Abstract
The use of direct oral anticoagulants (DOACs) is on the rise in the general population. However, data related to the safety of DOACs in patients with malignancy are limited. In this brief report, we present a series of three cases of haemorrhagic pericardial effusions and tamponade in patients receiving DOACs while undergoing cancer therapy. These three cases were all observed within a period of 6 weeks at a single institution and occurred shortly after the initiation of anticoagulation with one of the DOACs. Two of these patients had evidence of neoplastic pericardial process and were being treated with immunotherapy. The third patient was receiving targeted cancer therapy with a drug known to be associated with increased bleeding risks. Haemorrhagic pericarditis may represent a unique type of DOACs-related complications in subgroups of cancer patients with neoplastic pericardial disease and/or complex pharmacodynamics drug–drug interaction. The purpose of this report is to raise awareness of the lack of conclusive safety data of DOACs in certain cancer patients and to remind clinical providers of the National Comprehensive Cancer Network guidelines recommending against their use in patients with malignancy on the basis of limited safety data in patients undergoing cancer therapies.
Keywords: Tamponade, Haemorrhagic pericardial effusion, Direct oral anticoagulants, Case series, Neoplastic pericardial diseases
Introduction
Learning points
Haemorrhagic pericarditis may represent a unique type of DOACs-related complications in subgroups of cancer patients with neoplastic pericardial disease.
Caution is advised when using DOACs in cancer patients, especially when a pathological pericardial process is suspected or if they were to be combined with drugs that strongly interact with CYP3A4 or P-glycoprotein and when using chemotherapy drugs that are known to be associated with platelet dysfunction or increased bleeding risks.
Direct oral anticoagulants (DOACs) have been approved for the management of venous thrombo-embolism and atrial fibrillation. These drugs have been shown to have similar efficacy and bleeding risks to those of other standard anticoagulants.1,2 However, The National Comprehensive Cancer Network guidelines recommend against using DOACs in cancer patients3 on the basis of limited data regarding their safety in these patients.
In this report, we present three cases of spontaneous pericardial haemorrhages in cancer patients receiving DOACs while undergoing cancer therapy. The purpose of this report is to increase awareness about the potential bleeding complications of these drugs in patients with cancer.
Timeline
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Time 0 | |||
| Indication for anticoagulation | Deep vein thrombosis | Pulmonary embolism | Atrial fibrillation |
| Type of DOAC | Rivaroxaban | Rivaroxaban | Apixaban |
| Malignancy | Lung adenocarcinoma | Tongue squamous cell | CLL |
| Cancer therapy | Nivolumab | PDL-1 inhibitor | Ibrutinib |
| Time 1 | |||
| Onset of symptoms after DOAC | 2 days | 8 weeks | 2 days |
| Presenting symptoms | Chest pain and dyspnoea | Chest pain and dyspnoea | Shock |
| Time 3 | |||
| Echocardiographic findings | Cardiac tamponade | Cardiac tamponade | Cardiac tamponade |
| Symptoms after pericardiocentesis | Immediate relief | Immediate relief | Immediate relief |
| Volume drained (mL) | 540 | 610 | 700 |
| Pericardial fluid appearance | Haemorrhagic | Haemorrhagic | Haemorrhagic |
| Pericardial fluid cytology | Adenocarcinoma cells | Squamous cell carcinoma | No cancer cells |
Case series
Case 1
A 43-year-old woman was undergoing treatment with nivolumab for metastatic adenocarcinoma of the lung following chemoradiation therapy. Her primary tumour was adjacent to the pericardium, as indicated by a star in Figure 1. Around the time of her 11th cycle of nivolumab, she was started on rivaroxaban 20 mg once daily for asymptomatic deep vein thrombosis. Her baseline coagulation profile, platelet count, and hepatic and renal functions were all normal. Two days following the initiation of anticoagulation, she presented to the emergency department with symptoms of acute shortness of breath and a pleuritic, moderately severe, chest pain. A chest computed tomography (CT) scan revealed the presence of a large pericardial effusion that had not been present on a previous similar study 3 weeks earlier (Figure 1A and B). Transthoracic echocardiography confirmed the presence of a large pericardial effusion with associated tamponade physiological features. Urgent pericardiocentesis was performed, and 540 mL of haemorrhagic fluid was drained, with a cytological analysis demonstrating adenocarcinoma cells. The procedure provided the patient with an instant and a complete symptom resolution and both nivolumab and rivaroxaban were discontinued. The patient did not receive any other form of anticoagulation following this event.
Figure 1.
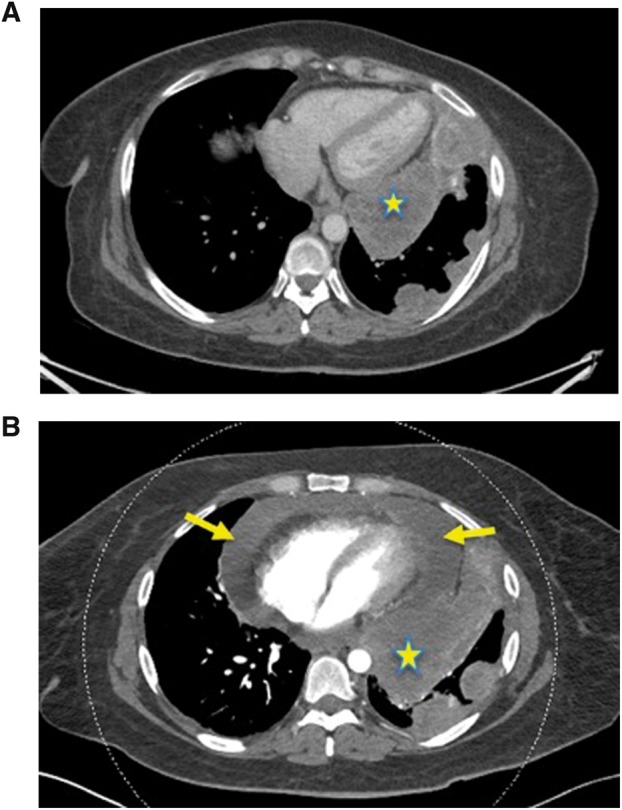
(A) Patient with lung cancer undergoing immunotherapy. Large tumour adjacent to the pericardium (yellow star), with no evidence of pericardial effusion. (B) Patient with lung cancer undergoing immunotherapy. Large pericardial effusion 48 h after the initiation of anticoagulation with rivaroxaban (arrows).
Case 2
A 47-year-old man with refractory squamous cell carcinoma of the tongue who was undergoing experimental immunotherapy with programmed death ligand 1 (PDL-1) inhibitor was incidentally found to have a small pulmonary embolism on a routine staging CT scan of the chest. He was started on rivaroxaban 15 mg twice daily for 1 week, followed by 20 mg once daily. His baseline coagulation profile, platelet count, and hepatic and renal functions were all normal. Eight weeks later, he presented to the emergency department with a history of progressively worsening dyspnoea and mild pleuritic chest pain for the last 4 days. A chest CT scan excluded pulmonary embolism but revealed a new pericardial effusion. Echocardiography confirmed the presence of a large pericardial effusion with tamponade physiological features. Urgent pericardiocentesis provided symptom relief after the removal of 610 mL of haemorrhagic fluid. Cytological studies confirmed the presence of malignant cells. The patient fully recovered and was able to resume his cancer therapy without rivaroxaban or any other form of anticoagulation.
Case 3
A 71-year-old man with a history of refractory B-cell chronic lymphocytic leukaemia was started on ibrutinib. He presented to the emergency room for palpitations 5 days after the initiation of ibrutinib and was diagnosed with the new-onset atrial fibrillation. His baseline echocardiogram was unremarkable. He underwent cardioversion within 24 h and was discharged home on apixaban 5 mg twice daily. His baseline coagulation profile, platelet count, and hepatic and renal functions were all normal. He was rehospitalized 48 h later for acute shortness of breath; he experienced rapid progression into shock with acute hepatic injury and renal failure. He was found to have a new large pericardial effusion on CT scan of the chest (Figure 2A and B) and confirmed by echocardiography. Emergent pericardiocentesis was performed, and 700 mL of haemorrhagic fluid was drained. Fluid cytological and flow cytometric studies did not reveal any malignant cells. The patient fully recovered and was discharged home. Ibrutinib was permanently discontinued, and the patient did not receive any other form of anticoagulation following this event.
Figure 2.
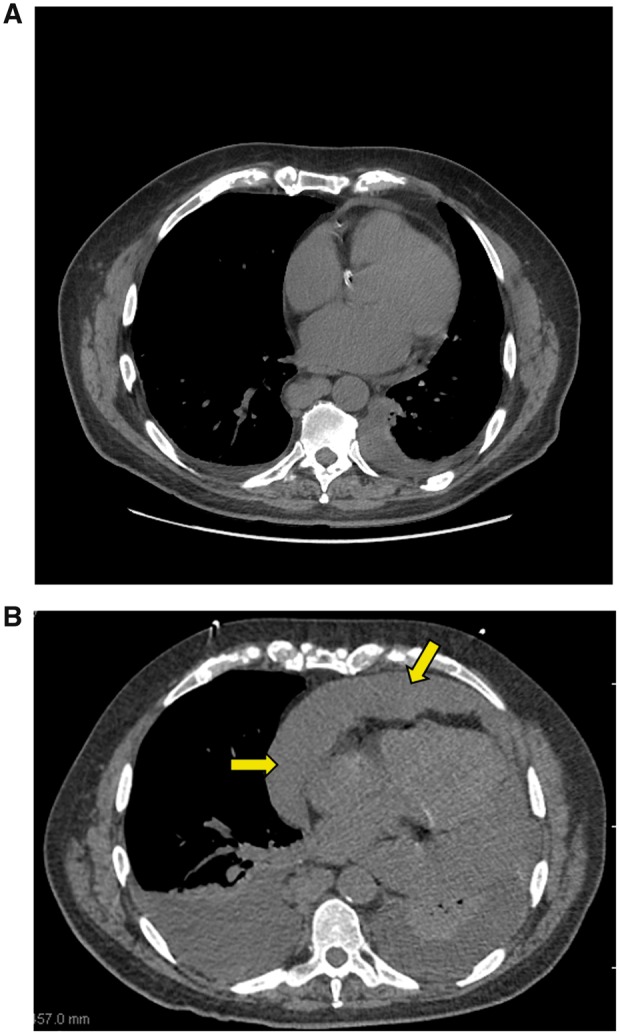
(A) Patient with chronic lymphocytic leukaemia receiving ibrutinib. Normal-appearing pericardium at baseline computed tomography scan. (B) Patient with chronic lymphocytic leukaemia receiving ibrutinib. Large pericardial effusion 48 h after the initiation of anticoagulation with apixaban (arrows) is shown.
Discussion
These cases, which all occurred within a period of 6 weeks from each other at a single institution, illustrate how patients undergoing cancer therapy may experience an adverse reaction to DOACs, leading to potentially life-threatening internal bleeding complications.
Because of the proven efficacy and safety of DOACs in the general population, their use is on the rise. Only a handful of cases of DOAC-induced pericardial haemorrhages in non-cancer patients have been reported in the literature.4–6 However, data on their use in patients with active malignancies, especially in those undergoing chemotherapy or immunotherapy, are limited. In fact, the scarce data on the safety and effectiveness of DOACs in cancer patients have been derived mainly from limited observational studies and several small subgroup analyses in large clinical trials of mainly non-cancer patients.7–9 These meta-analyses have the usual inherent limitations related to the heterogeneity of trial protocols, such as patient baseline clinical characteristics and pre-defined outcomes and complications.
Patients with cancer are not only at increased risk of thrombosis but also at increased risk of bleeding. Moreover, there are several clinical and metabolic features in cancer patients that can alter the DOACs pharmacodynamics with secondary unpredictable clinical response to these drugs: These features include altered renal and hepatic functions, cancer cachexia and malnutrition, coagulopathy and thrombocytopenia, and, more importantly, the unpredicted response caused by drug–drug interaction with cancer therapies. In fact, data on the combined use of chemotherapeutic agents and DOACs are rare. Direct oral anticoagulants interact with CYP3A4 and P-glycoprotein, making them theoretically susceptible to plasma concentration fluctuations when they are taken with inhibitors or inducers of these enzymes. Several categories of chemotherapeutic agents, including antimitotic microtubule inhibitors, tyrosine kinase inhibitors, and immune-modulating agents, are known substrates to CYP3A4 or P-glycoprotein.10,11 Theoretically, these types of pharmacodynamics drug–drug interactions can lead to the attenuation of the effects of DOACs, which increases the risk of thrombosis, or exacerbate the anticoagulation effects of DOACs, which leads to an increase in bleeding risks. The current National Comprehensive Cancer Network guidelines recommend against the use of DOACs in patients with active cancer.3 These recommendations are based mainly on the many reasons listed above and will likely hold true until more safety data are available. There are currently multiple ongoing randomized and observational trials investigating the safety and efficacy of these drugs in cancer patients (clinicaltrials.gov: NCT02048865, NCT02073682, NCT01708850 and NCT01727427) that will hopefully further clarify the role of these drugs in managing cancer patients.
The exact mechanism that led to these three cases of haemorrhagic pericardial effusions and tamponade is not well defined but perhaps can be partially explained by drug metabolism and pharmacodynamics concepts. Amplification of the DOACs effect leading to excessive anticoagulation, the degree of which cannot be adequately quantitated, should be considered. It is known that elevated levels of cytokines, interleukin 6 and tumour necrosis factor α are typically observed in cancer patients in general and even more so following immunotherapy.12 These cytokines have been shown to alter the pharmacokinetics of several drugs by down-regulating the expression and enzyme activity of the CYP3A4, the main enzyme responsible for the metabolism of rivaroxaban.
Similarly, the third case may be partially related to excessive anticoagulation because ibrutinib has been well documented to increase major bleeding risks,13 and in our case, this effect may have been potentiated by apixaban.
Other potential mechanisms that could explain haemopericardium in the first two cases include the presence of neoplastic pericardial disease with the evidence of pericardial malignant involvement, as demonstrated by chest imaging in the first case and by pericardial fluid cytological examination in the first two cases, and the possibility of subclinical pericarditis induced by immunotherapy (well-known and reported side effect of immunotherapy).14 These two conditions can evolve into haemorrhagic effusion when associated with DOAC-induced anticoagulation.
Conclusion
In conclusion, our purpose with this report is to raise awareness of the potential for increased internal bleeding risks in cancer patients receiving DOACs while undergoing certain cancer therapies. This is especially important because well-established alternative therapies, specifically low-molecular-weight heparin, have been proven to be effective and safe with even limited data, suggesting paraneoplastic effect with reduction in tumour progression of certain cancers such as melanoma and colon cancer.3,15 Until a better understanding of bleeding risks related to DOACs’ pharmacodynamics interaction with chemotherapy and a better evidence of clinical safety are both available, we recommend vigilance and caution when using DOACs in cancer patients, in particular when a pathological pericardial process is suspected or if they were to be combined with drugs that strongly interact with CYP3A4 or P-glycoprotein. We also caution against their use with chemotherapy drugs that are known to be associated with platelet dysfunction or increased bleeding risks.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Author Contributions: All authors had substantial contribution to this work and they all take full responsibility for the contents of the manuscript, including review and approval of this version.
References
- 1. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S.. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–2510. [DOI] [PubMed] [Google Scholar]
- 2. Agnelli G, Buller H, Cohen A, Gallus A, Raskob G, Weitz J, Prins M, Brandjes D, Kolbach D, Limburg M, Mac GM, Otten JM, Peters R, Roos Y, Segers A, Slagboom T, Bounameaux H, Hirsh J, Samama MM, Wedel H.. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 3. Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Somerfield MR, Falanga A.. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol 2015;33:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu B, MacIsaac A.. Life-threatening haemorrhagic pericarditis associated with rivaroxaban. Int J Cardiol 2014;174:e75–e76. [DOI] [PubMed] [Google Scholar]
- 5. Barton CA, McMillian WD, Raza SS, Keller RE.. Hemopericardium in a patient treated with dabigatran etexilate. Pharmacotherapy 2012;32:e103–e107. [DOI] [PubMed] [Google Scholar]
- 6. Sigawy C, Apter S, Vine J, Grossman E.. Spontaneous hemopericardium in a patient receiving apixaban therapy: first case report. Pharmacotherapy 2015;35:e115. [DOI] [PubMed] [Google Scholar]
- 7. Prins MH, Lensing AW, Brighton TA, Lyons RM, Ehm J, Trajanovic M, Davidson BL, Beyer-Westendorf J, Pap ÁF, Berkowitz SD, Cohen AT, Kovacs MJ, Wells PS, Prandoni P.. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 2014;1:e37–e46. [DOI] [PubMed] [Google Scholar]
- 8. Vedovati MC, Germini F, Agnelli G, Becattini C.. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest 2015;147:475–483. [DOI] [PubMed] [Google Scholar]
- 9. Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, Christiansen AV, Friedman J, Le Maulf F, Peter N, Kearon C. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764–772. [DOI] [PubMed] [Google Scholar]
- 10. Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet 2013;52:69–82. 10.1007/s40262-012-0030-9 [DOI] [PubMed] [Google Scholar]
- 11. Gnoth MJ, Buetehorn U, Muenster U, Schwarz T, Sandmann S.. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther 2011;338:372–380. [DOI] [PubMed] [Google Scholar]
- 12. Harvey RD, Morgan ET.. Cancer, inflammation, and therapy: effects on cytochrome p450-mediated drug metabolism and implications for novel immunotherapeutic agents. Clin Pharmacol Ther 2014;96:449–457. 10.1038/clpt.2014.143 [DOI] [PubMed] [Google Scholar]
- 13. Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Shaw Y, Bilotti E, Zhou C, James DF, O'brien S.. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015;125:2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S.. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med 2015;2015:794842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franchini M, Mannucci PM.. Low-molecular-weight heparins and cancer: focus on antitumoral effect. Ann Med 2015;47:116–121. 10.3109/07853890.2015.1004361 [DOI] [PubMed] [Google Scholar]


