Abstract
Background and Objectives
Childhood socioeconomic status (cSES) is found to predict later-life cognitive abilities, yet the mechanisms underlying these associations remain unclear. The objective of this longitudinal study was to examine the direct and indirect paths through which cSES influences late midlife cognitive outcomes.
Research Design and Methods
Participants were 1,009 male twins in the Vietnam Era Twin Study of Aging (VETSA). At mean ages 20 and 62, participants completed a standardized test for general cognitive ability (GCA). The age 62 cognitive assessment also included in-person tests of processing speed, episodic memory, abstract reasoning, working memory, verbal fluency, visual-spatial ability, and executive functions. At mean age 56, participants were interviewed regarding their own and their parents’ education and occupation, and completed questionnaires about cognitive leisure activities and sociodemographic information. Multiple mediation analyses were conducted to examine the direct path effects and indirect path effects of cSES through age 20 GCA, adult SES, and cognitive leisure activities on seven cognitive outcomes at age 62, adjusting for age, ethnicity, and non-independence of observations.
Results
Total (direct plus indirect) effects were significant for all measures with the exception of executive functions. Men from lower cSES backgrounds had poorer cognitive functioning in late midlife. The direct effect of cSES was partially mediated for abstract reasoning, and was fully mediated for the remaining six cognitive outcomes. Total indirect effects accounted for at least half of the total effects in each model, with paths through age 20 GCA explaining most of the total indirect effects.
Discussion and Implications
cSES predicted cognitive functioning in late middle age Using multiple mediation models, we show that lower cSES predicts poorer cognition in late midlife primarily through young adult cognitive ability and to a lesser extent through SES in adulthood and engagement in cognitively stimulating activities.
Keywords: Socioeconomic status, Cognition, Cognitive leisure activities, Adult development, Veterans
Translational Significance
This study highlights the contribution of parental socioeconomic status and engagement in adult cognitive leisure activities to adult cognitive performance. We show that parental socioeconomic status influences later outcomes due to its effect on the participants’ early cognitive abilities, their later adult socioeconomic status (i.e., education and occupation), and the kinds of leisure activities they engage in. These findings point to potential targets for early interventions in helping to prevent cognitive decline.
Childhood socioeconomic status (cSES) and cognitive ability have long been known to be correlated. In order to explain this association, schools of thought from developmental psychology-social epidemiology frameworks focus on “neo-materialist” pathways that emphasize the role of resource availability, or on psychosocial pathways that emphasize biological, psychological, and behavioral processes related to brain and cognitive health (Dunn, Veenstra, & Ross, 2006; Kahn & Pearlin, 2006; Pearlin, Schieman, Fazio, & Meersman, 2005). Models have been tested that implicate both direct and indirect effects of cSES on later-life cognitive outcomes, with longitudinal studies allowing for more thorough examination of pathways and accumulated risks.
From a neo-materialist perspective, cSES bestows different levels of material resources (i.e., economic, social, and cultural capital) and exposures for the developing child at individual, neighborhood, and school levels which then accumulate across the life course (S. Cohen, Janicki‐Deverts, Chen, & Matthews, 2010). For instance, families with lower SES tend to experience greater economic pressure, poorer quality schools, and health care (Conger, Conger, & Martin, 2010; Evans, 2004), and are exposed to higher levels of environmental hazards and pollutants (Conger et al., 2010; Evans, 2004). Lower SES parents are more likely to prioritize pressing needs (e.g., food, rent) over activities such as intellectual stimulation, healthy nutrition, and health care (Conger et al., 2010; Evans, 2004). The social and cultural capital of SES (i.e., access to resources such as social networks, habits, or lifestyle markers) also contribute to later outcomes (Lareau & Weininger, 2003; Walpole, 2003).
As would be expected from the neo-materialist perspective, cSES has been associated with multiple dimensions of mid- and later-life cognition (Hurst et al., 2013; Landy, Head, Richards, & Hardy, 2017; Melrose et al., 2015; Turrell et al., 2002). Associations between cSES and later-life cognitive outcomes, however, are substantially reduced or eradicated after accounting for adult SES (González, Tarraf, Bowen, Johnson-Jennings, & Fisher, 2013; Horvat et al., 2014; Luo & Waite, 2005; Lyu & Burr, 2016). In one of the few studies using formal mediation tests, the effect of mother education (but not other childhood SES indicators) on late adult cognitive functioning was partially mediated by adult education and economic amenities (Horvat et al., 2014). Thus, the role of adult SES in reducing the effect of cSES on adult cognitive status demonstrates empirical support for the pathway hypothesis of cSES: that cSES is mediated by adult SES.
Fewer studies have examined the role of social and cultural capital (i.e., types of activities or assets that can help promote social status) in cognitive outcomes (Lareau & Weininger, 2003). Engagement in cognitive/cultural leisure activities (CLA), one type of cultural capital, has been associated with better cognitive function in older adults (Jefferson et al., 2011; Vemuri et al., 2014). Children from higher SES families are more likely to have access to cultural resources (Hackman, Farah, & Meaney, 2010). They have higher rates of participation in or attendance at cultural events compared with their lower SES counterparts, even when cost is not a factor (Silber, Triplett, Iyengar, & National Endowment for the Arts, 2015). In general, then, in these models, childhood status is viewed in terms of the access to resources it provides that may promote or inhibit later cognitive outcomes.
Psychosocial pathway models traditionally focus more on other types of intervening variables on the path between cSES and cognitive outcomes (Kahn & Pearlin, 2006). These attributes may range from health behaviors to psychological mechanisms (i.e., depression and self-esteem), physiological responses (e.g., stress, allostatic load), and education achievement (Pearlin et al., 2005). For example, African-American adults who reported feelings of greater financial strain in both childhood and adulthood had higher levels of disability and depressive symptoms than those without financial strain, and cognitive functioning was inversely associated with memories of childhood financial strain (Szanton, Thorpe, & Whitfield, 2010). There is evidence suggesting that cSES can influence adult brain and cognitive development (Bradley & Corwyn, 2002; Evans, 2004; Hackman et al., 2010; Lyu & Burr, 2016; Schreiber et al., 2016), in part through health. One mechanism by which stress associated with childhood status may affect later outcomes could be through its influence on hypothalamic-pituitary-adrenal axis regulation, which has been linked to health, brain, and cognitive outcomes (Franz et al., 2013; McEwen, Nasca, & Gray, 2016).
Elements of the resource and psychosocial pathways clearly overlap and are often difficult to distinguish (S. Cohen et al., 2010; Zeki Al Hazzouri et al., 2017). For instance, poorer quality schools are associated with both fewer resources and with lower educational achievement, which—in turn—are predictive of cognitive function. Similarly, low SES families are exposed to more stressors, have fewer resources to cope with stress, and have higher symptoms of stress (Bradley & Corwyn, 2002; Evans, 2004), placing resources clearly in the pathway of stress exposure and stress responses.
Although SES is often considered as an environmental factor, it is important to note that genetic effects also play a role in SES and its association with cognition (Ericsson et al., 2017). Indeed, shared genetic influences explain associations between education, intelligence, childhood, and adult SES (Plomin & Deary, 2015). It is likely that parental SES may affect the extent to which a child is able to benefit from experiences that are consistent with his/her genetically influenced cognitive ability and interests, thereby improving cognitive outcomes (Tucker-Drob & Harden, 2012). Plomin and Deary (2015) point to cognitive ability as a crucial third variable in inequalities research, likely mediating the association between cSES and later-life cognitive function. Few studies, however, examine the role of early cognitive ability (i.e., ability during childhood or early adulthood) as a mediating factor between cSES and later-life cognitive performance.
A number of key gaps in our understanding of the relationship between cSES and later-life adult cognitive functioning emerge from the review of the literature. Most studies are cross-sectional or limited in their longitudinal data; SES and cognition tend to be assessed simultaneously. Remarkably few studies have examined childhood or young adult cognitive ability as a third variable mediating the association between cSES and later cognitive outcomes (Plomin & Deary, 2015), in part because most studies lack data on the participants’ cognitive ability when they were younger. Much of the focus has been on testing models of various SES indicators in order to identify specificity of associations, sensitive periods, and cumulative influences, rather than on the detailed assessment of cognitive ability. Also, studies have used cognitive screening instruments or just measured a few cognitive abilities. Furthermore, most studies use analytic designs that only allow them to infer mediation, rather than formally testing mediation models.
Here, we expand on these existing studies using unique data from the longitudinal Vietnam Era Twin Study of Aging (VETSA) that enables us to address some key gaps in the literature (Kremen, Franz, & Lyons, 2013). The VETSA includes an assessment of general cognitive ability (GCA) when the participants were inducted into the military (aged 20, on average), as well as in-depth cognitive assessments at age 62 (range 57–67). In addition, measures of childhood and adult SES were obtained 6 years prior to the age 62 cognitive assessments. We further evaluated the effects of adult engagement in CLA which is known to be associated with adult SES and cognitive function in later life and may be a pathway through which cSES is associated with later cognitive function. We performed multiple mediation models to formally examine the extent to which effects of cSES are directly or indirectly associated with cognitive outcomes in late midlife (Hayes, 2013). When multiple mediation models are used with longitudinal data, pathways among the variables can be more clearly discerned.
We predicted that the direct influence of cSES on multiple domains of late midlife cognition would be completely mediated by paths through age 20 cognitive ability, adult SES, and CLA. Because midlife is an important transitional period that is understudied in longitudinal designs (Finch & Shams, 2016), a better understanding of cognition prior to old age may provide insights into modifiable risk or protective factors against later cognitive decline.
Research Design and Methods
Participants
Participants were 1,009 men in the VETSA project. VETSA is an ongoing longitudinal study of genetic and environmental risk and protective factors for cognitive aging (Kremen et al., 2013). VETSA 1 participants were recruited from the Vietnam Era Twin Registry (VETR); VETR members are representative of all twins who served in the military during the Vietnam era, between 1965 and 1975 (Eisen, True, Goldberg, Henderson, & Robinette, 1987). Eligibility for VETSA 1 (2002–2008) included two criteria: participants had to be between ages 51–59 years at enrollment, and both members of a twin pair had agreed to participate. VETSA 2 (2009–2014) followed-up these participants 6 years later. Mean age was 56 years (SD = 2.48, range 51–61) in VETSA 1 and 62 years in VETSA 2 (SD = 2.43, range 57–67) (Kremen et al., 2013). Hereafter, these time-points are referred to as age 56 and 62.
The sample is similar in health and lifestyle characteristics to American men in their age range based on National Health Interview Survey data (Schoenborn & Heyman, 2009). Although this is a twin study, these analyses are non-twin. Nearly 80% of the sample reported no combat exposure and two-thirds of the sample did not serve in a war-zone. Of the 1,237 participants in VETSA 1,228 (18%) did not participate in VETSA 2, with the most common single reason for not participating being death (N = 43; 19% of non-returners) or being a brother of a deceased twin. Given that the study involved two to three nights travel to either University of California San Diego or Boston University for in-depth testing, this retention rate in a longitudinal study is excellent. The study was approved by the University of California San Diego and Boston University Institutional Review Boards. Written informed consent was obtained from all participants.
The sample comprises 1,009 individuals who participated in both VETSA 1 and 2; final mediation analyses were conducted on the approximately 971 participants who had complete data, as this is a requirement of multiple mediation analyses. This large longitudinal sample has more than adequate power to test the mediation models (Kremen et al., 2013). We have >80% power to detect small associations between variables (e.g., r =0.09 [n = 1,000] to r =0.12 [n = 600]).
Measures
Socioeconomic status
At VETSA 1, participants reported their own highest-attained lifetime occupation and education, as well as the highest occupation and education held by their parents/stepparents during their childhood (<18 years). Occupation and education were coded using the Hollingshead and Redlich scales (Hollingshead, 1975). Occupation was coded on a 0–9 scale (0 = homemaker/unemployed/retired; 1 = unskilled laborer; 9 = major professionals); major professionals included lawyers, engineers, professors. Education was coded on a 1–7 scale (1 = none to seventh grade; 7 = graduate professional training). An SES composite score was calculated as: (occupation score × 5) + (education score x 3) (Hollingshead, 1975); cSES was the average of parent’s SES if the mother was employed, or just father SES if the mother was a homemaker. Adult SES is that of the participant by age 56. This scoring has been shown to be a reliable and valid indicator of SES (Cirino et al., 2002).
General cognitive ability (GCA)
Participants took the same version of the Armed Forces Qualification Test (AFQT), a 100-item multiple-choice test of GCA, when inducted into the military at approximately age 20 and again as part of the study at age 62 (Uhlaner & Bolanovich, 1952). This test is a reliable and valid measure of GCA (Uhlaner & Bolanovich, 1952) and is highly correlated with other tests of GCA, such as Wechsler Adult Intelligence Scale (r = 0.84) (Lyons et al., 2009). Age 20 AFQT scores were obtained from military records by the VETR.
Dimensions of cognitive ability
Specific cognitive abilities were evaluated using 13 neuropsychological tests at age 62 (Kremen et al., 2014). When there were multiple measures of an ability, a composite score was created by standardizing measures and taking the average. These measures have been described in detail elsewhere (Franz et al., 2011). In brief, abstract reasoning was measured using the Wechsler Abbreviated Scale of Intelligence Matrix Reasoning subtest (Wechsler, 1997a). Verbal fluency/language combined scores from Delis–Kaplan Executive Function System (D-KEFS) Verbal Fluency letter (F-A-S) and Category Fluency (animals, boys names) conditions (Delis, Kaplan, & Kramer, 2001). Visual-spatial ability was measured using Card Rotations (Ekstrom, French, & Harmon, 1976) and Hidden Figures (Thurstone, 1944).
Processing speed was assessed with two tests: Stroop word and color conditions (Golden & Freshwater, 1978) and D-KEFS Trails number sequencing and letter sequencing (Trails conditions 2 and 3) (Delis et al., 2001). Stroop processing speed scores represented the number of items read correctly in 45 s. D-KEFS Trails tests are timed, scores were reversed so that high scores represent better performance. Episodic memory was measured with three tests: California Verbal Learning Test-II short-delay recall, long-delay recall, and total trials 1–5 scores (Delis, Kramer, Kaplan, & Ober, 2000), as well as the Wechsler Memory Scale (WMS-III) Logical Memory and Visual Reproduction subtests (Wechsler, 1997b). Working memory was measured using Reading Span (Daneman & Carpenter, 1980) and WMS-III Digit Span, Spatial Span, and Letter-Number Sequencing tests (Wechsler, 1997b). Digit and spatial span tests included both forward and backward conditions.
The executive function of inhibition was measured using the Stroop color/word interference condition adjusted for performance on the separate color and word conditions using regression (Golden & Shawna, 1978). The executive function of switching included two measures: D-KEFS Trails condition 4 (letter-number switching) adjusted for speed on the number and letter conditions (Trails conditions 2 and 3 respectively) using regression, and the Verbal Fluency category switching condition (fruit/furniture) adjusted for scores on category fluency using regression).
Cognitive leisure activity (CLA)
A composite CLA score at age 56 was created using total points earned on the 11 CLA items from Schaie’s life complexity inventory (Schaie, 2005). Items included: 1–4) time spent engaged in self-improvement activities/educational activities/cultural activities/reading in the past month; 5–6) number of books/magazines read in the past month, and 7–11) taking vocational training courses, adult education courses, extended university courses, correspondence courses, and/or on the job training in the previous 10 years. Rather than use a factor analysis derived score we created a CLA index. For items 1–6, a point was assigned to participants who engaged in the particular activity more often than the sample mean. For items 7–11, a point was assigned for each type of educational activity. The Cronbach’s Alpha for this index was 0.72.
Covariates
Multiple mediation model covariates included self-reported race/ethnicity (non-Hispanic white versus other), and age at VETSA 2. Although our literature review suggested that APOE genotype, physical activity, and health might comprise pathways between cSES and midlife cognitive functioning, measures of these constructs were not associated with cSES or cognitive outcome, and were trimmed from the multiple mediation analyses (see Supplementary Table 2).
Data Analyses
Multiple mediation analyses
Three criteria need to be satisfied to indicate a mediation relationship (Baron & Kenny, 1986): (1) The predictor variable needs to significantly predict the outcome variable, (2) the predictor variable must significantly predict the mediator variable(s), and (3) the mediator variable(s) must significantly predict the outcome variable while controlling for the predictor variable. If both direct and indirect effects remain significant, the association is said to be partially mediated (Hayes, 2013).
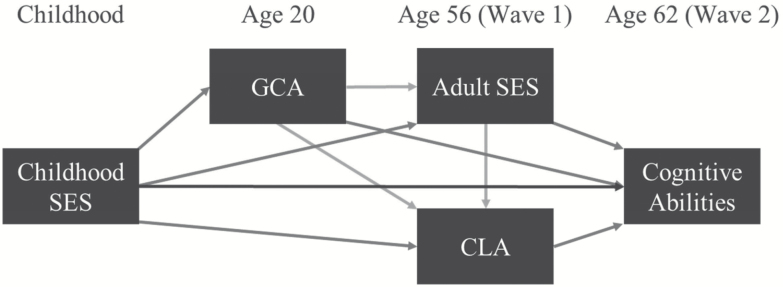
Multiple mediation analyses were based on 1000 bootstrapped samples using Hayes’ PROCESS Macro v2.15 modified for R (Hayes, 2013) allowing for formal tests of the total, direct, and indirect effects of cSES on age 62 cognitive abilities. The predictor variable was cSES, the three mediator variables were GCA at age 20, and adult SES and CLA at age 56; outcomes were cognitive abilities at age 62 (Figure 1). Because measurement of adult SES (highest attained education and occupation in lifetime) was considered temporally prior to CLA (activities in past month, past 10 years), it was regarded as antecedent to CLA in the models. Multiple mediation analyses estimated the total, direct and indirect effects of cSES in all eight (1 direct; 7 indirect) paths reflected in the model shown in Figure 1. Age and ethnicity were included as covariates in the multiple mediation models. Models also included a family identifier as a random effect to account for the non-independence of the twins. All measures were standardized with a mean of zero and standard deviation of one prior to analyses. Results are considered significant at p < .05, two-tailed.
Figure 1.
Illustration of direct and indirect effect paths in each multiple mediation model; variables arranged temporally. The direct effect path leads from childhood SES (socioeconomic status) to cognitive ability, and the seven indirect effect paths lead: (a) through GCA (general cognitive ability); (b) through adult SES; (c) through CLA (cognitive leisure activities); (d) through GCA and adult SES; (e) through GCA and CLA; (f) through adult SES and CLA; and (g) through GCA, adult SES, and CLA. Cognitive abilities tested include GCA and the six specific cognitive ability measures (abstract reasoning, episodic memory, processing speed, verbal fluency, working memory, and visual-spatial ability).
Calculation of overall effect sizes
Mediation analyses provide specific information about the longitudinal pathways through which cSES is associated with late mid-life cognitive abilities by parsing the total effect of cSES on cognitive outcomes into direct and indirect effects. What those analyses do not reveal is the overall combined influences of these measures on later cognitive ability and calculation of an overall effect size. In order to provide information on the total effect of the combined measures, we conducted post hoc generalized linear model analyses in SAS 9.4 (PROC GENMOD) to determine the contribution of cSES, age 20 GCA, adult SES, and CLA to age 62 cognitive abilities (see Supplementary Table 3). We included all covariates considered earlier: age, ethnicity, health, physical activity, and APOE genotype. Health was the total number of major chronic health conditions based on items from the Charlson Comorbity Index (Charlson, Szatrowski, Peterson, & Gold, 1994). Physical activity was the mean of two items reflecting the frequency of engagement in physical fitness and walking/hiking from the Schaie life complexity inventory (Schaie, 2005). APOE genotyping was conducted on blood samples collected in VETSA 1 by established methods (Lyons et al., 2009). Presence of any ε4 allele was coded as being ε4+ (29%) versus no ε4 alleles; presence of the APOE-ε4 allele is a major risk factor for cognitive decline/Alzheimer’s Disease (AD) in later life (Forero et al., 2016). In addition, we post-hoc calculated the contribution of cSES to age 20 GCA, adjusting for APOE genotype and ethnicity.
These analyses accounted for non-independence of the twin data by running linear regression analyses (PROC REG) on ‘A’ and ‘B’ twins separately, then calculating the proportion of residual sum of squares (SSR) to total sum of squares to find the multiple R2 for each outcome. Effect size, as indicated by Cohen’s f2, was calculated by dividing the resulting multiple R2 value by the variance of the outcome variable unexplained by all the measures in the model
Results
Demographics and Preliminary Analyses
Participants were mostly non-Hispanic white (90%); 78% were currently married; and 97% graduated high school. On average, participants had 13.88 years of formal education (SD = 2.09), an annual income of $57,500 (SD = $30,400), and were a small business owner or manager. Fathers’ average education was 10.63 years, and worked as a skilled manual worker; 52% graduated from high school. Mothers’ average education was 11.33 years, and tended to be homemakers (50%) or semi-skilled workers; 67% graduated high school. Demographic characteristics are presented in Supplementary Table 1.
In preliminary analyses, cSES, age 20 GCA, CLA, adult SES, age 62 GCA, and cognitive abilities at age 62 (with the exception of executive function measures) were all positively intercorrelated (ps < .001; see Supplementary Table 2) meeting the basic assumptions for conducting mediation analysis. Men from higher SES backgrounds were more likely to engage in CLAs as adults (β = 0.19, 95% CI [0.118, 0.255]), even when adjusted for age 20 GCA (β = 0.17, 95% CI [0.098, 0.237]). There was no main effect of APOE genotype or interaction between cSES and APOE status on age 62 cognitive outcomes indicating that APOE genotype did not moderate these associations. Given the lack of correlations with cSES and cognitive outcomes, physical activity, health, and APOE were trimmed from multiple mediation models.
Multiple Mediation Models
Results for the multiple mediation models are shown in Table 1. Total effects (direct plus indirect) were significant for age 62 GCA and all six specific cognitive ability outcomes tested. Only the direct effect of cSES for abstract reasoning remained significant along with a significant total indirect effect, indicating partial mediation. As shown by the significant total indirect effects and the non-significant direct effects (Table 1), associations between cSES and age 62 GCA, episodic memory, processing speed, verbal fluency, working memory, and visual-spatial ability outcomes were fully mediated by age 20 GCA, adult SES, and CLA engagement. The total (direct plus indirect) effects of cSES were strongest for Abstract Reasoning (0.168) and Visual-Spatial Ability (0.137).
Table 1.
Multiple Mediation Models: Total, Direct, and Indirect Effects from Childhood SES to Cognitive Outcomes at Age 62
| Effect | Age 62 GCA | Abstract Reasoning | Episodic Memory | Processing Speed |
Verbal Fluency |
Working Memory | Visual-Spatial Ability |
|---|---|---|---|---|---|---|---|
| Total effect: direct + indirect |
0.082
[0.046, 0.129] |
0.168
[0.112, 0.230] |
0.068
[0.026, 0.117] |
0.087
[0.044, 0.136] |
0.079
[0.024, 0.137] |
0.078
[0.036, 0.118] |
0.137
[0.082, 0.195] |
| Direct effect | 0.006 [−0.023, 0.046] |
0.071
[0.020, 0.140] |
0.004 [−0.040, 0.056] |
0.029 [−0.017, 0.087] |
0.001 [−0.054, 0.065] |
0.021 [−0.016, 0.064] |
0.046 [−0.005, 0.110] |
| Total indirect effect |
0.074
[0.044, 0.103] |
0.094
[0.051, 0.122] |
0.064
[0.026, 0.117] |
0.060
[0.032, 0.085] |
0.074
[0.044, 0.099] |
0.056
[0.032, 0.077] |
0.090
[0.053, 0.121] |
Note: Path estimates (β) provided for each. (95% confidence interval presented in brackets; Paths significantly different from 0 are bolded; SES = socioeconomic status; GCA = general cognitive ability; CLA = cognitive leisure activities. Age and ethnicity are included as covariates and family is included as a random effect.
Table 2 a–g shows results for each of the indirect paths tested by the multiple mediation models. The indirect effect of cSES through age 20 GCA (path a) was significant for all cognitive outcomes, accounting for 84% of the total indirect effect on age 62 GCA; 55% for abstract reasoning; 47% for episodic memory; 38% for processing speed; 24% for verbal fluency; 55% for working memory; and 56% for visual-spatial ability. The remaining paths through adult SES and CLA comprised less of the total indirect effect for each model, ranging from 9 to 41% for paths including adult SES, and from 2 to 15% for paths including CLA.
Table 2.
Multiple Mediation Models: Indirect Path Effects from Childhood SES to Cognitive Outcomes at Age 62
| Effect | Age 62 GCA | Abstract Reasoning | Episodic Memory | Processing Speed |
Verbal Fluency |
Working Memory | Visual-Spatial Ability |
|---|---|---|---|---|---|---|---|
| (a) Through age 20 GCA |
0.062
[0.036, 0.089] |
0.052
[0.027, 0.077] |
0.030
[0.016, 0.049] |
0.023
[0.010, 0.039] |
0.018
[0.006, 0.036] |
0.031
[0.016, 0.048] |
0.057
[0.030, 0.085] |
| (b) Through SES | 0.007 [−0.006, 0.018] |
0.028 [−0.001, 0.047] |
0.023
[0.002, 0.043] |
0.022 [−0.000, 0.041] |
0.030
[0.008, 0.048] |
0.023
[0.008, 0.039] |
0.017 [−0.004, 0.037] |
| (c) Through CLA | 0.002 [−0.002, 0.007] |
0.005 [−0.003, 0.013] |
0.004 [−0.003, 0.013] |
0.006 [−0.000, 0.017] |
0.011
[0.002, 0.023] |
−0.001 [−0.006, 0.005] |
0.007
[0.000, 0.017] |
| (d) Through age 20 GCA and SES | 0.001 [−0.001, 0.003] |
0.004 [−0.000, 0.007] |
0.003
[0.000, 0.006] |
0.003 [−0.000, 0.006] |
0.004
[0.001, 0.007] |
0.003
[0.001, 0.005] |
0.002 [−0.001, 0.005] |
| (e) Through age 20 GCA and CLA | 0.000 [−0.000, 0.001] |
0.001 [−0.001, 0.002] |
0.001 [−0.000, 0.002] |
0.001 [−0.000, 0.003] |
0.002
[0.000, 0.004] |
−0.000 [−0.001, 0.001] |
0.001 [−0.000, 0.003] |
| (f) Through SES and CLA | 0.002 [−0.001, 0.005] |
0.004 [−0.002, 0.009] |
0.003 [−0.002, 0.009] |
0.005 [−0.000, 0.011] |
0.009
[0.003, 0.016] |
−0.001 [−0.005, 0.003] |
0.006
[0.001, 0.012] |
| (g) Through age 20 GCA, SES, and CLA | 0.000 [−0.000, 0.001] |
0.001 [−0.000, 0.001] |
0.000 [−0.000, 0.001] |
0.001 [−0.000, 0.001] |
0.001
[0.000, 0.002] |
−0.000 [−0.001, 0.000] |
0.001
[0.000, 0.002] |
Note: 95% confidence interval presented in brackets; paths significantly different from 0 are bolded; CLA = cognitive leisure activities; GCA = general cognitive ability; SES = socioeconomic status. Age and ethnicity are included as covariates and family as a random effect. Seven separate indirect paths include: (a) through age 20 GCA; (b) through adult SES; (c) through CLA; (d) age 20 GCA and adult SES; (e) through age 20 GCA and CLA; (f) through adult SES and CLA; and (g) through age 20 GCA, adult SES, and CLA.
The multiple mediation models allow us to examine the contribution of each of the indirect paths to each midlife cognitive outcome (Table 2). The direct path from cSES was only significant for one cognitive outcome—abstract reasoning. Furthermore, only one indirect path to abstract reasoning—the path through age 20 GCA—significantly accounted for the remaining effect. The total effect for the remaining age 62 cognitive outcomes was largely due to indirect paths. For age 62 GCA and processing speed, only the indirect path through age 20 GCA was significant. For episodic memory, the pathway of cSES influence was primarily through two indirect paths—one through age 20 GCA and one through adult SES. Verbal fluency showed the most complex pattern of indirect effects, with multiple indirect paths reaching significance: cSES worked indirectly through age 20 GCA pathways, adult SES pathways, CLA pathways, and through the combined paths including age 20 GCA and adult SES; age 20 GCA and CLA; adult SES and CLA; and age 20 GCA, adult SES, and CLA (Table 2; paths a–g). cSES influenced working memory primarily through three indirect paths: paths through age 20 GCA, through adult SES; and through age 20 GCA to adult SES; (Table 2; paths a, b, d). Finally, visual-spatial ability was associated with cSES primarily through the age 20 GCA pathway, the CLA pathway, the adult SES and CLA pathway, and the age 20 GCA, adult SES, and CLA pathway (Table 2; paths a, c, f, g). These indirect paths highlight the fact that the role of SES is highly attenuated by age 20 GCA.
Estimates of Overall Effect Sizes
As described in the methods, we examined post hoc the association of cSES and age 20 GCA using generalized linear models. Both cSES and ethnicity were significantly associated with age 20 GCA. Men with higher GCA scores in young adulthood were more likely to come from higher SES families (β = 0.09, 95% CI [0.040, 0.146]) and were of non-Hispanic white ethnicity (β = 0.15, 95% CI [−0.199, −0.104]). Although the multiple R2 (0.10) was significant in this model, the effect size was small (Cohen’s f2 = 0.11) according to Cohen’s guidelines (J. Cohen, 1988).
Finally, in Supplementary Table 3 are the regression results for overall effect sizes for each cognitive outcome. Effect sizes for age 62 GCA and visual-spatial ability were large (Cohen’s f2 = 1.25 and 0.49, respectively). Effect sizes for abstract reasoning, episodic memory, processing speed, and working memory were in the moderate range (Cohen’s f2 = 0.35, 0.26, 0.24, 0.29, respectively). Verbal fluency had lowest overall effect size (Cohen’s f2 = 0.15), signifying a small but significant effect size. These effect sizes, and modest multiple R2 indicate that there are multiple sources of covariance as yet unidentified in predicting midlife cognition.
Discussion and Implications
Prior research finds that cSES contributes to an individual’s later cognitive abilities, but these relationships are seldom explored from a life-course perspective. We examined associations between cSES and multiple cognitive outcomes in late midlife. Childhood SES predicted both GCA as well as specific cognitive abilities in late middle age, with these effects largely working through the mediator of age 20 GCA. Much of neo-materialist research has focused on the role of adult SES in attenuating the effect of cSES on adult outcomes and on accumulated inequities (S. Cohen et al., 2010; Gonzalez et al., 2013; Horvat et al., 2014; Luo & Waite, 2005; Lyu & Burr, 2016) but has not examined early cognitive ability as mediator. Thus, consistent with evidence for the stability of cognitive function throughout most of the lifespan, cognitive functioning later in life is largely driven by early adult cognitive functioning (Deary, Whalley, Lemmon, Crawford, & Starr, 2000; Lyons et al., 2017; Lyons et al., 2009). Here we were able to show both direct and indirect influences of cSES on late midlife GCA, across more than four decades. This study is unique because of the variety of resource and cognitive measures collected longitudinally in the same sample that allowed us to formally test the pathways through which cSES mediates cognitive outcomes (Hackman et al., 2010).
Parental SES was correlated with engagement in CLAs, such that coming from a background with higher education and occupation was associated with increased likelihood of engaging in cognitively stimulating activities as an adult. Consistent with studies of older adults, SES, and age 20 cognitive ability were also associated with higher engagement in CLAs (Hackman et al., 2010; Jefferson et al., 2011; Silber et al., 2015; Vemuri et al., 2014). In five of seven mediation models, however, pathways including engagement in CLAs were not significant when the other mediators (age 20 GCA and adult SES) were included in the model. Similarly, significant influences of adult SES on later cognitive function were largely attenuated after accounting for age 20 GCA. For two abilities, however, pathways including engagement in CLA’s appeared to improve performance on later verbal fluency and visual-spatial ability tasks independently of the pathway through age 20 GCA thus demonstrating a potential area of intervention. We did not have a measure of childhood CLA. We do not know what accounts for the fact that the pattern of significant paths differed for different cognitive abilities. This might be a topic for future research.
Our results are also important for understanding cognitive reserve, a concept that is widely used in context of aging (Barulli & Stern, 2013). That is, engagement in CLAs is often thought to help maintain or enhance cognitive resources which would offset some age-related cognitive decline (Hughes, 2010). In contrast to most studies showing a positive association between CLAs and later-life cognition (Jefferson et al., 2011; Vemuri et al., 2014), we were able to include young adult GCA in our analyses. By doing so, we extended previous work by being able to clarify the cause and effect issue. Despite some support for a very small effect of paths including CLAs on verbal fluency and visual-spatial abilities, our results suggest that compared to CLAs, the level of GCA that one attains by early adulthood has a stronger influence on late middle age cognition. In our view, a direct measure of GCA such as the one used in the present study provides the best index of one’s cognitive resources. Thus, it highlights the importance of early GCA with respect to one’s cognitive reserve level. Although the association between engagement in CLAs, better cognition and reduced risk for dementia may seem to suggest that we should encourage greater engagement in such activities, our results are most consistent with reverse causation, i.e. that individuals with higher intellectual ability tend to more frequently engage in CLAs. Therefore, much further study is needed to determine whether and what type of engagement in CLAs might truly improve later adult cognitive outcomes.
Although there were no main or moderation effects of APOE-ε4 status that does not rule out other genetic influences. A meta-analysis found that genetic influences on cognitive abilities account for higher proportions of variance with age and stabilizes by early adulthood, suggesting a strong genetic basis for cognition in adults with heritability of adult cognitive ability estimated to range from 50 to 70% (Briley & Tucker-Drob, 2013). It has also been vigorously argued that the effects of parents on child intelligence are best explained by gene by environment correlations (Scarr & McCartney, 1983). We did not, however, have data on parental cognitive ability, which would have increased precision over parental education. A previous study by our group in a different sample of twins, for instance, found that parental education moderated the heritability of adult reading ability such that genetic influences on reading ability were lowest at the lowest parental education level and highest at the highest level of education (Kremen et al., 2005). Another study found similar gene by environment interplay, where heritability of IQ in children was highest in higher SES families, and close to zero in lower SES families (Turkheimer, Haley, Waldron, D’Onofrio, & Gottesman, 2003). Thus, even the small direct associations between cSES and later cognitive outcomes cannot be considered causal (Ericsson et al., 2017). In addition, gene by environment interplay may vary by other environmental influences such as culture (Tucker-Drob & Bates, 2016). However, the significant effect of cSES on age 20 cognitive ability, which likely reflects gene by environment interplay, emphasizes the importance of early influences and early interventions.
There are a variety of direct and indirect mechanisms by which adverse childhood SES can impair adult cognitive outcome while good childhood SES can promote healthy cognitive outcomes. One possible underlying mechanism may be an enduring sensitization of stress pathways. Recent research on the molecular biology underlying stress shows that stress mediators such as corticotropin releasing factor (CRF) regulate and exacerbate Alzheimer’s disease and related dementia relevant processes, potentially through activation of the CRFR1 receptor (Futch, Croft, Truong, Krause, & Golde, 2017; McEwen et al., 2016). CRF is widely expressed throughout the brain with broad implications for its effect on multiple cognitive functions. Chronic stress appears to be a risk factor for Alzheimer’s Disease and related dementias and recent research shows strong links between the CRF signaling pathway, beta amyloid production and plaque deposition, and tau pathology in animal models (Futch et al., 2017). Thus, research on overactive CRF signaling may provide insight into the biological link between stress, neurodegeneration, cognition, and potential risk for Alzheimer’s disease as well as a possible target for interventions.
The current investigation has several limitations. The sample included only men who had been in the US military during the Vietnam Era (1965–1975), and was predominantly Caucasian, so it is unclear if results are generalizable to women, minorities, or other older adult populations. As with virtually all studies of midlife and older adults, our measure of cSES was retrospective and might, therefore be subject to recall bias. However, there are several attributes of this sample that give us confidence that our results are applicable to larger populations. Demographic and health characteristics of this sample in midlife were similar to men in the same age group based on 2003 census and Centers for Disease Control and Prevention data (Boehmer, Flanders, McGeehin, Boyle, & Barrett, 2004; Schoenborn & Heyman, 2009). Midlife disease-related death rates from disease-related conditions have been found to be no different in Vietnam era veterans and other men their age (Boehmer et al., 2004). These studies have also shown that differences in socioeconomic status between veterans and non-veterans are modest. It may be that the lack of association with health was due to the fact that at the age 20 assessment participants were less likely to have major childhood diseases since health is part of military screening. Thus this sample was healthy at the age 20 cognitive assessment. This can also be considered a strength of the study since the majority of longitudinal studies on cSES and adult outcomes do not have information on childhood health. The sample is also relatively young (age 62) with few chronic conditions. The effects of health may be stronger at older ages.
We were unable to illuminate the role of other childhood characteristics on later outcomes due to lack of detailed information about participants’ childhood environments. Such influences could vary from parenting style and family activities, income, to household characteristics such as crowding or lack of resources (Hackman et al., 2010), health care that may have affected pregnancy or childhood illness, or exposure to environmental hazards such as pollution or parental smoking (Bradley & Corwyn, 2002). Participants’ self-reported on family-of-origin environment at age 56; however, none of the parenting styles were found to be significantly associated with cognitive function (data not shown). Under normal circumstances, parenting is likely to have low impact on intellectual outcomes of offspring as long as the familial environment is adequate (Scarr & McCartney, 1983). In this sample of blue-collar to upper middle class parents, it is likely that the range of cSES included few parents at extreme levels of poverty or adversity.
In summary, the effect of cSES on late midlife cognitive performance is relatively small but significant, and works primarily through its indirect association with early adult GCA and, to a much lesser extent, midlife SES and engagement in CLAs. The results have implications for theories of cognitive development and cognitive reserve across the life course; results suggest that, at least in late middle age, early adult GCA is a much stronger reserve factor compared with adult SES or engagement in CLAs. Also, the effects of parental SES on cognitive functioning are long lasting. Thus, the study has important implications for understanding the early origins of cognitive functioning and cognitive aging, and, potentially, cognitive decline. Because the pathological process in Alzheimer’s disease begins decades before the onset of clinical dementia (Golde, Schneider, & Koo, 2011; Sperling et al., 2011), understanding midlife cognition is also important for potentially improving early intervention or risk prediction.
Supplementary Material
Supplementary data are available at Innovation in Aging online.
Funding
This work was supported by grants from the National Institute on Aging at the National Institutes of Health (R01 AG 050595, R01 AG018386, R01 AG022381, R01 AG022982 to W.S.K., R01 AG018384 to M.J.L., and the R25 AG 043364 training grant to Dr. Cronan at SDSU supporting Mr. Beck).
Acknowledgements
Content of this manuscript is the responsibility of the authors and does not represent official views of NIH, or the Veterans’ Administration. U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University provided invaluable assistance in the conduct of the VET Registry. Mr. Beck was supported by the Advancing Diversity in Aging Research (ADAR) program at San Diego State University, for which Drs. Franz and Kremen are mentors. The authors gratefully acknowledge the continued cooperation of the twins and the efforts of many staff members.
References
- Baron R. M. & Kenny D. A (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. doi:10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Barulli D. & Stern Y (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17, 502–509. doi:10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R. H. & Corwyn R. F (2002). Socioeconomic status and child development. Annual Review of Psychology, 53, 371–399. doi:10.1146/annurev.psych.53.100901.135233 [DOI] [PubMed] [Google Scholar]
- Briley D. A. & Tucker-Drob E. M (2013). Explaining the increasing heritability of cognitive ability across development: A meta-analysis of longitudinal twin and adoption studies. Psychological Science, 24, 1704–1713. doi:10.1177/0956797613478618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer T. K., Flanders W. D., McGeehin M. A., Boyle C. A., & Barrett D. H (2004). Postservice mortality among Vietnam veterans: 30-year follow-up. Archives of Internal Medicine, 164, 1908–1916. doi:10.1001/archinte.164.17.1908 [DOI] [PubMed] [Google Scholar]
- Charlson M., Szatrowski T. P., Peterson J., & Gold J (1994). Validation of a combined comorbidity index. Journal of Clinical Epidemiology, 47, 1245–1251. doi:10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- Cirino P. T. Chin C. E. Sevcik R. A. Wolf M. Lovett M. & Morris R. D (2002). Measuring socioeconomic status: Reliability and preliminary validity for different approaches. Assessment, 9, 145–155. doi:10.1177/10791102009002005 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; doi:10.1016/B978-0-12-179060-8.50012-8. [Google Scholar]
- Cohen S. Janicki-Deverts D. Chen E. & Matthews K. A (2010). Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences, 1186, 37–55. doi:10.1111/j.1749-6632.2009.05334.x [DOI] [PubMed] [Google Scholar]
- Conger R. D. Conger K. J. & Martin M. J (2010). Socioeconomic status, family processes, and individual development. Journal of Marriage and the Family, 72, 685–704. doi:10.1111/j.1741-3737.2010.00725.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M., & Carpenter P. A (1980). Individual-differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior, 19, 450–466. doi:10.1016/S0022-5371(80)90312–6 [Google Scholar]
- Deary I. J., Whalley L. J., Lemmon H., Crawford J. R., & Starr J. M (2000). The stability of individual differences in mental ability from childhood to old age: Follow-up of the 1932 Scottish mental survey. Intelligence, 28, 49–55. doi:10.1016/S0160-2896(99)00031-8 [Google Scholar]
- Delis D. C., Kaplan E., & Kramer J. H (2001). Delis-Kaplan executive function system (D-KEFS). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., & Ober B. A (2000). California verbal learning test. (2nd ed). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Dunn J. R. Veenstra G. & Ross N (2006). Psychosocial and neo-material dimensions of ses and health revisited: Predictors of self-rated health in a canadian national survey. Social Science & Medicine, 62, 1465–1473. doi:10.1016/j.socscimed.2005.07.038 [DOI] [PubMed] [Google Scholar]
- Eisen S. True W. Goldberg J. Henderson W. & Robinette C. D (1987). The vietnam era twin (vet) registry: Method of construction. Acta Geneticae Medicae Et Gemellologiae, 36, 61–66. doi:10.1017/S0001566000004591 [DOI] [PubMed] [Google Scholar]
- Ekstrom R. B., French J. W., & Harmon H. H (1976). Kit of factor-referenced tests (manual). Princeton, NJ: Educational Testing Service. [Google Scholar]
- Ericsson M. Lundholm C. Fors S. Dahl Aslan A. K. Zavala C. Reynolds C. A. & Pedersen N. L (2017). Childhood social class and cognitive aging in the Swedish adoption/twin study of aging. Proceedings of the National Academy of Sciences of the United States of America, 114, 7001–7006. doi:10.1073/pnas.1620603114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. W. (2004). The environment of childhood poverty. The American Psychologist, 59, 77–92. doi:10.1037/ 0003-066X.59.2.77 [DOI] [PubMed] [Google Scholar]
- Finch C. E. & Shams S (2016). Apolipoprotein e and sex bias in cerebrovascular aging of men and mice. Trends in Neurosciences, 39, 625–637. doi:10.1016/j.tins.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero D. A., Lopez-Leon S., Gonzalez-Giraldo Y., Dries D. R., Pereira-Morales A. J., Jimenez K. M., & Franco-Restrepo J. E (2016). APOE gene and neuropsychiatric disorders and endophenotypes: A comprehensive review. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 9999, 1–17. doi:10.1002/ajmg.b.32516 [DOI] [PubMed] [Google Scholar]
- Franz C. E., O’Brien R. C., Hauger R. L., Mendoza S. P., Panizzon M. S., Prom-Wormley E.,…, Kremen W. S. (2011). Cross-sectional and 35-year longitudinal assessment of salivary cortisol and cognitive functioning: The Vietnam era twin study of aging. Psychoneuroendocrinology, 36, 1040–1052. doi:10.1016/j.psyneuen.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C. E., Spoon K., Thompson W., Hauger R. L., Hellhammer D. H., Jacobson K. C.,…, Kremen W. S. (2013). Adult cognitive ability and socioeconomic status as mediators of the effects of childhood disadvantage on salivary cortisol in aging adults. Psychoneuroendocrinology, 38, 2127–2139. doi:10.1016/j.psyneuen.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futch H. S. Croft C. L. Truong V. Q. Krause E. G. & Golde T. E (2017). Targeting psychologic stress signaling pathways in Alzheimer’s disease. Molecular Neurodegeneration, 12, 49. doi:10.1186/s13024-017-0190-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde T. E. Schneider L. S. & Koo E. H (2011). Anti-aβ therapeutics in Alzheimer’s disease: The need for a paradigm shift. Neuron, 69, 203–213. doi:10.1016/j.neuron.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden C. J., & Freshwater S. M (1978). Stroop color and word test. Chicago: Stoelting. [Google Scholar]
- González H. M. Tarraf W. Bowen M. E. Johnson-Jennings M. D. & Fisher G. G (2013). What do parents have to do with my cognitive reserve? Life course perspectives on twelve-year cognitive decline. Neuroepidemiology, 41, 101–109. doi:10.1159/000350723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D. A. Farah M. J. & Meaney M. J (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11, 651–659. doi:10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. F. (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Hollingshead A. B. (1975). Four-factor index of social status. Unpublished manuscript, Yale University, New Haven, CT.
- Horvat P., Richards M., Malyutina S., Pajak A., Kubinova R., Tamosiunas A.,…, Bobak M. (2014). Life course socioeconomic position and mid-late life cognitive function in Eastern Europe. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 69, 470–481. doi:10.1093/geronb/gbu014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T. F. (2010). Promotion of cognitive health through cognitive activity in the aging population. Aging Health, 6, 111–121. doi:10.2217/ahe.09.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst L. Stafford M. Cooper R. Hardy R. Richards M. & Kuh D (2013). Lifetime socioeconomic inequalities in physical and cognitive aging. American Journal of Public Health, 103, 1641–1648. doi:10.2105/AJPH.2013.301240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson A. L. Gibbons L. E. Rentz D. M. Carvalho J. O. Manly J. Bennett D. A. & Jones R. N (2011). A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. Journal of the American Geriatrics Society, 59, 1403–1411. doi:10.1111/j.1532-5415.2011.03499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. R. & Pearlin L. I (2006). Financial strain over the life course and health among older adults. Journal of Health and Social Behavior, 47, 17–31. doi:10.1177/002214650604700102 [DOI] [PubMed] [Google Scholar]
- Kremen W. S., Franz C. E., & Lyons M. J (2013). VETSA: The Vietnam era twin study of aging. Twin Research and Human Genetics, 16, 399–402. doi:10.1017/thg.2012.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen W. S. Jacobson K. C. Xian H. Eisen S. A. Waterman B. Toomey R.…Lyons M. J (2005). Heritability of word recognition in middle-aged men varies as a function of parental education. Behavior Genetics, 35, 417–433. doi:10.1007/s10519-004-3876-2 [DOI] [PubMed] [Google Scholar]
- Kremen W. S., Jak A. J., Panizzon M. S., Spoon K. M., Franz C. E., Thompson W. K., …Lyons M. J (2014). Early identification and heritability of mild cognitive impairment. International Journal of Epidemiology, 43, 600–610. doi:10.1093/ije/dyt242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy R. Head J. Richards M. & Hardy R (2017). The effect of life course socioeconomic position on crystallised cognitive ability in two large UK cohort studies: A structured modelling approach. BMJ Open, 7, e014461. doi:10.1136/bmjopen-2016-014461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lareau A., & Weininger E. B (2003). Cultural capital in educational research: A critical assessment. Theory and Society, 32, 567–606. doi:10.1023/B:RYSO.0000004951.04408.b0 [Google Scholar]
- Luo Y. & Waite L. J (2005). The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60, S93–S101. doi:10.1093/geronb/60.2.S93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons M. J. Panizzon M. S. Liu W. McKenzie R. Bluestone N. J. Grant M. D.…Xian H (2017). A longitudinal twin study of general cognitive ability over four decades. Developmental Psychology, 53, 1170–1177. doi:10.1037/dev0000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons M. J. York T. P. Franz C. E. Grant M. D. Eaves L. J. Jacobson K. C.…Kremen W. S (2009). Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological Science, 20, 1146–1152. doi:10.1111/j.1467-9280.2009.02425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J. & Burr J. A (2016). Socioeconomic status across the life course and cognitive function among older adults: An examination of the latency, pathways, and accumulation hypotheses. Journal of Aging and Health, 28, 40–67. doi:10.1177/0898264315585504 [DOI] [PubMed] [Google Scholar]
- McEwen B. S. Nasca C. & Gray J. D (2016). Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41, 3–23. doi:10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose R. J. Brewster P. Marquine M. J. MacKay-Brandt A. Reed B. Farias S. T. & Mungas D (2015). Early life development in a multiethnic sample and the relation to late life cognition. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 70, 519–531. doi:10.1093/geronb/gbt126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin L. I. Schieman S. Fazio E. M. & Meersman S. C (2005). Stress, health, and the life course: Some conceptual perspectives. Journal of Health and Social Behavior, 46, 205–219. doi:10.1177/002214650504600206 [DOI] [PubMed] [Google Scholar]
- Plomin R. & Deary I. J (2015). Genetics and intelligence differences: Five special findings. Molecular Psychiatry, 20, 98–108. doi:10.1038/mp.2014.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S., & McCartney K (1983). How people make their own environments: A theory of genotype greater than environment effects. Child development, 54, 424–435. doi:10.2307/1129703 [DOI] [PubMed] [Google Scholar]
- Schaie K. W. (2005). Developmental influences on adult intelligence: The Seattle longitudinal study. New York: Oxford University Press. [Google Scholar]
- Schoenborn C. A., & Heyman K. M (2009). Health characteristics of adults aged 55 years and over: United States, 2004–2007. National Health Statistics Reports, 16, 1–31. doi:10.1037/e623972009-001 [PubMed] [Google Scholar]
- Schreiber S. Vogel J. Schwimmer H. D. Marks S. M. Schreiber F. & Jagust W (2016). Impact of lifestyle dimensions on brain pathology and cognition. Neurobiology of Aging, 40, 164–172. doi:10.1016/j.neurobiolaging.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber B. G., Triplett T., Iyengar S.,& National Endowment for the Arts. (2015). A decade of arts engagement: Findings from the survey of public participation in the arts, 2002–2012. Washington, DC: National Endowment for the Arts. [Google Scholar]
- Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., …Phelps C. H (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7, 280–292. doi:10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanton S. L. Thorpe R. J. & Whitfield K (2010). Life-course financial strain and health in african-americans. Social Science & Medicine (1982), 71, 259–265. doi:10.1016/j.socscimed.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone L. L. (1944). A factorial study of perception. Chicago: University of Chicago Press. [Google Scholar]
- Tucker-Drob E. M. & Bates T. C (2016). Large cross-national differences in gene × socioeconomic status interaction on intelligence. Psychological Science, 27, 138–149. doi:10.1177/0956797615612727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob E. M. & Harden K. P (2012). Intellectual interest mediates gene × socioeconomic status interaction on adolescent academic achievement. Child Development, 83, 743–757. doi:10.1111/j.1467-8624.2011.01721.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E. Haley A. Waldron M. D’Onofrio B. & Gottesman I. I (2003). Socioeconomic status modifies heritability of IQ in young children. Psychological Science, 14, 623–628. doi:10.1046/j.0956-7976.2003.psci_1475.x [DOI] [PubMed] [Google Scholar]
- Turrell G. Lynch J. W. Kaplan G. A. Everson S. A. Helkala E. L. Kauhanen J. & Salonen J. T (2002). Socioeconomic position across the lifecourse and cognitive function in late middle age. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57, S43–S51. doi:10.1093/geronb/57.1.s43 [DOI] [PubMed] [Google Scholar]
- Uhlaner J. E., & Bolanovich D. J (1952). Development of the Armed Forces Qualification Test and Predecessor Army Screening Tests, 1946–1950 (No. PRS-976). Washington, D.C.: Personnel Research Section, Department of the Army. Retrieved February 2, 2018 from http://www.dtic.mil/dtic/tr/fulltext/u2/000191.pdf [Google Scholar]
- Vemuri P., Lesnick T. G., Przybelski S. A., Machulda M., Knopman D. S., Mielke M. M.,…, Jack C. R., Jr (2014). Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurology, 71, 1017–1024. doi:10.1001/jamaneurol.2014.963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walpole M. (2003). Socioeconomic status and college: How SES affects college experiences and outcomes. Review of Higher Education, 27, 45–73. doi:10.1353/rhe.2003.0044 [Google Scholar]
- Wechsler D. (1997a). Manual for the Wechsler Adult Intelligence Scale - Third Edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D. (1997b). Manual for the Wechsler Memory Scale - Third Edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Zeki Al Hazzouri A., Elfassy T., Sidney S., Jacobs D., Perez Stable E. J., & Yaffe K (2017). Sustained economic hardship and cognitive function: The coronary artery risk development in young adults study. American Journal of Preventive Medicine, 52, 1–9. doi:10.1016/j.amepre.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.