Abstract
Building on the weathering hypothesis, we advance health disparities research by assessing racial/ethnic differences in low-grade inflammation, a marker of chronic stress exposure, in young children. Using nationally representative data from NHANES (N=6652) and logistic regression, we find an increased risk of low-grade inflammation among Hispanic and African American children compared to white children. The risk of inflammation appears to be stronger for Hispanic and African American children with foreign-born parents compared to children of the same race/ethnicity with U.S.-born parents. Low parental education and elevated child body mass index (BMI) work as partial mediators of these associations. Our findings suggest the need to understand the social and psychological challenges faced by Hispanic and African American children, particularly those with foreign-born parents, if we are to make further progress in reducing health disparities.
INTRODUCTION
Racial and ethnic health disparities are critical social equity and public health issues in the United States. Research over decades has shown continued health disadvantages among minority populations, particularly for African Americans and Hispanics (Thomas, Quinn, Butler, Fryer, and Garza 2011). These health disparities are often theorized to reflect a weathering process; whereby, chronic stress and other disadvantages accumulate over the life course to produce premature biological aging and health disadvantages among African Americans. Although initially developed to explain observed black-white differences in birth outcomes among women at older childbearing ages (Geronimus, 1996; Geronimus, 1992), the theory has been extended to understanding how social status and inequalities can “get under the skin” and produce later-life racial health inequalities (Geronimus et al. 2006; Hayward et al. 2000). This latter research has focused on the use of biological markers to detect underlying physiological changes that result from chronic stress exposures and influence mental and physical health problems in the long term (Das 2013; McEwen 1998). Although chronic stress has been a focus of research on adult racial/ethnic health disparities, less attention has been paid to stress and related physiological changes that may produce racial health disparities in children.
Health disparities clearly begin well before adulthood, with minority children having more health problems than white children have, across a number of outcomes (Mehta, Lee, and Ylitalo 2013). At the same time, Hispanic and African American children’s higher risk of facing multiple family and neighborhood adversities, (Slopen, Shonkoff, Albert, Yoshikawa, Jacobs, Stoltz, and Williams 2016) suggests that chronic stress exposure may be an important part of the development of health disparities during childhood. However, the weathering hypothesis, and its prediction of higher physiological stress in disadvantaged racial/ethnic groups, has yet to be applied to children.
We aim to address this gap in research by estimating race/ethnic differences in children’s risk of low-grade inflammation, an immune-system marker that captures chronic stress exposure. We do so by assessing C-reactive protein (CRP) levels up to 10mg/L, which indicate the presence of low-grade inflammation (Black 2002; Cohen et al. 2012). Low-grade inflammation is the result of the body’s repeated response to physical and psychosocial stress, in which proinflammatory cytokines are produced and trigger the release of acute phase proteins (such as CRP) into the blood (Black 2002). Repeated stress exposure can lead to repeated activation of this process, as well as heightened glucocorticoid resistance and decreased immune cells’ sensitivity to glucocorticoids, which, in turn, contributes to the continuation of the inflammatory process (Cohen et al. 2012). Thus, exposure to psychosocial stress works through these multiple physiological processes to increase the risk of chronic, low-grade inflammation in children and adults. Low-grade inflammation in children, in turn, has been linked with depression (Kim, Szigethy, Melhem, Saghafi, and Brent 2014; Miller and Cole 2012), cardiovascular risk factors (Cook, Mendall, Whincup, Carey, Ballam, Morris, Miller, and Strachan 2000; Slopen, Koenen, and Kubzansky 2012), and cognitive disadvantages (Cullen et al. 2017). Thus, racial/ethnic disparities in inflammation during childhood may indicate differences in chronic stress that contribute to long-term racial/ethnic health disparities.
In this study, we pose the following research questions: (1) Are there racial/ethnic disparities in low-grade inflammation among young children in the U.S.? (2) Do family structural factors and child body mass index (BMI) mediate the associations between race/ethnicity and child inflammation? In assessing child race/ethnicity, we incorporate parental nativity to distinguish among minority children who have a foreign-born parent and may face additional stressors related to living in an immigrant family (Mendoza et al. 2017). We thus aim to estimate the relative risk of low-grade inflammation among racial/ethnic minority children with foreign-parents and racial/ethnic minority children with U.S.-born parents compared to white children with U.S.-born parents.
We further consider whether race/ethnic/parent nativity differences may work through social and biological processes to affect inflammation. Specifically, we posit that family structural conditions that disadvantage minority children and those in immigrant families more than white children (socioeconomic status, parental marital status, access to health insurance, and household size) may be key mediators explaining child race/ethnicity/parent nativity disparities in inflammation. We also evaluate the role of children’s BMI as a physiological mediator between race/ethnicity and inflammation, given the higher levels of BMI among minority and immigrant children (Ogden, Carroll, and Flegal 2008) and the links between BMI and inflammation (Dowd, Zajacova, and Aiello 2010).
Importantly, we assess disparities in inflammation in the early stage of childhood (ages 2–10 years). Early childhood is a critical developmental period when stress and related dysregulation of multiple physiological systems can become biologically embedded with implications for health in later-life (Berg et al. 2017; Hertzman 1999; Repetti, Robles, and Reynolds 2011; Wickrama, Bae, and O’Neal 2017). Further, inflammation in young children is less likely to be confounded by individual health behaviors (i.e. smoking, drinking), puberty, and advanced chronic diseases that can exacerbate inflammation (Shanahan et al. 2013).
We address our research questions using logistic regression and national data from the National Health and Nutrition Examination Survey (NHANES). NHANES includes a racially/ethnically-diverse sample of young children whose caregivers provided survey information about child race/ethnicity, parent nativity and family conditions. Blood-based biomarkers of CRP and anthropometric measurements were also assessed in children. These biosocial data allow for the study of how race/ethnicity/parent nativity may be associated with physiological disparities related to chronic stress exposure as measured by low-grade inflammation. The results of this study provide further insight into the development of racial/ethnic and parent nativity disparities in children’s stress exposure and related physiological effects that may have lasting health consequences.
BACKGROUND
Race/Ethnicity and Weathering
Empirical evidence provides support for the weathering hypothesis among adults, particularly African Americans (Das, 2013; Geronimus, Hicken, Keene, & Bound, 2006; Geronimus et al., 2010; Howard & Sparks, 2016; Mitchell & Aneshensel, 2017; Tomfohr, Pung, & Dimsdale, 2016). There is mixed evidence for stress-related weathering among Hispanics, in part due to the potential differences by immigrant status and migrant health selection that may confound the findings (Crimmins, Kim, Alley, et. al., 2007; Kaestner, Pearson, Keene, & Geronimus, 2009; Nazmi & Victora, 2007; Novak et al., 2017). An additional source of stress hypothesized to affect the health of Hispanic immigrants is acculturation stress, in which Hispanic immigrants experience stressors related to adapting to the U.S., particularly a low-SES position in the U.S. These stressors and related coping behaviors are theorized to worsen their health over time (Lara, Gamboa, Kahramanian, Morales, & Bautista, 2005).
Although extending the weathering hypothesis to children is a relatively new approach, biosocial research indicates that stress can exact physiological effects beginning in childhood (Drury et al., 2012; Repetti, Robles, & Reynolds, 2011; Wickrama, Bae, & O’Neal, 2017). Racial/ethnic status itself (independent of related socioeconomic disadvantages) may produce psychosocial stress in minority children through their experiences with discrimination and unfair treatment by others (Fisher, Wallace, & Fenton, 2000; Greene, Way, & Pahl, 2006), or via their parents’ experiences of discrimination that result in negative parenting and stressful parent-child interactions (McNeil, Harris-McKoy, Brantley, Fincham, & Beach, 2014; Simons et al., 2002).
Limited empirical research has documented inflammation differences by race/ethnicity in childhood. One study found African American and Hispanic children (ages 2–19) had higher mean levels of CRP compared to white children (Ford, Giles, Myers, Rifai, Ridker, and Mannino 2003). Another study using NHANES data found African American and Mexican American children ages 3–16 years had higher CRP levels compared to non-Hispanic white children (Dowd, Zajacova, and Aiello 2010). However, these studies assessed all levels of CRP and did not focus on low-grade inflammation, inflammation related to chronic stress exposure (Black, 2003), specifically. Further, the studies did not consider parent nativity within these racial/ethnic groups, which, as we discuss below, may be a critical aspect of minority children’s experiences of stress (and risk of low-grade inflammation) in combination with their race/ethnicity.
Parent Nativity
Going beyond race/ethnic status, we propose that parent immigrant status may affect minority children’s chronic stress exposures and evidence of weathering by race/ethnicity group. The role of parent nativity in predicting chronic stress and related physiological outcomes is uncertain. On the one hand, research finds that children with immigrant parents face more stressful living conditions than those with native-born parents, due to discrimination (Molina, Little, and Rosal 2016; Ornelas and Perreira 2011), lack of access to needed services (Huang, Yu, and Ledsky 2006), social or linguistic isolation (Shi, Zhang, van Meijgaard, MacLeod, and Fielding 2015), and economic hardship (Schmeer 2012). Further, parental or child undocumented status may contribute to stress in immigrant families (Roblyer, Carlos, Merten, Gallus, & Grzywacz, 2017). Reflecting, in part, these higher stress exposures, recent health studies indicate that immigrant children experience higher rates of obesity than non-immigrant children (Baker, Rendall, & Weden, 2015; Lawrence, Mollborn, & Riosmena, 2016).
Other studies have found that Hispanic children in immigrant families face fewer adverse childhood events than Hispanic children in U.S.-native families (Caballero, Johnson, Buchanan, & DeCamp, 2017), and that children in immigrant families fare better on some health outcomes than non-immigrant children (Logan, Alba, & Zhang, 2002; Riosmena, Kuhn, & Jochem, 2017; Vega & Amaro, 1994). This “immigrant health advantage” has been posited to be due to protective cultural factors, social ties, and parental health behaviors and has been most often found in infants born to Mexican immigrant mothers (Hummer, Powers, Pullum, Gossman, & Frisbie, 2007). Recent evidence suggests a potential health advantage among black immigrant compared with native-born black children (Hendi, Mehta, & Elo, 2015). Other research suggests that protective effects may exist only for recent immigrants, and that second-generation immigrant children (i.e., U.S.-born children with immigrant parents) are vulnerable to social and economic stressors that reduce their health status (Lara et al., 2005; Riosmena et al., 2017).
Empirical work on immigrant status and inflammation is limited, even for adults. One recent study found that job instability and financial strain predicted higher inflammation levels in adult Mexican immigrants (Steffen, Walker, Meredith, and Anderson 2016). In other research Chinese immigrant women had higher CRP when reporting higher levels of acculturation stress (Fang, Ross, Pathak, Godwin, and Tseng 2014). Regarding parent nativity, a small study found no association between mother’s place of birth and CRP in toddlers born to Puerto Rican mothers (Kannan, Acosta, Acevedo-Garcia, Divjan, Bracero, Perzanowski, and Chew 2013). In a larger, population-based study, Schmeer and Yoon (2016) found that Los Angeles children with U.S.-born primary caregivers had lower CRP than those with foreign-born caregivers.
Although a limited body of research suggests that racial/ethnic minority children may be at higher risk for low-grade inflammation than white children, studies of racial/ethnic disparities in inflammation have not considered the role of parental nativity or the specific developmental period of early childhood. We address these gaps by estimating the risk of low-grade inflammation among young children across racial/ethnic/parent nativity groups, controlling for infant health and other key background factors.
Family Structural Conditions
A second goal of this study is to assess whether race/ethnic/parent nativity disparities in inflammation work through family structural disadvantages that are higher among minority children and may contribute to stress and racial/ethnic disparities in inflammation. We assess the following family structure conditions as potential mediators of the race/ethnic/parent nativity associations with children’s low-grade inflammation: low socioeconomic status (SES), lack of health insurance, single-parent household, and large household size. These indicators of family structural conditions aim to capture family economic and social stressors that may increase the risk of low-grade inflammation in children.
Family resources of all types (economic, emotional, time, etc.) may be significantly reduced under these disadvantaged family conditions (low SES, medical hardship due to lack of health insurance, single-parent households and larger household size). The lack of resources may lead to increased stress among family members, with parents responding by increasing their coercive or authoritarian parenting styles and reducing time spent with their children (Conger et al., 2002; Conger, Ge, Elder, Lorenz, & Simons, 1994). Conflict and chaos may also increase under difficult structural conditions (Budescu & Taylor, 2013; Moore, Probst, Tompkins, Cuffe, & Martin, 2007). These family conditions may lead to reduced emotional support, positive relationships, and daily stability in routines, which, in turn, may result in increased psychosocial stress in young children (Chen, Brody, & Miller, 2017; Dush, Schmeer, & Taylor, 2013).
Related to racial/ethnic differences in our measures of family structural constraints, African American and Hispanic children are disproportionately situated in low SES families (Child Trends Data Bank 2015; National Center for Education Statistics 2005) and are less likely than white children to be covered by health insurance (Adams, Newacheck, Park, Brindis, and Irwin 2007; Van Wie, Ziegenfuss, Blewett, and Davern 2008). Children in immigrant families are at particularly high risk of being uninsured (Seiber 2014) and having a very low-educated parent (Van Hook, Brown, & Kwenda, 2004). Children in minority families are also more likely to be living in unmarried-parent and larger (more crowded) households (Blake, Kellerson, & Simic, 2007; Martin et al., 2011). Children in immigrant families are at higher risk of living in large households, although their parents are more likely to be married than children with native-born parents (Mendoza 2009).
Past research suggests that disadvantaged family structural conditions (low family SES and larger household size) are associated with increased risk of inflammation (as measured by CRP) in children (Dowd, Zajacova, and Aiello 2010; Schmeer and Yoon 2016). However, family structural conditions have not been theorized or tested as mediators of race/ethnic/parent nativity differences in assessing children’s risk of low-grade inflammation.
Physical Health Mediator: BMI
We also posit children’s body mass index (BMI) as a potentially important health mediator of the race/ethnicity-related weathering process. BMI is higher among African American children, Hispanic children (Haas, Lee, Kaplan, et. al. 2003) and children with foreign-born parents across multiple ethnic groups (Baker, Rendall, and Weden 2015; Van Hook, Baker, Altman, and Frisco 2012). Further, BMI is positively associated with increased levels of inflammation and research indicates that psychosocial stress contributes to increasing BMI, with this process beginning in childhood (Wilson & Sato, 2014). Past research has found evidence of BMI is a mediator between socioeconomic disadvantage and inflammation (Loucks et al., 2010), including in children (Schmeer & Yoon, 2016), but its role as a mediator between race/ethnicity and low-grade inflammation has not been assessed.
In sum, past research suggests that minority children (particularly African American and Hispanic) are exposed to more stressful social environments, with the potential for biological weathering that sets them up for current and later health disadvantages. We test this hypothesis using low-grade inflammation as a measure that includes, but is not limited to, biological embedding of chronic stress exposure in the immune system. We focus our analyses on young children (ages 2–10 years) to determine the extent of race/ethnic inflammation disparities during this critical developmental period and prior to the onset of risky health behaviors. We also incorporate parent nativity into the race/ethnic status of children to assess whether children with immigrant parents are at higher risk for inflammation than those with U.S.-born parents. Finally, we evaluate whether the risk of low-grade inflammation in minority children and those with immigrant parents is due to higher rates of family structural disadvantages or BMI in these children. Importantly, we estimate the race/ethnicity/parent nativity differences in low-grade inflammation net of birth conditions and other potential confounders.
METHODS
Data & Sample
We use data from the National Health and Nutrition Examination Survey (NHANES), a nationally representative survey conducted every two years by the National Center for Health Statistics (NCHS). We used the physical health exam and interview data linked across six waves, years 1999–2010. Blood samples were collected from children in a mobile laboratory, and a parent or guardian was interviewed to gather information about the child and the parent themselves. Using the household respondent data, we were able to link basic socio-demographic information about the household respondent (one parent) and their reports of family income and composition with the children’s biomarker data. NHANES does not publicly release data linking children to their parents, thus we are only able to assess the characteristics of the parent who served as the household respondent.
Because we are interested in low-grade inflammation as an indicator of a dysregulated immune system due to chronic stress exposure rather than inflammation as a response to illness, we excluded children with CRP>10mg/L (n=203). We further dropped children who were reported as non-Hispanic white with a foreign-born parent (n=112) because it was a small group with unclear origins. After dropping these cases and those with missing CRP data (n=5,350), our final sample size was 6,652. Those children missing CRP data were significantly more likely to be white, have a higher-educated parent respondent, and be in later waves (5 and 6) of NHANES. There was no statistical difference in missing CRP data by parent nativity.
Table 1 provides weighted descriptive statistics of our sample. Approximately 54% of sample children were non-white. The largest non-white subgroups were Hispanic children with foreign-born parents and African American children with U.S.-born parents. The smallest groups were the African American and “other race” children with foreign-born parents. Virtually all of the children were born in the U.S. (96%) and the mean age is 6.5 years.
Table 1:
Sample descriptive statistics. Children ages 2–10 years with valid CRP data ≤10 mg/L. NHANES 1999–2010. N=6652
| VARIABLES | Weighted Mean or % | Std. Dev. |
|---|---|---|
| Child race-ethnicity, parental nativity | ||
| Non-Hispanic white, U.S.-born parent | 54% | |
| Hispanic, foreign-born parent | 14% | |
| Hispanic, U.S.-born parent | 9% | |
| African American, foreign-born parent | 2% | |
| African American, U.S.-born parent | 13% | |
| Other race, foreign-born parent | 2% | |
| Other race, U.S.-born parent | 5% | |
|
Child characteristics Child U.S.-born |
96% | |
| Male child | 53% | |
| Child age in years | 6.5 | 2.2 |
| Recent head or chest cold | 27% | |
| Recent flu, pneumonia, ear infection | 7% | |
| Recent stomach/intestinal illness | 10% | |
| Low birthweight | 9% | |
| High birthweight | 11% | |
| Mother’s age at birth | 27.0 | 6.3 |
| Mother smoked during pregnancy | 17% | |
| Child BMI z-score | 0.4 | 1.2 |
| Ever told child had asthma | 14% | |
|
Parent characteristics Male |
51% | |
| Age | 36.8 | 10 |
| No high school degree | 23% | |
| High school degree | 24% | |
| Some college | 26% | |
| College Graduate | 27% | |
| Family income-to-poverty ratio | 2.2 | 1.4 |
| No health insurance (child) | 7% | |
| No partner in household | 25% | |
| Household size | 4.6 | 1.4 |
Measures
Our dependent variable is low-grade inflammation as measured by CRP 1–10mg/L as detected in whole blood. We coded values under 1 mg/L as no inflammation based on literature that has used 1mg/L as a minimum cut off of inflammation due to the associations of CRP <=1 mg/L with health risks (Järvisalo, Harmoinen, Hakanen, et. al. 2002; Ridker 2007b). Following this research, we created a dummy variable - no inflammation (CRP < 1 mg/L) vs low-grade inflammation (CRP 1–10mg/L).
Children’s race/ethnicity was reported by the parent respondent as Mexican-American, other Hispanic, non-Hispanic black, non-Hispanic white, and other race (which included multiracial children). Parent respondents were also asked about their own nativity, categorized as born in the U.S., Mexico, or in another (unspecified) country. We dichotomized this variable to indicate parent nativity (foreign-born=1, U.S.-born=0) and combined it with child race/ethnicity, resulting in the following categories: Mexican American child with a foreign-born parent; Mexican American child with a U.S.-born parent; other Hispanic child with a foreign-born parent, other Hispanic child with a U.S.-born parent; African American child with a foreign-born parent, African American child with a U.S.-born parent; other race child with a foreign-born parent; other race child with a U.S.-born parent; and non-Hispanic white child with a U.S.-born parent. Because early models revealed no significant differences in inflammation risk between Mexican and “other Hispanic” children, and due to the small sample sizes of “other Hispanic” children once disaggregated by parent nativity, we combined all Hispanic children differentiated only by parent nativity. We used non-Hispanic white children with U.S.-born parents as the reference category in all models.
Mediating Variables
We hypothesized several family structural factors as mediators: family SES (parent education and family income), lack of health insurance (child), parent marital status, and family size. All were reported by the parent respondent. Parent education was measured categorically as less than a high school, high school graduate, some college/associates degree, or graduated college (the reference category in regression models). Family income-to-poverty ratio was defined as total family income divided by the federal poverty line (FPL) for a given family size and year and capped at five times the FPL by NHANES. Lack of health insurance was coded as a dummy variable indicating whether the child was not currently covered by health insurance. Parent marital status was categorized in NHANES as single, cohabiting, separated/divorced, widowed and married. We dichotomized the marital status variable to “no partner” based on preliminary models that indicated no significant difference in child inflammation among the more disaggregated categories. Number of people in the household was measured as a continuous variable.
Our proposed health mediator, child BMI, was calculated using children’s height and weight, which was measured during the medical exams. These measures and the 2000 CDC growth charts for age and gender-adjusted scores were used to calculate each child’s BMI zscore (Cole, Bellizzi, Flegal, and Dietz 2000). The growth charts enable the calculation of zscores for children ages 2–20 years (Kuczmarski et al., 2000). Child BMI z-score was included in the model as a continuous variable.
Confounding Variables
We controlled for demographic, health and other factors that may be related to children’s risk of inflammation but do not reflect the weathering/stress process during childhood. First, we control for current child demographic characteristics: place of birth (U.S.=1 or abroad=0), age (in years), and gender (male=1). Child place of birth aims to control for differential exposures in infancy. Child age and gender were included to capture biological differences in inflammation, including physical maturation, which may differ by race/ethnicity (Sun, Schubert, Chumlea, Roche, Kulin, Lee, Himes, and Ryan 2002). We tested age and gender interactions with race/ethnicity and found no evidence of moderation by age or gender.
We also controlled for concurrent health status: three dummy variables capturing recent illnesses and diagnosed asthma. We controlled for recent illness (caregiver report of respiratory, stomach or infection in the 30 days) because higher CRP among recently ill children may reflect a normal immune response. We controlled for parent report of whether the child was ever diagnosed with asthma because African American and Puerto Rican children have higher rates of asthma than white children (Akinbami, Moorman, Garbe, and Sondik 2009); and, asthma is associated with increased inflammation in children (Michelson, Williams, Benjamin, & Barnato, 2009).
Finally, we controlled for maternal age at birth, maternal smoking during pregnancy, and birthweight (all parent-respondent reported) to reduce confounding by birth conditions. Maternal age at birth was measured linearly in years. Maternal age carries biological risk for infants, although there is little evidence on its links with inflammation. We also control for maternal smoking during pregnancy, which is more likely in white populations (Perreira and Cortes 2006) and may increase children’s inflammation given links between smoke exposure and inflammation (Wilkinson, Lee, and Arheart 2007). Maternal smoking was reported by the respondent as the mother smoked during pregnancy (=1) or did not (=0). Child’s weight at birth was used to create dummy variables of low birthweight (<5.5 pounds) and high birthweight (>9 pounds) (Walsh and McAuliffe 2012). The highest rates of low birthweight occur among African Americans and non-Mexican Hispanics (Sparks, 2009), while children of immigrant mothers are less likely to be born low birth weight than those with U.S.-born mothers (Hummer, Powers, Pullum, et al., 2007; Mendoza 2009). African American and Hispanic infants are also at risk for high birthweight (Kieffer, Alexander, Kogan, Himes, Herman, Mor, and Hayashi 1998; Silva, Kaholokula, Ratner, and Mau 2006). Both low and high birthweight are potentially related to inflammation, although research in children is inconsistent (Dowd, Zajacova, and Aiello, 2010; Trevisanuto, Doglioni, Altinier, et. al., 2007).
Statistical Analysis
Of the children ages 2–10 years of age who were included in the NHANES surveys, 5,350 were missing CRP data, either due to NHANES exclusion by age (which varied by wave) or medical issues, or due to refusal to participate in the phlebotomy portion of the medical exam. Our sample size after dropping those without CRP data was 6652 children. A further 32% of the sample had one or more missing independent variable value; of those with missing data, only 178 children were missing race/ethnicity/parent nativity. In order to address the missing data problem, we utilized multiple imputation with chained equations using the MI suite in Stata. The use of chained equations allowed us to make specific distributional assumptions for each covariate to ensure that plausible values were imputed for each variable. We created 25 imputed datasets for use in our analysis. The dependent variable was used in the imputation process but cases with missing CRP were dropped from the analysis (von Hippel 2007). This is the recommended method when more than 20% of the sample is missing on the dependent variable (Johnson and Young 2011), which was the case with our data. Similar results were found using complete case analysis.
We used logistic regression to test associations between child race/ethnicity and lowgrade inflammation, with no inflammation as the reference category. We weighted the descriptive statistics and regression analyses with individual medical exam weights calculated by NHANES for each survey year for those who participated in the medical exam (where the blood was drawn). We reported odds ratios (exponentiated coefficients), 95% confidence intervals, and statistical significance at p<0.05. In all models, we included survey wave dummy variables but do not show them in the tables for brevity. After estimating the base model (race/ethnicity/parent nativity and wave dummies only), we sequentially added the control variables, family structural variables, and then child BMI. We also calculated predicted probabilities of inflammation for the race/ethnic/parent nativity groups for models with the control variables and those with the family structural and BMI variables. Individual predictions (holding all other variables at their actual values) were calculated and then averaged across individuals in each group and are presented in figures to illustrate the changing probabilities of low-grade inflammation across models.
We conducted formal mediation tests using the methods outlined in Iacobucci (2012). This involved calculating Zmediation test statistics based on the results from relevant regression models (Iacobucci, 2012). Significance was assessed at p<.05. We also conducted supplementary regression models to show how the race/ethnicity/parent nativity categories were associated with the significant mediators.
RESULTS
Table 2 provides the CRP descriptive statistics for the sample children by race/ethnicity/parent nativity categories. In the full sample, mean CRP was 0.83 and 21% had CRP 1–10mg/L. Among white children with a U.S.-born parent, 17% had CRP 1–10mg/L. In contrast, 31% of Hispanic children with a foreign-born parent, and 26% of African American children with a foreign-born parent had CRP 1–10mg/L. Among African American children with a U.S.-born parent respondent, 22% had elevated CRP. CRP for children of “other race” with a U.S.-born parent was similar to that of white children with a U.S.-born parent (19% had CRP 1–10mg/L).
Table 2:
Weighted child CRP descriptive statistics for full sample and by race/ethnicity/parent nativity groups. Children ages 2–10 years with valid CRP data ≤10 mg/L, NHANES 1999–2010. N=6652
| C-Reactive Protein (CRP) | |||
|---|---|---|---|
| Mean (mg/L) |
Std. Error |
% low-grade inflammation (CRP 1–10mg/L) |
|
| Full sample | 0.83 | 0.02 | 21% |
| By child race/ethnicity, parent nativity | |||
| Non-Hispanic white, U.S.-born parent | 0.68 | 0.03 | 17% |
| Hispanic, foreign-born parent | 1.18 | 0.06 | 31% |
| Hispanic, U.S.-born parent | 0.93 | 0.05 | 26% |
| African American, foreign-born parent | 0.90 | 0.11 | 26% |
| African American, U.S.-born parent | 0.92 | 0.04 | 22% |
| Other race, foreign-born parent | 0.90 | 0.15 | 22% |
| Other race, U.S.-born parent | 0.76 | 0.11 | 19% |
Table 3 shows the results from the weighted logistic regression models, with white children with a U.S.-born parent as the reference category. Model 1, which controls only for the NHANES wave dummy variables shows substantially higher odds of inflammation among Hispanic and African American compared with white children. Hispanic children with a foreign-born parent had over two times the odds of low-grade inflammation compared with white children. Significance testing also indicated that Hispanic children with a foreign-born parent had higher odds of inflammation than those with a native-born parent (p<0.05). The odds of inflammation did not differ by immigrant status for African American or other race children in Model 1.
Table 3:
Results of logistic regression of low-grade inflammation (CRP >=1mg/L) regressed on child race/ethnicity/parent nativity for children ages 2–10 years with valid CRP data ≤10mg/L. NHANES 1999–2010. N=6652
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| VARIABLES | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
|
Child Race/Ethnicity, Parent Nativity (ref: non-Hispanic white child, U.S.-born parent) |
||||||||
| Hispanic, foreign-born parent | 2.24** | 1.85 – 2.72 | 2.41** | 1.97 – 2.95 | 1.97** | 1.56 – 2.49 | 1.70** | 1.34 – 2.15 |
| Hispanic, U.S.-born parent | 1.78** | 1.41 – 2.24 | 1.76** | 1.38 – 2.24 | 1.62** | 1.26 – 2.07 | 1.44** | 1.12 – 1.85 |
| African American, foreign-born parent | 1.82** | 1.24 – 2.68 | 2.10** | 1.42 – 3.11 | 1.89** | 1.28 – 2.79 | 1.71** | 1.16 – 2.53 |
| African American, U.S.-born parent | 1.42** | 1.17 – 1.72 | 1.36** | 1.11 – 1.68 | 1.21 | 0.96 – 1.53 | 1.12 | 0.88 – 1.41 |
| Other race, foreign-born parent | 1.45 | 0.83 – 2.53 | 1.53 | 0.84 – 2.77 | 1.47 | 0.80 – 2.70 | 1.70 | 0.91 – 3.18 |
| Other race, U.S.-born parent | 1.17 | 0.80 – 1.70 | 1.17 | 0.79 – 1.74 | 1.17 | 0.79 – 1.74 | 1.19 | 0.80 – 1.78 |
| Control Variables | ||||||||
| Child age in years | 1.13** | 1.08 – 1.17 | 1.13** | 1.09 – 1.18 | 1.12** | 1.08 – 1.17 | ||
| Male child | 0.73** | 0.62 – 0.85 | 0.73** | 0.62 – 0.85 | 0.69** | 0.59 – 0.82 | ||
| U.S.-born child | 1.54* | 1.05 – 2.24 | 1.52* | 1.03 – 2.22 | 1.44 | 0.96 – 2.15 | ||
| Recent head or chest cold | 1.94** | 1.63 – 2.30 | 1.93** | 1.63 – 2.30 | 2.04** | 1.71 – 2.44 | ||
| Recent flu, pneumonia, ear infection | 1.33* | 1.00 – 1.77 | 1.32 | 1.00 – 1.76 | 1.25 | 0.93 – 1.66 | ||
| Recent stomach/intestinal illness | 1.17 | 0.91 – 1.52 | 1.19 | 0.93 – 1.54 | 1.20 | 0.92 – 1.56 | ||
| Ever told child had asthma | 1.53** | 1.22 – 1.90 | 1.52** | 1.22 – 1.89 | 1.37** | 1.10 – 1.71 | ||
| Mother’s age at birth | 0.99 | 0.98 – 1.00 | 1.00 | 0.99 – 1.01 | 1.00 | 0.99 – 1.01 | ||
| Mother smoked during pregnancy | 1.31* | 1.04 – 1.66 | 1.16 | 0.92 – 1.46 | 1.05 | 0.83 – 1.33 | ||
| Low birthweight | 0.82 | 0.63 – 1.07 | 0.80 | 0.62 – 1.05 | 0.90 | 0.68 – 1.20 | ||
| High birthweight | 1.12 | 0.86 – 1.46 | 1.12 | 0.86 – 1.46 | 0.93 | 0.71 – 1.23 | ||
| Mediating Variables | ||||||||
| Parent < high school degree1 | 1.54** | 1.13 – 2.11 | 1.49* | 1.08 – 2.05 | ||||
| Parent high school degree1 | 1.33 | 0.99 – 1.79 | 1.30 | 0.95 – 1.76 | ||||
| Parent some college1 | 1.13 | 0.86 – 1.49 | 1.11 | 0.84 – 1.47 | ||||
| Family income-to-poverty ratio | 0.96 | 0.90 – 1.04 | 0.97 | 0.90 – 1.05 | ||||
| Covered by health insurance | 1.24 | 0.92 – 1.67 | 1.25 | 0.93 – 1.70 | ||||
| Parent no partner2 | 1.02 | 0.83 – 1.25 | 1.04 | 0.84 – 1.28 | ||||
| Household size | 0.98 | 0.92 – 1.05 | 1.01 | 0.95 – 1.08 | ||||
| Child BMI z-score | 0.98 | 0.92 – 1.05 | 1.56** | 1.44 – 1.69 |
Ref: Parent is college graduate or higher educ.
Ref: Parent cohabiting or married. Wave dummies included but not shown for brevity. OR=odds ratio, calculated by exponentiation of the coefficient; CI=confidence interval.
p<0.01
p<0.05 Analyses conducted with imputed data.
Model 2 includes the demographic, illness and birth conditions as control variables. Most of the control variables were associated with increased risk of inflammation. However, the only significant birth condition variable was maternal smoking during pregnancy, which increased the odds of low-grade inflammation in early/mid childhood by 31%. Even when including these potential confounding variables, Hispanic and African American children had significantly higher odds of low-grade inflammation than white children. Hispanic children with a foreign-born parent had almost a 2.5 times higher odds of low-grade inflammation than white children. Similarly, African American children with a foreign-born parent had over 2 times higher odds of low-grade inflammation than white children when controlling for these demographic and birth factors. Hispanic and African American children with U.S.-born parents had 76% and 36% higher odds of low-grade inflammation than white children, respectively.
Additional significance tests indicated that African American children with a foreign-born parent had significantly higher odds of inflammation than African American children with a U.S.-born parent (p<.05) (Model 2). Further, the higher odds of inflammation among Hispanic children with a foreign-was also significantly higher than for Hispanic children with a U.S.-born parent (p<0.01) in this model.
Mediation
Models 3 and 4 show the regression results when our proposed mediators were entered in the model, first family structural variables and then child BMI. As Model 3 shows, the only family structural aspect significantly associated with risk of inflammation was low parental education (less than a high school degree), which increased the odds of low-grade inflammation by 54% compared with parents who had a college degree. In this model, African American children with a U.S.-born parent were not significantly different from white children, and the difference between Hispanic children with foreign-born and U.S.-born parents was no longer significant. The inflammation disadvantage among black children with a foreign-born compared with U.S.-born parent, however, remained significant (p<0.05).
Model 4 indicates that child BMI was also significant; with each point higher z-score associated with a 56% increase in the odds of low-grade inflammation. Even with the inclusion of child BMI, however, Hispanic children (both those with foreign-born and U.S.-born parents) had significantly higher odds of low-grade inflammation, as did African American children with foreign-born parents, compared with the odds for white children.
Our formal mediation tests revealed that these variables were significant mediators (p<0.05) of the associations between Hispanic and African American children (both those with foreign-born and U.S.-born parents) and low-grade inflammation.
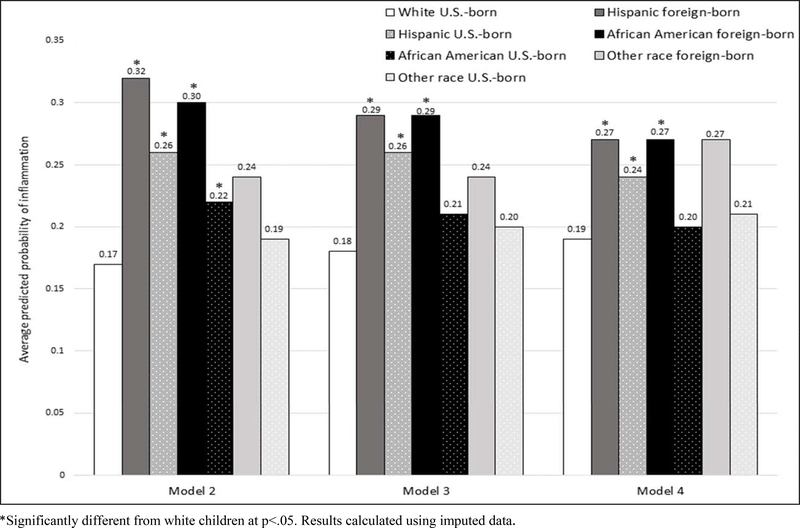
To illustrate the changes in the child race/ethnicity/parent nativity effects on low-grade inflammation across models 2–4, we calculated the predicted probabilities of low-grade inflammation for the significant race/ethnic/parent nativity groups. These predicted probabilities are presented in Figure 1 and are grouped by regression models (2, 3 and 4) shown in Table 3.
Figure 1:
Predicted probabilities of low-grade inflammation (CRP >1 mg/L) by child race/ethnicity/parent nativity. Calculated based on estimates from regression models 2–4 reported in Table 3. Children ages 2–10 years with valid CRP data ≤10mg/L. NHANES 1999–2010. N=6652
Comparing the first set of predicted probabilities (controlling for the demographic, illness and birth variables) with the second set (addition of family structural conditions), we can see that these factors resulted in a decrease in the predicted probability of low-grade inflammation mainly for Hispanic children with a foreign-born parent (dropping from 0.32 to 0.29). The probability of inflammation did not change for Hispanic children with a U.S.-born parent (0.26), and the difference between the two Hispanic groups’ predicted probabilities became insignificant. There was a slight decline in the predicted probabilities for African American children with foreign-born and U.S.-born parents. Although they both declined by 1 percentage point, the reduction for African American children with a U.S.-born parent was enough to reduce it to statistical insignificance when compared with the white children’s predicted probability (0.21 vs. 0.18). The difference in predicted probabilities between African American children with U.S.-born (0.21) and foreign-born (0.29) parents remained significant.
The final set of predicted probabilities (calculated based on Model 4), illustrate the leveling of the probabilities across race/ethnic/parent nativity groups when BMI was included. The predicted probability of inflammation for Hispanic children (foreign-born and U.S.-born parents) and African American children with a foreign-born parent decreased to 0.27, 0.24 and 0.27, respectively, when controlling for their BMI, but they remained significantly above the probability of inflammation for white children. The predicted probability of low-grade inflammation for African American children with U.S.-born parents decreased further, while the white child probability increased slightly; so that, these two groups had similar probabilities of low-grade inflammation (0.20 and 0.19, respectively) when BMI was accounted for.
As additional evidence of mediation, Table 4 below shows the associations between race/ethnicity/parent nativity with low parental education and children’s BMI z-scores. The results from the first model indicate that children across the Hispanic and African American groups have significantly higher risk of living with a parent who has less than a high school degree compared to white children. The results from the second model shows significantly higher BMI z-scores among Hispanic and African American (both with foreign-born and U.S.-born parents) compared with white children.
Table 4:
Results of regression models regressing parental education and child BMI z-scores on child race/ethnicity/parent nativity for children ages 2–10 years with valid CRP data ≤10mg/L. NHANES 1999–2010. N=6652
| Independent Variables | Dependent Variables | |||
|---|---|---|---|---|
| Parent education < high school vs. ≥ college degree1 |
Child BMI Z-score2 | |||
| RRR | 95% CI | β | 95% CI | |
| Child Race/Ethnicity, Parent Nativity | ||||
| Non-Hispanic white child, U.S.-born parent | REF | REF | ||
| Hispanic child, foreign-born parent | 11.8** | 7.82 – 17.9 | 0.39** | 0.28 – 0.51 |
| Hispanic child, U.S.-born parent | 3.08** | 2.14 – 4.42 | 0.30** | 0.19 – 0.41 |
| African American child, foreign-born parent | 3.43** | 1.88 – 6.26 | 0.23* | 0.035 – 0.43 |
| African American child, U.S.-born parent | 2.53** | 1.77 – 3.62 | 0.18** | 0.085 – 0.28 |
| Other race child, foreign-born parent | 0.65 | 0.30 – 1.45 | −0.30* | −0.56 - −0.031 |
| Other race child, U.S.-born parent | 0.72 | 0.40 – 1.29 | −0.041 | −0.20 – 0.12 |
RRR=relative risk ratio calculated by exponentiation of the coefficients; CI=confidence interval; β=beta coefficients
Regressions estimated using imputed data.
p<.01
p<.05
Multinomial logistic regression used for parental education model, results for less than high school category (only significant mediator) are reported; all covariates from Model 3, Table 3 included as covariates (except education variables) in the analysis; results are presented as relative risk ratios.
OLS regression used for BMI z-score model and included all covariates from Model 3, Table 3 included in the analysis.
DISCUSSION
In the U.S., minority populations often experience constrained social and economic conditions that result in health disadvantages compared to the majority white population. Increased physiological wear and tear among African American and Hispanic adults (“weathering”) due to higher exposure to chronic stress has been posited as one reason for racial/ethnic health disparities in adults (Geronimus et al. 2010). The goal of our study was to evaluate immune system weathering early in the life course by assessing racial/ethnic disparities in low-grade inflammation (CRP 1–10mg/L) in a nationally-representative sample of children ages 2–10 years. Early life weathering may contribute to the childhood racial health disparities, as well as adult racial health disparities, documented in prior research (Hayward & Gorman, 2004; Mehta, Lee, & Ylitalo, 2013). We incorporated parent nativity into our racial/ethnic groups to examine differences in risk of low-grade inflammation for minority children with and without a foreign-born parent. Our focus on young children allowed us to detect underlying physiological differences during a life course stage when CRP was less likely to be confounded by risky health behaviors, puberty, and chronic diseases that emerge in adolescence (Shanahan et al., 2013), and thus more likely to reflect chronic stress exposures.
In our descriptive statistics, we provided the first national estimates of the percent of young children with low-grade inflammation (21%). When disaggregated by race/ethnicity/parent nativity, 17% of young, white children with a U.S.-born parent had low-grade inflammation, compared to 22% and 26% for African American and Hispanic children, and 19% for other race children with a U.S.-born parent. Children with foreign-born parents had higher percentages − 31% for Hispanic, 26% for African American, and 22% for other race children with foreign-born parents.
These disparities persisted, and were statistically significant (except for children in the other race category compared with white children) even when accounting for demographic, illness and birth conditions. Importantly, African American and Hispanic young children with foreign-born parents had over 200% higher odds of inflammation than the odds for white children (with U.S.-born parents). Hispanic and African American children with U.S.-born parents had 76% and 36% higher odds of inflammation, respectively, than white children with U.S.-born parents.
Although we did not measure psychosocial stress directly, our measure of low-grade inflammation is indicative of immune system dysregulation due to, in part, chronic stress exposure (Black 2002; Cohen et al. 2012). This supports existing research on racial/ethnic differences in stressors, where African American and Hispanic children experience higher rates of family conflict, violence (Moore, Probst, Tompkins, Cuffe, and Martin 2007) and material hardship (Hernández, Aratani, and Jiang 2014; Nord 2009) than white children. Minority children also experience discrimination at higher rates than do white children (Cheng, Cohen, and Goodman 2015; Pachter, Bernstein, Szalacha, and García Coll 2010). Thus, exposure to multiple sources of difficult experiences may increase stress levels, and systemic inflammation, in minority children. Importantly, low-grade inflammation is associated with elevated risk of depression (Kim, Szigethy, Melhem, Saghafi, and Brent 2014; Miller and Cole 2012), cardiovascular risk factors (Cook, Mendall, Whincup, Carey, Ballam, Morris, Miller, and Strachan 2000; Slopen, Koenen, and Kubzansky 2012), and cognitive disadvantages (Cullen et al., 2017a) in children. Thus, these disparities in low-grade inflammation may have implications for disparities in other aspects of children’s physical and mental health.
Although past research has found an immigrant health advantage, we find no immigrant advantage here. Our findings are congruent with recent literature indicating that Hispanic children, including those with immigrant mothers, were more likely than white children to suffer negative health effects of prenatal stress (Bandoli, von Ehrenstein, Ghosh, et. al. 2016) and have higher rates of obesity that were not explained by health behaviors (Lawrence, Mollborn, & Riosmena, 2016). We add to this literature, suggesting that Hispanic and African American children with foreign-born parents may face higher levels of psychosocial stress, and related risk of inflammation, than minority children with U.S.-born parents. These physiological effects, which are not yet detectable as health problems, may underlie health disadvantages that emerge later in the life course (Hayward & Gorman, 2004).
Mediation by Family Structural Conditions and Child BMI
Our second study objective was to assess whether family social structural or children’s physical health (e.g. BMI) disadvantages mediated these race/ethnic/parent nativity disparities in inflammation. Of the structural factors, we found that low parental education (less than a high school degree) was a significant mediator of the associations between African American and Hispanic children’s (both with U.S.-born and foreign-born parents) risk of low-grade inflammation compared with white children. Further, with the addition of the family structural factors, the African-American/white gap in the predicted risk of inflammation for children with U.S.-born parents narrowed to three percentage points and became insignificant. Given the evidence of low parental education as the one key family structural mediator, our findings suggest that low parental education may account for the elevated chronic stress exposure (as indicated by low-grade inflammation) for African American children with a U.S.-born parent. This is consistent with other research in the adult population that finds racial/ethnic health disparities are due, in part, to lower SES among African Americans (Hayward, Crimmins, Miles, and Yang 2000).
Children’s current BMI was also hypothesized to be part of the explanation for racial/ethnic disparities in inflammation, as psychosocial stress is associated with children’s obesity (Wilson & Sato, 2014) and BMI gain (Tomiyama, Puterman, Epel, et. al. 2013), and higher BMI is associated with elevated inflammation (Visser, Bouter, McQuillan, Wener, & Harris, 2001). Our findings indicated that BMI was a statistically significant mediator of race/ethnic/parent nativity differences in low-grade inflammation, particularly for African American and Hispanic compared with white children. However, the addition of BMI in our models did not substantially alter the predicted inflammation differences between children with foreign-born and U.S.-born parents for either African American or Hispanic children.
In sum, although there was evidence of attenuation in disparities with the inclusion of our hypothesized mediators, the predicted risks of low-grade inflammation remained significantly higher for Hispanic (those with foreign- and U.S.-born parents) and African American children with foreign-born parents compared to white children. This suggests that other aspects of children’s social contexts not measured here may be contributing to stress exposures for children in these minority groups.
Limitations
There are several limitations to this study. First, as previously indicated, several of our racial/ethnic/parent nativity groups had small sample sizes. This calls for caution in interpreting the findings of significant associations found for African American children with a foreign-born parent (n=196), and the lack of significant effects found for the small and heterogeneous “other race” group.
A further limitation is our use of cross-sectional data, which did not allow us to assess how inflammation changed over time or to determine whether elevated CRP reflected a chronic, long-lasting dysregulation of the immune system. We were also lacking information on children’s (and their families’) experiences of stress and discrimination as potential explanations for the racial/ethnic/parental nativity disparities we observed in low-grade inflammation.
Finally, we only had data on one parent (half of whom were fathers), which limits our ability to assess the relevance of the immigrant health advantage that has been found mainly for children with immigrant mothers (Hendi, Mehta, & Elo, 2015; Hummer, Powers, Pullum, Gossman, & Frisbie, 2007; Padilla, Hamilton, & Hummer, 2009). However, additional testing found no difference in inflammation whether the mother or father was the parent respondent. Relatedly, we cannot ascertain from the NHANES data the acculturation status or duration in the U.S. of the foreign-born parents. Thus, we do not claim to refute the immigrant health advantage, but rather to provide evidence of the potential health challenges and chronic stress exposures faced by minority children in immigrant families.
Conclusion
Notwithstanding these limitations, our study contributes to the growing body of biosocial research indicating physiological changes due to psychosocial stress can begin during childhood, and that chronic stress may contribute to the higher risk of inflammation in African American and Hispanic children (Dowd, Zajacova, and Aiello 2010; Ford 2003). Our results further research in this area by providing evidence that these inequalities begin early in childhood, and that African American and Hispanic children with foreign-born parents have especially high risks of low-grade inflammation. Importantly, we estimated these race/ethnicity/parent nativity disparities in inflammation net of birth conditions, illness and demographic characteristics. Most of these disparities persisted after accounting for family structural conditions and children’s BMI. This suggests that other sources of psychosocial or physical stress, such as exposures to discrimination, family processes or environmental exposures, may explain the remaining inequalities in the risk of low-grade inflammation among minority children.
Our use of a biomarker of inflammation to assess physiological disparities was an advantage since it did not rely on parental reports or medical diagnosis of child health problems. These physiological disparities provide evidence that biological weathering may begin early in the life course. The heightened risk for low-grade inflammation among minority children and those with foreign-born parents, in turn, has potentially important health consequences. Future research should further explicate these biosocial processes across race/ethnic and immigrant groups and over time to improve our ability to reduce health and developmental disparities during childhood and across the life course.
Contributor Information
Kammi K. Schmeer, The Ohio State University.
Jacob Tarrence, The Ohio State University.
REFERENCES
- Adams Sally H., Newacheck Paul W., Jane Park M, Claire D. Brindis, and Charles E. Irwin. 2007. “Health Insurance Across Vulnerable Ages: Patterns and Disparities From Adolescence to the Early 30s.” Pediatrics 119:e1033. [DOI] [PubMed] [Google Scholar]
- Akinbami L, Moorman J, Garbe P, and Sondik E. 2009. “Status of Childhood Asthma in the United States, 1980–2007.” Pediatrics 123:S131–S145. [DOI] [PubMed] [Google Scholar]
- Baker E, Rendall M, and Weden M. 2015. “Epidemiological Paradox or Immigrant Vulnerability? Obesity Among Young Children of Immigrants.” Demography 52:1295–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoli G, von Ehrenstein O, Ghosh J, Flores M, Schetter C, and Ritz B. 2016. “Prenatal Maternal Stress and the Risk of Lifetime Wheeze in Young Offspring: An Examination by Stressor and Maternal Ethnicity.” Journal of Immigrant and Minority Health 18:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittoni Marisa, Wexler Randy, Spees Colleen K., Clinton Steven K., and Taylor Christopher A.. 2015. “Lack of private health insurance is associated with higher mortality from cancer and other chronic diseases, poor diet quality, and inflammatory biomarkers in the United States.” Preventive medicine 81:420–426. [DOI] [PubMed] [Google Scholar]
- Black P 2002. “Stress and the inflammatory response: A review of neurogenic inflammation.” Brain Behavior and Immunity 16:622–653. [DOI] [PubMed] [Google Scholar]
- Blake Kevin, Kellerson Rebecca L., and Simic Aleksandra. 2007. “Measuring Overcrowding in Housing.” U.S Department of Housing and Urban Development Office of Policy Development and Research. [Google Scholar]
- Brody G, Lei M, Chae D, Yu T, Kogan S, and Beach S. 2014. “Perceived Discrimination Among African American Adolescents and Allostatic Load: A Longitudinal Analysis With Buffering Effects.” Child Development 85:989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G, Yu T, and Beach S. 2016. “Resilience to adversity and the early origins of disease.” Development and Psychopathology 28:1347–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles Stephanie T., Staiano Amanda E., Drazba Kathryn T., Gupta Alok K., Sothern Melinda, and Katzmarzyk Peter T.. 2012. “Elevated C-Reactive Protein in Children from Risky Neighborhoods: Evidence for a Stress Pathway Linking Neighborhoods and Inflammation in Children.” Plos One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Erika R., Cohen Alyssa, and Goodman Elizabeth. 2015. “The Role of Perceived Discrimination during Childhood and Adolescence in Understanding Racial and Socioeconomic Influences on Depression in Young Adulthood.” The Journal of Pediatrics 166:370–377.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child Trends Data Bank. 2015. “Parental Education.”
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, and Turner RB. 2012. “Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk.” Proceedings of the National Academy of Sciences of the United States of America 109:5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole Tim J., Bellizzi Mary C., Flegal Katherine M., and Dietz William H.. 2000. “Establishing a standard definition for child overweight and obesity worldwide: international survey.” BMJ : British Medical Journal 320:1240–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, Miller GJ, and Strachan DP. 2000. “C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors.” Atherosclerosis 149:139–50. [DOI] [PubMed] [Google Scholar]
- Das A 2013. “How does race get “under the skin”?: Inflammation, weathering, and metabolic problems in late life.” Social Science & Medicine 77:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heredia F, Gomez-Martinez S, and Marcos A. 2012. “Obesity, inflammation and the immune system.” Proceedings of the Nutrition Society 71:332–338. [DOI] [PubMed] [Google Scholar]
- Dowd J, Zajacova A, and Aiello A. 2010. “Predictors of Inflammation in U.S. Children Aged 3–16 Years.” American Journal of Preventive Medicine 39:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S, Theall K, Gleason M, Smyke A, De Vivo I, Wong J, Fox N, Zeanah C, and Nelson C. 2012. “Telomere length and early severe social deprivation: linking early adversity and cellular aging.” Molecular Psychiatry 17:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed A and Galea S. 2011. “Maternal Immigrant Status and High Birth Weight: Iplications for Childhood Obesity.” Ethnicity & Disease 21:47–51. [PubMed] [Google Scholar]
- Fagundes C, Glaser R, and Kiecolt-Glaser J. 2013. “Stressful early life experiences and immune dysregulation across the lifespan.” Brain Behavior and Immunity 27:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Ross E, Pathak H, Godwin A, and Tseng M. 2014. “Acculturative Stress and Inflammation Among Chinese Immigrant Women.” Psychosomatic Medicine 76:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES 2003. “C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999–2000.” Circulation 108:1053–8. [DOI] [PubMed] [Google Scholar]
- Ford E, Giles W, Myers G, Rifai N, Ridker P, and Mannino D. 2003. “C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999–2000.” Clin Chem 49:1353–7. [DOI] [PubMed] [Google Scholar]
- Fraser A, Brockert J, and Ward R. 1995. “Association of Young Maternal Age with Adverse Reproductive Outcomes.” New England Journal of Medicine 332:1113–1117. [DOI] [PubMed] [Google Scholar]
- Geronimus A, Hicken M, Keene D, and Bound J. 2006. ““Weathering” and age patterns of allostatic load scores among blacks and whites in the United States.” American Journal of Public Health 96:826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus A, Hicken M, Pearson J, Seashols S, Brown K, and Cruz T. 2010. “Do US Black Women Experience Stress-Related Accelerated Biological Aging?” Human Nature-an Interdisciplinary Biosocial Perspective 21:19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus Arline T. 1992. “The weathering hypothesis and the health of African-American women and infants: Evidence and speculations.” Ethnicity and Disease 2:207–221. [PubMed] [Google Scholar]
- Haas J, Lee L, Kaplan C, Sonneborn D, Phillips K, and Liang S. 2003. “The association of race, socioeconomic status, and health insurance status with the prevalence of overweight among children and adolescents.” American Journal of Public Health 93:2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward M, Crimmins E, Miles T, and Yang Y. 2000. “The significance of socioeconomic status in explaining the racial gap in chronic health conditions.” American Sociological Review 65:910–930. [Google Scholar]
- Hendi A, Mehta N, and Elo I. 2015. “Health Among Black Children by Maternal and Child Nativity.” American Journal of Public Health 105:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández Diana, Aratani Yumiko, and Jiang Yang. 2014. “Energy Insecurity among Families with Children” National Center for Children in Poverty, Columbia University Mailman School of Public Health, New York. [Google Scholar]
- Huang Zhihuan, Yu Stella, and Ledsky Rebecca. 2006. “Health Status and Health Service Access and Use Among Children in U.S. Immigrant Families.” American Journal of Public Health 96:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer R, Powers D, Pullum S, Gossman G, and Frisbie W. 2007. “Paradox found (again): Infant mortality among the Mexican-origin population in the United States.” Demography 44:441–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvisalo Mikko., Aimo Harmoinen, Maarit Hakanen, Ulla Paakkunainen, Jorma Viikari, Jaakko Hartiala, Terho Lehtimäki, Olli Simell, and Olli T. Raitakari. 2002. “Elevated Serum CReactive Protein Levels and Early Arterial Changes in Healthy Children.” Arteriosclerosis, Thrombosis, and Vascular Biology 22:1323–1328. [DOI] [PubMed] [Google Scholar]
- Johnson D and Young R. 2011. “Toward Best Practices in Analyzing Datasets with Missing Data: Comparisons and Recommendations.” Journal of Marriage and Family 73:926–945. [Google Scholar]
- Kannan Srimathi, Luis Acosta, Dolores Acevedo-Garcia, Adnan Divjan Luis Bracero, Matthew Perzanowski, and Ginger Chew. 2013. “Sociocultural characteristics, obesity and inflammatory biomarkers in Puerto Rican toddlers born in New York City.” Pediatric Allergy & Immunology 24:487–492. [DOI] [PubMed] [Google Scholar]
- Khoshnood B, Wall S, and Lee K. 2005. “Risk of low birth weight associated with advanced maternal age among four ethnic groups in the United States.” Maternal and Child Health Journal 9:3–9. [DOI] [PubMed] [Google Scholar]
- Kieffer E, Alexander G, Kogan M, Himes J, Herman W, Mor J, and Hayashi R. 1998. “Influence of diabetes during pregnancy on gestational age-specific newborn weight among US black and US white infants.” American Journal of Epidemiology 147:1053–1061. [DOI] [PubMed] [Google Scholar]
- Kim J, Szigethy E, Melhem N, Saghafi E, and Brent D. 2014. “Inflammatory Markers and the Pathogenesis of Pediatric Depression and Suicide: A Systematic Review of the Literature.” Journal of Clinical Psychiatry 75:1242–1253. [DOI] [PubMed] [Google Scholar]
- Logan J, Alba R, and Zhang W. 2002. “Immigrant enclaves and ethnic communities in New York and Los Angeles.” American Sociological Review 67:299–322. [Google Scholar]
- Martin Joyce, Hamilton Brady, Ventura Stephanie, Osterman Michelle, Kirmeyer Sharon, Mathews T, and Elizabeth Wilson. 2011. “Births: final data for 2009.” National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 60:1–70. [PubMed] [Google Scholar]
- Mehta N, Lee H, and Ylitalo K. 2013. “Child health in the United States: Recent trends in racial/ethnic disparities.” Social Science & Medicine 95:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza Fernando S. 2009. “Health disparities and children in immigrant families: a research agenda.” Pediatrics 124 Suppl 3:S187–95. [DOI] [PubMed] [Google Scholar]
- Michelson P, Williams L, Benjamin D, and Barnato A. 2009. “Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004.” Annals of Allergy Asthma & Immunology 103:381–385. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, and Parker K. 2011. “Psychological Stress in Childhood and Susceptibility to the Chronic Diseases of Aging: Moving Toward a Model of Behavioral and Biological Mechanisms.” Psychological Bulletin 137:959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Gregory and Cole Steve. 2012. “Clustering of Depression and Inflammation in Adolescents Previously Exposed to Childhood Adversity.” Biological Psychiatry 72:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina K, Little T, and Rosal M. 2016. “EVERYDAY DISCRIMINATION, FAMILY CONTEXT, AND PSYCHOLOGICAL DISTRESS AMONG LATINO ADULTS IN sTHE UNITED STATES.” Journal of Community Psychology 44:145–165. [Google Scholar]
- Moore C, Probst J, Tompkins M, Cuffe S, and Martin A. 2007. “The prevalence of violent disagreements in US families: Effects of residence, race/ethnicity, and parental stress.” Pediatrics 119:S68–S76. [DOI] [PubMed] [Google Scholar]
- National Center for Education Statistics; 2005. “Percentage of children ages 6 to 18, by parent’s highest educational attainment and race/ethnicity: 2005.” [Google Scholar]
- Nazmi Aydin and Victora Cesar. 2007. “Socioeconomic and racial/ethnic differentials of Creactive protein levels: a systematic review of population-based studies.” BMC Public Health 7:212–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord Mark. 2009. “Food Insecurity in Households with Children: Prevalence, Severity, and Household Charactersistics “ US Department of Agriculture [Google Scholar]
- Ornelas I and Perreira K. 2011. “The role of migration in the development of depressive symptoms among Latino immigrant parents in the USA.” Social Science & Medicine 73:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachter Lee, Bernstein Bruce, Szalacha Laura, and García Coll Cynthia. 2010. “Perceived Racism and Discrimination in Children and Youths: An Exploratory Study.” Health & Social Work 35:61–70. [DOI] [PubMed] [Google Scholar]
- Perreira Krista and Cortes Kalena. 2006. “Race/Ethnicity and Nativity Differences in Alcohol and Tobacco Use During Pregnancy.” American Journal of Public Health 96:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D 2013. “Paradox Revisited: A Further Investigation of Racial/Ethnic Differences in Infant Mortality by Maternal Age.” Demography 50:495–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P 2007a. “C-Reactive protein and the prediction of cardiovascular events among those at intermediate risk - Moving an inflammatory hypothesis toward consensus.” Journal of the American College of Cardiology 49:2129–2138. [DOI] [PubMed] [Google Scholar]
- Ridker Paul 2007b. “C-Reactive Protein and the Prediction of Cardiovascular Events Among Those at Intermediate Risk: Moving an Inflammatory Hypothesis Toward Consensus.” Journal of the American College of Cardiology 49:2129–2138. [DOI] [PubMed] [Google Scholar]
- Schmeer K 2012. “Early childhood economic disadvantage and the health of Hispanic children.” Social Science & Medicine 75:1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeer Kammi and Yoon Aimee. 2016. “Socioeconomic status inequalities in low-grade inflammation during childhood.” Archives of Disease in Childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook Jamie and Avison William. 2015. “Family Structure and Children’s Socioeconomic Attainment: A Canadian Sample.” Canadian Review of Sociology/Revue canadienne de sociologie 52:66–88. [DOI] [PubMed] [Google Scholar]
- Seiber Eric. 2014. “Covering the remaining uninsured children: almost half of uninsured children live in immigrant families.” Medical care 52:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Zhang D, van Meijgaard J, MacLeod K, and Fielding J. 2015. “The Interaction Between an Individual’s Acculturation and Community Factors on Physical Inactivity and Obesity: A Multilevel Analysis.” American Journal of Public Health 105:1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., Kaholokula J, Ratner R, and Mau M. 2006. “Ethnic differences in perinatal outcome of gestational diabetes mellitus.” Diabetes Care 29:2058–2063. [DOI] [PubMed] [Google Scholar]
- Slopen N, Koenen K, and Kubzansky L. 2012. “Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: A systematic review.” Brain Behavior and Immunity 26:239–250. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky L, McLaughlin K, and Koenen K. 2013. “Childhood adversity and inflammatory processes in youth: A prospective study.” Psychoneuroendocrinology 38:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Shonkoff J, Albert M, Yoshikawa H, Jacobs A, Stoltz R, and Williams D. 2016. “Racial Disparities in Child Adversity in the US Interactions With Family Immigration History and Income.” American Journal of Preventive Medicine 50:47–56. [DOI] [PubMed] [Google Scholar]
- Sparks Johnelle P. 2009. “One Size Does Not Fit All: An Examination of Low Birthweight Disparities Among a Diverse Set of Racial/Ethnic Groups.” Maternal and Child Health Journal 13:769–779. [DOI] [PubMed] [Google Scholar]
- Steffen PR, Walker J, Meredith R, and Anderson C. 2016. “The Effects of Job Instability and Financial Strain on C-Reactive Protein in a Sample of Mexican Immigrants.” Ethnicity & Disease 26:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SMS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, and Ryan AS. 2002. “National estimates of the timing of sexual maturation and racial differences among US children.” Pediatrics 110:911–919. [DOI] [PubMed] [Google Scholar]
- Taylor CAL and Sarathchandra D. 2016. “Migrant Selectivity or Cultural Buffering? Investigating the Black Immigrant Health Advantage in Low Birth Weight.” Journal of Immigrant and Minority Health 18:390–396. [DOI] [PubMed] [Google Scholar]
- Thomas SB, Quinn SC, Butler J, Fryer CS, and Garza MA. 2011. “Toward a Fourth Generation of Disparities Research to Achieve Health Equity” Pp. 399–416 in Annual Review of Public Health , Vol 32, vol. 32, Annual Review of Public Health, edited by Fielding JE, Brownson RC, and Green LW. Palo Alto: Annual Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama AJ, Puterman E, Epel ES, Rehkopf DH, and Laraia BA. 2013. “Chronic Psychological Stress and Racial Disparities in Body Mass Index Change Between Black and White Girls Aged 10–19.” Annals of Behavioral Medicine 45:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisanuto D, Doglioni N, Altinier S, Zaninotto M, Plebani M, and Zanardo V. 2007. “High-Sensitivity C-Reactive Protein in Umbilical Cord of Small-for-Gestational-Age Neonates.” Neonatology 91:186–189. [DOI] [PubMed] [Google Scholar]
- Van Hook J, Baker E, Altman CE, and Frisco ML. 2012. “Canaries in a coalmine: Immigration and overweight among Mexican-origin children in the US and Mexico.” Social Science & Medicine 74:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wie Van, Alissa Jeanette Ziegenfuss, Blewett Lynn A., and Davern Michael. 2008. “Persistent Disparities in Health Insurance Coverage: Hispanic Children, 1996 to 2005.” Journal of Health Care for the Poor and Underserved 19:1181–1191. [DOI] [PubMed] [Google Scholar]
- Vega William and Amaro Hortensia. 1994. “Latino outlook: good health, uncertain prognosis.” Annual review of public health 15:39–67. [DOI] [PubMed] [Google Scholar]
- von Hippel PT 2007. “Regression with Missing Ys: an Improved Strategy for Analyzing Multiply Imputed Data” Pp. 83–117 in Sociological Methodology 2007, Vol 37, vol. 37, Sociological Methodology. Oxford: Blackwell Publishing. [Google Scholar]
- Walsh Jennifer and Fionnuala McAuliffe. 2012. “Prediction and prevention of the macrosomic fetus.” European Journal of Obstetrics & Gynecology and Reproductive Biology 162:125–130. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Lee D, and Arheart K. 2007. “Secondhand smoke exposure and C-reactive protein levels in youth.” Nicotine & Tobacco Research 9:305–307. [DOI] [PubMed] [Google Scholar]
- Williams D, Mohammed SA, Leavell J, and Collins C. 2010. “Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities” Pp. 69–101 in Biology of Disadvantage: Socioeconomic Status and Health, vol. 1186, Annals of the New York Academy of Sciences, edited by Adler NE and Stewart J. Malden: Wiley-Blackwell. [DOI] [PMC free article] [PubMed] [Google Scholar]