Abstract
Background
Libman–Sacks endocarditis (LSE) is an infrequently recognized pathogenesis of embolic cerebrovascular disease. Patients often have asymptomatic valvular dysfunction which if not recognized promptly, can lead to serious complications such as heart failure, arrhythmias, cerebroembolic phenomena with increased neurocognitive disability, and even death. It can be associated with systemic lupus erythematosus and/or antiphospholipid antibody syndrome (APLS).
Case summary
Previously very healthy and active, 49-year-old Caucasian female with past medical history of mild lupus, for which she stopped treatment 10 year ago, saw a primary care physician complaining of intermittent double vision of 2 months duration. Urgent brain magnetic resonance imaging revealed multiple embolic infarcts of the brain stem. Further comprehensive work-up led to diagnosis of mitral LSE and APLS. After 2 months of systemic anticoagulation with warfarin and immunosuppressive therapy with hydroxychloroquine sulfate, repeat imaging demonstrated resolution of the mitral valve vegetation with no clinical recurrence of thromboembolic events at 6 months.
Discussion
Mild, often silent, autoimmune disease as described in our case can lead to significant cerebrovascular disease. Patients who present with cryptogenic strokes with high suspicion of underlying autoimmune disease should be worked up thoroughly for possible valvular heart disease associated with lupus, APLS, or both. Acquisition of transoesophageal images proved superior to transthoracic approach and it should be implemented in these subsets of patients. With this case report, we highlight the importance of early recognition of cardiac manifestations, amelioration of risk factors, as well as close follow-up of lupus or APLS patients, as crucial steps in reducing their morbidity and mortality along with preventing recurrence or progression of cerebrovascular disease.
Keywords: Non-bacterial endocarditis, Systemic lupus erythematosus, Antiphospholipid syndrome, Case report
Learning points
Libman–Sacks lesions have a tendency to cause thromboembolic events.
Cryptogenic strokes with high suspicion of underlying autoimmune disease should be worked up thoroughly for underlying valvular heart disease.
Transoesophageal images should be implemented in patients presenting with thromboembolic phenomena.
Background
Libman–Sacks endocarditis (LSE), characterized by non-infective inflammatory and/or thrombotic vegetations, may be a common and under-recognized pathogenesis of cerebral macroemboli or microemboli.1 It was first described in patients with lupus by Libman and Sacks.2 It can be associated with antiphospholipid antibody syndrome (APLS).3 Positive antiphospholipid antibodies on two or more occasions at least 12 weeks apart, along with the clinical criteria of vascular thrombosis or foetal loss during pregnancy are used for the diagnosis of APLS.4 Antiphospholipid antibody syndrome can occur as a primary disorder or secondary to an underlying disease such as lupus or other systemic autoimmune diseases.5 Between 20% and 30% of patients with SLE have persistent moderate-to-high-risk antiphospholipid-antibody profiles.6 These patients tend to have higher prevalence of thrombotic events, obstetric complications, valve disease, pulmonary hypertension, haematological and renal complications, along with moderate or severe cognitive impairment than the patients who are negative for such antibodies.6
Autoimmune disease associated valvulopathy is initially asymptomatic and often underdiagnosed.5 Patients with vegetations tend to have poor outcomes, with reduced event-free time to cerebrovascular events, cognitive disability, or death.1
Transoesophageal echocardiography (TOE), a method with higher sensitivity and specificity than transthoracic echocardiography (TTE), is the best antemortum diagnostic modality for LSE established so far.7,8 It should be implemented when there is a high suspicion for an underlying rheumatological disorder, especially in patients presenting with thromboembolic phenomena.
Timeline
| 1996 | Diagnosed with systemic lupus erythematosus Hydroxychloroquine sulfate initiated |
| 2007 | Stopped taking hydroxychloroquine sulfate |
| September 2017 | Intermittent double vision |
| November 2017 | Internal medicine office visit |
| 11 November 2017 | Urgent magnetic resonance imaging brain suggestive of multiple ischaemic strokes |
| 12 November 2017 | Hospitalization |
| 13 November 2017 | Transthoracic echocardiogram with no abnormalities. Continued to have symptoms. Autoimmune work up concerning for antiphospholipid syndrome |
| 16 November 2017 | Transoesophageal echocardiogram significant for mitral valve vegetation. Systemic anticoagulation initiated along with Hydroxychloroquine sulfate |
| 18 November 2017 | Discharged home with no neurological deficits with uneventful outpatient follow-up 2 weeks later |
| 3 January 2017 | Repeat transoesophageal echocardiography showed complete resolution of previously described mitral vegetation |
Case summary
A 49-year-old Caucasian female saw a primary care physician complaining of intermittent double vision of 2 months duration. The double vision came and went, sometimes vertical and sometimes horizontal. Each episode lasted for a few hours to several hours. She was sent to see a neurologist, who urgently sent her for a magnetic resonance imaging (MRI) of the head, which revealed multiple embolic infarcts of the brain stem. Thus, she was advised for urgent admission to a hospital. She is a previously generally healthy woman who takes non-steroidal agents for arthritis, but otherwise she takes no other regular medications and works as a child care provider at home. She is married and has two healthy children, and she denies any previous miscarriage or spontaneous abortion. She denies fever, chills, headache, night sweats, weight loss, nausea, or vomiting.
On further questioning, she stated that 21 years ago, she had inflammatory arthropathy, and was diagnosed with lupus by a rheumatologist. Hydroxychloroquine sulfate (unknown dose) treatment was implemented and it was continued for several years. However, she stopped taking it 10 years ago of her own decision as she had no symptoms other than mild chronic arthritic pain, for which she continued to take non-steroidal agents over the counter. She denies history of rash, photosensitivity, renal problems, seizures, pleurisy, pericarditis, or anaemia. She admits occasional canker sores sometimes and mild thinning of the hair.
On arrival, the patient was haemodynamically stable. Cardiovascular examination was unremarkable with an undisplaced point of maximum impulse, normal S1 and S2, no murmurs or friction rub, and normal breath sounds on auscultation. Pertinent neurological examination findings include a mild weakness in adduction of the left eye with no nystagmus. Finger-to-nose testing however, revealed mild dysmetria on past-pointing on the right side only. She also demonstrated a mild dysmetria on heel-to-shin testing on the right. Gait assessment revealed mild truncal ataxia with difficulty walking and tandem gait without assistance. Deep tendon reflexes were symmetrical 1+/5 in biceps, triceps, 1/5 ankle jerks.
Outpatient MRI, reviewed by a neurologist, was significant for multiple subacute ischaemic strokes in the brain stem area. Normal intracranial branching pattern without aneurysm or vasculitis was demonstrated on MRA. Carotid Doppler ultrasound showed no significant atherosclerotic lesions.
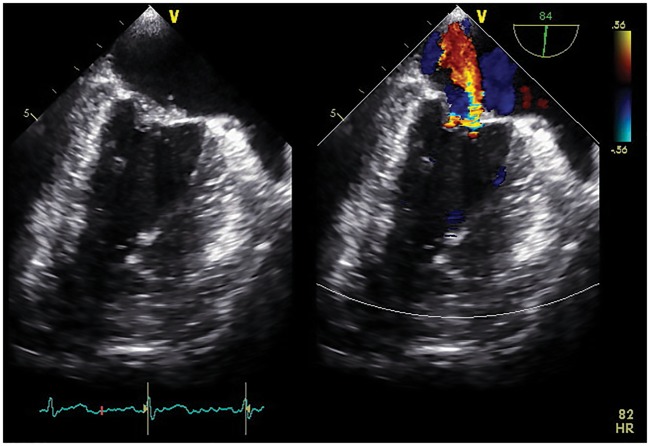
Initial transthoracic echocardiogram showed preserved ejection fraction and no significant abnormalities. However, because of cryptogenic stroke at a young age, transoesophageal echocardiogram was recommended, which demonstrated no intracardiac shunt, but it did show mildly thickened mitral valve leaflets with several fixed vegetations and moderate eccentric regurgitation (Figure 1).
Figure 1.
Initial transoesophageal two-chamber view demonstrating several small sessile, loosely organized, fixed mitral vegetations with moderate regurgitation directed eccentrically.
Inflammatory parameters were not suggestive of infection, white blood cell count 7.7 × 109/L (4.0–11.0 × 109/L), C-reactive protein 7.5 mg/dL (0.0–10.0 mg/dL) and erythrocyte sedimentation rate 64 mm/h (0.0–20.0 mm/h). Blood cultures showed no growth. C3 was normal and C4 level 14 mg/dL (16–38 mg/dL) was mildly decreased. C and S protein levels were normal. Rheumatological evaluation yielded elevated double-stranded DNA, anticardiolipin antibody IgG >112 m GPL-U/m, IgM 50.9 MPL-U/mL (0.0–19.9 MPL-U/mL), and a positive lupus anticoagulant test, elevated beta 2 glycoprotein Ab IgG> 112.0 unit/mL, IgM 34.9 unit/mL (0.0–9.9 unit/mL), high RNP of 2.4 AI (0.0–0.9 AI), and SSA of greater than 8.0 AI (0.0–0.9 AI) and normal SSB. All of the above are suggestive as significant for lupus, with secondary APLS.
We assumed a diagnosis of LSE and the patient started anticoagulation therapy (enoxaparin to warfarin bridging) along with appropriate rheumatological therapy for lupus using hydroxychloroquine 200 mg twice a day.
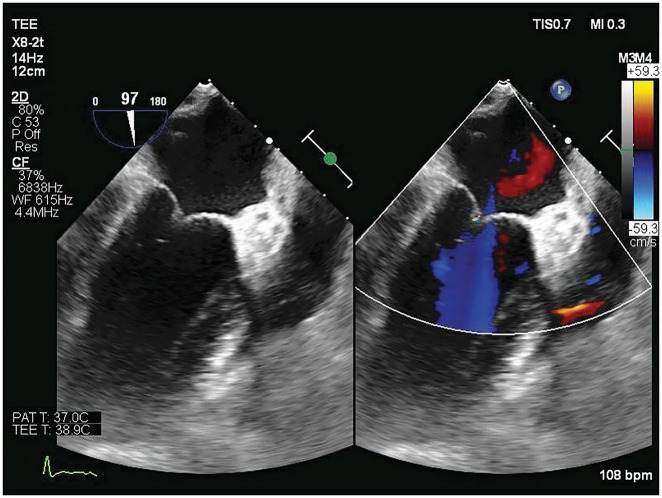
A follow-up transoesophageal echocardiogram showed complete resolution of the previously described vegetation (Figure 2). The patient has had several multidisciplinary follow-up visits since discharge with most recent one being 6 months from hospitalization with no clinical recurrence of thromboembolic events.
Figure 2.
Follow-up transoesophageal two-chamber view shows resolution of previous mitral valve vegetation and regurgitation.
Discussion
This case describes a patient with multiple brainstem strokes with what appears to be mitral LSE associated with history of mild lupus, positive anticardiolipin antibody, and positive lupus anticoagulant. Libman–Sacks vegetations develop mainly on the mitral valve and the aortic valve, but may affect any other valves, or even subvalvular apparatus. Isolated tricuspid valve endocarditis is rarely reported.9 Libman–Sacks endocarditis presentation can range from asymptomatic valvular thickening to heart failure from valvular dysfunction along with generalized symptoms of fatigue, fevers, night sweats, and weight loss. As demonstrated in our case, a stroke in ‘silent’ autoimmune disorder is certainly possible and APLS should be ruled out. Catastrophic APLS is most feared and often fatal complication. The diagnosis is challenging especially if there is no history of APLS and should be suspected in patients presenting with acute renal failure and the respiratory distress syndrome, diffuse alveolar haemorrhage, encephalopathy, and adrenal haemorrhage syndrome.10
Secondary infective endocarditis should be considered in lupus patients with acute refractory heart failure and fever of unknown origin.11
A significant proportion of patients with lupus have LSE detected in autopsy studies (30–50%). Nevertheless, the real prevalence of LSE remains unknown since most patients with Libman–Sacks vegetations have asymptomatic valve abnormalities.12 Moyssakis et al.12 described 11% incidence of LSE by TTE and an association with lupus duration, cerebrovascular events, disease activity, presence of anticardiolipin antibody, and manifestations of antiphospholipid syndrome over a 4-year period in 342 patients. Roldan et al.13 studied 69 patients with lupus by TOE and found a 43% incidence of LSE, which suggests a possibility of higher prevalence of undiagnosed LSE in patients with lupus. It is of paramount importance to integrate clinical findings with those of TOE, transcranial Doppler, and brain MRI in patients with acute, recent (within 2–4 weeks), or recurrent stroke or transient ischaemic attack (TIA); cognitive dysfunction, or seizures, focal brain abnormalities as it should lead to a prompt and accurate diagnosis and treatment of LSE.1
The pathogenesis of valve lesions in LSE involves endothelial damage by autoimmune activity on the valvular surface that leads to fibrin-platelet formation. Further deposition of immunoglobulins and complement components leads to cusp fibrosis, thickening, and scarring, and ultimately to valvular vegetation, deformation, or dysfunction. Regurgitant jet effects are more pronounced and prevalent in the left-sided heart valves. Valvular pathological conditions tend to be more severe in patients with both lupus and APLS.14
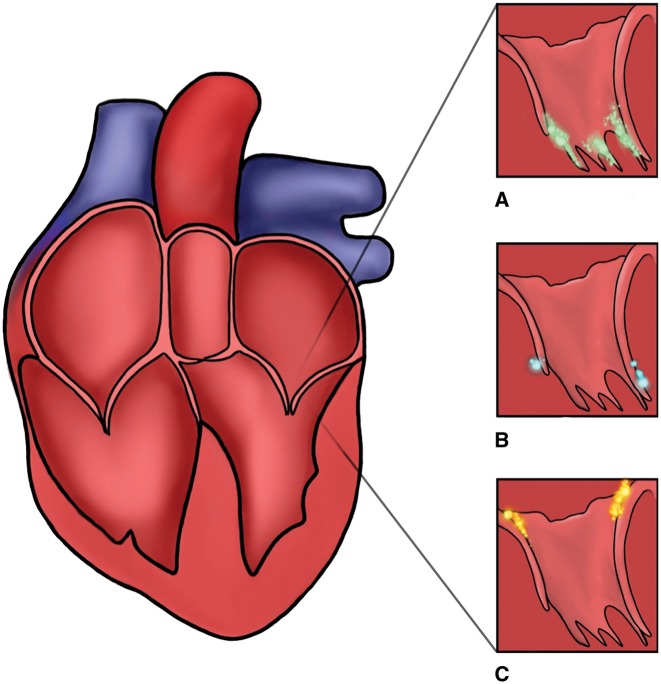
Although often challenging, it is important to distinguish between infectious and non-infectious vegetations as treatment differs significantly. Certain subtle echocardiographic findings can be utilized along with clinical/infectious correlation to achieve correct diagnosis (Figure 3). Infective endocarditis lesions involving mitral valves are often more mobile and found at the leaflet closure line.15 Cardiac masses due to papillary fibroelastomas may mimick vegetations, but they are typically located on the LV side of the valve. In contrast, LSE lesions are more echogenic and can affect any part of the leaflet, predominantly base.13
Figure 3.
(A) Mitral valve infectious vegetation most commonly located on atrial coaptation side. (B) Cardiac masses such as papillary fibroelastoma may mimic vegetation, but they are typically located on the left ventricular side of mitral valve. (C) Non-infectious mitral valve vegetations can be variable in shape and size typically smaller than infectious and can affect any part of the leaflets, but with tendency to occur more often near the leaflet base.
The incidence of thromboembolic cerebrovascular events in patients with LSE has been reported as 10–20% and a cardio embolic origin was assumed in most cases.12 Roland et al.1 presented the first fully integrated study linking Libman–Sacks vegetations with cerebromicroembolism, cerebral hypoperfusion, ischaemic brain injury, stroke/TIA, neurocognitive dysfunction, and death.
Patients who present with mild valvular thickening and vegetations can be successfully treated with anticoagulation therapy along with appropriate lupus therapy as demonstrated in our case.16 There is experimental and clinical evidence that hydroxychloroquine may reduce the risk of thrombosis in patients with SLE,17,18 a trial of steroids in addition to standard lupus therapy medication may be given. However, steroids themselves may lead to fibrosis later.19 It is important to note that recurrent venous thrombosis despite warfarin use is a well-recognized complication of the antiphospholipid syndrome.20 When warfarin therapy fails despite a therapeutic INR, options include higher-intensity warfarin therapy (target INR, 3–4); the addition of low-dose aspirin, hydroxychloroquine, or a statin; use of low-molecular-weight heparin; and a combination of these approaches. In addition, antiphospholipid antibodies can cause artifactual prolongation of the prothrombin time, leading to falsely elevated INR results and a subtherapeutic warfarin dose which is typically seen with point-of-care devices21; For now, there is insufficient evidence to determine the relative efficacy and safety of direct oral anticoagulants in this patient population.22
However, haemodynamically significant valvular lesions or thromboembolic phenomena are indications for consideration of the surgery similar to that for infective endocarditis.23 In a prospective clinical and echocardiographic study, approximately 10% of SLE patients developed severe valvular regurgitation associated with high levels of anticardiolipin antibody.24 Vianna et al. reported that valve lesions in LSE are more severe in patients with secondary APLS than in patients with APLS alone. Patients with secondary APLS needed surgery more often, and they had a higher perioperative risk. They also had a higher incidence of autoimmune haemolytic anaemia, neutropenia, low-complement component four levels, thrombocytopenia, and antiphospholipid manifestations.25 There are some recent case reports indicating need for redo bioprostetic mitral valve replacement secondary to underlying APLS with LSE. It is concerning that some of these patients already had undiagnosed APLS prior to surgery.26,27
Improvement with current therapy of Libman–Sacks vegetations, cerebromicroembolism, brain perfusion and injury, and neurocognitive dysfunction further support a causal association of LSE and cerebrovascular disease.1
Even though anticoagulation is still the cornerstone of treatment in these cases, it is usually not effective for non-thrombotic manifestations of antiphospholipid antibodies, nephropathy, and microthrombosis. Thus, immunomodulatory treatment strategies targeting these additional targets have been used and increasingly investigated (new). Finally, addressing traditional risk factors for cardiovascular disease, as well as active systemic autoimmune diseases, is not to be forgotten.10
Conclusion
Cryptogenic stroke patients with high suspicion of underlying autoimmune disease should be worked up thoroughly for possible valvular heart disease associated with lupus, APLS, or both. Acquisition of transoesophageal images should be implemented in these subsets of patients because transthoracic echocardiogram alone may miss the diagnosis of LSE as shown in our case. Early recognition of cardiac manifestation, timely management, amelioration of risk factors, as well as close follow-up of lupus or APLS patients, can reduce their mortality and provide adequate prevention of recurrence and progression of cerebrovascular disease.
Patient perspective
Patient was satisfied with her treatment.
Acknowledgements
The authors thank Dr Tara Deryavoush, DO for her illustration.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Roldan CA, Sibbitt WL, Qualls CR, Jung RE, Greene ER, Gasparovic CM, Hayek RA, Charlton GA, Crookston K.. Libman-Sacks endocarditis and embolic cerebrovascular disease. JACC Cardiovasc Imaging 2013;6:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Libman E, Sacks B.. A hitherto undescribed form of valvular and mural endocarditis. Arch Intern Med 1924;33:701–737. [Google Scholar]
- 3. Blank M, Shani A, Goldberg I, Kopolovic J, Amigo MC, Magrini L, Shoenfeld Y.. Libman-Sacks endocarditis associated with antiphospholipid syndrome and infection. Thromb Res 2004;114:589–592. [DOI] [PubMed] [Google Scholar]
- 4. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA.. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 5. Menard GE. Establishing the diagnosis of Libman-Sacks endocarditis in systemic lupus erythematosus. J Gen Intern Med 2008;23:883–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ünlü O, Zuily S, Erkan D.. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol 2016;3:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roldan CA, Qualls CR, Sopko KS, Sibbitt WL Jr. Transthoracic versus transesophageal echocardiography for detection of Libman-Sacks endocarditis: a randomized controlled study. J Rheumatol 2008;35:224–229. [PubMed] [Google Scholar]
- 8. Omdal R, Lunde P, Rasmussen K, Mellgren SI, Husby G.. Transesophageal and transthoracic echocardiography and Doppler-examinations in systemic lupus erythematosus. Scand J Rheumatol 2001;30:275–281. [DOI] [PubMed] [Google Scholar]
- 9. Bai Z, Hou J, Ren W, Guo Y.. Diagnosis and surgical treatment for isolated tricuspid libman-sacks endocarditis: a rare case report and literatures review. J Cardiothorac Surg 2015;10:93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia D, Erkan D.. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018;378:2010–2021. [DOI] [PubMed] [Google Scholar]
- 11. Lin G-M, Chang F-Y, Wang W-B.. Coagulase-negative staphylococcus infective endocarditis in a lupus patient with Libman-Sacks endocarditis. J Heart Valve Dis 2015;24:2236–2238. [PubMed] [Google Scholar]
- 12. Moyssakis I, Tektonidou MG, Vasilliou VA, Samarkos M, Votteas V, Moutsopoulos HM.. Libman–Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med 2007;120:636–642. [DOI] [PubMed] [Google Scholar]
- 13. Roldan CA, Shively BK, Crawford MH.. An echocardiographic study of valvular heart disease associated with systemic lupus erythematosus. N Engl J Med 1996;335:1424–1430. [DOI] [PubMed] [Google Scholar]
- 14. Ziporen L, Goldberg I, Arad M, Hojnik M, Ordi-Ros J, Afek A, Blank M, Sandbank Y, Vilardell-Tarres M, de Torres I, Weinberger A, Asherson RA, Kopolovic Y, Shoenfeld Y.. Libman-Sacks endocarditis in the antiphospholipid syndrome: immunopathologic findings in deformed heart valves. Lupus 1996;5:196–205. [DOI] [PubMed] [Google Scholar]
- 15. Hojnik M, George J, Ziporen L, Shoenfeld Y.. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation 1996;93:1579–1587. [DOI] [PubMed] [Google Scholar]
- 16. Moaref AR, Afifi S, Rezaian S, Rezaian GR.. Isolated tricuspid valve Libman-Sacks endocarditis and valvular stenosis: unusual manifestations of systemic lupus erythematosus. J Am Soc Echocardiogr 2010;23:341.e3–345. [DOI] [PubMed] [Google Scholar]
- 17. Rand JH, Wu X-X, Quinn AS, Ashton AW, Chen PP, Hathcock JJ, Andree HAM, Taatjes DJ.. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood 2010;115:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM.. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum 2009;61:29–36. [DOI] [PubMed] [Google Scholar]
- 19. Lee JL, Naguwa SM, Cheema GS, Gershwin ME.. Revisiting Libman–Sacks endocarditis: a historical review and update. Clin Rev Allergy Immunol 2009;36:126–130. [DOI] [PubMed] [Google Scholar]
- 20. Ruiz-Irastorza G, Khamashta MA, Hunt BJ, Escudero A, Cuadrado MJ, Hughes GR.. Bleeding and recurrent thrombosis in definite antiphospholipid syndrome: analysis of a series of 66 patients treated with oral anticoagulation to a target international normalized ratio of 3.5. Arch Intern Med 2002;162:1164–1169. [DOI] [PubMed] [Google Scholar]
- 21. Tripodi A, Chantarangkul V, Clerici M, Negri B, Galli M, Mannucci PM.. Laboratory control of oral anticoagulant treatment by the INR system in patients with the antiphospholipid syndrome and lupus anticoagulant: results of a collaborative study involving nine commercial thromboplastins. Br J Haematol 2001;115:672–678. [DOI] [PubMed] [Google Scholar]
- 22. Andrade D, Cervera R, Cohen H, Crowther M, Cuadrado MJ, Canaud G, Garcia DA, Gerosa M, Ortel T, Pengo V, Rahman A. 15th International Congress on Antiphospholipid Antibodies Task Force on Antiphospholipid Syndrome treatment trends report In: Erkan D, Lockshin MD, eds. Antiphospholipid Syndrome: Current Research Highlights and Clinical Insights. New York: Springer; 2017. pp. 317–338. [Google Scholar]
- 23. Bhimani AA, Hoit BD.. Extensive nonbacterial thrombotic endocarditis isolated to the tricuspid valve in primary antiphospholipid syndrome. J Am Echocardiogr 2010;23:107.e5–106. [DOI] [PubMed] [Google Scholar]
- 24. Perez-Villa F, Font J, Azqueta M, Espinosa G, Pare C, Cervera R, Reverter JC, Ingelmo M, Sanz G.. Severe valvular regurgitation and antiphospholipid antibodies in systemic lupus erythematosus: a prospective, long-term, followup study. Arthritis Rheum 2005;53:460–467. [DOI] [PubMed] [Google Scholar]
- 25. Vianna JL, Khamashta MA, Ordi-Ros J, Font J, Cervera R, Lopez-Soto A, Tolosa C, Franz J, Selva A, Ingelmo M, Vilardell M, Hughes GRV.. Comparison of the primary and secondary antiphospholipid syndrome: a European multicenter study of 114 patients. Am J Med 1994;96:3–9. [DOI] [PubMed] [Google Scholar]
- 26. Samura T, Toda K, Yoshioka D, Nakamura T, Miyagawa S, Yoshikawa Y, Saito S, Domae K, Sawa Y.. Libman-Sacks endocarditis due to systemic lupus erythematosus activation after mitral valve plasty. Ann Thorac Surg 2017;104:e109–e111. [DOI] [PubMed] [Google Scholar]
- 27. Sladek EH, Accola KD.. Antiphospholipid syndrome and Libman-Sacks endocarditis in a bioprosthetic mitral valve. Ann Thorac Surg 2016;101:e29–e31. [DOI] [PubMed] [Google Scholar]