and Table 2).
Table 1.
List of strains used in this study
Table 2.
Strain Growth Ratesa (h−1)
Transcriptome analysis
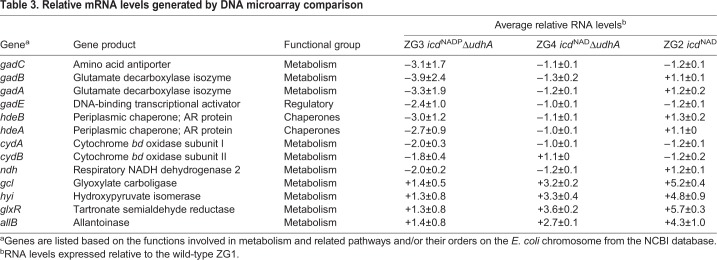
To investigate the poor growth rate of ZG3 on acetate, the transcript profiles of the wild-type strain ZG1 and the mutant strains ZG2, ZG3 and ZG4 were performed during growth on acetate. The total RNAs of all strains were collected at the time of OD600=0.6. Transcriptome analysis showed that the differences in the expression of most genes were small (<twofold) and were not statistically significant. The most significant differences were reductions in expression observed in the mutants (Table 3). Genes involved in the respiratory chain and acid resistance system were downregulated in ZG3. In addition, four genes involved in glyoxylate metabolism were upregulated in ZG2 and ZG4. To confirm the gene expression data from the DNA microarray, reverse transcription polymerase chain reaction (RT-PCR) confirmed that compared with the wild-type ZG1, the transcript levels of NADH dehydrogenase-2 (NDH-2, encoded by ndh) and isocitrate dehydrogenase (IDH, encoded by icdA) were similar to those obtained through microarray results (Fig. S1).
Table 3.
Relative mRNA levels generated by DNA microarray comparison
Genes involved in acid resistance
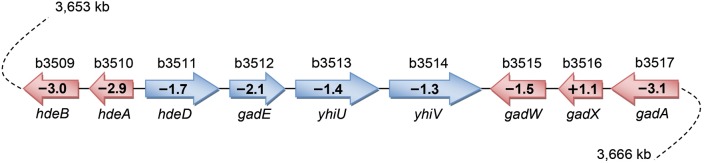
During growth on acetate, the expression of six genes involved in AR2 (gadA, gadB, gadC, gadE, hdeA and hdeB) was evidently reduced in ZG3 compared with wild-type ZG1 (Table 3). The gadA and gadB genes encode isoforms of glutamate decarboxylase, and gadC encodes a γ-aminobutyrate (GABA) antiporter (Castanie-Cornet et al., 1999, 2007). The GadC antiporter together with the GadA and GadB decarboxylases maintain the intracellular pH in cells subjected to extreme acid stress (Arnold et al., 2001). The product of gadE regulates the acid-induced expression of gadA, gadB and gadC (Ma et al., 2003). In addition, hdeA and hdeB encode two acid stress chaperones that prevent periplasmic protein aggregation at low pH (Kern et al., 2007). Four genes, gadA, gadE, hdeA and hdeB, cluster with gadW, gadX, hdeD, yhiU and yhiV between 3650 and 3666 kb in the E. coli genome (Fig. 1). The expression of each gene except for gadX was reduced to some extent in ZG3 (Table 3 and Fig. 1). In all, eleven genes (including the gadBC operon) involved in bacterial acid resistance were repressed.
Fig. 1.
The cluster of nine genes involved in the acid resistance system in the E. coli genome. The arrows represent the genes and the direction of transcription. Gene names are shown below the gene, and their entry numbers are shown above the gene. The fold-change of each gene in ZG3 (icdANADPΔudhA) is provided inside the arrows.
Acid resistance assays
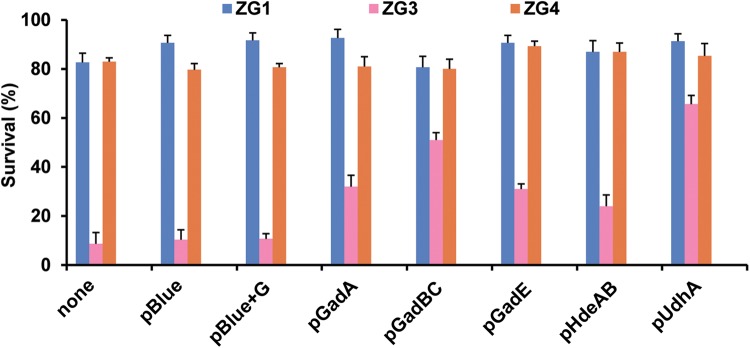
To verify whether ZG3 was sensitive to the acid stress which may be caused by reduced expression of acid resistance genes, the genes including udhA, gadA, the gadBC operon, gadE and the hdeAB operon were cloned and expressed in various genetic backgrounds. It was found that 80% of ZG1 and ZG4 cells, either with or without plasmids, survived for 1 h at pH 4.9 (Fig. 2). By contrast, only 9% of ZG3 cells survived. In the presence of pUdhA, ZG3 survival was increased to 66%. Expressing acid-resistance genes such as gadA, the gadBC operon, gadE or the hdeAB operon also improved ZG3 survival, although not as strongly as pUdhA (Fig. 2). However, the survival of ZG3 harboring pBlue was not increased, regardless of whether glutamate was present. These results indicated that UdhA plays an important role in affecting the Gad acid resistance system of E. coli.
Fig. 2.
Survival of acid-challenged strains. The percentage survival of strains challenged at pH 4.9 for 1 h was calculated compared with the count at time zero. Plasmids are denoted below the x-axis: none, no plasmid; pBlue, empty vector; pBlue+G, empty vector with glutamate in the medium, pGadA, pGadBC, pGadE, pHdeAB and pUdhA. IPTG and glutamate were supplemented in the culture media. The survival of ZG3 (icdANADPΔudhA) was significantly increased (P<0.05) by the expression of pHdeAB, pGadA, pGadBC, pGadE or pUdhA compared with ZG3 with or without pBlue. The survival of ZG1 (icdANADP, WT) and ZG4 (icdANADΔudhA) was not significantly affected by the plasmids (P>0.05). Data are presented as the mean±s.d. of three independent experiments.
Respiratory chain genes
The aerobic respiratory chain of E. coli is composed of a number of dehydrogenases and two quinol oxidase complexes. The microarray data showed that during growth on acetate, no significant changes occurred in the expression of either the sdhCDAB operon, which encodes succinate dehydrogenase, or the cyoABCDE operon, which encodes the cytochrome bo quinol oxidase (data not shown). Only the expression of ndh, which encodes a NADH dehydrogenase (NDH-2), and the cydAB operon, which encodes the heterodimeric cytochrome bd quinol oxidase (Zhang et al., 2004), were reduced twofold in ZG3 (Table 3).
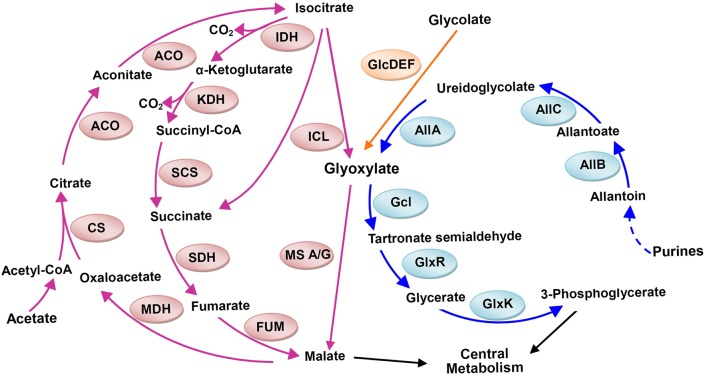
Genes involved in glyoxylate metabolism
When acetate is the sole available source of carbon and energy for E. coli, it is first activated to acetyl-CoA and then metabolized through the tricarboxylic acid cycle and glyoxylate shunt (Oh et al., 2002). Genes (allB, gcl and glxR) involved in glyoxylate metabolism (Cusa et al., 1999) showed increased expression in ZG2 and ZG4 (Table 3). Gene allB, which encodes the allantoinase that converts allantoin to allantoate, was upregulated by 4.3-fold in ZG2 and 2.7-fold in ZG4. Gene gcl, which encodes glyoxylate carboligase, and glxR, which encodes tartronate semialdehyde reductase, were upregulated by 5.2-fold and 5.7-fold in ZG2 and by 3.2-fold and 3.6-fold in ZG4, respectively. In addition, hyi, located downstream of glxR, encodes hydroxypyruvate isomerase and was upregulated 4.8-fold in ZG2 and 3.3-fold in ZG4.
Growth of deletion strains
Nine mutants (Table 1) were constructed to explore how deleting udhA and changing IDH coenzyme specificity influence the glyoxylate metabolism pathways (Fig. 3). Without allantoinase (encoded by allB), glyoxylate carboligase (encoded by gcl) or malate synthase A/G (encoded by aceB and glcB), the growth rates of all constructed strains (ZG5-ZG16) were lower than that of ZG1, ZG2, ZG3 and ZG4, correspondingly (Table 2). Deleting allB had the least effect on the growth rate. Deleting gcl further reduced the growth rates, particularly in ZG9 (icdANADΔgcl) and ZG12 (icdANAD/ΔudhA Δgcl). The greatest reductions in the growth rate were observed when aceB and glcB in the glyoxylate bypass were deleted. Surprisingly, ZG7 (icdANADPΔaceB ΔglcB) grew more slowly on acetate than either ZG10 (icdANADΔaceBΔglcB) or ZG13 (icdANADΔudhA ΔaceB ΔglcB). These results indicated that glyoxylate metabolism played an important role in E. coli during growth on acetate, which was probably regulated by the NADH pool as identified in followings.
Fig. 3.
Pathways involved in glyoxylate metabolism. Three pathways generate glyoxylate: glycolate metabolism, acetate metabolism and purine degradation. Glyoxylate enters the central metabolism through two pathways: direct conversion to malate by malate synthase G (GlcB) and/or malate synthase A (AceB) or conversion to 3-phosphoglycerate by glyoxylate carboligase (Gcl), tartronate semialdehyde reductase (GlxR) and glycerate kinase (GlxK). Genes with blue backgrounds were upregulated in ZG2 (icdANAD) and ZG3 (icdANADPΔudhA). ACO, aconitase; AllB, allantoinase; AllC, allantoate amidohydrolase; AllA, ureidoglycolate hydrolase; CS, citrate synthase; FUM, fumarase; Gcl, glyoxylate carboligase; GlxK, glycerate kinase; GlxR, tartronic semialdehyde reductase; ICL, Isocitrate lyase; KDH, α-Ketoglutarate dehydrogenase; MDH, malate dehydrogenase; SCS, Succinyl-CoA synthetase; SDH, succinate dehydrogenase.
The effect of NADH on growth and acid resistance
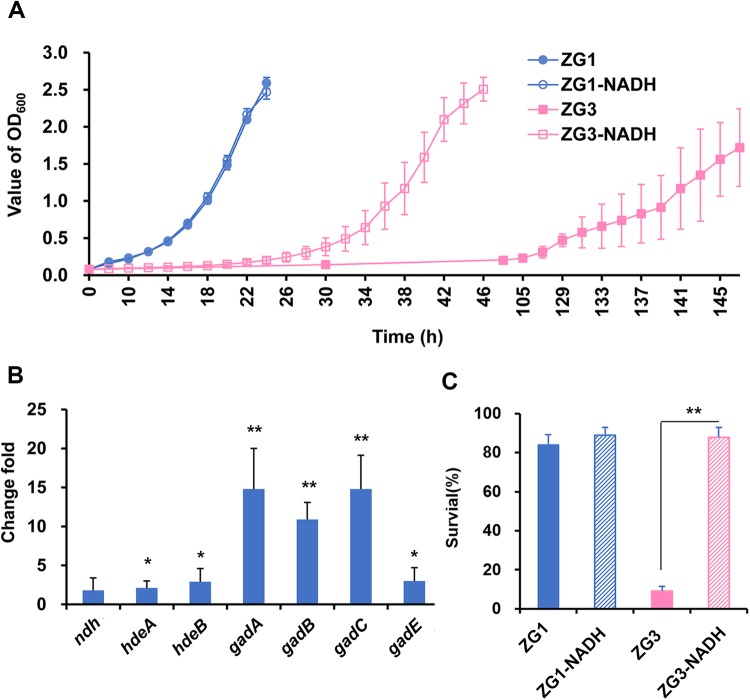
In order to verify the effect of UdhA on the production of NADH, the concentrations of NADH in ZG1, ZG2, ZG3, ZG4, ZG7, ZG10 and ZG13 were determined by HPLC. The NADH concentrations in all strains were not significantly different when glucose was used the carbon source (Table S1). However, when acetate was used as the carbon source, the NADH concentrations were approximately 8.26, 7.51, 2.63, 6.88, 3.88, 6.82 and 5.82 μmol per gram of cells, respectively (Table 4). The NADH concentration in ZG7 was significantly lower than that of ZG1, which was in accord with the low growth rate of ZG7. In ZG3, the concentration of NADH was the lowest among these strains, about 2.63 μmol per gram of cells (Table 4). It was proposed that NADH may play a key role in ZG3. After the addition of 5 mM NADH to the culture medium at the beginning of culture, the growth rate of ZG1 was nearly the same with or without NADH supplementation (Fig. 4A), whereas the growth rate of ZG3 was significantly increased (P<0.01). Furthermore, NADH significantly increased the expression of genes involved in acid resistance, especially the genes (gadA, gadB and gadC) in AR2 (Fig. 4B). The acid resistance assay confirmed that NADH significantly increased the survival rate of ZG3 from 10% to the same level as ZG1 (P<0.001), whose survival rate was not affected by NADH supplementation (Fig. 4C). These results demonstrated that UdhA could affect growth rate and survival of ZG3 in a NADH-dependent manner.
Table 4.
The concentrations of NADH measured by HPLCa.
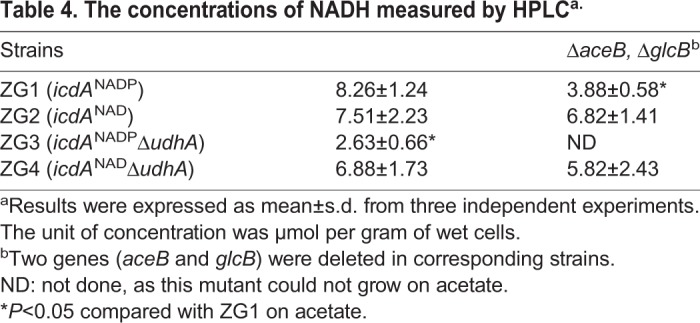
Fig. 4.
The effect of NADH on the WT and ZG3 strains. (A) Growth curves for ZG1 (icdANADP, WT) and ZG3 (icdANADPΔudhA) with or without NADH supplementation. ZG1-NADH and ZG3-NADH indicate that NADH was added to the culture medium at the beginning. The dashed line represents the long lag phase of ZG3. The growth rate of ZG3-NADH was significantly faster than that of ZG3 (P<0.05). (B) Q-PCR results regarding gene expression levels in ZG3-NADH compared with ZG1-NADH. (C) Acid resistance assay for ZG1 and ZG3 cells with or without NADH supplementation. Data are presented as the mean±s.d. of three independent experiments. *P<0.05. **P<0.01.
DISCUSSION
Gram-negative bacteria exhibit several adaptations for survival and growth at low pH. The most effective acid-resistance system in E. coli is AR2 (Ma et al., 2003; Tucker et al., 2002). Intracellular glutamate is decarboxylated to 4-aminobutyrate and CO2, with stoichiometric consumption of protons, by either of two isozymes, GadA or GadB. Intracellular 4-aminobutyrate is then exchanged for external glutamate by GadC. The combined activity of these three enzymes efficiently consumes protons, increasing the intracellular pH to levels compatible with survival (Foster, 2004; Richard and Foster, 2004, 2003). The DNA array data showed that the expression of gadA and gadBC was lower in ZG3 (icdANADPΔudhA) than in wild-type ZG1 (icdANADP) during growth on acetate. These results are consistent with the acid resistance experiments. More than 80% of ZG1 and ZG4 cells survived for 1 h at pH 4.9, whereas less than 10% of ZG3 cells survived (Fig. 2). Transforming ZG3 with expression vector, pGadA or pGadBC, improved survival to 35% or 50%, respectively (Fig. 2), whereas transforming ZG1 or ZG4 with the same vectors had little effect (Fig. 2).
In addition to the decarboxylase, other acid resistance genes expressing the periplasmic acid chaperones hdeA and hedB were downregulated in ZG3 (Table 3). Chaperones provide another adaptation to low pH (Bearson et al., 1997). In E. coli, chaperones HdeA and HdeB suppress the aggregation of periplasmic proteins at low pH (Kern et al., 2007). The expression of hdeAB, expressed under gad operon, is HN-S repressed, RpoS dependent and acid induced (Richard and Foster, 2004). As in an earlier study (Kern et al., 2007), survival improved when hdeAB is overexpressed in ZG3 (Fig. 2). Lower expression of hdeAB may be caused by lower expression of GadE, which is believed to bind directly to hdeAB operon promoter and induce expression. Survival of ZG3 was found to improve dramatically, from about 12% to 66%, when ZG3 cells harbored pUdhA (Fig. 2). These results indicate that UdhA is important to acid resistance through affecting gene expression of the Gad acid resistance system in E. coli.
Furthermore, our developing model indicates that UdhA is an important source of NADH during bacterial growth on acetate. The concentration of NADH in ZG3 is quite low. The lower expression of acid resistance genes in ZG3 is attributed directly to a shortage of NADH. Replacing the NADP-specific icdANADP with the NAD-specific icdANAD in ZG4 and the direct supplementary of NADH in ZG3 replenish the NADH pool, increase the expression of acid resistance genes (such as gadA and gadBC) and improve growth to almost wild-type levels. NADH could increase gadABC expression, which may affect gadE expression and subsequently regulate GAD operon (Ma et al., 2003). Another possibility of UdhA deletion affecting acid resistance of E. coli in acetate is that the low NADH pool could not provide enough production of ATP, which affects the acid resistance system (Sauer et al., 2004; Sun et al., 2011). More experiments are needed to verify these possibilities.
NDH-2, the primary dehydrogenase during aerobic respiration, catalyzes the re-oxidation of NADH, transferring the electrons to ubiquinone-1 (Calhoun and Gennis, 1993; Gyan et al., 2006). NDH-2 is tightly regulated by global transcription factors and links the major catabolic and energy-producing pathways (Jaworowski et al., 1981). The down-regulation of ndh in ZG3 might be a mechanism for bacterial adaption to a poor NADH status. The effect of NADH on ndh expression was further confirmed by the NADH supplementation experiment. The concentration of NADH in ZG3 cells measured via HPLC was the lowest among all of the tested strains, whereas the supplemental NADH restored the expression of ndh in ZG3 to a level similar to that in the wild-type strain ZG1 cells (Fig. 4B). Thus, UdhA is an important source of NADH. The depletion of the NADH pool, as observed in ZG3, reduces the expression of respiratory chain genes and, hence, growth.
Even though udhA was deleted in ZG4 and ZG13, the growth rates of these two strains were similar to the corresponding strains (ZG4 to ZG2; ZG13 to ZG10). The concentration of NADH did not differ significantly among these four strains. These results illustrate that in the icdNAD background, UdhA does not play an important role in supplying the NADH pool. By contrast, udhA was very important in the wild-type (ZG1-icdNADP) background, as the growth rate of ZG3 and NADH concentration in ZG3 were significantly lower compared with ZG1, and it was important for bacterial adaption to acid stress. Taken together, the soluble transhydrogenase, UdhA, is an important source of NADH of E. coli growth on acetate, and plays roles in affecting the expression of genes involved in the Gad acid resistance system under acetate stress.
MATERIALS AND METHODS
Materials
Restriction enzymes were purchased from New England Biolabs (Beverly, USA). Plasmids and genomic DNA were extracted using Wizard® purification kits from Promega (Madison, USA). Herculase™ enhanced DNA polymerase (Stratagene, California, USA) and PrimeStar™ HS DNA polymerase from TaKaRa (Dalian, China) were used for PCR. The GeneChip® E. coli genome 2.0 array was employed for genome-wide expression profiling (Affymetrix, California, USA). The SV Total RNA Isolation System for total RNA extraction was purchased from Promega, and Superscript II reverse transcriptase was used for reverse transcription (Invitrogen). The LightCycler-FastStart DNA Master SYBR Green I Kit for RT-PCR was obtained from Roche Applied Science (Indiana, USA). Oligonucleotides were synthesized by Sangon (Shanghai, China).
Strains and plasmids
All experiments were conducted with strains derived from E. coli MG1655 (ATCC. 700926) (Table 1). Two strains, ZG1 (icdANADP) and ZG2 (icdANAD), containing NADP+-dependent isocitrate dehydrogenase (NADP-IDH) and an engineered NAD+-dependent IDH (NAD-IDH), and two strains in which udhA was deleted, ZG3 (icdANADPΔudhA) and ZG4 (icdANADΔudhA), which also contained NADP-IDH and an engineered NAD-IDH, were constructed previously (Zhu et al., 2005). All other strains were constructed specifically for this study. The plasmids used in this study are listed in Table S2.
Culture conditions
Lysogeny broth (LB), Super Optimal broth (SOB), Super Optimal broth with catabolite repression (SOC) and MOPS-based minimal medium were prepared as previously described (Zhu et al., 2005). MOPS-based minimal medium (250 ml, pH 7.4) included 20.925 g MOPS, 1.800 g Tricine, 0.007 g FeSO4·7H2O, 1.275 g NH4Cl, 0.120 g K2SO4, 7.300 g NaCl, 0.137 g CaCl2, 0.124 g MgCl2, 2.5 mg Vitamin B1. LB and SOB media were supplemented with 100 μg ml−1 ampicillin, 10-15 μg ml−1 tetracycline, 30 μg ml−1 kanamycin, or 20 μg ml−1 chloramphenicol, as required.
Growth rates
Strains were grown in 25 ml of MOPS-based minimal medium containing 2% glucose or 2% acetate as a carbon source in a 250 ml flask in an orbital shaker (200 rpm) at 37°C. Samples were taken every 45 min (glucose) or 2 h (acetate), and culture densities were determined spectrophotometrically at 600 nm (UV-2102 spectrophotometer, UNICO Co. Ltd). Growth rates were calculated using linear regression on semi-logarithmic plots of OD600 against time and processed by JMPIN software. The growth rates were from at least three independent experiments.
RNA extraction
RNA was isolated and purified by using the SV Total RNA Isolation System according to the manufacturer's instructions. After purification, genomic DNA was removed by treatment with RNase-free DNase I (Promega), followed by RNA repurification with an RNeasy column. The quantity and purity of RNA were determined by measuring A260 and the A260/280 ratio, respectively. Purified total RNA was precipitated with ethanol and stored at −80°C until needed.
Microarray analysis
All protocols were conducted as described in the Affymetrix GeneChip Expression Analysis Technical Manual and produced by CapitalBio Corporation (Beijing, China). The GeneChip® E. coli genome 2.0 array, containing 20,366 genes, was used to study the differential expression profiles of strains ZG2 (icdANAD), ZG3 (icdANADPΔudhA) and ZG4 (icdANADΔudhA), compared with ZG1 (icdANADP, wild-type MG1655). Total RNA (10 µg) was reverse transcribed with random primers using SuperScript II reverse transcriptase. The resulting cDNA was fragmented into sequences ranging from 50–200 bp and labeled at the 3′ termini using the GeneChip® DNA Labeling Reagent (Affymetrix, P/N 900542). Biotin-labeled cDNAs were hybridized to an Affymetrix GeneChip® E. coli genome 2.0 array at 45°C for 16 h at 60 rpm using a Hybridization Oven 640 (Affymetrix). The array was washed and stained on an Affymetrix Fluidics Station 400. The microarray was scanned using the default settings on a GeneChip Scanner 3000. The scanned images were analyzed with GeneChip Operating Software (GCOS 1.4). Intensities were scaled using dChip software to allow direct comparisons between strains. All experiments were performed in two biological replicates, and transcripts showing a ≥twofold change in expression and P<0.05 were considered significant. The full microarray data are accessed by NCBI BioProject (PRJNA382597).
Real-time PCR
Real-time PCR was performed to validate the microarray data. The cDNAs used for microarray analysis were used as the templates in Real-time PCR. The LightCycler-FastStart DNA Master SYBR Green I Kit was employed for real-time RT-PCR. The RT-PCR assays were performed using an initiation step of 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 5 s at 58°C and 15 s at 72°C, with fluorescence data collection at 76°C using LightCycler Software Version 3.5. Controls were used to ensure that there was no contaminating DNA in the RNA sample. Quantification of the ndh and icdA genes relative to a housekeeping gene (16S rRNA) was performed using a mathematical model by Paffl (Pfaffl, 2001), which accounts for real-time RT-PCR efficiencies and the crossing points for the transcripts of each sample.
Plasmid constructions
The downregulated genes (hdeAB operon, gadA, gadBC operon, gadE) identified with the microarrays and the udhA gene were amplified via PCR using E. coli MG1655 genomic DNA as a template. The forward primers contained a HindIII or XbaI site, and the reverse primers contained an XhoI or HindIII site (Table S3). The PCR products were digested and ligated into digested pBluescript SK(+) II (pBlue) and then transformed into ZG1, ZG3 and ZG4 cells. The plasmid constructs (Table S2) were confirmed through DNA sequencing.
Acid resistance assay
The ability of the strains to survive acid stress was evaluated as previously described (Abdul-Raouf et al., 1993; Castanie-Cornet et al., 1999; Sainz et al., 2005). Cells were grown in MOPS-acetate medium with antibiotics, 1 mM IPTG or 1.5 mM glutamate, as required. After the bacteria were inoculated into acetate medium, the cells were grown exponentially to an A600 of 0.6 and then diluted 1:100 into fresh acetate medium, pH 4.9, followed by incubation for 1 h at 37°C. The acid challenge was stopped through dilution. The samples were serially diluted and plated onto LB plates. The percentage of surviving cells was estimated from the number of colonies at 1 h relative to the number at time zero. Each experiment was repeated at least three times.
Construction of allB, gcl, aceB and glcB deletions
The genes were disrupted via the method of Datsenko and Wanner (Datsenko and Wanner, 2000). Briefly, primers for the deletions (Table S4) were used to amplify the kanamycin resistance gene from pKD13 (Datsenko and Wanner, 2000). Strains harboring pKD46 were grown in SOB medium containing 200 μg ml−1 ampicillin and 10 mM L-arabinose at 30°C to mid-log phase. Linearized PCR products containing kan and flanking sequences on either side of allB, gcl, aceB or glcB were introduced into competent cells via CaCl2 transformation. Cells, shocked at 42°C and recovered at 37°C in SOC, were spread on LB plates containing kanamycin. The desired disruption was confirmed via sequencing PCR-amplified regions spanning 0.5∼1 kb of the flanking DNA on either side of the inserted kan gene. Next, the kan genes were eliminated from the strains, and ampicillin-resistant transformants carrying pCP20 were isolated and grown at 42°C on LB plates (Datsenko and Wanner, 2000). Colonies were screened for sensitivity to ampicillin and loss of pCP20. Multiple deletions were constructed sequentially. Finally, icd alleles (icdANADP, which encodes NADP-IDH, or icdANAD, which encodes NAD-IDH) were introduced via P1 cotransduction with adjacent tetr cassettes. All together twelve strains were constructed (Table 1), four with single deletions (ZG5, ZG6, ZG8, ZG9), five with double deletions (ZG7, ZG10, ZG11, ZG12) and two with a triple deletion (ZG13). Chromosome-integrated regions were sequenced to ensure that no other mutations had been inadvertently introduced during construction.
NADH extraction and quantification through HPLC
To extract NADH, 250 ml cell cultures were centrifuged at 4600 rcf for 5 min and resuspended in extraction buffer [1 ml of 0.5 M Tris-HCl, pH 7.0 containing 1 mM EDTA and 1 ml of methanol (Heuser et al., 2009)]. After the addition of 2 ml of chloroform, NADH extraction was performed by shaking the tube for 1.5 h at 25°C. Prior to extraction, 1 mM and 100 mM NADH was added to a parallel sample as an extraction control. The separation of NADH in the aqueous phase was carried out via centrifugation for 5 min at 3200 rcf to separate the aqueous phase from the chloroform phase. The aqueous phase was filtered through a 0.22 µm filter to remove denatured protein. HPLC quantification of NADH was performed with a Prominence LC20 (SHIMADZU) on a column (VP-ODS C18 15X0.2). Buffer A (pH 6.0) contained 0.1 M potassium phosphate buffer and 4 mM tetrabutylammonium hydrogen sulfate, and buffer B (pH 7.5) contained 70% buffer A and 30% methanol. The two buffers were applied by mixing 100% buffer A and 0% buffer B at the beginning, followed by 70% buffer A and 30% buffer B after 12.5 min, 40% buffer A and 60% buffer B after 25 min and 0% buffer A and 100% buffer B after 32.5 min. The total separation time was 45 min. The flow rate was 0.2 ml min−1.
Statistical analysis
Statistical analyses were performed with the two-tailed Student’s t-test using Stata statistical software. The P value <0.05 was considered as statistically significant.
Supplementary Material
Acknowledgements
We are grateful to Prof. Antony M. Dean (University of Minnesota, USA) for discussing and helping us in revising the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: H.Z.; Methodology: H.Z., F.Z., Q.X., Z.C., J.L.; Software: H.Z.; Validation: H.Z., F.Z., Q.X., Z.C., J.L.; Formal analysis: H.Z., G.Z.; Investigation: H.Z., F.Z., Q.X., Z.C., J.L., G.Z.; Resources: G.Z.; Data curation: H.Z., G.Z.; Writing - original draft: H.Z., G.Z.; Writing - review & editing: H.Z., G.Z.; Visualization: H.Z., G.Z.; Supervision: H.Z., G.Z.; Project administration: H.Z., G.Z.; Funding acquisition: G.Z.
Funding
This work was supported by the National Natural Science Foundation of China [31570010], Innovation Team of Scientific Research Platform in Anhui University, Anhui Provincial Key Laboratory of the Conservation and Exploitation of Biological Resources and Key Laboratory of the Biotic Environment and Ecological Safety in Anhui Province and Institute of Molecular Biology and Biotechnology in Anhui Normal University.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.031856.supplemental
References
- Abdul-Raouf U. M., Beuchat L. R. and Ammar M. S. (1993). Survival and growth of Escherichia coli O157:H7 in ground, roasted beef as affected by pH, acidulants, and temperature. Appl. Environ. Microb. 59, 2364-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C. N., McElhanon J., Lee A., Leonhart R. and Siegele D. A. (2001). Global analysis of Escherichia coli gene expression during the acetate-induced acid tolerance response. J. Bacteriol. 183, 2178-2186. 10.1128/JB.183.7.2178-2186.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearson S., Bearson B. and Foster J. W. (1997). Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 147, 173-180. 10.1111/j.1574-6968.1997.tb10238.x [DOI] [PubMed] [Google Scholar]
- Bi H., Sun L., Fukamachi T. and Saito K. H. (2009). HU participates in expression of a specific set of genes required for growth and survival at acidic pH in Escherichia coli. Curr. microbiol. 58, 443-448. 10.1007/s00284-008-9340-4 [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Plunkett G. III, Bloch C. A., Perna N. T., Burland V., Riley M., Collado-Vides J., Glasner J. D., Rode C. K., Mayhew G. F. et al. (1997). The complete genome sequence of Escherichia coli K-12. Science 277, 1453-1462. 10.1126/science.277.5331.1453 [DOI] [PubMed] [Google Scholar]
- Boonstra B., Bjorklund L., French C. E., Wainwright I. and Bruce N. C. (2000a). Cloning of the sth gene from Azotobacter vinelandii and construction of chimeric soluble pyridine nucleotide transhydrogenases. FEMS Microbiol. Lett. 191, 87-93. 10.1111/j.1574-6968.2000.tb09323.x [DOI] [PubMed] [Google Scholar]
- Boonstra B., Rathbone D. A., French C. E., Walker E. H. and Bruce N. C. (2000b). Cofactor regeneration by a soluble pyridine nucleotide transhydrogenase for biological production of hydromorphone. Appl. Environ. Microb. 66, 5161-5166. 10.1128/AEM.66.12.5161-5166.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun M. W. and Gennis R. (1993). Demonstration of separate genetic loci encoding distinct membrane-bound respiratory NADH dehydrogenases in Escherichia coli. J. Bacteriol. 175, 3013-3019. 10.1128/jb.175.10.3013-3019.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonaco F., Hess T. A., Heri S., Wang T., Szyperski T. and Sauer U. (2001). Metabolic flux response to phosphoglucose isomerase knock-out in Escherichia coli and impact of overexpression of the soluble transhydrogenase UdhA. FEMS Microbiol. Lett. 204, 247-252. 10.1111/j.1574-6968.2001.tb10892.x [DOI] [PubMed] [Google Scholar]
- Castanie-Cornet M. P. and Foster J. W. (2001). Escherichia coli acid resistance: cAMP receptor protein and a 20 bp cis-acting sequence control pH and stationary phase expression of the gadA and gadBC glutamate decarboxylase genes. Microbiology 147, 709-715. 10.1099/00221287-147-3-709 [DOI] [PubMed] [Google Scholar]
- Castanie-Cornet M. P., Penfound T. A., Smith D., Elliott J. F. and Foster J. W. (1999). Control of acid resistance in Escherichia coli. J. Bacteriol. 181, 3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanie-Cornet M.-P., Treffandier H., Francez-Charlot A., Gutierrez C. and Cam K. (2007). The glutamate-dependent acid resistance system in Escherichia coli: essential and dual role of the His-Asp phosphorelay RcsCDB/AF. Microbiology 153, 238-246. 10.1099/mic.0.29278-0 [DOI] [PubMed] [Google Scholar]
- Cusa E., Obradors N., Baldoma L., Badia J. and Aguilar J. (1999). Genetic analysis of a chromosomal region containing genes required for assimilation of allantoin nitrogen and linked glyoxylate metabolism in Escherichia coli. J. Bacteriol. 181, 7479-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A. and Wanner B. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640-6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W. (1991). Salmonella acid shock proteins are required for the adaptive acid tolerance response. J. Bacteriol. 173, 6896-6902. 10.1128/jb.173.21.6896-6902.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W. (2004). Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2, 898-907. 10.1038/nrmicro1021 [DOI] [PubMed] [Google Scholar]
- Gong S., Richard H. and Foster J. W. (2003). YjdE (AdiC) is the arginine:agmatine antiporter essential for arginine-dependent acid resistance in Escherichia coli. J. Bacteriol. 185, 4402-4409. 10.1128/JB.185.15.4402-4409.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyan S., Shiohira Y., Sato I., Takeuchi M. and Sato T. (2006). Regulatory loop between redox sensing of the NADH/NAD(+) ratio by Rex (YdiH) and oxidation of NADH by NADH dehydrogenase Ndh in Bacillus subtilis. J. Bacteriol. 188, 7062-7071. 10.1128/JB.00601-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A.-M., Qiu Y., Yeh N., Blattner F. R., Durfee T. and Jin D. J. (2005). SspA is required for acid resistance in stationary phase by downregulation of H-NS in Escherichia coli. Mol. Microbiol. 56, 719-734. 10.1111/j.1365-2958.2005.04567.x [DOI] [PubMed] [Google Scholar]
- Heuser F., Marin K., Kaup B., Bringer S. and Sahm H. (2009). Improving d-mannitol productivity of Escherichia coli: impact of NAD, CO2 and expression of a putative sugar permease from Leuconostoc pseudomesenteroides. Metab. Eng. 11, 178-183. 10.1016/j.ymben.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Iyer R., Williams C. and Miller C. (2003). Arginine-agmatine antiporter in extreme acid resistance in Escherichia coli. J. Bacteriol. 185, 6556-6561. 10.1128/JB.185.22.6556-6561.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski A., Campbell H. D., Poulis M. I. and Young I. G. (1981). Genetic identification and purification of the respiratory NADH dehydrogenase of Escherichia coli. Biochemistry 20, 2041-2047. 10.1021/bi00510a047 [DOI] [PubMed] [Google Scholar]
- Kern R., Malki A., Abdallah J., Tagourti J. and Richarme G. (2007). Escherichia coli HdeB is an acid stress chaperone. J. Bacteriol. 189, 603-610. 10.1128/JB.01522-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif H., Li H. J., Charusanti P., Palsson B. O. and Aziz R. K. (2014). A gapless, unambiguous genome sequence of the enterohemorrhagic Escherichia coli O157:H7 strain EDL933. Genome Announc 2, e00821-e00814 10.1128/genomeA.00821-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Smith M. P., Chapin K. C., Baik H. S., Bennett G. N. and Foster J. W. (1996). Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microb. 62, 3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochowska A., Iwanicka-Nowicka R., Zaim J., Witkowska-Zimny M., Bolewska K. and Hryniewicz M. M. (2004). Identification of activating region (AR) of Escherichia coli LysR-type transcription factor CysB and CysB contact site on RNA polymerase alpha subunit at the cysP promoter. Mol. Microbiol. 53, 791-806. 10.1111/j.1365-2958.2004.04161.x [DOI] [PubMed] [Google Scholar]
- Ma Z., Gong S., Richard H., Tucker D. L., Conway T. and Foster J. W. (2003). GadE (YhiE) activates glutamate decarboxylase-dependent acid resistance in Escherichia coli K-12. Mol. Microbiol. 49, 1309-1320. 10.1046/j.1365-2958.2003.03633.x [DOI] [PubMed] [Google Scholar]
- Masuda N. and Church G. M. (2002). Escherichia coli gene expression responsive to levels of the response regulator EvgA. J. Bacteriol. 184, 6225-6234. 10.1128/JB.184.22.6225-6234.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S. Y. and Bennett G. N. (1992). Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH. J. Bacteriol. 174, 2659-2669. 10.1128/jb.174.8.2659-2669.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk J. M., Charusanti P., Aziz R. K., Lerman J. A., Premyodhin N., Orth J. D., Feist A. M. and Palsson B. O. (2013). Genome-scale metabolic reconstructions of multiple Escherichia coli strains highlight strain-specific adaptations to nutritional environments. Proc. Natl. Acad. Sci. USA 110, 20338-20343. 10.1073/pnas.1307797110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.-K., Rohlin L., Kao K. C. and Liao J. C. (2002). Global expression profiling of acetate-grown Escherichia coli. J. Biol. Chem. 277, 13175-13183. 10.1074/jbc.M110809200 [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic. Acids. Res. 29, e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. B., Cheng C.-M., Kaspar C. W., Wright J. C., DeGraves F. J., Penfound T. A., Castanie-Cornet M.-P. and Foster J. W. (2000). Role of rpoS in acid resistance and fecal shedding of Escherichia coli O157:H7. Appl. Environ. Microb. 66, 632-637. 10.1128/AEM.66.2.632-637.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. B., Wright J. C., DeGraves F. J., Castanie-Cornet M.-P. and Foster J. W. (2004). Acid resistance systems required for survival of Escherichia coli O157:H7 in the bovine gastrointestinal tract and in apple cider are different. Appl. Environ. Microb. 70, 4792-4799. 10.1128/AEM.70.8.4792-4799.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard H. T. and Foster J. W. (2003). Acid resistance in Escherichia coli. Adv. Appl. Microbiol. 52, 167-186. 10.1016/S0065-2164(03)01007-4 [DOI] [PubMed] [Google Scholar]
- Richard H. and Foster J. W. (2004). Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J. Bacteriol. 186, 6032-6041. 10.1128/JB.186.18.6032-6041.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz T., Pérez J., Villaseca J., Hernandez U., Eslava C., Mendoza G. and Wacher C. (2005). Survival to different acid challenges and outer membrane protein profiles of pathogenic Escherichia coli strains isolated from pozol, a Mexican typical maize fermented food. Int. J. Food. Microbiol. 105, 357-367. 10.1016/j.ijfoodmicro.2005.04.017 [DOI] [PubMed] [Google Scholar]
- Sauer U., Canonaco F., Heri S., Perrenoud A. and Fischer E. (2004). The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J. Biol. Chem. 279, 6613-6619. 10.1074/jbc.M311657200 [DOI] [PubMed] [Google Scholar]
- Sayed A. K., Odom C. and Foster J. W. (2007). The Escherichia coli AraC-family regulators GadX and GadW activate gadE, the central activator of glutamate-dependent acid resistance. Microbiology 153, 2584-2592. 10.1099/mic.0.2007/007005-0 [DOI] [PubMed] [Google Scholar]
- Stincone A., Daudi N., Rahman A. S., Antczak P., Henderson I., Cole J., Johnson M. D., Lund P. and Falciani F. (2011). A systems biology approach sheds new light on Escherichia coli acid resistance. Nucleic. Acids. Res. 39, 7512-7528. 10.1093/nar/gkr338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Fukamachi T., Saito H. and Kobayashi H. (2011). ATP requirement for acidic resistance in Escherichia coli. J. Bacteriol. 193, 3072-3077. 10.1128/JB.00091-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramonti A., Visca P., De Canio M., Falconi M. and De Biase D. (2002). Functional characterization and regulation of gadX, a gene encoding an AraC/XylS-like transcriptional activator of the Escherichia coli glutamic acid decarboxylase system. J. Bacteriol. 184, 2603-2613. 10.1128/JB.184.10.2603-2613.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramonti A., De, Canio M., Delany I., Scarlato V. and De Biase D. (2006). Mechanisms of transcription activation exerted by GadX and GadW at the gadA and gadBC gene promoters of the glutamate-based acid resistance system in Escherichia coli. J. Bacteriol. 188, 8118-8127. 10.1128/JB.01044-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker D. L., Tucker N. and Conway T. (2002). Gene expression profiling of the pH response in Escherichia coli. J. Bacteriol. 184, 6551-6558. 10.1128/JB.184.23.6551-6558.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Juarez R. C., Kuriakose J. A., Rasko D. A., Ritchie J. M., Kendall M. M., Slater T. M., Sinha M., Luxon B. A., Popov V. L., Waldor M. K. et al. (2008). CadA negatively regulates Escherichia coli O157:H7 adherence and intestinal colonization. Infect. Immun. 76, 5072-5081. 10.1128/IAI.00677-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voordouw G., van der Vies S. M. and Themmen A. P. (1983). Why are two different types of pyridine nucleotide transhydrogenase found in living organisms? Eur. J. Biochem. 131, 527-533. 10.1111/j.1432-1033.1983.tb07293.x [DOI] [PubMed] [Google Scholar]
- Yoon S. H., Han M.-J., Jeong H., Lee C. H., Xia X.-X., Lee D.-H., Shim J. H., Lee S. Y., Oh T. K. and Kim J. F. (2012). Comparative multi-omics systems analysis of Escherichia coli strains B and K-12. Genome. Biol. 13, R37 10.1186/gb-2012-13-5-r37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Hellwig P., Osborne J. P. and Gennis R. B. (2004). Arginine 391 in subunit I of the cytochrome bd quinol oxidase from Escherichia coli stabilizes the reduced form of the hemes and is essential for quinol oxidase activity. J. Biol. Chem. 279, 53980-53987. 10.1074/jbc.M408626200 [DOI] [PubMed] [Google Scholar]
- Zhao H. J., Wang P., Huang E. Q., Ge Y. D. and Zhu G. P. (2008). Physiologic roles of soluble pyridine nucleotide transhydrogenase Escherichia coli as determined by homologous recombination. Ann. Microbiol. 58, 275 10.1007/BF03175329 [DOI] [Google Scholar]
- Zhu G. P., Golding G. B. and Dean A. M. (2005). The selective cause of an ancient adaptation. Science 307, 1279-1282. 10.1126/science.1106974 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.