Abstract
Background
Implantable cardioverter defibrillator (ICD) therapy is indicated in patients with structural heart disease who have had an aborted cardiac arrest (ACA). After atrial repair of d-transposition of the great arteries (d-TGA, Mustard repair) patients seem to be at a higher risk of failing intraoperative subcutaneous ICD (S-ICD) shock testing.
Case summary
We report the case of a 45-year-old patient with congenital heart disease (CHD) who suffered a cardiac arrest from ventricular fibrillation and was subsequently implanted with a S-ICD. We describe the challenges of ICD therapy in patients after Mustard procedure for d-TGA, with the additional challenge of concomitant AAI pacemaker therapy. In this patient, we opted for the implantation of an S-ICD, and detail the necessary considerations and operative technique employed in this patient. A right parasternal electrode position was chosen and intraoperative shock testing was successful.
Discussion
Patients after atrial switch surgery for d-TGA and ACA require careful consideration of the appropriate type of ICD therapy. Subcutaneous ICD implantation with right parasternal electrode position may be a viable option in these patients.
Keywords: Subcutaneous ICD, Congenital heart disease, d-TGA, Case report, Sudden cardiac death
Learning points
Congenital heart disease patients after aborted sudden cardiac death require an individualized approach to implantable cardioverter defibrillator (ICD) therapy.
Subcutaneous ICD (S-ICD) therapy is a feasible and safe alternative in certain cases, but electrode placement requires special considerations in patients with d-transposition of the great arteries.
Although concomitant S-ICD therapy in patients with a pacemaker is not recommended, it is feasible and safe in individual cases.
Introduction
In patients with abnormal vascular access to the right ventricle, subcutaneous implantable cardioverter defibrillator (S-ICD) therapy has become a viable alternative to conventional transvenous ICD implantation.1,2 Patients with d-transposition of the great arteries (d-TGA) after atrial correction carry a high risk of sudden cardiac death due to malignant arrhythmia.3–5 In these patients, transvenous ICD implantation presents unique challenges and is associated with a high risk of lead failure.6,7 Subcutaneous ICD implantation may be an alternative in this population, but some evidence suggests, that the shock efficacy may be lower in these patients.8
Timeline
| Date | Events |
|---|---|
| 1973 | Atrial correction for d-transposition of the great arteries (Mustard) |
| 1986 | Implantation of a single chamber AAI pacemaker due to sinus node dysfunction |
| 2016 | Implantation of an additional atrial lead because of lead dysfunction |
| March 2018 | Out of hospital cardiac arrest because of ventricular fibrillation, with successful resuscitation |
| April 2018 | Implantation of a subcutaneous implantable cardioverter defibrillator with successful shock testing at 65 J |
Case presentation
We report a case of S-ICD implantation with right-parasternal electrode position in a 45-year-old male congenital heart disease (CHD) patient after atrial switch surgery, due to d-TGA, with a concomitant AAI pacemaker.
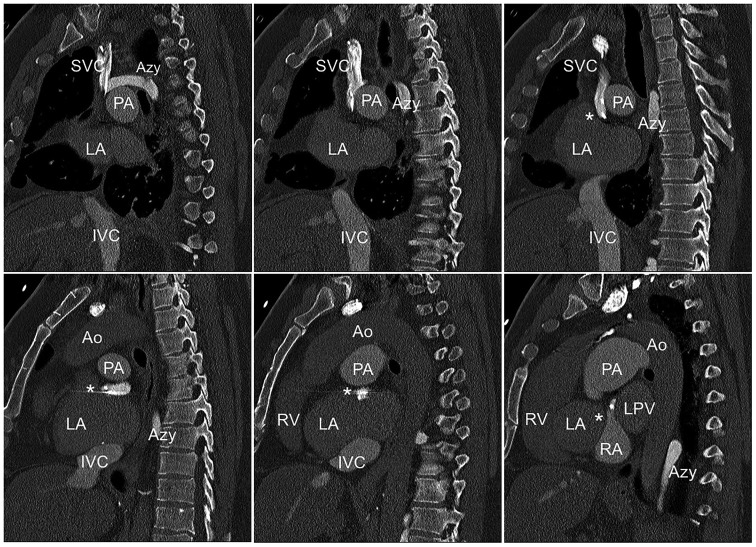
The patient had undergone atrial switch surgery at the age of one and an AAI pacemaker was implanted at age 14 years, following atrial asystole. An additional atrial lead was implanted at age 44 years, because of lead failure. The patient had no other pre-existing conditions beyond being overweight and was not taking any regular medication. He had suffered an out of hospital cardiac arrest due to ventricular fibrillation on 8 March 2018 and was successfully resuscitated by emergency medical services. After spontaneous circulation was re-established by external defibrillation (360 J) of ventricular fibrillation, the patient regained consciousness. On the 12-lead ECG, the patient was in sinus rhythm and did not show signs of myocardial ischaemia. On physical examination at admission to our institution, the patient was alert and oriented without neurologic impairment. He complained of chest pain at inspiration, which could be reproduced by rib compression. Vital signs were SpO2 95% under 5 L/min O2, RR 120/70 mmHg, heart rate 70 b.p.m., and regular. Myocardial ischaemia was excluded by high-sensitive troponin T assay and pulmonary embolism and aortic dissection were excluded in a thoracic computed tomography (CT) angiography. Laboratory testing revealed no relevant electrolyte imbalances. Furthermore, coronary artery disease and coronary anomalies were excluded by coronary angiogram. As the patient did not show any neurological sequelae, secondary prevention ICD implantation was indicated. On CT coronary angiography, which was performed to better assess the complex anatomy prior to ICD implantation, access to the subpulmonary ventricle was revealed to be hindered by the two atrial leads and the narrow diameter of the venous baffle connecting the vena cava superior with the subpulmonary ventricle (Figure 1). Also, the projected shock path of a transvenous ICD would not have included the systemic ventricle, which was suspected to be the arrhythmogenic ventricle. Taking into consideration the young age of the patient, the anatomical challenges, the shock vector of a transvenous ICD implantation, and the potential future need for stent implantation within the narrow baffle, we decided to recommend implantation an S-ICD. The patient gave his consent after detailed consultation.
Figure 1.
Reconstruction of a pre-operative computed tomography scan showing the narrow baffle (asterisk) and the two atrial leads. Ao, aorta; Azy, azygos vein; IVC, inferior vena cava; LA, left atrium; PA, pulmonary artery; RA, right atrium; SVC, superior vena cava.
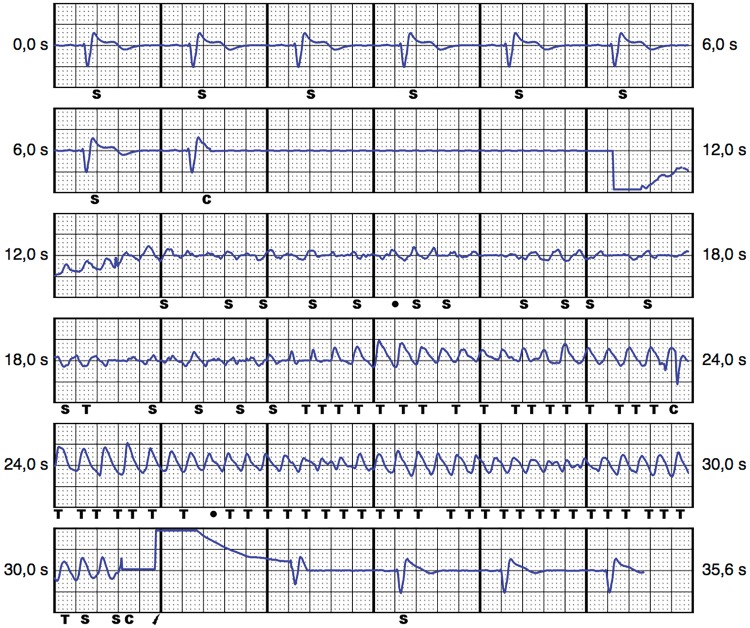
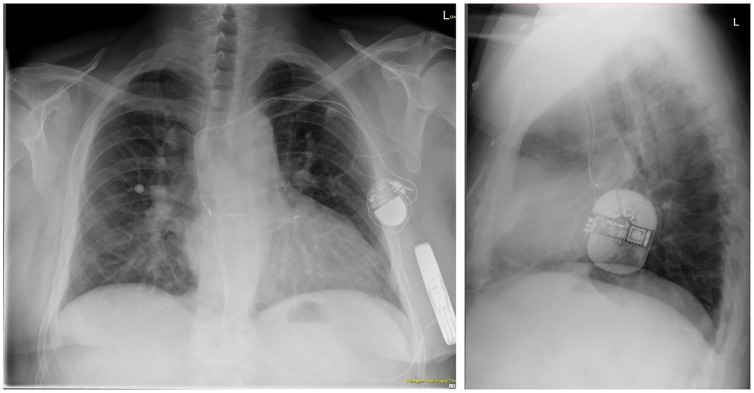
To include as much of the anatomical right ventricle as possible, a right parasternal electrode position was evaluated in the pre-operative screening. The primary and secondary vectors were sufficient in the supine and standing position. The S-ICD system (Boston Scientific Emblem S-ICD) was implanted and tested successfully under general anaesthesia on 27 March. Due to the right parasternal electrode location and patient’s build (1.81 m, 96 kg); the length of the electrode was the limiting factor in achieving an even more dorsal position of the S-ICD generator. Still, a sufficiently dorsal intermuscular position of the S-ICD between the M. latissimus dorsi and M. serratus anterior was achieved. Intraoperative automatic vector set-up of the S-ICD revealed no oversensing during bipolar and unipolar atrial pacing. After 50 Hz induction of ventricular fibrillation, a 65 J S-ICD shock terminated to stable sinus rhythm, with a shock impedance of 82 Ohm (Figure 2). In the post-operative interrogation, both bi- and unipolar atrial pacing did not lead to oversensing in any of the vectors in the supine and standing position. The pacemaker was programmed to bipolar pacing. A post-operative chest X-ray confirmed the dorsal position of the S-ICD (Figure 3). The patient was started on beta blockers and discharged on the first post-operative day. At the 3-month follow-up visit in June 2018, the patient was well and had not suffered from further ventricular arrhythmia or S-ICD shocks.
Figure 2.
Subcutaneous implantable cardioverter defibrillator EGM of the secondary vector during intraoperative shock testing. C, charging; S, sensed event; T, tachycardia detection.
Figure 3.
Post-operative chest X-ray in posteroanterior and left-lateral projection, showing the right parasternal lead position (left panel, patient not centred note processi spinosi projection).
Discussion
This case addresses several rare challenges of S-ICD therapy in a CHD patient not eligible for conventional transvenous ICD implantation.
The combination of S-ICD therapy with a pacemaker is generally not recommended by the S-ICD manufacturer, but in selected cases, it may be the only option for the patient. Several case reports and small case series have been published and report no relevant interaction between the two systems.9,10 It is recommended to programme the pacemaker to bipolar pacing, to prevent oversensing of pacing artefacts by the S-ICD systems. In certain pacemaker models, a power-on-reset may be induced by an S-ICD shock, which could lead to a reversion to unipolar pacing. In our patient, unipolar atrial pacing did not cause oversensing.
Special considerations apply to CHD patients after corrected d-TGA. In a meta-analysis of the cohort of CHD patients in the IDE study and the EFFORTLESS registry, only two patients could not be defibrillated successfully with a 65 J shock.8 Both were patients with d-TGA, out of a group of six patients with d-TGA in the entire cohort. The mode of corrective surgery in these d-TGA patients was unknown. After an atrial switch surgery, the anatomical right ventricle becomes the systemic ventricle. The risk of malignant arrhythmia is substantially higher after atrial switch, as opposed to arterial switch.3,4,11 As a result of the atrial switch surgery, a left parasternal electrode position may not sufficiently include the dilated systemic (right) ventricle in the shock path (Figure 4). Whether this was the reason for the unsuccessful shock testing at 65 J in the study remains unknown.
Figure 4.
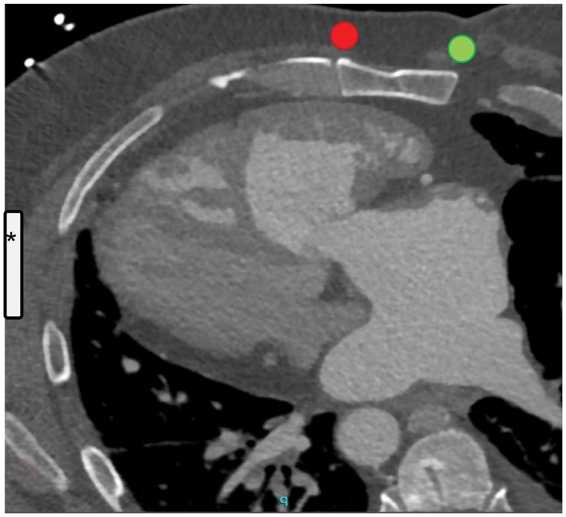
Pre-operative computed tomography scan showing the dilated right ventricle and its relation to the sternum and the left hemithorax. The left atrium drains into the right ventricle after the atrial switch surgery. Superimposed are the estimated locations of the subcutaneous implantable cardioverter defibrillator (asterisk) and a right (green) or left (red) parasternal lead position.
A recent study involving patients with tetralogy of fallot, a condition that also results in dilation of the right ventricle, found a high S-ICD screening failure rate for the left parasternal electrode position.12
While atrial switch surgery has long been replaced by arterial switch as the standard of corrective d-TGA surgery, older patients after atrial switch surgery may still present with malignant arrhythmia. To include the anatomical right ventricle in the S-ICD shock vector in these patients, in addition to a dorsal S-ICD pocket, which is recommended in all patients, a right parasternal lead position seems to be favourable (Figure 4). This needs to be confirmed in larger case series or studies.
Acknowledgements
We thank Z. Arica, A. Hof, D. Stern (University Heart Center, Department of Electrophysiology), and M. Stukenberg (Boston Scientific) for their involvement and valuable input in this clinical case.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL.. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 2. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ.. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 3. Schwerzmann M, Salehian O, Harris L, Siu SC, Williams WG, Webb GD, Colman JM, Redington A, Silversides CK.. Ventricular arrhythmias and sudden death in adults after a mustard operation for transposition of the great arteries. Eur Heart J 2009;30:1873–1879. [DOI] [PubMed] [Google Scholar]
- 4. Kammeraad JAE, van Deurzen CHM, Sreeram N, Bink-Boelkens MTE, Ottenkamp J, Helbing WA, Lam J, Sobotka-Plojhar MA, Daniels O, Balaji S.. Predictors of sudden cardiac death after mustard or senning repair for transposition of the great arteries. J Am Coll Cardiol 2004;44:1095–1102. [DOI] [PubMed] [Google Scholar]
- 5. Gelatt M, Hamilton RM, McCrindle BW, Connelly M, Davis A, Harris L, Gow RM, Williams WG, Trusler GA, Freedom RM.. Arrhythmia and mortality after the mustard procedure: a 30-year single-center experience. J Am Coll Cardiol 1997;29:194–201. [DOI] [PubMed] [Google Scholar]
- 6. Backhoff D, Kerst G, Peters A, Lüdemann M, Frische C, Horndasch M, Hessling G, Paul T, Krause U.. Internal cardioverter defibrillator indications and therapies after atrial baffle procedure for d-transposition of the great arteries: a multicenter analysis. Pacing Clin Electrophysiol 2016;39:1070–1076. [DOI] [PubMed] [Google Scholar]
- 7. Buber J, Ackley TJ, Daniels CJ, Roble SL, Mah ML, Kamp AN, Kertesz NJ.. Outcomes following the implantation of cardioverter-defibrillator for primary prevention in transposition of the great arteries after intra-atrial baffle repair: a single-centre experience. Europace 2016;18:1016–1022. [DOI] [PubMed] [Google Scholar]
- 8. D’Souza BA, Epstein AE, Garcia FC, Kim YY, Agarwal SC, Belott PH, Burke MC, Leon AR, Morgan JM, Patton KK, Shah M.. Outcomes in patients with congenital heart disease receiving the subcutaneous implantable-cardioverter defibrillator. JACC Clin Electrophysiol 2016;2:615–622. [DOI] [PubMed] [Google Scholar]
- 9. Kuschyk J, Stach K, Tülümen E, Rudic B, Liebe V, Schimpf R, Borggrefe M, Röger S.. Subcutaneous implantable cardioverter-defibrillator: first single-center experience with other cardiac implantable electronic devices. Heart Rhythm 2015;12:2230–2238. [DOI] [PubMed] [Google Scholar]
- 10. Huang J, Patton KK, Prutkin JM.. Concomitant use of the subcutaneous implantable cardioverter defibrillator and a permanent pacemaker. Pacing Clin Electrophysiol 2016;39:1240–1245. [DOI] [PubMed] [Google Scholar]
- 11. Khairy P, Clair M, Fernandes SM, Blume ED, Powell AJ, Newburger JW, Landzberg MJ, Mayer JE.. Cardiovascular outcomes after the arterial switch operation for d-transposition of the great arteries. Circulation 2013;127:331–339. [DOI] [PubMed] [Google Scholar]
- 12. Alonso P, Osca J, Cano O, Pimenta P, Andrés A, Yagüe J, Millet J, Rueda J, Sancho-Tello MJ.. The role of conventional and right-sided ECG screening for subcutaneous ICD in a tetralogy of fallot population. Pacing Clin Electrophysiol 2017;40:145–153. [DOI] [PubMed] [Google Scholar]