Abstract
Introduction
Patients who receive or have received anti-programmed cell-death-1 (PD-1) monoclonal antibodies can develop immune-related adverse events due to activation of the immune system.
Case presentation
We report a case of a patient who received pembrolizumab and presented with cardiac tamponade. Despite pericardial drainage, she persisted with refractory arterial hypotension due to secondary adrenal insufficiency. After initiating corticosteroid therapy, the patient recovered successfully.
Discussion
The association of pericarditis, hypophysitis and thyroid dysfunction support the diagnosis of a life-threatening immune-related adverse event due to pembrolizumab. In case of immune-related adverse events secondary to anti-PD-1 monoclonal antibodies, corticosteroid therapy should be promptly initiated in order to avoid major complications.
Keywords: Pericarditis, Cardiac tamponade, Adrenal insufficiency, Pembrolizumab, Corticosteroids, Case report
Learning points
Immune-checkpoint inhibitors (ICIs) can induce several immune-related adverse events, including thyroid dysfunction, hypophysitis, and pericarditis.
Immune-mediated pericarditis secondary to ICIs is rare, but can be the first clinical manifestation of a wide spectrum of immune-related adverse events.
Prompt recognition of immune-related adverse events and the early use of corticosteroid therapy are crucial in order to avoid major complications.
Introduction
Immune-checkpoint inhibitors (ICIs) have demonstrated antitumor response in melanoma, non-small cell lung cancer and renal cell cancer, and are being tested in other tumours. However, a wide spectrum of immune-related adverse events has been observed, including pneumonitis, colitis, hypophysitis, hepatitis, or hypothyroidism. Occasional cases of cardiotoxicity, including myocarditis, heart block or pericarditis, have also been reported.
Although corticosteroids are not recommended as first-line treatment for acute pericarditis because of the risk of favouring a chronic evolution of the disease and promoting drug dependence,1 immune-mediated cardiotoxicity secondary to ICIs needs prompt steroid treatment to ensure favourable clinical outcomes.2
Timeline
| Time | Events |
|---|---|
| March–September 2016 | The patient received treatment with pembrolizumab |
| March 2017: Day 1 | Patient presented with cardiac tamponade. |
| Pericardiocentesis—260 mL of pericardial fluid was drained. | |
| Day 2 | Maintained hypotension despite pericardiocentesis. |
| Echocardiogram showed signs of constrictive pericarditis | |
| Day 2 (12 a.m.) | Anterior pericardiectomy |
| Day 2 (1 p.m.) | Noradrenaline was started due to refractory hypotension |
| Day 4 (12 a.m.) | Negative pericardial cultures: a bacterial infection of the pericardium was ruled out. |
| First dose of corticosteroids (2 mg/kg) | |
| Day 4 (6 p.m.) | Noradrenaline stopped—blood pressure recovered |
| Day 6 | A hormonal study of the pituitary confirmed a secondary adrenal insufficiency |
Case presentation
A 55-year-old woman was admitted to the Acute Cardiac Care Unit with the diagnosis of cardiac tamponade. She was a current smoker and was diagnosed with an infiltrating ductal carcinoma of the left breast cT2cN1cM0 in February 2016. Soon after, she enrolled in a clinical trial and received neoadjuvant chemotherapy based on five cycles of pembrolizumab (200 mg/tw), nab-paclitaxel (100 mg/m2) and carboplatin and four cycles of adriamycin (60 mg/m2) and cyclophosphamide (600 mg/m2), finishing the treatment in September 2016. The patient did not exhibit any relevant toxicity during chemotherapy. In October 2016, a lumpectomy and an axillary-lymph-node dissection were performed, followed by radiation therapy.
In March 2017, the patient presented to the emergency department with pericardial chest pain. She did not report shortness of breath or fever. On admission, blood pressure (BP) was 90/55 mmHg, pulse was 105 b.p.m., baseline oxygen saturation was 97% and temperature was 36°C. A pericardial rub was present, and lungs were clear. An electrocardiogram showed sinus tachycardia and decreased electrocardiographic voltage. During the first hours, the patient had transient episodes of severe hypotension, requiring fluid therapy. Pulsus paradoxus was present and an urgent transthoracic echocardiogram (TTE) showed moderate pericardial effusion and right atrial systolic collapse. Left ventricular ejection fraction (LVEF) was 55%, and troponin I levels were in the normal range. Emergent pericardiocentesis was performed, and 260 mL of pericardial fluid was drained. Then, BP increased transiently to 112/64 mmHg. Analysis of the fluid showed an exudate with 4.3 g/dL of proteins, 36 mg/dL of glucose, a lactate-dehydrogenase value of 1543 UI/L, and abundant cellularity (19 × 109 cells/L) with a predominant acute inflammatory component (93% neutrophils).
Despite pericardiocentesis, episodes of arterial hypotension (BP 65/45 mmHg) persisted during the following 12 h. However, pericardial drainage flow rate was low, urine output was preserved, and lactate levels were <2 mmol/L. Another TTE showed mild pericardial effusion and ventricular septal shift with preserved LVEF. The Doppler registry showed increased hepatic vein flow reversal with expiration (Figure 1 and Supplementary material online, Video S1). Due to the persistence of haemodynamic instability and the suspicion of constrictive pericarditis, an emergent anterior pericardiectomy was performed. The macroscopic evaluation showed a slightly thickened pericardium but without inflammation of the visceral layer so visceral pericardiectomy was not necessary. Histologic examination of the pericardium showed a huge infiltration of neutrophils, but no fibrous tissue or granulomatous inflammation (Figure 2).
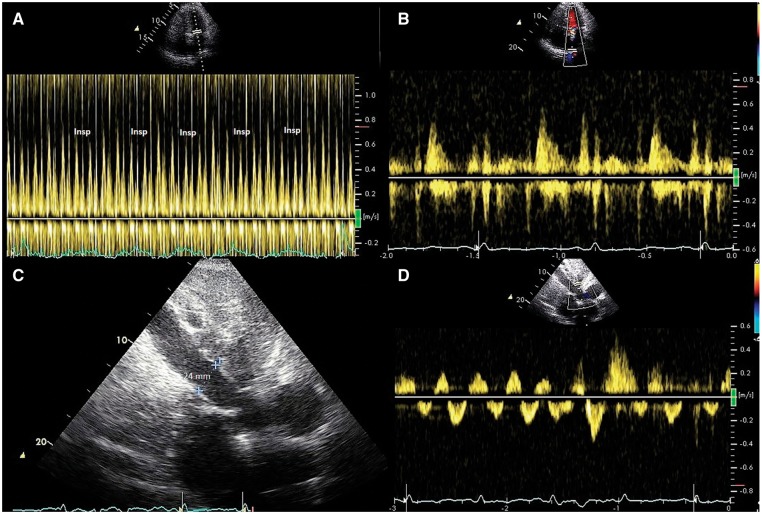
Figure 1.
(A) Pulsed Doppler recording of left ventricular inflow showing reduced early diastolic filling with inspiration. (B) Pulsed Doppler recording of pulmonary vein flow showing a prominent diastolic filling phase. (C) Subcostal view showing a dilated inferior vena cava reflecting the elevated right atrial pressure. (D) Pulsed Doppler recording of hepatic vein flow showing increased hepatic vein flow reversal with expiration.
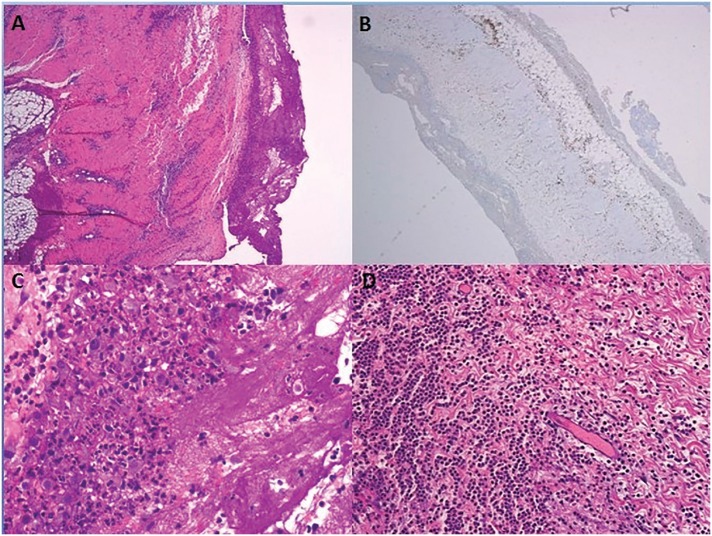
Figure 2.
(A) Acute pericarditis, haematoxylin, and eosin staining. (B) CD3+ infiltrated in the pericardium. (C, D) Huge infiltration in pericardium with predominance of neutrophils, although there are some lymphocytes.
Despite cardiac surgery, severe hypotension persisted requiring increasing doses of noradrenaline up to 0.5 mcg/kg/min. Moreover, acute pancytopenia appeared and haemoglobin, leucocytes and platelets fell from 11.9 to 7.6 g/dL (range 12–15 g/dL), 7.44 to 1.29 × 109/L (range 4–11 × 109/L) and 189 to 105 × 109/L (range 140–400 × 109/L), respectively. C-reactive protein increased to 28 mg/dL (normal < 0.5 mg/dL). Other blood investigations showed a low sodium level of 132.3 mmol/L (range 136–146 mmol/L) and a normal serum potassium of 4.32 mmol/L (range 3.5–5.1 mmol/L).
As cultures of the pericardial fluid, as well as antinuclear and antineutrophil cytoplasmic antibodies, were negative and no malignant cells were detected in the pericardial fluid, an immune-mediated pericarditis associated with adrenal insufficiency secondary to pembrolizumab was suspected and empiric treatment with intravenous corticosteroids 2 mg/kg/day was started.
Response to corticosteroids treatment was rapid. Blood pressure recovered and noradrenaline could be promptly stopped. Prior to steroid treatment, a hormonal study of the pituitary confirmed a secondary adrenal insufficiency [cortisol 0.93 μg/dL (range 5.27–22.45 μg/dL) and corticotropin <1.6 pg/mL (range 4.7–48.8 pg/mL)] and primary hypothyroidism [TSH 16.819 mU/L (range 0.55–4.78 mU/L) and T4 0.79 pg/mL (range 0.8–1.76 pg/mL)]. There was no other impaired pituitary secretion and a brain magnetic resonance ruled out metastasis to the pituitary gland. Adrenal insufficiency was treated with physiologic steroid replacement. A cardiac magnetic resonance (Figure 3) showed normal LVEF and diffuse pericardial thickening with diffuse pericardial enhancement, indicating pericardial inflammation. No myocardial delayed-contrast enhancement was observed. The patient was discharged a few days later. Six months after discharge, she has not developed any other immune-related adverse event and is doing well on low doses of corticosteroids.
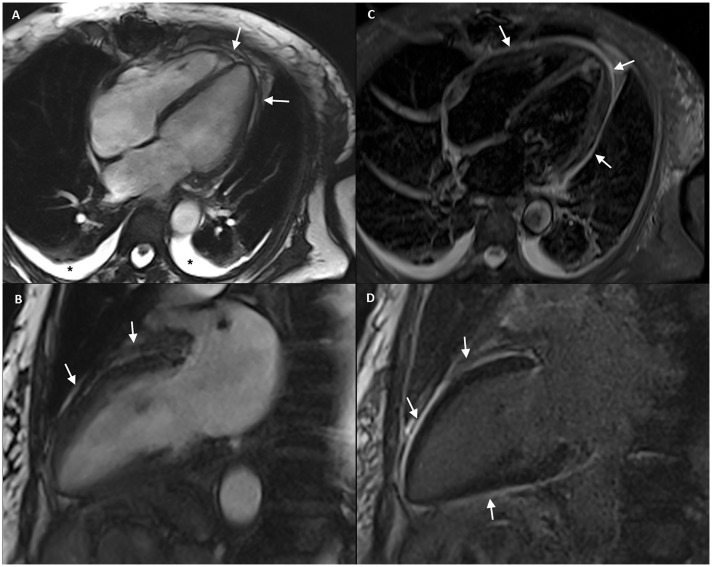
Figure 3.
(A) Four-chamber and (B) two-chamber end-diastole cine-RM images show a thickened pericardium without pericardial effusion (arrows). Note mild bilateral pleural effusion (asterisks). (C) Four-chamber short-tau inversion recovery (STIR) T2-weighted imaging shows diffuse pericardial hyperintensity suggesting pericardial oedema (arrows) and (D) two-chamber delayed-contrast-enhanced imaging depicts diffuse pericardial enhancement (arrows) indicating pericardial inflammation.
Discussion
Pericardial disease in oncological patients is relatively common. It may be secondary to cardiac metastases, as a consequence of radiotherapy, chemotherapy, or immunotherapy or it could also be bacterial or viral.3
Our report describes a case of acute pericarditis and profound hypotension in a patient who has been previously treated with pembrolizumab. As the patient presented with pericardial chest pain and pericardial effusion, hypotension was firstly attributed to cardiac tamponade. However, hypotension persisted after pericardiocentesis. The association between pericardial effusion, profound hypotension, and the analysis of the pericardial fluid made us consider a bacterial infection, but cultures of the pericardial fluid were negative. Troponin I levels were normal and LVEF remained preserved following pericardial drainage, so myopericarditis and pericardial decompression syndrome were ruled out. Recent radiotherapy made us also consider constrictive pericarditis. Although the echocardiogram showed evidence of constrictive physiology, this might have been a temporary form of constriction.4 The absence of inflammation of the visceral layer of the pericardium in the surgical examination and the absence of pericardial fibrosis in the histological examination ruled out constrictive pericarditis.
Hypotension was finally explained by a secondary adrenal insufficiency, which was probably worsened by the stress of cardiac surgery. In this scenario, corticosteroid therapy was successful in treating both adrenal insufficiency and acute pericarditis. The association of pericarditis, adrenal insufficiency secondary to hypophysitis, and hypothyroidism support the diagnosis of an immune-related adverse event due to pembrolizumab.
Immune-checkpoint inhibitors can induce several immune-related endocrinopathies, including thyroid dysfunction and hypophysitis.5 In addition, haematologic disorders, such as anaemia or thrombocytopenia, have also been described.6 Cardiotoxicity of ICIs is rare. Few cases of cardiac tamponade related to anti-programmed cell-death-1 immunotherapy have been reported,7–10 but none involve pembrolizumab. All cases were treated with pericardiocentesis and corticosteroids.7 Two cases of recurrent pleural effusion and cardiac tamponade secondary to nivolumab have been published. Both of them had a history of malignant pleural and pericardial effusions prior to treatment with nivolumab, mimicking disease progression.8 Moreover, two cases of late-onset cardiac tamponade related to ipilimumab (anti-CTLA4 antibody) have been published.9,10 In both cases, diagnosis was supported by the association of other immune-related adverse events.
To our knowledge, this is the first case report of cardiac tamponade and adrenal insufficiency secondary to pembrolizumab. Physicians treating patients with ICIs should be aware of their potentially late-onset cardiac and extracardiac effects. The early use of corticosteroids is crucial in order to avoid major complications. In our case, cardiac surgery might have been avoided.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Funding
This work was funded by CIBERCV and co-financed by the European Regional Development Fund (ERDF-FEDER).
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Imazio M, Gaita F.. Diagnosis and treatment of pericarditis. Heart 2015;101:1159–1168. [DOI] [PubMed] [Google Scholar]
- 2. Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ.. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 2017;19:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM.. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 4. Gentry J, Klein AL, Jellis CL.. Transient constrictive pericarditis: current diagnostic and therapeutic strategies. Curr Cardiol Rep 2016;18:41.. [DOI] [PubMed] [Google Scholar]
- 5. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M.. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017;13:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pföhler C, Eichler H, Burgard B, Krecké N, Müller CSL, Vogt T.. A case of immune thrombocytopenia as a rare side effect of an immunotherapy with PD1-blocking agents for metastatic melanoma. Transfus Med Hemother 2017;44:426–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kushnir I, Wolf I.. Nivolumab-induced pericardial tamponade: a case report and discussion. Cardiology 2017;136:49–51. [DOI] [PubMed] [Google Scholar]
- 8. Kolla BC, Patel MR.. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy—a report of two cases. J Immunother Cancer 2016;4:80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S.. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med 2015;2015:794842.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dasanu CA, Jen T, Skulski R.. Late-onset pericardial tamponade, bilateral pleural effusions and recurrent immune monoarthritis induced by ipilimumab use for metastatic melanoma. J Oncol Pharm Pract 2017;23:231–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.