Abstract
Objective
Behavioral and psychological symptoms of dementia (BPSD) refer to the often distressing, noncognitive symptoms of dementia. BPSD appear in up to 90% of persons with dementia and can cause serious complications. Reducing the use of antipsychotic medications to treat BPSD is an international priority. This review addresses the following questions: What nonpharmacological interventions work to manage BPSD? And, in what circumstances do they work and why?
Method
A realist review was conducted to identify and explain the interactions among context, mechanism, and outcome. We searched electronic databases for empirical studies that reported a formal evaluation of nonpharmacological interventions to decrease BPSD.
Results
Seventy-four articles met the inclusion criteria. Three mechanisms emerged as necessary for sustained effective outcomes: the caring environment, care skill development and maintenance, and individualization of care. We offer hypotheses about how different contexts account for the success, failure, or partial success of these mechanisms within the interventions.
Discussion
Nonpharmacological interventions for BPSD should include consideration of both the physical and the social environment, ongoing education/training and support for care providers, and individualized approaches that promote self-determination and continued opportunities for meaning and purpose for persons with dementia.
Keywords: Alzheimer disease, Antipsychotics, Behaviour management
Translational Significance
To be effective, initiatives to address BPSD should include consideration of both the physical and the social environment, ongoing education/training and support for care providers, and individualized approaches that promote self-determination and continued opportunities for meaning and purpose for persons with dementia.
Behavioral and psychological symptoms of dementia (BPSD) refer to the often distressing, noncognitive symptoms of dementia (e.g., aggression, apathy, psychomotor agitation) (1). BPSD appear in up to 90% of persons with dementia and can cause serious complications (e.g., increased emergency department visits, caregiver distress and illness, early institutionalization, and diminished quality of life) (2). Antipsychotic medications are often prescribed to manage BPSD, especially in long-term care (LTC) settings (3). However, a clear association has been demonstrated between treatment with antipsychotic medications and increased morbidity and mortality in people with dementia (3,4). Thus, implementing initiatives aimed at reducing the use of antipsychotic medications for people with dementia has become an international priority (5,6). A central feature of these initiatives is the development of guidelines that recommend (or require) the use of nonpharmacological treatment modalities prior to initiating pharmacotherapy (5–7).
Nonpharmacological management of BPSD can be grouped into two categories: (a) indirect interventions aimed at decreasing BPSD through working with caregivers or adapting the environment (e.g., caregiver training, multidisciplinary team approaches, individualized treatment plans, and modifying environmental factors) and (b) direct interventions targeted directly at individuals with dementia to decrease BPSD (e.g., individualized recreation therapy, sensory-based therapy, exercise, music therapy, massage) (8).
Review of the literature indicates that little is known about the feasibility and effectiveness of direct and indirect nonpharmacological interventions for the management of BPSD. A recent systematic review of interventions to reduce inappropriate prescribing of antipsychotic medications demonstrated that most interventions focus primarily on education of care staff, physicians, and pharmacists (7). The authors of this review found that some of these indirect interventions may be effective in the short term (i.e., reduced prescribing levels immediately following the intervention) but that the culture and nature of care settings, in addition to the availability and feasibility of additional nonpharmacological alternatives (e.g., beyond education), needs to be addressed to sustain reduced antipsychotic prescribing over the long term (7).
The complexity of BPSD in dementia suggests that an integrated approach is required for effective intervention and management. Approaches that do not take into consideration the complex biological, psychosocial, psychological, and environmental factors will likely produce results that are sporadic, inconsistent, and short lived (8,9). Consequently, a comprehensive and integrated approach, which addresses the needs of the individual with dementia, the caregivers, and the context (e.g., physical and psychosocial environment) is required (8,9).
Objectives and Aims
The objective of this study is to improve our understanding of effective nonpharmacological treatment modalities of BPSD by identifying and examining the interactions among context (something that can enable or modify or block a mechanism; the context may be provided by the intervention or it might relate to a broader contextual backdrop—the organizational and system setting in which complex interventions are delivered), mechanisms (the processes that operate in particular contexts that ultimately lead to the outcomes), and outcomes (both intended and unintended).
Method
We conducted a realist review to address the following question: What nonpharmacological interventions work to manage BPSD? And, in what circumstances do they work and why? The Realist and Meta-narrative Evidence Syntheses: Evolving Standards (RAMESES) criteria guided the conduct and reporting of this review (10). A realist review seeks to explicate the mechanism(s) of how complex interventions work, or why they fail, in particular contexts. This contextually bound approach to causality is represented as follows: context + mechanism = outcome (10).
The steps in a realist review include (a) clarifying the scope of the review; (b) searching for evidence; (c) appraising the primary studies and extracting their relevant data; (d) synthesizing the evidence and drawing conclusions; and (e) disseminating, and possibly implementing and evaluating, the recommendations (11). Following a realist review approach, candidate theories with potential explanatory value for outcomes of nonpharmacological interventions for BPSD were identified and their key principles were integrated into our data extraction matrix.
The identified theories and models included the following: Needs-driven Dementia-compromised Behavior (NDB) (12), Progressively Lowered Stress Threshold Model (PLST) (13); Sense of Belonging (14); Value, Individual Approach, Perspective, And Positive Social Psychology (VIPS) (15); Psychologically Based Service Delivery Model For Therapeutic Recreation (PBTRSD) (16); and Activity Theory (17). See Supplementary Appendix A for brief description of each theory.
Inclusion Criteria
A broad range of studies were included in this review, with a focus not only on indirect interventions designed for the care staff and the environment but also on direct interventions designed for persons with dementia. Studies meeting the following criteria were chosen for inclusion:
1 The population of interest was persons with dementia in any setting (i.e., residential care or community).
2. A primary focus of the study was the use of nonpharmacological interventions to address BPSD.
3. There was evident evaluation of the intervention with empirical data provided (i.e., the article described a primary study as opposed to a review and presented full evaluations, not preliminary results).
4. The description of the intervention was detailed enough to enable the reviewers to categorize it based on the key principles of the selected candidate theories or models.
5. The study was published in an English-language journal.
6. The publication date was between 2000 and 2016.
Identification of the Primary Studies
A search of ALOIS was conducted on April 28, 2016 by D. Scott (a health sciences librarian). ALOIS is a comprehensive, open access register of dementia studies created and maintained by the Cochrane Dementia and Cognitive Improvement Group (CDCIG). Developed for the use of researchers and practitioners engaged in evidence-based dementia care, the register is continually updated through monthly searches of MEDLINE, Embase, PsycINFO, CINAHL, LILACS, and a wide range of trials registers and other gray literature sources. ALOIS is study based, meaning that records refer to studies rather than the articles or reports written about them. Consequently, a single record may include references to multiple articles. Because of its relatively comprehensive coverage of literature pertaining to dementia, we agreed that a search conducted in ALOIS alone would be sufficient for the purposes of this review. As the review process progressed, it was determined that no additional searches of ALOIS were needed.
The search in ALOIS was designed to optimize sensitivity while maintaining feasibility. An advanced search of intervention studies was conducted with results filtered to include only nonpharmacological interventions. Using the register’s controlled vocabulary, the population was limited to people with dementia. No limits pertaining to study design or aim were applied.
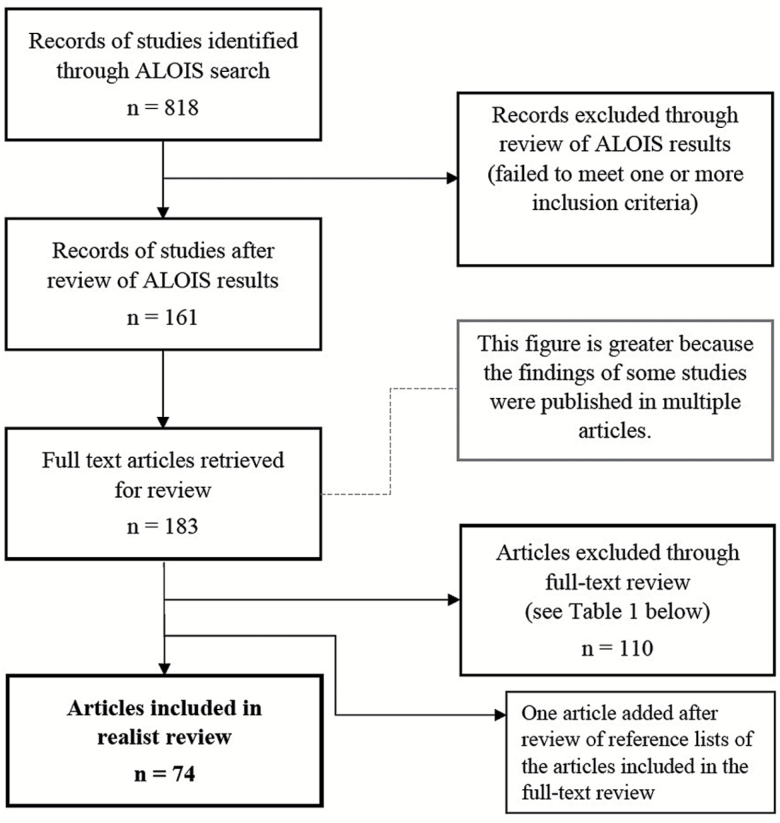
The search returned 818 results (i.e., records of studies; see Figure 1). Because only one database was searched, no deduplication of results was required. The results were reviewed, and 161 were identified for potential inclusion in the study; the remaining 657 were found not to meet the inclusion criteria detailed above. A total of 183 full-text articles reporting the findings of the 161 studies that were retrieved. Bibliographic records for these articles were created in an EndNote X7 library. A second, full-text screen was then completed, which removed a further 110 articles because they failed to meet the study’s inclusion criteria, as detailed in Table 1.
Figure 1.
Flow diagram for the realist review process.
Table 1.
Results of Full-Text Review
| Full-text articles reviewed | 183 | |
| Inclusion criteria | ||
| The population of interest was persons with dementia in any setting (i.e., residential care or community) | 14a | |
| A primary focus of the study was the use of nonpharmacological interventions to address BPSD | 18a | |
| There was evident evaluation of the intervention with empirical data provided (i.e., the article described a primary study as opposed to a review and presented full evaluations, not preliminary results) | 41a | |
| The description of the intervention was detailed enough to enable the reviewers to categorize it based on the key principles of the selected candidate theories or models | 37a | |
| Articles removed from consideration | −110 | |
| Article added after review of reference lists of full-text articles | +1 | |
| Articles included in the study | 74 | |
Note: aArticles failed to meet the inclusion criterion.
The full-text review identified 73 articles for inclusion, and, after review of the reference lists of these studies, one more article was included. This resulted in a total of 74 articles included in this study. It is important to note that three articles examined two different interventions within one study (18–20). For example, Lin and colleagues (18) examined the effectiveness of the intervention “Montessori-based activities” and compared it to the effectiveness of implementing the intervention “acupressure” within the same study. Because our aim was to examine the effectiveness of interventions for the treatment of BPSD, we entered these interventions separately into our matrix. As a result, although 74 studies were included in the review, we evaluated the effectiveness of 77 interventions.
Data Management, Analysis, and Synthesis
Phase one: appraisal and data extraction
Two authors (E. Davis and A. Douziech) independently extracted the relevant data from each study with a structured extraction matrix organized with the categories: location and date of study, sample size and description, details of the intervention, results and findings (including whether follow-up occurred, and if so, over what period), and inclusion of key principles of candidate theories and models. The two coders then independently evaluated the methodological quality of each study by assessing the quality of the evidence and the risk of bias to determine an effectiveness rating (further description is provided below). The first author read select studies and reviewed the coding imputed by E. Davis and A. Douziech to assess for consistency and accuracy. Following completion of the data extraction phase, the completed matrix was reviewed in detail; any discrepancies were discussed and resolved by discussion and consensus.
The quality of the published evidence was categorized as the level of evidence generated from (a) properly randomized controlled trials (RCT), (b) quasi-experimental designs (i.e., well-designed controlled trials without randomization) (QE), or (c) descriptive case studies or case reports (DCS). Follow-up evaluation of the intervention (i.e., whether the researchers evaluated the sustainability of practice change over time) was noted to better understand the typical length of follow-up used in intervention studies, to assist in the evaluation of the intervention effectiveness, and to determine whether it was sustained.
The Cochrane Collaboration’s tool for assessing the risk of bias (21,22) was used to evaluate risks related to five key domains for each of the randomized studies (i.e., selection, performance, detection, attrition, and reporting bias). The Risk of Bias Assessment for Non-randomized Studies (RoBANS) (22) was used to evaluate risks in the nonrandomized studies related to five key domains: (a) selection, (b) confounding variables, (c) performance, (d) inadequate blinding, and (e) incomplete outcome data. The risk of bias was classified as follows: “1” = low risk of bias in all key domains with plausible bias unlikely to seriously alter the results; “2” = unclear risk of bias for one or more key domains with plausible bias that raised some doubt about the results; and “3” = high risk of bias for one or more key domains with plausible bias that seriously weakened confidence in the results.
The effectiveness of each intervention was evaluated independently by the authors. The effectiveness rating was based on a combined overview of the sample size, effect size, outcomes and impact, level of evidence, follow-up, and risk of bias. Following the format utilized by Aylward and colleagues (23), the effectiveness was rated as follows: “A” = good evidence to support a recommendation of effectiveness, “B” = fair evidence to support a recommendation of effectiveness, “C” = insufficient evidence to recommend for or against effectiveness, “D” = fair evidence to support a recommendation of ineffectiveness, and “E” = good evidence to support a recommendation of ineffectiveness.
Phase two: synthesis and interpretation
On completion of the data extraction, descriptive statistics were computed for each item in the matrix. Next, we identified recurrent contextual features that might have acted as barriers to, or enablers of, the success of the interventions aimed at decreasing BPSD, and tested the explanatory ability of our candidate theories against these. Throughout this process, we sought and highlighted disconfirming data.
Results
Search Results and Study Characteristics
Seventy-four studies met the selection criteria and, as previously described, 77 interventions were analyzed. Studies from 20 countries were included in this review with the majority having been conducted in the United States (n = 25; 33%) and using RCT as their study design (n = 56; 73%). Of the interventions reviewed, 34% were evaluated as fair to good evidence to support a recommendation for effectiveness (“A” + “B”; n = 26), 44% were evaluated as insufficient evidence to support a recommendation for effectiveness (“C”; n = 34), and 22% were evaluated a fair to good evidence to support a recommendation for ineffectiveness (“D” + “E”; n = 17; see Supplementary Appendix B).
In total, 14 different types of nonpharmacological interventions were included in our sample. They ranged from specific indirect interventions (e.g., training and education, light focused) to specific direct interventions (e.g., massage, music based). Similar to previous reviews, we found the effectiveness of these interventions varied significantly depending on the study (see Table 2). This finding further highlighted the need to examine more closely how different contexts may account for the successes, failures, or partial successes of these interventions.
Table 2.
Effectiveness Rating by Interventions
| Effectiveness | ||||
|---|---|---|---|---|
| Interventions tested | n | A & B | C | D & E |
| Training and education | 13 | 39% | 30% | 31% |
| Music focused | 15 | 47% | 26% | 27% |
| Individualized | 5 | 60% | 20% | 20% |
| Physical | 8 | 50% | 38% | 13% |
| Therapeutic recreation | 2 | 100% | — | — |
| Light focused | 3 | — | 67% | 33% |
| Aromatherapy | 1 | — | 100% | — |
| Massage | 3 | 34% | 33% | 33% |
| Cognitive and functional | 6 | — | 83% | 17% |
| Art and theatre | 2 | — | 100% | — |
| Reminiscence | 6 | — | 67% | 33% |
| Montessori | 3 | 33% | 67% | — |
| Animal (robotic and alive) | 2 | 50% | — | 50% |
| Multisensory (including thermal bath and combinations of other interventions) | 8 | 25% | 62% | 13% |
Note: A & B = Fair to good evidence to support effectiveness; C = Insufficient evidence to support effectiveness; D & E = Fair to good evidence to support ineffectiveness.
The duration of the interventions varied widely, ranging from 5 to 92 weeks (mean = 15 weeks, SD = 17 weeks). Sample sizes also varied widely, ranging from 14 to 624 (mean = 110). The majority of interventions did not include any follow-up (n = 47; 63%) and less than a quarter included follow-up lasting longer than 8 weeks (n = 16; 21%). The majority of interventions were implemented in residential care facilities (n = 63; 82%) as opposed to the community (n = 13; 17%) and included persons with dementia who were at any stage of the disease process (n = 56; 73%) as opposed to specifying the stage (e.g., mild, moderate, or severe). Importantly, the effectiveness rating of the interventions was not found to be associated with the participants’ stage in the disease process (see Table 3).
Table 3.
Effectiveness Rating by Severity of Diagnosis
| Effectiveness | ||||
|---|---|---|---|---|
| Severity of diagnosis | n | A & B | C | D & E |
| Mild | 2 | — | 6% | — |
| Mild–Moderate | 9 | 19% | 6% | 12% |
| Moderate–Severe | 7 | 4% | 14% | 6% |
| Severe | 3 | 12% | — | — |
| Mild–Severe | 56 | 65% | 74% | 82% |
Note: A & B = Fair to good evidence to support effectiveness; C = Insufficient evidence to support effectiveness; D & E = Fair to good evidence to support ineffectiveness.
From Candidate Theories Toward a Model for Understanding Nonpharmacological Interventions to Manage BPSD
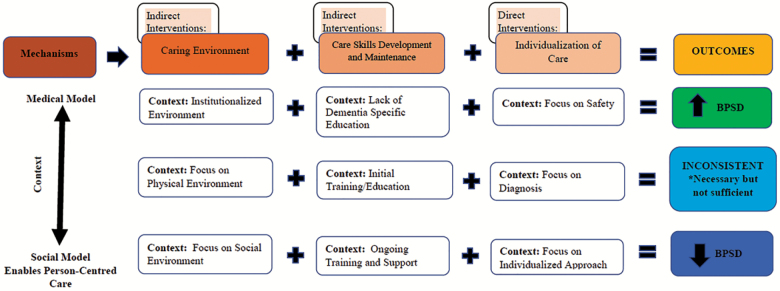
Of the selected candidate theories, none emerged as able to describe conclusively the interactions among context, mechanisms, and outcomes within interventions aimed at decreasing BPSD. As a result, we examined the specific principles within each candidate theory and a preliminary thematic analysis was undertaken to identify aspects of each study that were key to the intervention’s success (or which explained its partial success or failure). Following this, we identified three mechanisms by which an intervention to decrease BPSD might achieve its goals and developed a unifying heuristic model to explain the influence of context on these mechanisms with the resulting outcomes (see Figure 2). Two of the mechanisms were indirect interventions—these included the caring environment and care skill development and maintenance. The third mechanism was individualization of care, which is considered a direct intervention. As represented in the model, we assert that all three of these mechanisms, and the contexts within which they occur, must be included and addressed for successful and sustainable outcomes related to the reduction of BPSD to occur.
Figure 2.
Heuristic model for clinical intervention implementation or future research.
The key contextual influences were then grouped according to those that caused the mechanisms to be unsuccessful in decreasing BPSD (and, in fact, could potentially lead to an increase in symptoms), those that produced inconsistent outcomes and we described as “necessary but not sufficient,” and those that helped to ensure the mechanisms resulted in the successful decrease in BPSD. Once placed into the model, we found that these contextual influences could be further categorized as those that were more consistent with the medical model (i.e., focused almost exclusively on objective medical and clinical outcomes), to those that were more consistent with a social model of care (i.e., focused on tailoring care to an individual’s needs and abilities while also promoting choice and enhanced relationships; see Figure 2). We describe the mechanisms and these contextual influences below.
Mechanism 1: caring environment
We considered the caring environment to include both the physical built environment and the social environment (e.g., caregiver staff stability, supports for the development of positive interpersonal relationships between care giver and care recipient). However, most interventions in this review primarily emphasized the importance of adapting the physical environment (n = 59; 77%). This is very likely due to the extensive literature that has demonstrated that not attending to physical environmental attributes (e.g., room temperature, light levels, noise levels, depersonalized and institutionalized ambience) leads to increased prevalence of BPSDs (24,25). Thus, within our model, we asserted that not attending to these important environmental adaptations leads to an increase in the prevalence of BPSD. However, analysis of our matrix indicated that focusing solely on adapting the physical environment, without attention to the social environment, results in inconsistent outcomes. Of the 32 (40%) interventions that attended to physical environment alone, only seven were found to support recommendations for effectiveness (26–32). These findings led to our assertion that adapting the physical environment may be necessary, but it is not sufficient when addressing BPSD. Alternatively, of the 26 (33%) interventions that included adaptation to both the social and physical environments, 12 (46%) were found to have good evidence to support recommendation for being effective (19,33–42). This led to our conclusion that the physical environment needs to be considered (and adapted if necessary); however, adaptations to the social environment should also be considered and addressed for more successful outcomes to occur related to BPSD. Focus on the social environment included addressing such things as whether or not there was effective communication between all stakeholders (i.e., care providers, care recipients, and family members) (19,37,42), creation of supportive relationships (43), the presence of supportive and responsive leadership (42,44), and the cultivation of care teams that trust (42).
Mechanism 2: care skill development and maintenance
To our surprise, only 22 (27%) of the interventions in our sample specified the inclusion of an education and training component (19,20,26,29,37,41,42,44–57). When reviewing the literature, it is clear that continuing professional development via education and training courses for care providers related to understanding dementia is necessary for the implementation of best practices in dementia care (58) and that the lack of these opportunities can lead to increased prevalence of, and negative outcomes related to, BPSD (59). This empirical evidence is reflected in our model.
When examining our matrix to further elucidate the contextual factors that influence outcomes related to the presence of care skill development we found that 22 (28%) interventions included initial training to educate staff in dementia care and/or the benefits of the specific interventions. Of these studies, eight (36%) received an effectiveness rating “A” or “B” (19,26,29,37,41,42,55), nine (40%) received an effectiveness rating “C” (45–50,52–54), and five (23%) received an effectiveness rating “D” or “E” (20,42,51,56,57). Thus, our review found that providing initial training on these essential topics may be necessary, but it is not sufficient in producing substantive or sustained improvements in decreasing BPSD.
When we compared these findings to those studies that included ongoing training and support (n = 9; 12%), we found that the majority (n = 6; 67%) received an effectiveness rating “A” or “B” (19,26,29,37,41). This led to our conclusion that the successful reduction in BPSD requires ongoing education and training combined with supports. An exemplary example of this comes from McCabe and colleagues (19), who found that initial training and structured clinical protocols successfully decreased BPSD behaviors only [my own italics] when they were combined with ongoing training and clinical supports. Review of our matrix indicated that, in addition to having ongoing opportunities for training, the supports that were most influential included being adaptable to the needs of care providers (42), ensuring that appropriate resources are available to enable the implementation of the intervention (19), and implementing system (or organizational) processes that support and reinforce the intervention (26).
Mechanism 3: individualization of care
Individualizing care practices and approaches is widely recognized as essential to both quality of care for persons with dementia and also as a successful strategy in the management of BPSD (60). In our review, 45 (58%) interventions were individualized to the unique needs and preferences of the care recipients. Of these, 17 (22%) included maximizing safety as a form of individualization of care; however, all but two included other methods of individualization. Of these two, both received an effectiveness rating of “C” (54,61).
Historically, care of persons with dementia has primarily been focused on meeting safety and physiological needs (62). This emphasis is highlighted in the ever increasing (and some would argue constricting) number of regulations developed for the long-term, residential care industry. Banerjee and colleagues (63) asserted that, “these regulations, and the reporting they require, take valuable time away from care, often fail to account for the relational aspects of care, and disempower residents while empowering paperwork (p. 7). The focus on safety in these regulations may lead to the disempowerment of persons with dementia by limiting their choices, removing their control, and ensuring that the dignity of risk is not extended to persons with dementia. It is for this reason that individualizing care based only on maximizing safety was specifically included within our model as a mechanism that is not effective in decreasing BPSD.
Those studies that focused solely on individualizing the interventions based on diagnosis or neurological assessment (i.e., stage of dementia, functional abilities) (n = 10, 13%) had mixed results (n = 9, 12%, rated as “C”) or were ineffective (n = 1, 1.3%, rated as “D” or “E”) in decreasing BPSD (64–67). Alternatively, we found that individualizing the interventions based on individual preferences and personal characteristics, which is a fundamental tenet of person-centered care, was significantly associated to effectiveness of the intervention. That is, of all interventions that received an effectiveness rating of “A” or “B” (n = 26, 34%), 22 (85%) included individualization of the intervention based on personal preferences and characteristics.
Additionally, and we feel importantly, those interventions that included individualized approaches that promoted self-determination, desire for meaning, intrinsic motivation, and value were most effective. For example, Guétin and colleagues (38) not only enabled individuals living with dementia to chose music that they enjoyed when creating play lists for the purpose of reducing anxiety but also created a computer program that enabled them to produce their own playlists, based on their personal subjective experiences and moods, on a daily basis. Providing interventions based primarily on preferences increase the opportunity for enjoyment and entertainment, while interventions that honor choice, control, and empowerment promote true engagement. Our review indicates that the latter is a fundamental principle for the successful reduction in BPSD.
Discussion
This review elucidated the interactions among contexts, mechanisms, and outcomes associated with nonpharmacological interventions designed to manage BPSD. Three mechanisms emerged as essential—caring environment, care skill development and maintenance, and individualization of care. We developed a unifying heuristic model to explain the influence of context on these mechanisms with the resulting outcomes. Within this model, we assert that all three of these mechanisms, and the contexts within which they occur, must be included and addressed for successful and sustainable outcomes related to the reduction of BPSD to occur. This adds to the body of literature demonstrating that a comprehensive and integrated approach is required for effective intervention and management of BPSD.
Although all of the mechanisms and contextual factors included in our model were identified primarily from the studies includes in this review, they also align with a wider literature. When examining the caring environment, we found that adapting the physical environment may be necessary, but is not sufficient in managing BPSD. To be effective, the social environment must also be considered. Attempting to create “homelike” care environments requires much more than simplistic alterations of the physical environment (e.g., the absence of ceiling mounted fluorescent light, the presence of noninstitutional furniture, carpeting on the floor). To make a place feel like “home,” the patterns of activities and interactions, the ways meals are prepared and served, and opportunities for greater privacy and control (which are typical at home but exist to a much lesser degree in residential LTC settings) must also be considered (68). Furthermore, the structure of the relationships between the care providers and care recipients (e.g., whether staff sit down and eat meals with residents or put trays down in front of them) has a significant effect on the extent to which a setting is experienced as being “like home” (68).
A key feature of the social environment is the development of therapeutic relationships between the care providers, the care recipients and the family members. Therapeutic relationships are mutually supportive and are fostered by effective communication and listening techniques, giving support, and ensuring care is person centered as opposed to task orientation (69). According to Doherty and Thompson (70), the development of a therapeutic relationship with care recipients is integral to the accurate assessment and understanding of patients’ needs and the ensuing delivery of effective, person-centered care. Unfortunately, our findings indicate that, although attending to and adapting the social environment to meet the needs of persons with dementia is necessary, it is not yet the norm in interventions aimed at managing BPSD.
Care skill development and maintenance was found to be another essential principle in interventions aimed at reducing BPSD. However, education alone is not sufficient; it must be combined with ongoing training and support for the interventions to be effective and sustainable. This finding is consistent with other reviews of interventions to change practice in LTC settings (9,23). The literature consistently notes that successful implementation of education and training must also include organizational and system changes and that lack of administrative support likely explains the failure of education, training, and practice change initiatives (9,23,71). Furthermore, Caspar and colleagues (9) found that resources in the form of direct human involvement and interaction (e.g., on-the-job coaching, hands-on practice, supportive mentoring, increased supervision, and team meetings) were essential to the success of interventions to change care practices in LTC settings. However, similar to our findings, the literature also indicates that, although the provision of ongoing training and support is necessary for effective practice change, it is not yet the norm (9,23,71).
Individualization of care is the final mechanism included in our model. Of all of the mechanisms, this was most influential on the outcomes. This is likely because it is a direct intervention and it embodies, more than the others, the central tenets of person-centered care. Person-centered care has been described as a care philosophy in which a positive relationship is established that respects care recipients’ preferences and life histories, honors their identities, enables their engagement in meaningful activities, and encourages an overall sense of well-being (72). Furthermore, person-centered care takes care recipient individuality into account and incorporates their participation into decision making (73,74). Our review found that these principles were essential to the success of interventions aimed at decreasing BPSD.
Like the others, this mechanism was placed on a range of contextual factors that were based on the medical model (e.g., focused on meeting safety and physiological needs) to those that were based on the social model (e.g., focused on person-centered care). Critics of the medical model state that it “justifies control as appropriate treatment for the good of the patient” (75). Furthermore, they contend that it creates a hierarchy of power within care environments and promotes care that is primarily custodial in nature (72,75). We found that the effectiveness of individualization of care is significantly impacted by contextual factors that either support or infringe upon the person with dementia’s sense of self and autonomy. However, as was so eloquently stated by Gawande (76), “We want autonomy for ourselves and safety for those we love.” (p. 106). This may be why the focus on safety in our aged-care environments continues to be such a high priority, regardless of the unintended negative consequences this has on the person with dementia’s ability to exert choice, control, and empowerment in these settings. Thus, though our review found that honoring the self-determination and autonomy of a person with dementia is essential for the successful management of BPSD, it is certainly not yet the norm.
Conclusions
Nonpharmacological interventions for BPSD should include consideration of the caring environment, care skill development and maintenance, and individualization of care. However, when considering the environment, adapting the physical environment is necessary but not sufficient. To effectively address BPSD, the social environment must also be incorporated into the intervention. Also, when considering care skill development related to initiatives aimed at decreasing BPSD, providing initial training is necessary but not sufficient. Instead, ongoing education and support of care providers in the form of effective enabling and reinforcing factors must also be included. Finally, individualized approaches that promote self-determination and autonomy of persons with dementia should also be included in any interventions aimed at decreasing BPSD. Though we found that the combination of each of these was supportive of the success of these interventions, we also found that they are not the norm. This may help to explain why so many nonpharmacological interventions designed to address BPSD result in inconsistent outcomes. It is our hope that this also provides direction for future research and initiatives aimed at successful and sustainable nonpharmacological management of BPSD in persons with dementia.
Supplementary Material
Supplementary data is available at The Gerontologist online.
Funding
This study was funded by the 2017 Alberta Innovates Health Solutions Summer Student Ship Award.
Conflict of Interest
None reported.
References
- 1. Robert PH, Verhey FR, Byrne EJ et al. Grouping for behavioral and psychological symptoms in dementia: clinical and biological aspects. Consensus paper of the European Alzheimer disease consortium. Eur Psychiatry. 2005;20:490–496. doi:10.1016/j.eurpsy.2004.09.031 [DOI] [PubMed] [Google Scholar]
- 2. Steinberg M, Corcoran C, Tschanz JT, et al. Risk factors for neuropsychiatric symptoms in dementia: the cache county study. Int J Geriatr Psychiatry. 2006;21:824–830. doi:10.1002/gps.1567 [DOI] [PubMed] [Google Scholar]
- 3. Foebel A, Ballokova A, Wellens NI et al. A retrospective, longitudinal study of factors associated with new antipsychotic medication use among recently admitted long-term care residents. BMC Geriatr. 2015;15:128. doi:10.1186/s12877-015-0127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballard C, Creese B, Corbett A, Aarsland D. Atypical antipsychotics for the treatment of behavioral and psychological symptoms in dementia, with a particular focus on longer term outcomes and mortality. Expert Opin Drug Saf. 2011;10:35–43. doi:10.1517/14740338.2010.506711 [DOI] [PubMed] [Google Scholar]
- 5. Banerjee S. The Use of Anitpsychotic Medication for People With Dementia: Time for Action. London, UK: Department of Health; 2009. [Google Scholar]
- 6. Valiyeva E, Herrmann N, Rochon PA, Gill SS, Anderson GM. Effect of regulatory warnings on antipsychotic prescription rates among elderly patients with dementia: a population-based time-series analysis. CMAJ. 2008;179:438–446. doi:10.1503/cmaj.071540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coon JT, Abbott R, Rogers M et al. Interventions to reduce inappropriate prescribing of antipsychotic medications in people with dementia resident in care homes: a systematic review. J Am Med Dir Assoc. 2014;15:706–718. doi.10.1016/j.jamda.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 8. Grand JH, Caspar S, Macdonald SW. Clinical features and multidisciplinary approaches to dementia care. J Multidiscip Healthc. 2011;4:125–147. doi:10.2147/JMDH.S17773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caspar S, Cooke HA, Phinney A, Ratner PA. Practice change interventions in long-term care facilities: what works, and why?Can J Aging. 2016;35:372–384. doi:10.1017/S0714980816000374 [DOI] [PubMed] [Google Scholar]
- 10. Wong G Greenhalgh T Westhorp G Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med. 2013;11:21. doi:10.1186/1741-7015-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pawson R, Greenhalgh T, Harvey G Walshe K. Realist review – a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy. 2005;10(suppl 1):21–34. doi:10.1258/1355819054308530 [DOI] [PubMed] [Google Scholar]
- 12. Algase DL, Beck C, Kolanowski A et al. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. Am J Alzheimers Dis Other Demen. 1996;11:10–19. doi:10.1177/153331759601100603 [Google Scholar]
- 13. Hall G, Buckwalter K. Progressively lowered stress threshold: a conceptual model for care of adults with Alzheimer’s disease. Arch Psychiatr Nurs. 1987;1:399–406. [PubMed] [Google Scholar]
- 14. Hagerty BM, Williams RA, Coyne JC, Early MR. Sense of belonging and indicators of social and psychological functioning. Arch Psychiatr Nurs. 1996;10:235–244. doi:10.1016/S0883-9417(96)80029-X [DOI] [PubMed] [Google Scholar]
- 15. Brooker D. What is person-centred care in dementia?Rev Clin Gerontol. 2004;13:215–222. doi:10.1017/S095925980400108X [Google Scholar]
- 16. Dattilo J, Kleiber D, Williams R. Self-determination and enjoyment enhancement: a psychologically-based service delivery model for therapeutic recreation. Ther Recreat J. 1998;33:258. [Google Scholar]
- 17. Longino CF, Kart CS. Explication activity theory: a formal replication. J Gerontol. 1982;37:713–722. [DOI] [PubMed] [Google Scholar]
- 18. Lin LC, Yang MH, Kao CC, Wu SC, Tang SH, Lin JG. Using acupressure and Montessori-based activities to decrease agitation for residents with dementia: a cross-over trial. J Am Geriatr Soc. 2009;57:1022–1029. doi:10.1111/j.1532-5415.2009.02271.x [DOI] [PubMed] [Google Scholar]
- 19. McCabe MP, Bird M, Davison TE et al. An RCT to evaluate the utility of a clinical protocol for staff in the management of behavioral and psychological symptoms of dementia in residential aged-care settings. Aging Ment Health. 2015;19:799–807. doi:10.1080/13607863.2014.967659 [DOI] [PubMed] [Google Scholar]
- 20. Rokstad AM Røsvik J Kirkevold Ø, Selbaek G, Saltyte Benth J, Engedal K. The effect of person-centred dementia care to prevent agitation and other neuropsychiatric symptoms and enhance quality of life in nursing home patients: a 10-month randomized controlled trial. Dement Geriatr Cogn Disord. 2013;36:340–353. doi:10.1159/000354366 [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim SY, Park JE, Lee YJ et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. doi:10.1016/j.jclinepi.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 23. Aylward S Stolee P, Keat N, Johncox V. Effectiveness of continuing education in long-term care: a literature review. Gerontologist. 2003;43:259–271. doi:10.1093/geront/43.2.259 [DOI] [PubMed] [Google Scholar]
- 24. Fleming R, Goodenough B, Low L-F, Chenoweth L, Brodaty H. The relationship between the quality of the built environment and the quality of life of people with dementia in residential care. Dementia. 2014;15:663–680. doi:10.1177/1471301214532460 [DOI] [PubMed] [Google Scholar]
- 25. Marquardt G, Bueter K, Motzek T. Impact of the design of the built environment on people with dementia: an evidence-based review. Herd. 2014;8:127–157. doi:10.1177/193758671400800111 [DOI] [PubMed] [Google Scholar]
- 26. Deudon A, Maubourguet N, Gervais X et al. Non-pharmacological management of behavioural symptoms in nursing homes. Int J Geriatr Psychiatry. 2009;24:1386–1395. doi:10.1002/gps.2275 [DOI] [PubMed] [Google Scholar]
- 27. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867–873. doi:10.1016/j.jamda.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 28. Raglio A, Bellelli G, Traficante D et al. Efficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementia. Alzheimer Dis Assoc Disord. 2008;22:158–162. doi:10.1097/WAD.0b013e3181630b6f [DOI] [PubMed] [Google Scholar]
- 29. Rolland Y, Pillard F, Klapouszczak A et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–165. doi:10.1111/j.1532-5415.2007.01035.x [DOI] [PubMed] [Google Scholar]
- 30. Sung HC, Chang SM, Lee WL, Lee MS. The effects of group music with movement intervention on agitated behaviours of institutionalized elders with dementia in Taiwan. Complement Ther Med. 2006;14:113–119. doi:10.1016/j.ctim.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 31. Ward-Smith P Llanque SM, Curran D. The effect of multisensory stimulation on persons residing in an extended care facility. Am J Alzheimers Dis Other Demen. 2009;24:450–455. doi:10.1177/1533317509350153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams CL, Tappen RM. Effect of exercise on mood in nursing home residents with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2007;22:389–397. doi:10.1177/1533317507305588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buettner LL, Fitzsimmons S. AD-venture program: therapeutic biking for the treatment of depression in long-term care residents with dementia. Am J Alzheimers Dis Other Demen. 2002;17:121–127. doi:10.1177/153331750201700205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen-Mansfield J, Libin A, Marx MS. Nonpharmacological treatment of agitation: a controlled trial of systematic individualized intervention. J Gerontol B Psychol Sci Soc Sci. 2007;62:908–916. [DOI] [PubMed] [Google Scholar]
- 35. Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M, Freedman L. Efficacy of nonpharmacologic interventions for agitation in advanced dementia: a randomized, placebo-controlled trial. J Clin Psychiatry. 2012;73:1255–1261. doi:10.4088/JCP.12m07918 [DOI] [PubMed] [Google Scholar]
- 36. Garland K, Beer E, Eppingstall B, O’Connor DW. A comparison of two treatments of agitated behavior in nursing home residents with dementia: simulated family presence and preferred music. Am J Geriatr Psychiatry. 2007;15:514–521. doi:10.1097/01.JGP.0000249388.37080.b4 [DOI] [PubMed] [Google Scholar]
- 37. Gerdner LA, Buckwalter KC, Reed D. Impact of a psychoeducational intervention on caregiver response to behavioral problems. J Nurs Res. 2002;51:363–374. doi:10.1097/ 00006199-200211000-00004 [DOI] [PubMed] [Google Scholar]
- 38. Guétin S, Portet F, Picot MC et al. Effect of music therapy on anxiety and depression in patients with Alzheimer’s type dementia: randomised, controlled study. Dement Geriatr Cogn Dis. 2009;28:36–46. doi:10.1159/000229024 [DOI] [PubMed] [Google Scholar]
- 39. Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT Jr. A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. J Am Geriatr Soc. 2011;59:1032–1041. doi:10.1111/j.1532-5415.2011.03449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Politis AM, Vozzella S, Mayer LS, Onyike CU, Baker AS, Lyketsos CG. A randomized, controlled, clinical trial of activity therapy for apathy in patients with dementia residing in long-term care. Int J Geriatr Psychiatry. 2004;19:1087–1094. doi:10.1002/gps.1215 [DOI] [PubMed] [Google Scholar]
- 41. Sakamoto M, Ando H, Tsutou A. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. Int Psychogeriatr. 2013;25:775–784. doi:10.1017/S1041610212002256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verkaik R Francke AL van Meijel B Spreeuwenberg PM Ribbe MW, Bensing JM. The effects of a nursing guideline on depression in psychogeriatric nursing home residents with dementia. Int J Geriatr Psychiatry. 2011;26:723–732. doi:10.1002/gps.2586 [DOI] [PubMed] [Google Scholar]
- 43. Lam LCW, Lui VWC, Luk DNK et al. Effectiveness of an individualized functional training program on affective disturbances and functional skills in mild and moderate dementia—a randomized control trial. Int J Geriatr Psychiatry. 2010;25:133–141. doi:10.1002/gps.2309 [DOI] [PubMed] [Google Scholar]
- 44. Visser SM, McCabe MP, Hudgson C, Buchanan G Davison TE, George K. Managing behavioural symptoms of dementia: effectiveness of staff education and peer support. Aging Ment Health. 2008;12:47–55. doi:10.1080/13607860701366012 [DOI] [PubMed] [Google Scholar]
- 45. Baker R, Bell S, Baker E, et al. A randomized controlled trial of the effects of multi-sensory stimulation (MSS) for people with dementia. Br J Clin Psychol. 2001;40:81–96. doi:10.1348/014466501163508 [DOI] [PubMed] [Google Scholar]
- 46. Ballard CG, O’Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with melissa. J Clin Pharmacol. 2002;63:553–558. [DOI] [PubMed] [Google Scholar]
- 47. Bowles EJ, Griffiths DM, Quirk L, Brownrigg A, Croot K. Effects of essential oils and touch on resistance to nursing care procedures and other dementia-related behaviours in a residential care facility. Int J Aromath. 2002;12:22–29. doi:10.1054/ijar.2001.0128 [Google Scholar]
- 48. Finnema E Dröes RM, Ettema T et al. The effect of integrated emotion-oriented care versus usual care on elderly persons with dementia in the nursing home and on nursing assistants: a randomized clinical trial. Int J Geriatr Psychiatry. 2005;20:330–343. doi:10.1002/gps.1286 [DOI] [PubMed] [Google Scholar]
- 49. Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304:983–991. doi:10.1001/jama.2010.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Haight BK, Gibson F, Michel Y. The Northern Ireland life review/life storybook project for people with dementia. Alzheimers Dement, 2006;2:56–58. doi:10.1016/j.jalz.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 51. Leone E, Deudon A, Bauchet M et al. Management of apathy in nursing homes using a teaching program for care staff: the STIM-EHPAD study. Int J Geriatr Psychiatry. 2013;28:383–392. doi:10.1002/gps.3836 [DOI] [PubMed] [Google Scholar]
- 52. Magai C, Cohen CI, Gomberg D. Impact of training dementia caregivers in sensitivity to nonverbal emotion signals. Int Psychogeriatr. 2002;14:25–38. doi:10.1017/S1041610202008256 [DOI] [PubMed] [Google Scholar]
- 53. McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer’s disease: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:793–802. doi:10.1111/j.1532-5415.2005.53252.x [DOI] [PubMed] [Google Scholar]
- 54. Remington R. Calming music and hand massage with agitated elderly. Appl Nurs Res. 2002;51:317–323. doi:10.1097/00006199-200209000-00008 [DOI] [PubMed] [Google Scholar]
- 55. Särkämö T, Tervaniemi M, Laitinen S et al. Cognitive, emotional, and social benefits of regular musical activities in early dementia: randomized controlled study. Gerontologist. 2014;54:634–650. doi:10.1093/geront/gnt100 [DOI] [PubMed] [Google Scholar]
- 56. van de Ven G, Draskovic I, Adang EMM et al. Effects of dementia-care mapping on residents and staff of care homes: a pragmatic cluster-randomised controlled trial. PLoS One. 2013;8. doi:10.1371/journal.pone.0067325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Woods RT, Bruce E, Edwards RT et al. REMCARE: reminiscence groups for people with dementia and their family caregivers – effectiveness and cost-effectiveness pragmatic multicentre randomised trial. Health Technol Assess. 2012;16:v–xv, 1. doi:10.3310/hta16480 [DOI] [PubMed] [Google Scholar]
- 58. Pulsford D, Hope K, Thompson R. Higher education provision for professionals working with people with dementia: a scoping exercise. Nurse Educ Today. 2007;27:5–13. doi:10.1016/j.nedt.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 59. Brodaty H Draper B, Low LF. Nursing home staff attitudes towards residents with dementia: strain and satisfaction with work. J Adv Nurs. 2003;44:583–590. doi:10.1046/j.0309-2402.2003.02848.x [DOI] [PubMed] [Google Scholar]
- 60. Brooker D. Person-centred Dementia Care: Making Services Better. London, UK: Jessica Kingsley Publishers; 2007. [DOI] [PubMed] [Google Scholar]
- 61. McGilton KS, Rivera TM, Dawson P. Can we help persons with dementia find their way in a new environment?Aging Ment Health. 2003;7:363–371. doi:10.1080/1360786031000150676 [DOI] [PubMed] [Google Scholar]
- 62. Brownie S, Nancarrow S. Effects of person-centered care on residents and staff in aged-care facilities: a systematic review. Clin Interv Aging. 2013;8:1–10. doi:10.2147/CIA.S38589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Banerjee A, Armstrong P, Daly T, Armstrong H, Braedley S. “Careworkers don’t have a voice”: epistemological violence in residential care for older people. J Aging Stud. 2015;33:28–36. doi:10.1016/j.jaging.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 64. Ferrero-Arias J Goñi-Imízcoz M González-Bernal J, Lara-Ortega F, da Silva-González A, Díez-Lopez M. The efficacy of nonpharmacological treatment for dementia-related apathy. Alzheimer Dis Assoc Disord. 2011;25:213–219. doi:10.1097/WAD.0b013e3182087dbc [DOI] [PubMed] [Google Scholar]
- 65. Hattori H, Hattori C, Hokao C, Mizushima K, Mase T. Controlled study on the cognitive and psychological effect of coloring and drawing in mild Alzheimer’s disease patients. Geriatr Gerontol Int. 2011;11:431–437. doi:10.1111/j.1447-0594.2011.00698.x [DOI] [PubMed] [Google Scholar]
- 66. Lee J, Lee B, Park Y, Kim Y. Effects of combined fine motor skill and cognitive therapy to cognition, degree of dementia, depression, and activities of daily living in the elderly with Alzheimer’s disease. J Phys Ther Sci. 2015;27:3151–3154. doi:10.1589/jpts.27.3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Telenius EW, Engedal K, Bergland A. Effect of a high-intensity exercise program on physical function and mental health in nursing home residents with dementia: an assessor blinded randomized controlled trial. PLoS One. 2015;10:e0126102. doi:10.1371/journal.pone.0126102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Calkins MP. The physical and social environment of the person with Alzheimer’s disease. Aging Ment Health. 2001;5(suppl 1):74–78. doi:10.1080/713650003 [DOI] [PubMed] [Google Scholar]
- 69. Bach S, Grant A.. Communication and Interpersonal Skills in Nursing. Thousand Oaks, CA: Sage; 2011. [Google Scholar]
- 70. Doherty M, Thompson H. Enhancing person-centred care through the development of a therapeutic relationship. Br J Community Nurs. 2014;19:504–507. doi:10.12968/bjcn.2014.19.10.502 [DOI] [PubMed] [Google Scholar]
- 71. Nolan M, Davies S, Brown J et al. The role of education and training in achieving change in care homes: a literature review. J Res Nurs. 2008;13:411–433. doi:10.1177/1744987108095162 [Google Scholar]
- 72. Fazio S. Person-centered care in residential settings: taking a look back while continuing to move forward. Alzheimers Care Today. 2008;9:155–161. doi:10.1097/01.ALCAT.0000317200.58816.a3 [Google Scholar]
- 73. Happ MB, Williams CC, Strumpf NE, Burger SG. Individualized care for frail elders: theory and practice. J Gerontol Nurs. 1996;22:6–14. [DOI] [PubMed] [Google Scholar]
- 74. Suhonen R, Välimäki M, Katajisto J. Individualized care in a Finnish healthcare organization. J Clin Nurs. 2000;9:218–227. doi:10.1046/j.1365-2702.2000.00362.x [DOI] [PubMed] [Google Scholar]
- 75. Lynman KA. Brining the social back in: a critique of the biomedicalization of dementia. Gerontologist. 1989;29:597–605. [DOI] [PubMed] [Google Scholar]
- 76. Gawande A. Being Mortal: Medicine and What Matters in the End. New York, NY: Metropolitan Books-Henry Holt and Company; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.