Abstract
Background
Lower muscle and higher fat mass are characteristics of older adults; their physical function is also characterized by slower gait speed and weaker strength. However, the association between specific body composition and physical function is unclear.
Methods
We examined the association between body composition and physical performance using combined cross-sectional data of 1,821 participants from 13 clinical studies at Wake Forest University that used a consistent battery of tests. All participants were ≥60 years old and had one of the following conditions: healthy, osteoarthritis, coronary artery disease, obesity, heart failure, at elevated risk for disability, renal transplantation candidates, heart failure with preserved ejection fraction, moderate self-reported disability, hypertension, diabetes, or coronary artery disease, at high risk for cardiovascular disease. Data at enrollment from each study using uniform tools including body mass index (BMI), dual energy x-ray absorptiometry, physical performance assessment using 4 m walk speed, five chair rise time, handgrip strength, short physical performance battery (17), and Pepper Assessment Tool for Disability were analyzed.
Results
Increased BMI was associated with slower walk speed, lower short physical performance battery, and higher Pepper Assessment Tool for Disability score. Increased percentage of body fat was associated with slower walk speed, lower hand grip strength, lower short physical performance battery scores, and higher Pepper Assessment Tool for Disability scores. Percent appendicular lean mass was associated with faster walk speed, higher handgrip strength, higher short physical performance battery, and lower Pepper Assessment Tool for Disability score. There were no significant discrepancies in relationship between body composition and physical function by gender except gender and BMI on chair-rise time.
Conclusions
Higher BMI and percent body fat were associated with poor physical function while percent appendicular lean mass was associated with better physical function. There was no significant discrepancy in the by gender.
Keywords: Body composition, BMI, Body percent (%) fat, Physical function, Percent appendicular lean mass, SPPB, 4 m walk speed
Translational Significance
This study, which used data from 1821 older adult participants with various comorbidities, found association between high percent body fat and high BMI with poor strength and limitation in mobility and daily activities. We also found that lean body mass was associated with better strength, mobility and performance in daily activities. The results suggest that preventing adiposity and increasing muscle mass in older people may be an effective strategy to delay loss of physical function.
The number of people who were 65 years and older in the United States was 41.4 million, accounting for 13.3% of the total population in 2011. It is projected that the population of people aged 65 years and older will become 92 million by year 2060, representing one in five U.S. residents (United States Census Bureau, 2013).
Aging causes changes in body composition such as decrease in muscle mass, increase in adiposity, and muscle fat infiltration (Gallagher et al., 2000; Goodpaster et al., 2006; Newman et al., 2003). Physical performance also declines with aging (Onder et al., 2002; Seeman et al., 1994). In the United States, 17% of adults aged 50–59 years have one or more physical limitations and the prevalence increases to 23%, 31%, and 43% in age groups of 60–69, 70–79, and 80 years and over, respectively (Holmes, Powell-Griner, Lethbridge-Cejku, & Heyman, 2009). Physical limitations can impair independent lifestyle and quality of life (Kaplan, 1992). With the projected increase in the older population, it is predicted that a significant number of individuals will experience declines in physical function and disability.
Exercise has been shown to be an effective intervention to prevent disability and the focus has been on aerobic activities. The U.S. Department of Health and Human Services recommended at least 150 min of aerobic activity every week and suggested walking as an activity with health benefits and a low risk of injury (National Heart, Lung, and Blood Institute, 2016) for all Americans aged 6 years and older. However, in a systematic review, progressive resistance training has been shown to result in improvement in physical function (Liu & Latham, 2009). Resistance training is known to cause increase in muscle strength as well as reduction in fat percentage (Nunes et al., 2016) and it is superior to aerobic exercise in increasing muscle mass and strength and reducing fat percentage (Chen, Chung, Chen, Ho, & Wu, 2017).
Declines in physical performance with aging and changes in body composition occur simultaneously. Nonetheless, it is not clear if certain body composition changes are responsible for the decline in physical performance. Although some studies have reported a positive association between muscle mass and physical performance in older adults (Reid, Naumova, Carabello, Phillips, & Fielding, 2008; Visser, Deeg, Lips, Harris, & Bouter, 2000), other studies reported no association between muscle mass and physical performance but a clear negative relationship between fat mass and physical performance (Bouchard, Beliaeff, Dionne, & Brochu, 2007; Jankowski et al., 2008; Visser et al., 1998; Woo, Leung, & Kwok, 2007). Older women are known to have higher fat mass, lower skeletal muscle mass, and poorer physical function and higher prevalence of disability compared to men (Chen & Guo, 2008; Holmes et al., 2009; Jankowski et al., 2008), and there is a question of interaction of gender and body composition on physical function: some studies reported different relationships between body composition and physical performance by gender (Newman et al., 2003; Valentine, Misic, Rosengren, Woods, & Evans, 2009; Visser et al., 2000).
Possible reasons for conflicting results include: (a) various methods of measuring body composition and physical performance used in prior studies, (b) undetected interactions between gender and body composition on physical function (prior studies were not large enough to test possible interactions), and (c) possible interactions among comorbid conditions affecting associations between body composition and physical function. Therefore this study used combined data from 13 different clinical studies that enrolled participants with various aging-related comorbidities, all of whom completed a consistent battery of relevant tests. These included body mass index (BMI), body composition analysis using dual energy x-ray absorptiometry (DXA), and physical performance assessment using 4 m walk speed, five chair rise time, hand grip strength, short physical performance battery (SPPB) (Guralnik et al., 1994), and Pepper Assessment Tool for Disability (PAT-D). We analyzed the relationship between specific body composition and physical performance, using uniform physical performance and body composition measurement tools, in populations with various comorbidities from enrollment assessment data of 13 clinical studies.
Methods
Subjects
We analyzed data from participants in 13 clinical studies conducted at Wake Forest University (WFU) between 1996 and 2010. These studies assessed body composition and physical function in a total of 1,821 older individuals (see Table 1) at enrollment of each study. For current analyses, we included participants who were aged 60 years at the time of enrollment. All of the subjects signed an informed consent form and these studies were approved by the WFU Institutional Review Board.
Table 1.
Summary of Participants Included in This Report Per Study (N = 1,821)
| Study name and acronym | Condition(s) | N | Female (%) | Whites (%) | Mean age, years (SD) |
|---|---|---|---|---|---|
| Combined | 1,821 | 1,183 (65) | 1,460 (80) | 69.8 (6.5) | |
| Arthritis, Diet, and Activity Promotion Trial (ADAPT) | Knee osteoarthritis | 265 | 187 (71) | 203 (77) | 68.4 (6.0) |
| Cooperative Lifestyle Intervention Program (CLIP) | Evidence of myocardial infarction (MI), percutaneous coronary transluminal intervention (PCTI), chronic stable angina, cardiovascular surgery, or an ATP III diagnosis of metabolic syndrome | 287 | 192 (67) | 235 (82) | 67.1 (4.8) |
| Diet, Exercise and Metabolism in Older Women (DEMO) | Obese women | 46 | 46 (100) | 37 (80) | 64.9 (2.6) |
| Leg blood flow, sarcopenia and physical function (FLOW) | 15 with heart failure vs 15 healthy | 23 | 11 (48) | Unknown | 68.8 (6.8) |
| Database of determinants of physical function in healthy older persons (HEALTHY) | Healthy—free of chronic diseases | 60 | 37 (62) | 57 (95) | 69.8 (7.4) |
| Intensive Diet and Exercise for Arthritis (IDEA) | Knee osteoarthritis | 342 | 239 (70) | 283 (83) | 67.4 (5.4) |
| Lifestyle Interventions and Independence for Elders-Pilot (LIFE-P) | Elevated risk of disability | 269 | 194 (72) | 215 (80) | 76.7 (4.1) |
| Optimizing Body Composition in Older Adults (OPTIMA) | At risk for disability and with indications for weight loss | 88 | 40 (45) | 78 (89) | 70.6 (3.6) |
| Impact of a Physical Activity Intervention on Physical Function and Quality of Life in Aging Candidates for Renal Transplantation (PART) | Renal transplant candidates | 24 | 7 (29) | 15 (63) | 65.1 (4.6) |
| Pharmacological Intervention in the Elderly (PIE) | Heart failure with normal ejection fraction | 48 | 39 (81) | 42 (88) | 71.2 (7.2) |
| Resistance Training to Increase Muscle POWER in Older Adults (POWER) | Moderate self-reported disability | 45 | 34 (76) | 40 (89) | 75.3 (5.7) |
| Vascular Stiffness and Pulmonary Congestion (PREDICT) | History of diabetes, hypertension, or prior coronary artery disease | 109 | 62 (57) | 87 (80) | 72.7 (7.5) |
| ACE Inhibition and Novel Cardiovascular Risk Factors (TRAIN) | High cardiovascular risk profile | 215 | 95 (44) | 168 (78) | 68.9 (6.2) |
Measurement
Body composition
Body mass index was calculated using body weight in kilograms divided by height in meters squared. Body composition was measured using DEXA on a Hologic scanner (Hologic, Bedford, MA). A whole body scan was used to determine total body fat mass. Percent body fat was calculated by dividing total body fat by the sum of bone, lean, and fat mass. Regional analyses were performed and mineral-free lean mass of the arms and legs were summed to calculate appendicular lean mass. Percent appendicular lean mass (%ALM) was calculated by dividing total appendicular lean mass in kilograms by weight in kilograms.
Physical function
Hand grip strength was measured in both hands using an adjustable grip strength dynamometer (Jamar Model No. BK7498; Fred Sammons, Inc., Burr Ridge, IL). Participants performed the test three times with each hand, and the maximum overall value was used in the analyses.
The SPPB consists of three timed measures: a 4 m walk, repeated chair rise, and a balance test (Guralnik et al., 1994). To measure walking speed, the participants were asked to walk at their usual pace over a 4-m course. Duplicate measurements were done, and the faster measure was used to compute walking speed. For the repeated chair rise, participants were asked to stand from a sitting position without using their arms. Those who could do so were asked to stand up and sit down five times at their fastest speed. Balance was measured by asking the participants to maintain balance in three positions with a progressive narrowing of the base of support: side by side, semitandem, and tandem. Each task was scored from 0 to 4, with 4 indicating the highest performance and 0 inability to perform the task, based on the rubric from the Established Populations for Epidemiologic Studies of the Elderly (Guralnik et al., 2000). A total score was calculated and ranged from 0 to 12.
The Pepper Assessment Tool for Disability (PAT-D) is a 19-item self-administered questionnaire to assess mobility, activities of daily living (ADL) and instrumental activities of daily living (IADL). Responses are made on a five-point Likert scale ranging from 1 (“usually did with no difficulty”) to 5 (“unable to do”), or a box can be checked that reads “usually did not do for other reasons” (Rejeski, Ip, Marsh, Miller, & Farmer, 2008). The summary score, a mean of the three domain scores that ranges from 1 to 5, is an indication of a person’s overall perceived disability.
Statistical Analysis
Multiple linear regression was used to characterize the strength of the relationships between physical function measures (4-m walk speed, repeated chair rise time, grip strength, SPPB, and PAT-D) and body composition (BMI, percentage of body fat, and percent appendicular lean mass) within each study while controlling for age, gender, and race (individual study model). Multiple linear regression models were then used to determine the associations between physical function and body composition in all participants combined, adjusted for study effect using dummy variables, in addition to age, gender, and race (combined model). Residual plots were produced for the combined analyses to examine the patterns across studies. We found the residual patterns were consistent across different studies for all the physical function measure outcomes and body composition predictors. We investigated the consistency of relationships across gender by testing for interactions between gender and body composition. This was done by adding an interaction term in the combined model described above. If the test for the interaction was statistically significant, it indicated that the relationship between physical function measure and body composition depended on gender; otherwise, we concluded that the relationship was consistent across both genders. A p value ≤ .05 was considered statistically significant for all comparisons except for the tests of interaction (p ≤ .10). All analyses were performed with SAS 9.3 (Cary, NC).
Results
There were 1,821 participants from 13 studies included in the analyses. The mean age of the participants was 69.8 (±6.5), 1,183 (65%) were female, and 1,460 (80.2%) were White. Table 2 describes body composition and physical performance of the participants by studies and combined at enrollment of each study.
Table 2.
Body Composition and Physical Function of Participants Per Study
| Study | BMI, kg/m2 (SD) | % body fat (SD) | %ALM (SD) | 4-m walk speed, m/s (SD) | Chair-rise, seconds (SD) | Grip strength, kg (SD) | SPPB score, 0–12 (SD) | PAT-D (SD) |
|---|---|---|---|---|---|---|---|---|
| Combined | 31.3 (5.3) | 37.1 (7.9) | 25.8 (4.1) | 1.0 (0.2) | 15.4 (6) | 30.2 (11) | 9.6 (2) | 1.5 (0.5) |
| ADAPT | 33.1 (5.0) | 1.9 (0.6) | ||||||
| CLIP | 32.8 (3.9) | 1.1 (0.2) | 14.7 (4) | 9.9 (2) | 1.4 (0.4) | |||
| DEMO | 33.8 (4.1) | 43.9 (3.5) | 22.6 (1.2) | 1.2 (0.2) | 13.3 (3) | 30.6 (7) | 10.7 (1) | 1.2 (0.3) |
| FLOW | 26.9 (4.2) | 1.2 (0.3) | 12.3 (2) | 35.9 (12) | 10.9 (1) | 1.4 (0.7) | ||
| HEALTHY | 25.9 (4.9) | 31.5 (8.5) | 28.3 (4.4) | 1.2 (0.2) | 11.3 (3) | 34.2 (12) | 11.2 (1) | 1.1 (0.2) |
| IDEA | 33.5 (3.9) | 39.8 (6.7) | 24.3 (3.5) | 1.0 (0.2) | 12.4 (4) | 28.6 (10) | 10.8 (1) | 1.7 (0.5) |
| LIFE-P | 30.1 (6.3) | 37.7 (7.3) | 24.9 (3.6) | 0.8 (0.2) | 19.8 (9) | 24.3 (9) | 7.6 (1) | |
| OPTIMA | 32.7 (5.4) | 36.7 (7.5) | 26.7 (3.7) | 1.0 (0.2) | 17.7 (5) | 33.4 (10) | 9.0 (1) | 1.2 (0.2) |
| PART | 30.8 (5.5) | 0.9 (0.2) | 14.8 (3) | 34.3 (10) | 9.1 (2) | 1.3 (0.4) | ||
| PIE | 29.6 (4.8) | 38.1 (7.8) | 25.6 (4.0) | 1.2 (0.2) | 15.3 (4) | 28.4 (9) | 9.7 (2) | 1.9 (0.6) |
| POWER | 30.3 (5.8) | 38.2 (6.2) | 25.0 (3.1) | 1.0 (0.3) | 18.7 (8) | 9.1 (2) | 1.9 (0.5) | |
| PREDICT | 28.9 (5.1) | 1.1 (0.2) | 14.3 (3) | 29.9 (12) | 9.9 (2) | 1.5 (0.5) | ||
| TRAIN | 27.8 (4.6) | 33.3 (8.2) | 28.4 (4.3) | 1.2 (0.2) | 14.4 (4) | 37.6 (12) | 10.2 (2) | 1.4 (0.5) |
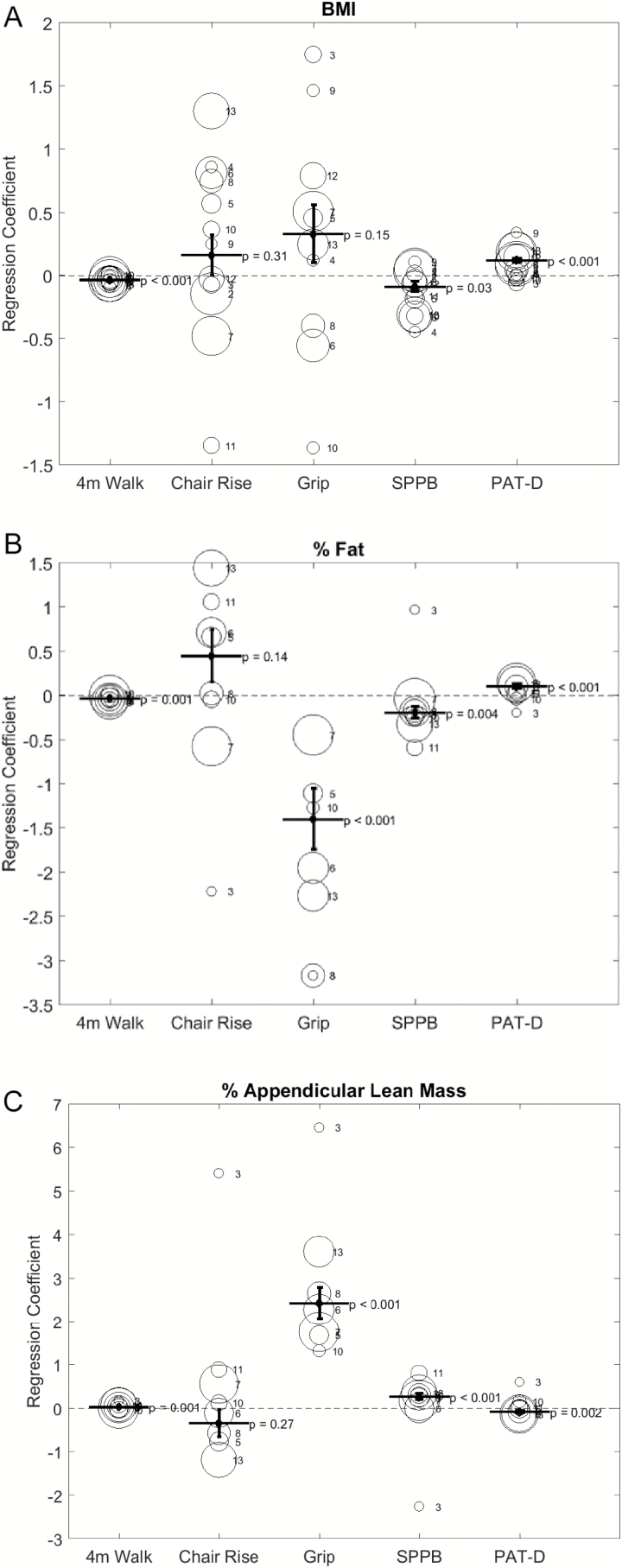
We examined associations between body composition and physical performance measure using multiple linear regression analyses for each study and for all studies combined. Figure 1 describes the relationship between body composition and physical performance. In the combined analysis, after adjusting for age, gender, race, and study, higher BMI was associated with slower walking speed (p < .001), lower SPPB score (p = .03), and higher PAT-D score(p < .001) (Figure 1A). Higher percent body fat was associated with slower walking speed (p = .001), lower handgrip strength (p < .001), lower SPPB score (p = .004), and higher PAT-D score (p < .001) (Figure 1B). Percent appendicular lean mass (appendicular lean mass/weight) was associated with faster walking speed (p = .001), stronger hand grip strength (p < .001), higher SPPB score (p < .001), and lower PAT-D score (p = .002) (Figure 1C). Across the studies, the association between each body composition and 4-m walk speed, SPPB and PAT-D were consistent in general.
Figure 1.
Regression Coefficient of each body composition for physical function assessments. 4-m walk = 4-m walk speed; Chair Rise = Repeated chair rise time; Grip = Hand grip strength; SPPB = Short Physical Performance Battery score; PAT-D = The Pepper Assessment Tool for Disability. Each bubble denotes each with the area of the bubble represents the size of the study. 1 = ADAPT, 2 = CLIP, 3 = DEMO, 4 = FLOW, 5 = HEALTHY, 6 = IDEA, 7 = LIFE-P, 8 = OPTIMA, 9 = PART, 10 = PIE, 11 = POWER, 12 = PREDICT, 13 = TRAIN.
Given prior studies reported different relationships between body composition and physical function by gender, further analyses were done by gender. In women, a higher BMI was associated with slower walking speed (p < .001), lower SPPB score (p = .05), and higher PAT-D score (p < .001) (Supplementary Figure 1A). In addition, percent body fat was associated with slower 4-m walk speed (p = .004), lower handgrip strength (p = .002), and higher PAT-D score (p = .001) (Supplementary Figure 1B). Women also showed an association between percent appendicular lean mass and faster 4-m walk speed (p = .01), stronger hand grip strength (p < .001), and higher SPPB score (p = .01) (Supplementary Figure 1C). In men, a higher BMI was associated with a higher PAT-D score (p < .001) (Supplementary Figure 2A). In addition, a higher percent body fat in men was associated with longer repeated chair rise time (p = .05), lower handgrip strength (p = .01), and lower SPPB score (p = .03) (Supplementary Figure 2B). Percent appendicular lean mass was associated with stronger handgrip strength (p < .001), higher SPPB score (p = .01), and lower PAT-D score (p = .02) (Supplementary Figure 2C). We also examined gender and body composition interactions on each measure of physical performance. We found significant interactions between gender and BMI only for the multiple chair rise test (p = .017).
Discussion
In this report, we combined information from 13 previous clinical studies, using a consistent battery of tests administered in 1,821 participants. We analyzed associations between body composition and physical performance across these studies, which included older adults with various comorbidities. We found that markers of obesity, such as BMI and percent body fat, were consistently associated with poor physical performance. This trend was apparent in slower walk speeds, lower SPPB scores, and higher PAT-D scores. On the other hand, increased muscle mass (i.e., percent appendicular lean mass) was associated with faster walk speed, stronger grip strength, higher SPPB score, and lower PAT-D score. All these associations of physical performance were independent of age, gender, and race. Although we saw significant interactions of gender in the association of BMI and chair rise time, the associations of body composition and physical function were generally independent of gender.
Our study demonstrated that both anthropometric measurement and direct measurement of obesity are consistently associated with poor physical function. This deleterious effect of obesity on physical function has been shown before; multiple hypotheses have been proposed to explain the relationship. The first theory is that obesity creates low-grade, chronic inflammation (Lumeng & Saltiel, 2011). Adipose tissue produces inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6 (IL-6) (Coppack, 2001). Adipose tissue also demonstrates increased activation of intracellular kinases, such as c-jun N-terminal kinase (an inhibitor of κ kinases) and protein kinase R, which can induce inflammation (Nakamura et al., 2010; Solinas & Karin, 2010). Activators of inflammation such as inflammasome and the Toll-like receptors are activated in adipose tissue (Schroder, Zhou, & Tschopp, 2010; Shi et al., 2006). In addition, inflammatory cells like macrophages and T cells infiltrate into adipose tissues (Feuerer et al., 2009; Weisberg et al., 2003). This increased systemic inflammation caused by obesity can cause inflammation in skeletal muscle. In a study of datasets that were included in our group analyses, Brinkley and colleagues demonstrated that higher levels of C-reactive protein and IL-6 were associated with lower grip strength, lower SPPB scores, and longer times to complete the 4-m walk test and repeated chair stands test (Brinkley et al., 2009). Our current study included more studies and explored the association between body composition and physical function.
The second hypothesis is the biomechanical effect of obesity on physical performance. Although obesity is associated with increased muscle mass, obese subjects have relative muscle weakness for their weight and lower fatigue resistance (Maffiuletti et al., 2007), resulting in altered knee and pelvis kinematics (Lerner, Board, & Browning, 2014). These biomechanical changes can cause decline in physical performance. Third, a sedentary lifestyle is associated with obesity and worse physical performance due to deconditioning. Fourth, obesity is associated with certain musculoskeletal diseases, such as osteoarthritis and gout (Magliano, 2008); these conditions in turn can cause decline in physical function. Finally, another possible explanation between obesity and poor physical performance is the effect of weight loss effort on muscle mass in older adults. Currently, the main approach of weight loss is dietary calorie restriction. However, dietary caloric restriction not only results in reduction in fat mass but also induces decrease in lean muscle mass, although consumption of a protein supplement might attenuate the weight loss-induced muscle loss (Smith & Mittendorfer, 2015). Weight regain is common after intentional weight loss, and 80% of individual who lose >10% body weight will regain the weight within one year (Wing & Hill, 2001). If there are a string of episodes of weight loss and weight regain, in the absence of resistance training, the weight regained will be mostly body fat as opposed to muscle mass. Over time, body composition would become worse (e.g., higher percent fat, lower percent muscle), making traditional weight loss an iatrogenic cause for poor physical function. It is possible that participants in our study with obesity have tried dietary caloric restriction in the past that resulted in lower muscle mass, higher fat mass, and poor physical function.
While the negative association between body fat content and physical function has been consistent (Baumgartner et al., 1998; Lebon, Barsalani, Payette, Brochu, & Dionne, 2016; Visser et al., 1998), the association between muscle mass and physical function has been inconsistent (Bouchard et al., 2007; Janssen, Baumgartner, Ross, Rosenberg, & Roubenoff, 2004; Woo, Leung, Sham, & Kwok, 2009). Also, there are reports of different relationships between muscle mass and physical performance by gender (Valentine et al., 2009). Bioelectrical impedance analysis (BIA) or DXA are common ways to measure body composition. Prior studies used several methods of quantifying relative muscle mass; skeletal muscle index (SMI) is calculated by dividing total skeletal muscle measured by BIA by weight (Janssen, Heymsfield, & Ross, 2002); appendicular skeletal muscle index (ASMI) or appendicular lean muscle index (ALMI) is defined as appendicular skeletal muscle mass (ASM) or appendicular lean mass (ALM), obtained by DXA divided by height squared (Baumgartner et al., 1998). An analysis of The Third National Health and Nutrition Examination Survey 1988–1994 of 4,449 older adults (≥60 years) reported higher rate of physical disability in participants with low skeletal muscle mass index, with J-shaped relations between SMI and physical disability in women (increased disability in both low and high SMI) (Janssen et al., 2004). In another study of 4,000 older adults, a U-shaped relationship was observed between ASMI and physical limitation (Woo et al., 2009). Our study demonstrated a positive relationship between percent appendicular lean mass (appendicular lean mass/body weight) and walking speed, hand grip strength, SPPB score, and a negative relationship with PAT-D. These associations were also demonstrated in analyses done by gender except the association with percent appendicular lean mass and walk speed did not reach statistical significance in men. When we used ALMI as a marker of muscle mass, we found positive association between ALMI and hand grip strength, and PAT-D score, while there was negative association with walking speed (data not shown) in combined analysis. ALMI tends to underestimate the muscle mass in tall subjects, whereas it overestimates the muscle mass in short and obese subjects, explaining the counterintuitive positive association between relative muscle mass and disability as well as negative association with walking speed. Our analysis demonstrated that percent appendicular lean mass is consistently associated with physical function in older adult participants, across genders.
Multiple studies have reported discrepancies between the relationship of body composition and physical function per gender (Newman et al., 2003; Valentine et al., 2009; Visser et al., 2000). In our analysis, we observed a generally consistent relationship between body composition and physical function. In the analyses of gender interactions on the associations between body composition and physical function, we found consistent association between body composition and measures of physical function (4-m walk speed, SPPB and PAT-D) across gender and studies in this analysis of 1,821 participants.
Strengths and Limitations
A major strength of this study is the use of reliable objective measures of body composition and physical function across 13 studies. Furthermore, combining 13 cohorts with over 1,800 participants afforded sufficient statistical power to examine different effects of body composition on physical function by gender.
However, our study also has some limitations. Since it was a cross-sectional analysis, we cannot draw any conclusions regarding causality. In addition, longitudinal studies might show different relationships between body composition and physical function, although there are reports that suggest associations similar to those we report here. Second, we included participants with various comorbidities who may not be representative of the general population of older people, so our findings may be difficult to generalize. As described in Table 1, our data include multiple studies that enrolled participants with various conditions and the two largest studies (IDEA and ADAPT studies) (Messier et al., 2013; Messier et al., 2004) enrolled participants with knee osteoarthritis, resulting over-representation of knee osteoarthritis to the general population (33% compared to 10%–13%) (Zhang & Jordan, 2010). This difference in comorbidity prevalence may have affected our findings and caution should be exercised in interpreting our findings. It is also possible that individuals with more severe disease conditions did not participate in the studies, so our sample may be skewed toward those with less severe illnesses and disabilities.
There are multiple studies that defined cutoff thresholds to identify sarcopenia (Bahat et al., 2016; Newman et al., 2003) including the European consensus on definition and diagnosis of sarcopenia (Cruz-Jentoft et al., 2010). Our study did not use any cutoff threshold to define sarcopenia but rather treated percent appendicular muscle mass as a continuous variable and if we divided the group to sarcopenia/nonsarcopenia, we might have seen different associations between body composition and physical function. However, the purpose of our study was not to define sarcopenia or to examine the association of sarcopenia and physical function.
In summary, this study confirmed a positive association between muscle mass and better physical function, and a negative association between obesity and physical function, using data from 13 previous clinical studies in 1,821 older adult participants with various comorbidities. Using combined data from 13 different studies to reduce bias associated with a particular study sample, we found association between high percent body fat and high BMI, which is partly determined by body fat with poor strength, limitation in mobility and daily activities. We also identified that lean body mass was associated with better strength, mobility and performance in daily activities. The results suggest that preventing adiposity and increasing muscle mass in older persons may be an effective strategy to delay loss of physical function.
Funding
This study was funded in part by the Grants for Early Medical/Surgical Specialists’ Transition to Aging Research (GEMSSTAR, R03 AG050919) and the Claude D. Pepper Center Older Americans Independence Center (P30 AG21332) (S. Kim), Wake Forest School of Medicine, Winston-Salem, NC.
Conflict of Interest
None reported.
Supplementary Material
References
- Bahat G., Tufan A., Tufan F., Kilic C., Akpinar T. S., Kose M., … Cruz-Jentoft A. J (2016). Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clinical Nutrition. doi:10.1016/j.clnu.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Baumgartner R. N., Koehler K. M., Gallagher D., Romero L., Heymsfield S. B., Ross R. R., … Lindeman R. D (1998). Epidemiology of sarcopenia among the elderly in New Mexico. [Comparative Study Research Support, U.S. Gov’t, P.H.S.]. American Journal of Epidemiology, 147, 755–763. [DOI] [PubMed] [Google Scholar]
- Bouchard D. R., Beliaeff S., Dionne I. J., & Brochu M (2007). Fat mass but not fat-free mass is related to physical capacity in well-functioning older individuals: Nutrition as a determinant of successful aging (NuAge)--the Quebec Longitudinal Study. [Research Support, Non-U.S. Gov’t]. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 62, 1382–1388. [DOI] [PubMed] [Google Scholar]
- Brinkley T. E., Leng X., Miller M. E., Kitzman D. W., Pahor M., Berry M. J., … Nicklas B. J (2009). Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. [Comparative Study Research Support, N.I.H., Extramural]. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 64, 455–461. doi:10.1093/gerona/gln038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., & Guo X (2008). Obesity and functional disability in elderly Americans. [Comparative Study Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural]. Journal of the American Geriatrics Society, 56, 689–694. doi:10.1111/j.1532-5415.2007.01624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. T., Chung Y. C., Chen Y. J., Ho S. Y., & Wu H. J (2017). Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. Journal of the American Geriatrics Society, 65, 827–832. doi:10.1111/jgs.14722 [DOI] [PubMed] [Google Scholar]
- Coppack S. W. (2001). Pro-inflammatory cytokines and adipose tissue. [Review]. Proceedings of the Nutrition Society, 349–356. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft A. J., Baeyens J. P., Bauer J. M., Boirie Y., Cederholm T., Landi F., … Zamboni M (2010). Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. [Consensus Development Conference Practice Guideline Research Support, Non-U.S. Gov’t]. Age Ageing, 39, 412–423. doi:10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., … Mathis D (2009). Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Nature Medicine, 15, 930–939. doi:10.1038/nm.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D., Ruts E., Visser M., Heshka S., Baumgartner R. N., Wang J., … Heymsfield S. B (2000). Weight stability masks sarcopenia in elderly men and women. [Clinical Trial Research Support, U.S. Gov’t, P.H.S.]. American Journal of Physiology-Endocrinology and Metabolism, 279, EC6–375. [DOI] [PubMed] [Google Scholar]
- Goodpaster B. H., Park S. W., Harris T. B., Kritchevsky S. B., Nevitt M., Schwartz A. V., … Newman A. B (2006). The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. [Research Support, N.I.H., Intramural]. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 61, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Guralnik J. M., Ferrucci L., Pieper C. F., Leveille S. G., Markides K. S., Ostir G. V., … Wallace R. B (2000). Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 55, M221–231. [DOI] [PubMed] [Google Scholar]
- Guralnik J. M., Simonsick E. M., Ferrucci L., Glynn R. J., Berkman L. F., Blazer D. G., … Wallace R. B (1994). A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. [Research Support, U.S. Gov’t, P.H.S.]. The Journals of Gerontology, 49, M85–M94. [DOI] [PubMed] [Google Scholar]
- Holmes J., Powell-Griner E., Lethbridge-Cejku M., & Heyman K (2009). Aging differently: Physical limitations among adults aged 50 years and over: United States, 2001–2007. [Comparative Study]. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Jankowski C. M., Gozansky W. S., Van Pelt R. E., Schenkman M. L., Wolfe P., Schwartz R. S., & Kohrt W. M (2008). Relative contributions of adiposity and muscularity to physical function in community-dwelling older adults. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Obesity (Silver Spring), 16, 1039–1044. doi:10.1038/oby.2007.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I., Baumgartner R. N., Ross R., Rosenberg I. H., & Roubenoff R (2004). Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. American Journal of Epidemiology, 159, 413–421. [DOI] [PubMed] [Google Scholar]
- Janssen I., Heymsfield S. B., & Ross R (2002). Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Journal of the American Geriatrics Society, 50, 889–896. [DOI] [PubMed] [Google Scholar]
- Kaplan G. A. (1992). Maintenance of functioning in the elderly. Annals of Epidemiology, 2, 823–834. [DOI] [PubMed] [Google Scholar]
- Lebon J., Barsalani R., Payette H., Brochu M., & Dionne I. J (2016). Inflammation and fat mass as determinants of changes in physical capacity and mobility in older adults displaying a large variability in body composition: The NuAge Study. Experimental Aging Research, 42, 403–417. doi:10.1080/0361073X.2016.1224649 [DOI] [PubMed] [Google Scholar]
- Lerner Z. F., Board W. J., & Browning R. C (2014). Effects of obesity on lower extremity muscle function during walking at two speeds. [Research Support, N.I.H., Extramural]. Gait Posture, 39, 978–984. doi:10.1016/j.gaitpost.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. J., & Latham N. K (2009). Progressive resistance strength training for improving physical function in older adults. [Meta-Analysis Review]. Cochrane Database of Systematic Reviews, CD002759. doi:10.1002/14651858.CD002759.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng C. N., & Saltiel A. R (2011). Inflammatory links between obesity and metabolic disease. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. Journal of Clinical Investigation, 121, 2111–2117. doi:10.1172/JCI57132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffiuletti N. A., Jubeau M., Munzinger U., Bizzini M., Agosti F., De Col A., … Sartorio A (2007). Differences in quadriceps muscle strength and fatigue between lean and obese subjects. [Research Support, Non-U.S. Gov’t]. European Journal of Applied Physiology, 101, 51–59. doi:10.1007/s00421-007-0471-2 [DOI] [PubMed] [Google Scholar]
- Magliano M. (2008). Obesity and arthritis. [Review]. Menopause International, 14, 149–154. doi:10.1258/mi.2008.008018 [DOI] [PubMed] [Google Scholar]
- Messier S. P., Loeser R. F., Miller G. D., Morgan T. M., Rejeski W. J., Sevick M. A., … Williamson J. D (2004). Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. [Clinical Trial Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S.]. Arthritis & Rheumatology, 50, 1501–1510. doi:10.1002/art.20256 [DOI] [PubMed] [Google Scholar]
- Messier S. P., Mihalko S. L., Legault C., Miller G. D., Nicklas B. J., DeVita P., … Loeser R. F (2013). Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. [Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. JAMA, 310, 1263–1273. doi:10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Furuhashi M., Li P., Cao H., Tuncman G., Sonenberg N., … Hotamisligil G. S (2010). Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Cell, 140, 338–348. doi:10.1016/j.cell.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (2016). Recommendations for Physical Activity Retrieved May 1, 2017, from https://www.nhlbi.nih.gov/health/health-topics/topics/phys/recommend. [PubMed]
- Newman A. B., Kupelian V., Visser M., Simonsick E., Goodpaster B., Nevitt M., … Harris T. B (2003). Sarcopenia: Alternative definitions and associations with lower extremity function. [Comparative Study Research Support, U.S. Gov’t, P.H.S.]. Journal of the American Geriatrics Society, 51, 1602–1609. [DOI] [PubMed] [Google Scholar]
- Nunes P. R., Barcelos L. C., Oliveira A. A., Furlanetto Junior R., Martins F. M., Orsatti C. L., … Orsatti F. L (2016). Effect of resistance training on muscular strength and indicators of abdominal adiposity, metabolic risk, and inflammation in postmenopausal women: Controlled and randomized clinical trial of efficacy of training volume. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. Age (Dordr), 38, 40. doi:10.1007/s11357-016-9901-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Penninx B. W., Lapuerta P., Fried L. P., Ostir G. V., Guralnik J. M., & Pahor M (2002). Change in physical performance over time in older women: The Women’s Health and Aging Study. [Research Support, U.S. Gov’t, P.H.S.]. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 57, M289–293. [DOI] [PubMed] [Google Scholar]
- Reid K. F., Naumova E. N., Carabello R. J., Phillips E. M., & Fielding R. A (2008). Lower extremity muscle mass predicts functional performance in mobility-limited elders. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. Journal of Nutrition Health and Aging, 12, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski W. J., Ip E. H., Marsh A. P., Miller M. E., & Farmer D. F (2008). Measuring disability in older adults: The International Classification System of Functioning, Disability and Health (ICF) framework. [Research Support, N.I.H., Extramural]. Geriatrics & Gerontology International, 8, 48–54. doi:10.1111/j.1447-0594.2008.00446.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Zhou R., & Tschopp J (2010). The NLRP3 inflammasome: a sensor for metabolic danger? [Research Support, Non-U.S. Gov’t Review]. Science, 327, 296–300. doi:10.1126/science.1184003 [DOI] [PubMed] [Google Scholar]
- Seeman T. E., Charpentier P. A., Berkman L. F., Tinetti M. E., Guralnik J. M., Albert M., … Rowe J. W (1994). Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. [Multicenter Study Research Support, Non-U.S. Gov’t]. The Journals of Gerontology, 49, M97–108. [DOI] [PubMed] [Google Scholar]
- Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., & Flier J. S (2006). TLR4 links innate immunity and fatty acid-induced insulin resistance. [Research Support, N.I.H., Extramural]. Journal of Clinical Investigation, 116, 3015–3025. doi:10.1172/JCI28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. I., & Mittendorfer B (2015). Slimming down in old age. [Comment Editorial Research Support, N.I.H., Extramural]. American Journal of Clinical Nutrition, 101, 247–248. doi:10.3945/ajcn.114.103564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G., & Karin M (2010). JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. [Research Support, Non-U.S. Gov’t Review]. FASEB Journal, 24, 2596–2611. doi:10.1096/fj.09-151340 [DOI] [PubMed] [Google Scholar]
- United States Census Bureau (2013). Profile America facts for features Retrieved January 15, 2014, from http://www.census.gov/newsroom/releases/archives/facts_for_features_special_editions/cb13-ff07.html.
- Valentine R. J., Misic M. M., Rosengren K. S., Woods J. A., & Evans E. M (2009). Sex impacts the relation between body composition and physical function in older adults. [Research Support, N.I.H., Extramural]. Menopause, 16, 518–523. doi:10.1097/gme.0b013e31818c931f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M., Deeg D. J., Lips P., Harris T. B., & Bouter L. M (2000). Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. Journal of the American Geriatrics Society, 48, 381–386. [DOI] [PubMed] [Google Scholar]
- Visser M., Harris T. B., Langlois J., Hannan M. T., Roubenoff R., Felson D. T., … Kiel D. P (1998). Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 53, M214–221. [DOI] [PubMed] [Google Scholar]
- Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., & Ferrante A. W. Jr (2003). Obesity is associated with macrophage accumulation in adipose tissue. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Journal of Clinical Investigation, 112, 1796–1808. doi:10.1172/JCI19246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing R. R., & Hill J. O (2001). Successful weight loss maintenance. [Research Support, U.S. Gov’t, P.H.S. Review]. Annual Review of Nutrition, 21, 323–341. doi:10.1146/annurev.nutr.21.1.323 [DOI] [PubMed] [Google Scholar]
- Woo J., Leung J., & Kwok T (2007). BMI, body composition, and physical functioning in older adults. [Research Support, Non-U.S. Gov’t]. Obesity (Silver Spring), 15, 1886–1894. doi:10.1038/oby.2007.223 [DOI] [PubMed] [Google Scholar]
- Woo J., Leung J., Sham A., & Kwok T (2009). Defining sarcopenia in terms of risk of physical limitations: A 5-year follow-up study of 3,153 chinese men and women. [Research Support, Non-U.S. Gov’t]. Journal of the American Geriatrics Society, 57, 2224–2231. doi:10.1111/j.1532-5415.2009.02566.x [DOI] [PubMed] [Google Scholar]
- Zhang Y., & Jordan J. M (2010). Epidemiology of osteoarthritis. [Review]. Clinics in Geriatric Medicine, 26, 355–369. doi:10.1016/j.cger.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.