Abstract
Introduction
Congenital long-QT (LQT) syndrome can lead to torsades de pointes (TdP), which can deteriorate into ventricular fibrillation resulting in sudden death. Thus far, more than 16 genes have been linked to the LQT syndrome. We report an orgasm-induced TdP in a patient with LQT syndrome type 2 with a novel mutation in the KCNH2 gene.
Case presentation
A 24-year-old Caucasian woman with a medical history of depression, no medication use and no family history of sudden death, presented with recurrent syncope during sexual activity. Immediately after achieving orgasm during sexual intercourse she lost consciousness. Baseline 12-lead electrocardiogram revealed a wide based T-wave with a prolonged QTc-interval of 507 ms. During hospital admission runs of TdP were recorded. The patient was treated with magnesium, an oral beta-blocker, and an implantable cardioverter-defibrillator. Genetic testing (Sanger sequencing) revealed a novel mutation (c.361del) in the KCNH2 gene (chromosome 7q36).
Discussion
To date, orgasm-induced TdP as a first symptom in a patient with LQT2 has not been published previously. In studies with continuous blood sampling in healthy volunteers, large peaks in plasma epinephrine levels during orgasm were observed with fast post-orgasmic decline. However, in a large cohort study (402 patients of which 129 with LQT2), no patients experienced cardiac events during sexual activity, suggesting that these are indeed very rare. Nevertheless, the high levels of sympathetic adrenal hormones during orgasm may explain the timing of the TdP in our patient. The patient has remained free of syncope at 6 months of follow-up.
Keywords: Case report, Torsades des pointes, Long-QT syndrome, Novel mutation
Learning points
Orgasm-induced torsades des points, although extremely rare, could be a first symptom in a patient with long-QT (LQT) syndrome type 2.
A ‘c.361del-mutation’ in the KCNH2 gene, encoding the alpha-subunit of the delayed rectifier potassium channel current, is associated with the LQT syndrome type 2 with the establishment of genotype-fenotype (torsades des pointes) correlation.
Introduction
Congenital long-QT (LQT) syndrome can lead to torsades de pointes (TdP) and is characterized by the occurrence of transient syncope. TdP or polymorphic ventricular tachycardia can deteriorate into ventricular fibrillation, which causes cardiac arrest resulting in sudden death.1 Thus far, more than 16 genes have been linked to the LQT syndrome.1,2 Although deaths are mainly provoked by sympathetic activation during emotional or physical stress, some deaths occur during sleep.3 Patients with LQT2 are not expected to be at special risk during physical activity.4 Sympathetic stimulation produces a greater increase in both transmural dispersion of repolarization and spatial dispersion of repolarization in LQT1 than in LQT2 syndrome, which may explain why LQT2 patients are less sensitive to sympathetic stimulation.3 Nevertheless, a large proportion of LQT2 patients experience a first cardiac event associated with an acute arousal or exercise trigger.1 We report an orgasm-induced TdP in a patient with LQT syndrome type 2 with a novel mutation in the KCNH2 gene.
Timeline
| Day | Events |
|---|---|
| 1 | Patient experiences syncope after sexual activity on reaching orgasm. After tapping her face, consciousness was recovered within seconds, and she was transferred to our Cardiology unit by the emergency services |
| Physical examination and laboratory investigations revealed no abnormalities | |
| Baseline 12 lead electrocardiogram revealed a wide based T-wave with a prolonged QT interval of 520 ms with a heart rate of 56 b.p.m. | |
| 3 | Patient became tachypneuic, dizzy and was unresponsive for a few seconds. Runs of torsades de pointes were recorded simultaneously on telemetry |
| Magnesium was administered intravenously | |
| A beta-blocker (metoprolol) was started | |
| 8 | Patient was treated with an implantable cardioverter-defibrillator |
| 35 | Genetic testing revealed a mutation in the KCNH2 gene encoding the alpha-subunit of the delayed rectifier potassium channel |
Case presentation
A 24-year-old Caucasian woman with a medical history of depression and no medication use presented with recurrent syncope during sexual activity. There was no family history of sudden death. Immediately after achieving orgasm during sexual intercourse she became tachypneuic and her whole body was shaking. Subsequently, she lost consciousness. Her partner noticed an absent pulse and started mouth-to-mouth resuscitation. She regained consciousness within minutes, and she was transferred to our Cardiology unit by the emergency services. She had experienced a similar episode several months earlier during orgasm. Physical examination revealed no abnormalities. Baseline 12-lead electrocardiogram (ECG) revealed a wide based T-wave with a prolonged QTc-interval of 507 ms (corrected QTc according to the Framingham formula; Figure 1). Laboratory investigations of our patient revealed a sodium level of 141 mmol/L (normal range 135-145 mmol/L), a potassium level of 3.7 mmol/L (normal range 3.5–5 mmol/L), and a calcium level of 2.22 mmol/L with a normal albumin level. A chest X-ray was completely normal. Bicycle exercise testing revealed exercise dependent prolonging of the PQ-interval. Also, her family underwent ECG testing. Her father’s ECG showed also an abnormally LQT-interval, but he never experienced a cardiac event (syncope or cardiac arrest).
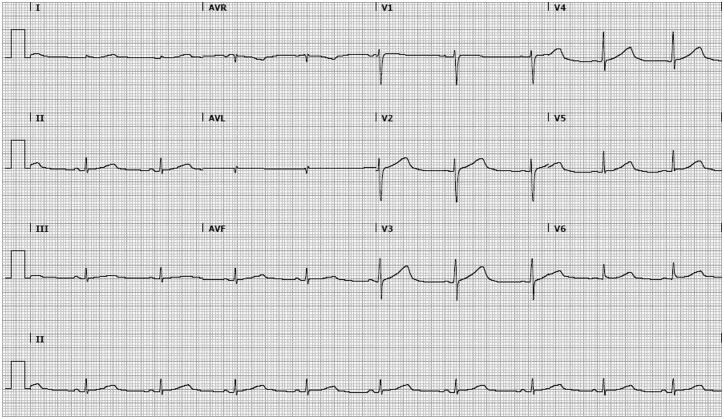
Figure 1.
Electrocardiographic findings in our patient with long-QT syndrome type 2. A standard 12-lead electrocardiogram shows a wide based T-wave and a prolonged QTc-interval of 507 ms (heart rate of 56 b.p.m., paper speed 25 mm/s).
During visiting hours in the hospital our patient again became tachypneuic, dizzy and was unresponsive for a few seconds. Runs of TdP were recorded simultaneously on telemetry as shown in Figure 2. This episode was similar to the previous episode during sexual intercourse. The patient was treated with magnesium intravenously after which sinus rhythm returned. Also an oral beta-blocker (metoprolol 100 mg once daily) was started. Subsequently, she was treated with a dual chamber pacemaker defibrillator (DDD-ICD). Genetic testing (Sanger sequencing) revealed a novel mutation (c.361del) in the KCNH2 gene. This mutation leads to a change in the reading frame at codon Ala121 and the introduction of a premature stop codon (p.[Ala121Leufs*12]). The KCNH2 gene is located on chromosome 7q36 encoding the alpha-subunit of the delayed rectifier potassium (K+) channel current (Ikr). Ikr is one of the major determinants of phase 3 repolarization of the cardiac action potential in ventricular cardiomyocytes.4
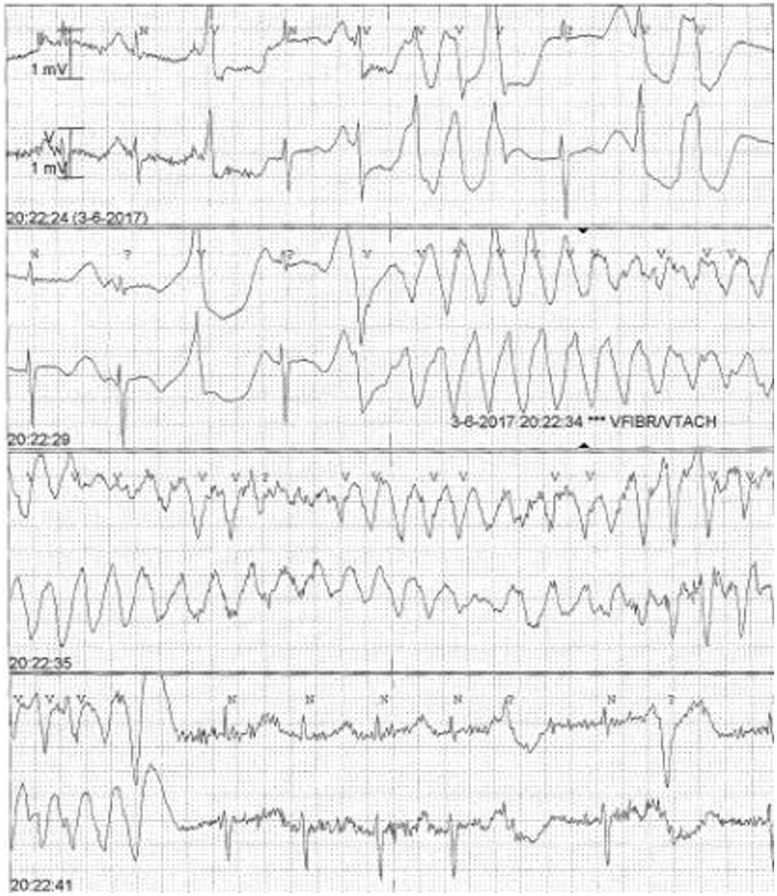
Figure 2.
Electrocardiographic findings in our patient with long-QT syndrome type 2. Torsades de pointes are shown initiated by a short-long-short RR interval due to premature ventricular beats.
One month after presentation the patient was doing very well as assessed in the outpatient clinic. She performed an outpatient cardiac rehabilitation program after discharge. Goal of this programme was to restore her quality of life and to maintain functional capacity.5 Components of the rehabilitation program include patient assessment, physical activity counselling, exercise training, and psychosocial management. Both the metoprolol and the implantable cardioverter-defibrillator (ICD) were well tolerated during daily life. In addition, an ECG was performed (during treatment with a beta-blocker) in the outpatient clinic as shown in Figure 3. This ECG showed an atrial paced rhythm with a QTc-interval of 444 ms. The patient has remained free of syncope at 8 months of follow-up. ICD follow-up showed 56% atrial pacing and no ventricular pacing at 8 months of follow-up. No premature ventricular contractions or ventricular tachyarrhythmias were recorded. Also, no ventricular therapy was observed.
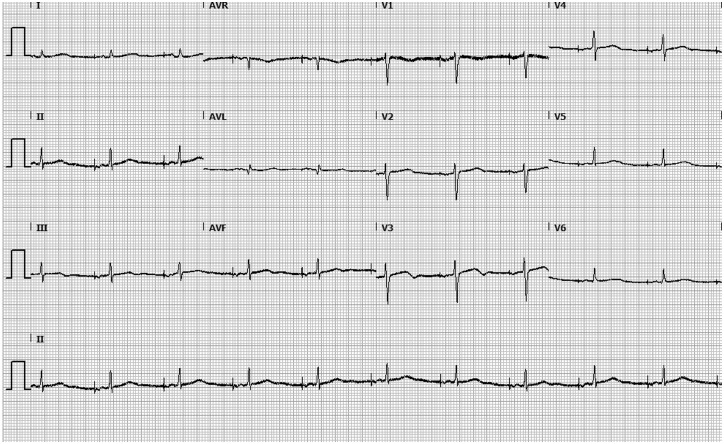
Figure 3.
Electrocardiographic findings in our patient with long-QT syndrome type 2 during treatment with a beta-blocker and an implantable cardioverter-defibrillator. A standard 12-lead electrocardiogram shows an atrial paced rhythm with a QTc-interval of 440 ms.
Discussion
LQTS may be either congenital or acquired. Several factors could lead to a prolonged QT-interval which may trigger TdP including electrolyte imbalance (most importantly hypokalaemia) and drug therapy. Also, increased risk of TdP, which may result in sudden cardiac death, is associated with ischaemic heart disease. The clinical presentation of patients with LQTS is variable and is influenced by genotype, drug therapy and sex.6
In this case, a mutation in the KCNH2 gene is reported with the establishment of genotype-fenotype correlation. This mutation has been identified before.7 To date however, orgasm-induced TdP as a first symptom in a patient with LQT2 with a ‘c.361del-mutation’ in the KCNH2 gene has not been published previously. In studies with continuous blood sampling in healthy volunteers, large peaks in plasma epinephrine levels during orgasm were observed with fast post-orgasmic decline.8 However, in a large cohort study (402 patients of which 129 with LQT2), no patients experienced cardiac events during sexual activity, suggesting that these are indeed very rare.9 Nevertheless, the high levels of sympathetic adrenal hormones during orgasm may explain the timing of the TdP in our patient.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Morita H, Wu J, Zipes DP.. The QT syndromes: long and short. Lancet 2008;372:750–763. [DOI] [PubMed] [Google Scholar]
- 2. Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med 2008;358:169.. [DOI] [PubMed] [Google Scholar]
- 3. Tanabe Y, Inagaki M, Kurita T, Nagaya N, Taguchi A, Suyama K, Aihara N, Kamakura S, Sunagawa K, Nakamura K, Ohe T, Towbin JA, Priori SG, Shimizu W.. Sympathetic stimulation produces a greater increase in both transmural and spatial dispersion of repolarization in LQT1 then LQT2 forms of congenital long-QT syndrome. J Am Coll Cardiol 2001;37:911–919. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz PJ, Ackerman MJ, George AL, Wilde AAM.. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol 2013;62:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corra U, Piepoli MF, Carre F, Heuschmann P, Hoffmann U, Verschuren M, Halcox J, Giannuzzi P, Saner H, Wood D, Piepoli MF, Corra U, Benzer W, Bjarnason-Wehrens B, Dendale P, Gaita D, McGee H, Mendes M, Niebauer J, Zwisler A-DO, Schmid J-P.. Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training: key components of the position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur Heart J 2010;31:1967–1974. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locate EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A, Robinson JL, Benhorin J, Choi S.. The long QT syndrome: prospective longitudinal study of 328 families. Circulation 1991;84:1136–1144. [DOI] [PubMed] [Google Scholar]
- 7. https://www.ncbi.nlm.nih.gov/clinvar/RCV000182054/#evidence (29 January 2018).
- 8. Krüger TH, Hartmann U, Schedlowski M.. Prolactinergic and dopaminergic mechanisms underlying sexual arousal and orgasm in humans. World J Urol 2005; 23:130–138. [DOI] [PubMed] [Google Scholar]
- 9. Loar RW, Bos JM, Cannon BC, Ackerman MJ.. Sudden cardiac arrest during sex in patients with either catecholaminergic polymorphic ventricular tachycardia or long-QT syndrome: a rare but shocking experience. J Cardiovasc Electrophysiol 2015; 26:300–304. [DOI] [PubMed] [Google Scholar]