Abstract
Individual studies have assessed the association between TNF-α-308G>A and TNF-α-238 G>A polymorphisms and severity of dengue infection. However, the results are inconclusive and most studies had small sample sizes. The objective of this study was to summarize the evidence of association between TNF-α-308 G>A and TNF-α-238 G>A and severity of dengue infection.
This study follows the preferred reporting items for systematic reviews and meta- analyses of genetic association studies, recommended by PLOS One. We calculated pooled odds ratio and its 95% confidence interval (CI) to estimate the association between TNF-α-308 G>A or TNF-α-238 G>A and the risk of severe dengue infections. To determine the information size required for this meta-analysis study, a trial sequential analysis (TSA) was done. Eight studies (640 cases and 1275 controls), which assessed the association of TNF-α-308 G>A or TNF-α-238 G>A and the risk of DHF were included. Overall, we found no significant association between TNF-α-308 G>A and the DHF risk in the allelic model (OR, 0.91; 95% CI, 0.51–1.63), the recessive model (OR,1.32;95%CI,0.73–2.37), the dominant model (OR,0.93;95%CI:0.59–1.47) or the additive model (OR,1.43,95;95%CI:0.79–2.59). There was also no significant association between TNF-α-238 G>A and DHF risk under the allele contrast model (OR:1.51;95%CI:0.88–2.58), the recessive model (OR,1.48,95% CI:0.33–6.58), the dominant model (OR,1.48;95%CI:0.56–3.92), or the additive model (OR:1.5;95%CI:0.34–6.69). On subgroup analysis, neither the Asian population nor the non-Asian population showed significant association between TNF-α-308 G>A/TNF-α-238 G>A and the DHF risk under any genetic models. Leave-one-out meta-analysis showed stability of the results. TSA plots suggested that the sample size in this meta-analysis study was below the required information size.
The findings suggest an inclusive evidence of the association between TNF-α-308/ TNF-α-238 G>A and the risk of developing severe dengue infection. Large studies with evidence of Hardy-Weinberg equilibrium, assessing gene-gene interactions are recommended.
Introduction
Dengue fever is endemic in the tropics and sub-tropics and it is the most common arboviral infection caused by dengue viruses (DENV-1, -2, -3, and -4), which are transmitted primarily by the bite of Aedes aegypti and Ae. albopictus mosquitoesThe clinical manifestations of DENV infection vary from mild (asymptomatic, undifferentiated fever, dengue fever) to the severe form of dengue haemorrhagic fever (DHF). It is estimated that 2.5 billion people worldwide are at risk for DENV infection. About 5% of those at risk will have severe forms such as DHF and dengue shock syndrome (DSS), which are potentially fatal [1,2].
The exact pathogenesis of progression from mild to severe forms of dengue infections is not known. A number of studies have suggested that leakage of plasma, which differentiates DHF from DF is attributed to the direct and indirect effects of cytokines or chemical mediators on the vascular endothelial cells [3–5]. In animal models, TNF-α has been implicated in the process of plasma leakage and shock [6].
There is an array of polymorphisms regulating the TNF-α gene, of which the two most frequently investigated are at position -308 [7] and at -238 in the promoter region [8]. Meta-analyses have documented the association between TNF-α and the increased risk of colorectal cancer under the homozygote model [9], and for pulmonary tuberculosis [10].
Individual studies assessing the association between the TNF-α-308 G>A or TNF-α-238 G>A promoter polymorphism and the risk of dengue infections in different populations are available in the literature. However, the results are inconsistent. Most of these studies are based on small sample sizes, with low statistical power. A meta-analysis of available individual genetic association studies is valuable to ascertain whether the polymorphisms of TNF-α-308 G>A and TNF-α-238 G>A gene affect the risk of severe dengue infections. Published meta-analyses in this field are also available, but there are concerns related to the selection of studies included [11], the SNPs included [12], the accuracy of data extracted [12] and genetic models undertaken [11]. Taken together, the objective of this review was to summarize the evidence of association between TNF-α-308 G>A or TNF-α-238 G>A and severity of dengue infection.
Materials and methods
The current study conformed to the checklist for meta-analysis of genetic association studies specified for PLOS One approach [13] (S1 Table).
Study search
The potentially relevant studies were searched in the health-related databases of PubMed, Ovid Medline, google scholar and web of science and with the combination of the keywords with Boolean operators: “dengue” OR “DHF” OR “dengue haemorrhagic fever” OR “DSS” OR “dengue shock syndrome” AND “tumour necrosis factor- alpha-308” OR “rs1800629” OR “TNF-α-308 G>A” OR “tumour necrosis factor-alpha” OR “TNF-α-238 G>A”. The details of search in PubMed database is provided in S2 Table.
The search was restricted to the publications in English until January 2018. We also searched manually the references of articles retrieved and relevant systematic reviews to find any additional studies.
Inclusion criteria
Human studies of any sample size that assessed severe dengue infection were included, if (1) TNF-α-308 G>A (including rs 1800629), TNF-α-238 G/A or both were investigated; (2) they were case-control design (retrospective or nested case-control) with an outcome of severe dengue infections (DHF/DSS) measured as incidence or prevalence, and (3) there was sufficient information to extract the genotype frequency both in cases and controls.
Severe dengue infection in the current analysis encompassed DHF and/or DSS as defined in the primary studies. Studies, which did not meet the inclusion criteria were excluded. Studies based on family or sibling-pairs were not considered.
Data extraction
Two investigators independently screened the titles and abstracts, and then selected the relevant full-text articles, according to the inclusion criteria. The two investigators independently extracted data, using a piloted data extraction form. Information collected from each study included: first author, publication year, country, study setting (e.g. hospital-based or population-based), the frequency of cases and controls, the racial descent (Asian or non-Asian), method of genotyping and genotype/allele frequencies in cases and controls. If allele frequency was zero, then we added 1 to all allele, following the Laplace approximation [14]. Any discrepancy between the two investigators were resolved by consensus.
Assessment of the methodology quality
The two investigators independently evaluated the methodological quality of studies with the use of the Newcastle-Ottawa Scale (NOS) [15]. These scores are based on three factors such as the selection of the study groups (4 points), the comparability of the groups (2 points) and the ascertainment of the exposure (3 points). The total score for each study ranged between 0 (the worst) and 9 (the best). As described in a published systematic review [16], we adapted the cut-off score points as good (≥7), moderate (≥5) and poor (≤4) for study quality assessment. Any discrepancy between the two investigators was resolved by consensus.
Statistical analysis
An evidence of HWE in the control population was assessed (if not provided in the study included) or re-assessed (if already provided) in this meta-analysis with the use of the goodness-of-fit test (p>0.05) [17].
Following a method described [18], the strength of the association between TNF-α-308 G>A or TNF-α-238 G>A and the risk of developing DHF/DSS was estimated using odds ratios (ORs) and its 95% confidence intervals (CIs) for individual studies included. Heterogeneity was evaluated with the use of I2 test. The I2 test values indicates the percentage of total variation across studies that is attributable to the heterogeneity rather than chance. I2 values lie between 0% and 100%, with greater than 50% regarded as having substantial heterogeneity [19]. For pooling of the estimates across studies included, the summary ORs and its 95% CIs were calculated with random-effect model (The Der Simonian and Laird method), based on the presence of statistical heterogeneity of the studies. Otherwise, fix-effect model (the Mental-Haenszel method) was used. We calculated the summary ORs and its 95% CIs in four genetic models: the allelic contrast model (A vs G), the dominant model (AA+GA vs GG), the recessive model (AA vs GA+GG), and the additive model (AA vs GG). To investigate the stability of results, a sensitivity analysis was done with leave-one-out meta-analysis by omitting one study at a time [20]. All eight studies included used different case definitions of dengue severity. Hence, a sensitivity analysis was done with the studies that used the WHO 1997 definition for dengue severity. We did not assess the publication bias by visual inspection of funnel plots in the absence of a minimum 10 studies required for this assessment [21,22].
Trial sequential analysis (TSA), a tool for adjustment of random error risk, was done for estimation of the required information size [23]. It is classified as ‘firm evidence of effect’ or ‘potentially spurious evidence of effect’; this classification depends on the cumulative Z-curve that cross the monitoring boundaries or not [24].
Forest plots and leave-one-out meta-analysis were done with RevMan 5.3 (The Cochrane collaboration, Copenhagen) and meta package in R 3.4.3 software (The R Foundation), respectively. TSA plot was done with TSA software (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen).
Results
Study search results
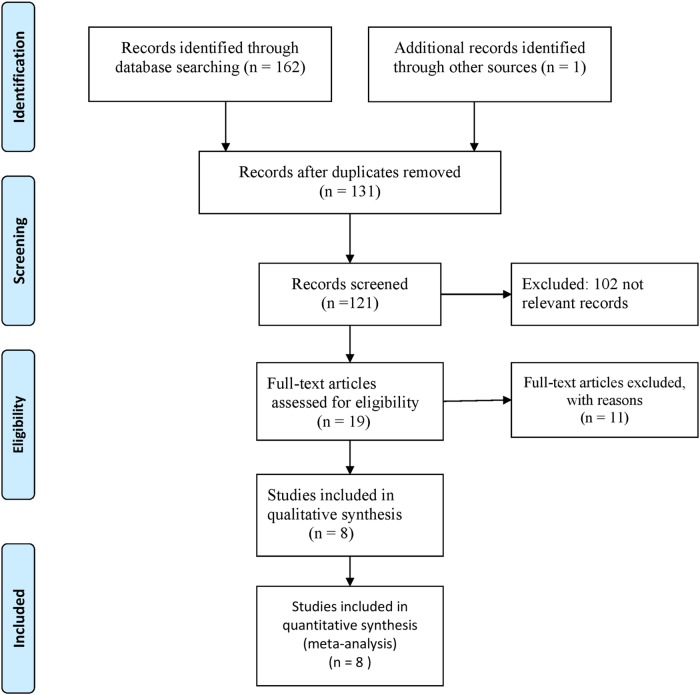
Fig 1 shows a four-phase study selection process in this meta-analysis. A total of 167 records were found in the initial search, of which 32 were duplicates. After screening of abstracts, 19 full-text articles were retrieved, based on the inclusion criteria. Finally, eight studies (with 640 cases and 1275 controls) were identified for this review [25–32]. All these eight studies assessed TNF-α-308 G>A. Only 3 studies assessed both TNF-α-308 G>A and TNF-α 238 G>A [26,30,32] in the risk of severe dengue. Summary of the 11 excluded studies [33–43] were provided in S3 Table.
Fig 1. PRISMA flow diagram showing the study selection process.
Study characteristics
Table 1 presents the characteristics of the nine included studies, along with their genotype and allele frequency distribution of TNF-α-308 G>A and TNF-α-238 G>A gene polymorphisms. Two studies each were from Brazil [27, 31] and Mexico [26,32], while one study each from Cuba [25], India [28], Malaysia [30] and Sri Lanka [29]. Study samples varied from 43 cases [25] to 196 [30]. The years of publication spanned from 2010 and 2017. The single nucleotide polymorphisms (SNPs) in two studies deviated from HWE [25, 26]. All these eight studies were with moderate to good methodological quality, based on the NOS criteria (S4 Table).
Table 1. Characteristics of the studies included in the meta-analysis.
| Study | Yr | Ref # | Country | Setting | Diagnostic criteria | Genotyping | DHF cases/ Healthy controls |
Age group | Study quality score | Cases | Controls | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/G | AA/GA/GG | A/G | AA/GA/GG | ||||||||||
| Perez | 2010 | 25 | Cuba | population based | 1997 WHO criteria | PCR-SSP | 43/99 | adults | 7 | 29/57 | 9/11/23 | 21/145 | 14/7/69 |
| García-Trejo | 2011 | 26 | Mexico | hospital based; | 1997 WHO criteria | PCR-RFLP | 45/169 | adults | 7 | 0/64 | 0/0/32 | 23/315 | 1/21/147 |
| Xavier-Carvalho | 2013 | 27 | Brazil | hospital based; | 2009 WHO/TDR | Real-time PCR | 88/335 | children | 8 | 20/144 | 2/16/64 | 73/585 | 6/66/271 |
| Alagarasu | 2015 | 28 | India | hospital based | 1999 WHO criteria | ARMS-PCR | 45/108 | adults | 8 | 6/84 | 0/6/39 | 13/203 | 0/13/95 |
| Fernando | 2015 | 29 | Sri Lanka | hospital based | 2011 WHO criteria | ARMS-PCR | 107/52 | adults | 5 | 14/200 | 2/10/95 | 28/107 | 2/13/47 |
| Sam | 2015 | 30 | Malaysia | hospital based | 1997 WHO criteria | PCR-RFLP | 196/120 | Mostly adults | 8 | 17/373 | 0/17/178 | 24/216 | 1/22/97 |
| dos Santos | 2017 | 31 | Brazil | hospital based | 1997 WHO criteria | Real-time PCR | 49/135 | adults | 7 | 10/88 | 0/10/39 | 39/231 | 3/33/99 |
| Sanchez-Leyva | 2017 | 32 | Mexico | hospital based | 1997 WHO criteria | PCR-RFLP | 67/257 | adults | 9 | 12/122 | 3/6/58 | 35/497 | 2/31/224 |
Quantitative estimates
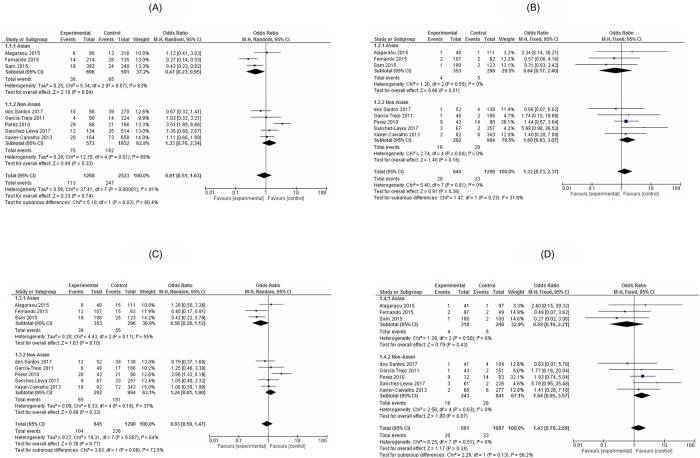
Overall, there were no significant associations between TNF-α-308 G>A and the DHF risk under the allelic model (OR,0.91;95% CI,0.51–1.63,I2:81%), the recessive model (OR,1.32, 95%CI; 0.73–2.37,I2:0%), the dominant model (OR,0.93;95%CI:0.59–1.47,I2:64%) and the additive model (OR,1.43;95%CI:0.79–2.59, I2:0%). On stratification, the Asian population or the non-Asian population showed no significant relationships between TNF-α-308 G>A and the DHF risk under any genetic models, except a recessive model with the Asian population (0.47, 95%CI: 0.23–0.85, I2: 67%) (Fig 2A, 2B, 2C and 2D).
Fig 2. Forest plot of the associated TNF-α-308 G>A and dengue haemorrhagic fever (A: Allele contrast model, B: Recessive model, C: Dominant model, D: Additive model).
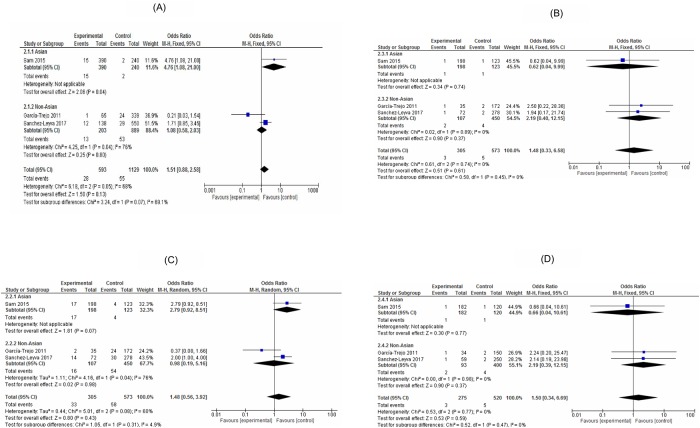
For TNF-α-238 G>A, overall there was no significant association with the DHF risk in the allelic model (OR,1.51;95%CI,0.88–2.58,I2:68%), the recessive model (OR,1.48,95%CI,0.33–6.58,I2:0%), the dominant model (OR,1.48;95%CI:0.56–3.92,I2:60%) and the additive model (OR,1.5;95%CI:0.34–6.69,I2:0%). Also, a stratified analysis with the Asian population or the non-Asian population did not show significant association between TNF-α-238 G>A polymorphism and the risk of DHF in any genetic models (Fig 3A, 3B, 3C and 3D).
Fig 3. Forest plot of the associated TNF-α-238 G>A with dengue haemorrhagic fever (A: Allele contrast model; B: Recessive model, C: Dominant model, D: Additive model).
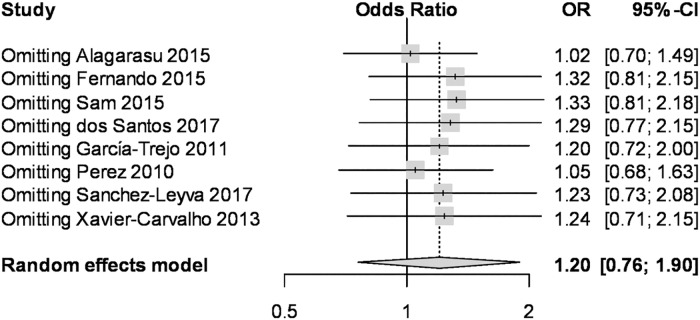
To evaluate the impact of individual data on the pooled ORs, sensitivity analysis was performed using leave-one-out meta-analysis. For instance, the overall estimate of dominant model of TNF-α-238 G>A remained as having no statistical significance, on omitting any single study (Fig 4), indicating that the results are stable. Another sensitivity analysis was done with the studies that used the WHO 1997 definition for dengue sensitivity. We also found no significant association between TNF-α-308 G>A polymorphism and the risk of DHF in allelic genetic models (OR: 1.08, 95%CI: 0.49–2.37, I2: 83%) (S5 Table). This was also true for the other genetic models.
Fig 4. Leave-one-out meta-analysis for the TNF-α-308 G>A under the dominant model.
Trial sequential monitoring
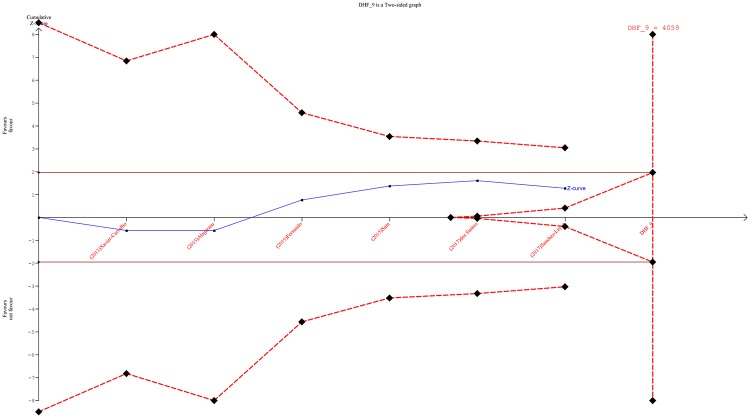
We performed TSA with six studies that followed evidence of HWE [27–32]. We plotted with the use of an overall type I error of 5% and type II error of 20%. The currently included total participants in this meta-analysis was below the required information size of 4059. As the cumulative Z-curve (blue full line with quadratic indications of each trial) touches the boundary for futility, it will be unlikely to reach a statistical significance p <0.05, even the required information size of 4059 is obtained (Fig 5). This was also true for TNF- α -238 G>A.
Fig 5. Trial sequential monitoring plot of TNF-α-308 G>A in severe dengue infection.
Discussion and conclusions
The current study provides evidence on the relationship between TNF-α-308 G>A or TNF-α-238 G>A and the risk of DHF, and the major observations are as follows;
In a combined population as well as subgroups of Asian and non-Asian groups, both SNPs (TNF-α-308 G>A and TNF-α-238 G>A were not significantly associated with the DHF risk in almost all genetic models investigated.
The TSA plot revealed that the required information size for evidence of effect was insufficient to draw a firm conclusion.
The plasma leakage in DHF occurs up to several days after viremia has been reduced or disappeared, suggesting the role of an immune-mediated mechanism in DHF [6, 7]. TNF-α gene was first cloned in 1985 and it is expressed in diverse cells including macrophages and tumour cells. Although an exact mechanism is not fully understood, the action of TNF-α includes stimulating the acute phase response that lead to increase in the C-reactive protein levels, and producing IL-1 oxidant and inflammatory lipid prostaglandin E-2 [7]. An in vitro study has reported that acute dengue sera had significantly high TNF-α levels, and the endothelial activation was inhibited more than 70% with pre-treatment of monoclonal antibodies against TNF-α. This could explain why the transient plasma leakage occurred mainly at serosal tissues in dengue cases [4,44].
A published meta-analysis has documented that TNF- α -308 G>G genotype and allele G confer susceptibility to symptomatic dengue, while TNF- α -308 G>A genotype and allele A confer protection [12]. The current analysis, which focussed on the severe form of infection with potential fatality such as DHF/DSS, showed no significant relationship between TNF-α-308 G>A and the DHF risk. This difference is related to variation in immunopathology of the target conditions as the clinical outcome of symptomatic dengue and severe dengue, are not at the same degree of importance. The findings in this analysis were comparable with another meta-analysis of seven individual studies (n = 1533), suggesting that TNF-α-308 G>A was not associated with DHF [12]. Although there are more participants in this analysis the results retained the evidence of no association. Moreover, the current review assessed more than one SNP.
The lack of association either protective or enhancement of the DHF risk in the current analysis might be due to insufficient number of participants, which is supported by the TSA plot. It showed that the included eight studies in this meta-analysis could only contribute to 65.7% of the required information size. This implied there was insufficient information to provide conclusive results.
Another possible reason of a lack of association in the present analysis may be due to the limited role of TNF-α in the development of DHF risk. In animal models, IFN-γ does not directly induce plasma leakage, but promotes TNF-α production through activated monocytes [45] and interactions with TNF-α to activate endothelial cells [46]. As such, it is likely that the combined cytokines including TNF-α-308 G>A and other cytokines are important to establish the significant role in the DHF risk. This was supported by a study in Malaysia that showed a significant correlation between interactions of TNF-α-308G>A genotype and IL10 non-GCC haplotypes, IL12B pro homozygotes (pro1/pro1, pro2/pro2) and IL-12B 3’UTR AC and the protective effects against DHF/DSS [30]. It has been suggested that the interactions of TNF-α, IFN- γ and activated complement proteins enhance plasma leakage of endothelial cells in secondary dengue infection [42]. A small number of studies did not allow to perform pooled analysis with these combined cytokines. The studies identified for the current meta-analysis used different case definition of dengue severity. Some used 2009 WHO definition, some used 1997 definition. Both definitions of the WHO 1997 and 2009 may include the patients with different disease severity, which may not relate to vascular permeability. However, a sensitivity analysis solely on the five studies that used WHO 1997 definition remained the non-significant association between TNF-α-308 G>A and the risk of severe dengue. This implied that the criteria for dengue severity had no impact on the direction of association.
Study limitations
We acknowledge the limitations of the present review. Approximately 75% of the global population residing in the Asia Pacific Region are exposed to DENV [47]. Only 36% of the included studies were carried out in the Asia Pacific Region; there is a likely selection bias with geographical imbalance. The current analysis was done with the unadjusted raw data provided in the primary studies, whereas most of the estimations in the individual studies might have been adjusted with common factors (i.e. age, gender). Hence, the pooled ORs in this meta-analysis may be slightly differed from what was reported in the primary adjusted studies. If cases and controls have been genotyped in separate batches in the primary studies, a bias related to differential misclassification of exposure is a concern. Due to the small sample sizes in most of the included studies, type II errors had limited to find the significant differences between the cases and controls or amongst the ethnic groups. However, meta-analysis is a retrospective pooling of published studies, and type II errors are less likely than in individual studies [48]. We planned to do stratified analysis by age group. This was not possible in view of only one study with children. Moreover, there might be some extent of interaction with TNF-α-238 G>A or TNF-α-308 G>A and other genes (gen-gen interaction/synergism) or other potential confounding factors such as nutritional status of the patients included [16]. Hence, findings in this meta-analysis should be interpreted with cautions.
There are some strengths in this meta-analysis compared with other reviews in this field. To be comprehensive, we have added more than one SNP in the present analysis. We performed this review, following the guideline for meta-analysis of genetic association studies [13]. Moreover, the TSA technique was useful to adjust random-error risk. An add-on TSA approach to this field will highlight to researchers about the optimal sample size to adopt. This will help the researchers and policy makers to reach a more balanced conclusion on the estimates [24], which is the case in this study. In summary, the findings suggest that it is inconclusive to determine whether any of these two SNPs is associated with the risk of severe dengue infection. Additional case-control studies on these two SNP may likely to change the estimates. Large studies with evidence of HWE, assessing these SNPs and other SNPs as gene-gene interactions are recommended.
Supporting information
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
The authors thank the participants and researchers of the primary studies identified for this meta-analysis. We are grateful to the editors and the anonymous reviewers for the comments provided and the valuable inputs. We thank our institutions for allowing us to perform this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.CDC. Dengue virus infections. Case Definition.2015.
- 2.World Health Organization (WHO). Dengue: guidelines for diagnosis, treatment, prevention and control—New edition. 2009. WHO/HTM/NTD/DEN/2009.1 [PubMed]
- 3.Halstead S. Pathogenesis of dengue: challenges to molecular biology. Science. 1988; 239:476e81. [DOI] [PubMed] [Google Scholar]
- 4.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990; 70:427e51. [DOI] [PubMed] [Google Scholar]
- 5.Tracey K J, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993; 9: 317–343. 10.1146/annurev.cb.09.110193.001533 [DOI] [PubMed] [Google Scholar]
- 6.Rothman AL, Ennis FA. Immunopathogenesis of Dengue hemorrhagic fever. Virology. 1999; 257:1–6. 10.1006/viro.1999.9656 [DOI] [PubMed] [Google Scholar]
- 7.Wilson AG, di Gioine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumor necrosis factor a (TNF-a) gene detected by NcoI restriction of PCR product. Hum Mol Genet. 1992; 5:353. [DOI] [PubMed] [Google Scholar]
- 8.Kaluza W, Reuss E, Hug R, Galle PR, Maerker-Hermann E, Hoehler T, et al. Different transcriptional activity and in vitro TNF-α production in psoriasis patients carrying the TNF-α 238A promoter polymorphism. Journal of Investigative Dermatology. 2000; 114:1180–1183. 10.1046/j.1523-1747.2000.00001.x [DOI] [PubMed] [Google Scholar]
- 9.Min L, Chen D, Qu L, Shou C. Tumor necrosis factor-a polymorphisms and colorectal cancer risk: a meta-analysis. PLoS One. 2014; 9: e85187 10.1371/journal.pone.0085187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan HM, Wang Z, Feng FM, Zhang KL, Yuan JX, Sui H, et al. Association of TNF-alpha-238G/A and 308 G/A gene polymorphisms with pulmonary tuberculosis among patients with coal worker’s pneumoconiosis. Biomed Environ Sci. 2010; 23:137–145. 10.1016/S0895-3988(10)60043-8 [DOI] [PubMed] [Google Scholar]
- 11.dos Santos ACM, de Moura EL, Ferreira JM, dos Santos BRC, de Medeiros Alves V, Fiqueiredo EVS et al. Meta-analysis of the relationship between TNF-α (-308G/A) and IL-10 (-819C/T) gene polymorphisms and susceptibility to dengue. Immunological Investigations. 2017. 10.1080/08820139.2016.1248560 [DOI] [PubMed] [Google Scholar]
- 12.Pabalan N, Chaisri S, Tabunhan S, Tarasuk M, Jarjanazi H, Steiner T. Associations of tumor necrosis factor-α-308 polymorphism with dengue infection: A systematic review and meta-analysis. Acta Tropica. 2017; 173: 17–22. 10.1016/j.actatropica.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 13.Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, Khoury MJ, et al. Strengthening the Reporting of Genetic Association Studies (STREGA)—an extension of the STROBE statement. PLOS Med. 2009; 6(2): e1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthold VMR, Hoppner BC, Klawonn F. How to Intelligently Make Sense of Real Data, Descriptive Statistics: Guide to Intelligent Data Analysis. Springer, Germany, 2010, 315. [Google Scholar]
- 15.Wells BS GA, O’Connell D, Peterson J, Welch V, Losos MP. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16.Zulkipli MS, Dahlui M, Jamil N, Peramalah D, Wai HVC, Bulgiba A, et al. The association between obesity and dengue severity among pediatric patients: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018; 12(2): e0006263 10.1371/journal.pntd.0006263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992; 48:361–372. [PubMed] [Google Scholar]
- 18.Thakkinstian A, McElduff P, D’Este C, Duffy D’ Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2005; 24:1291–1306. 10.1002/sim.2010 [DOI] [PubMed] [Google Scholar]
- 19.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, ed. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011.
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001; 54:1046–1055. [DOI] [PubMed] [Google Scholar]
- 23.Thorlund K, Wetterslev J, Brok J, Imberger G, Gluud G: User Manual For Trial Sequential Analysis (TSA). Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research; 2011;1–115. [Google Scholar]
- 24.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive—Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009; 38: 287–298. 10.1093/ije/dyn188 [DOI] [PubMed] [Google Scholar]
- 25.Perez AB, Sierra B, Garcia G, Aguirre E., Babel N, Alvarez M, et al. Tumor necrosis factor–alpha, transforming growth factor–β1, and interleukin-10 gene polymorphisms: implication in protection or susceptibility to dengue hemorrhagic fever. Hum Immunol. 2010; 71:1135–1140. 10.1016/j.humimm.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Trejo AR, Falcon-Lezama JA, Juarez-Palma L, Granados J, Zuniga-Ramos J, Rangel H et al. Tumor necrosis factor alpha promoter polymorphisms in Mexican patients with dengue fever. Acta Trop. 2011; 120:67–71. 10.1016/j.actatropica.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 27.Xavier-Carvalho C, Gibson G, Brasil P, Ferrierira RX, de Santos DFR, Goncalves CO et al. Single nucleotide polymorphisms in candidate genes and dengue severity in children: A case–control, functional and meta-analysis study. Infect Genet Evol. 2013; 20:197–205. 10.1016/j.meegid.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 28.Alagarasu K, Bachal RV, Tillu H, Mulay AP, Kakade MB, Shah PS.et al. Association of combinations of interleukin-10 and proinflammatory cytokine gene polymorphisms with dengue hemorrhagic fever. Cytokine 2015; 74:130–136. 10.1016/j.cyto.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 29.Fernando AN, Malavige GN, Perera KLN, Premawansa S, Ogg GS, Silva ADD et al. Polymorphisms of transporter associated with antigen presentation, tumor necrosis factor-α and interleukin-10 and their implications for protection and susceptibility to severe forms of dengue fever in patients in Sri Lanka. J Global Infectious Dis. 2015; 7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sam SS, Teoh BT, Chinna K, AbuBakar S. High producing tumor necrosis factor alpha gene alleles in protection against severe manifestations of dengue. Int J Med Sci. 2015; 12:177 10.7150/ijms.8988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.dos Santos ACM, de Moura EL, Ferreira JM, de Moura AWA, Ferreira ADS, Bezerra RP, et al. Association of TNFA (-308G/A), IFNG (+874A/T) and IL-10 (-819 C/T) polymorphisms with protection and susceptibility to dengue in Brazilian population. Asian Pac J Trop Med. 2017; 10:1065–1071. 10.1016/j.apjtm.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Leyva M, Sanchez-Zazueta JG, Osuna-Ramos JF, Horacio Rendon-Aguilar H, Felix-Espinoza R, Becerra-Loaiza DS, et al. Genetic polymorphisms of tumor necrosis factor alpha and susceptibility to dengue virus infection in a Mexican population. Viral Immunology. 2017; 1–7. [DOI] [PubMed] [Google Scholar]
- 33.Vitarana T, de Silva H, Withana N, Gunasekera C. Elevated tumour necrosis factor in dengue fever and dengue haemorrhagic fever. Ceylon Med. J 1991; 36:63–65. [PubMed] [Google Scholar]
- 34.Fernandez-Mestre MT, Gendzekhadze K, Rivas-Vetencourt P, Layrisse Z TNF-α-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens. 2004; 64:469–472. 10.1111/j.1399-0039.2004.00304.x [DOI] [PubMed] [Google Scholar]
- 35.Huan-Yao L, Kao-Jean H, Yee-Shin L, Yeh TM, Liu HS, Hsiao-Sheng L et al. Immunopathogenesis of dengue hemorrhagic fever. Am J Infect Dis. 2008; 4: 1–9. [Google Scholar]
- 36.Restrepo BN, Ramirez RE, Arboleda M, Alvarez G, Ospina M, Diaz FJ. Serum levels of cytokines in two ethnic groups with dengue virus infection. Am J Trop Med Hyg. 2008; 79:673–677. [PubMed] [Google Scholar]
- 37.Nascimento EJM, Braga-Neto U, Calzavara-Silva CE, Gomes ALV, Abath FGC, Brito CA, et al. Gene expression profiling during early acute febrile stage of dengue infection can predict the disease outcome. PLoS ONE. 2009; 4: e7892 10.1371/journal.pone.0007892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vejbaesya S, Luangtrakool P, Luangtrakool K, Kalayanarooj S, Vaughn DW, Endy TP et al. TNF and LTA gene, allele, and extended HLA haplotype associations with severe dengue virus infection in ethnic Thais. J Infect Dis. 2009; 199:1442–1448. 10.1086/597422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alagarasu K, Mulay AP, Singh R, Gavade VB, Shah PS, Cecilia D. Association of HLA-DRB1 and TNF genotypes with dengue hemorrhagic fever. Hum Immunol. 2013; 74:610–617. 10.1016/j.humimm.2013.01.027 [DOI] [PubMed] [Google Scholar]
- 40.Chuansumrit A, Anantasit N, Sasanakul W, Chaiyaratana W, Tangnararatchakit K, Butthep P et al. Tumour necrosis factor gene polymorphism in dengue infection: association with risk of bleeding. Paediatr Int Child Health. 2013; 33:97–101. 10.1179/2046905512Y.0000000049 [DOI] [PubMed] [Google Scholar]
- 41.Mathew A, Townsley E, Ennis FA. Elucidating the role of T cells in protection against and pathogenesis of dengue virus infections Future Microbiol. 2014; 9: 411–425. 10.2217/fmb.13.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dettogni RS, Tristao-Sa R, dos Santos M, da Silva FF, Louro ID. Single nucleotide polymorphisms in immune system genes and their association with clinical symptoms persistence in dengue infected persons. Hum Immunol. 2015; 76: 717–723. 10.1016/j.humimm.2015.09.026 [DOI] [PubMed] [Google Scholar]
- 43.Feitosa RN, Vallinoto AC, Vasconcelos PF, Azevedo R do S, Azevedo VN, Machado LF, et al. Gene polymorphisms and serum levels of pro- and anti-inflammatory markers in dengue viral infections. Viral Immunol. 2016; 29:379–388 10.1089/vim.2016.0026 [DOI] [PubMed] [Google Scholar]
- 44.Cardier JE, Marino E, Romano E, Taylor P, Liprandi F, Bosch N, et al. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine. 2005; 306: 359–365. [DOI] [PubMed] [Google Scholar]
- 45.Nedwin GE, Svedersky LP, Bringman TS, Palladino MA Jr, Goeddel DV. Effect of interleukin 2, interferon-g, and mitogens on the production of tumor necrosis factors and b. J Immunol. 1985; 135: 2492–2497. [PubMed] [Google Scholar]
- 46.Burke-Gaffney A, Keenan AK. Modulation of human endothelial cell permeability by combinations of the cytokines interleukin-1 alpha/beta, tumor necrosis factor-alpha and interferongamma. Immunopharmacology. 1993; 25: 1–9. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization (WHO). Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever. Revised and Expanded Edition. SERO Technical Publication Series No. 60. WHO, New Delhi. 2011. http://www.searo.who.int/entity/vector_borne_tropical_diseases/documents/SEAROTPS60/en/
- 48.Zintzaras E, Lau J. Synthesis of genetic association studies for pertinent gene-disease associations requires appropriate methodological and statistical approaches. J Clin Epidemiol. 2008; 61: 634–645. 10.1016/j.jclinepi.2007.12.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.