Abstract
The World Health Organization (WHO) has been on front line to encourage developing countries to identify medicinal plants that are safe and easily available to patients. Traditional medicine represents the first-treatment choice for the healthcare of approximately 80% of people living in developing countries. Also, its use in the United States has increased by 38% during within the last decade of the 20th century alone. Therefore, the aim of the present study was to explore the efficacy of a medicinal plant, Vernonia amygdalina Delile (VAD), as a new targeted therapy for the management of acute promyelocytic leukemia (APL), using HL-60 cells as a test model. To address our specific aim, HL-60 promyelocytic leukemia cells were treated with VAD. Live and dead cells were determined by acridine orange and propidium iodide (AO/PI) dye using the Cellometer Vision. The extent of DNA damage was evaluated by the comet assay. Cell apoptosis was evaluated by flow cytometry assessment. Data obtained from the AO/PI assay indicated that VAD significantly reduced the number of live cells in a dose-dependent manner, showing a gradual increase in the loss of viability in VAD-treated cells. We observed a significant increase in DNA damage in VAD-treated cells compared to the control group. Flow cytometry data demonstrated that VAD induced apoptosis in treated cells compared to the control cells. These results suggest that induction of cell death, DNA damage, and cell apoptosis are involved in the therapeutic efficacy of VAD. Because VAD exerts anticancer activity in vitro, it would be interesting to perform clinical trials to confirm its effectiveness as an anticancer agent towards the treatment of APL patients.
Keywords: Vernonia amygdalina Delile, promyelocytic leukemia (HL-60) cells, DNA damage, apoptosis
INTRODUCTION
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) that accounts for approximately 10–15% of nearly 12,330 adults diagnosed with AML each year in the USA [1]. The signs and symptoms of APL are nonspecific and may include the following: fatigue or feeling tired, minor infections, tendency to bleed or hemorrhagic diathesis. There is usually pancytopenia with low levels of red blood cells or anemia, low levels of the white blood cells and platelets, dyspnea, fever, thrombocytopenia, leukocytosis and coagulopathy [2]. The current treatment choice for APL is arsenic trioxide (Trisenox). Arsenic trioxide has been shown to induce complete remission in 85–86% of patients who did not respond to conventional therapy [3, 4]. Although arsenic trioxide is currently a new treatment for APL patients, the possible side effects associated with arsenic trioxide treatment includes dermatologic symptoms such as skin itching and gastrointestinal symptoms such as nausea, vomiting, and loss of appetite reported in 26.7% of patients [4]. Therefore, the development of new chemotherapeutic or chemopreventive drugs from natural products is considered important. The World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) have reported that fruit and vegetable diets highly reduce the risk of several cancers including those of the mouth, pharynx, esophagus, breast, lung, pancreas, stomach, colon, rectum, and bladder [5]. Vernonia amygdalina Delile (VAD) known as bitter leaf and a species of Vernonia amygdalina (family of asteraceae) is a valuable medicinal plant that is widespread in East and West Africa [6, 7]. It has been used for many years as ethnomedicine under the recommendation of herbalists for the treatment of many diseases and disorders including stomach discomfort, vomiting, diarrhea, and intestinal illnesses in Cameroon [8, 9]. Interestingly, some of these ailments may stop in less than few hours and/or to a day after oral intake. These vegetables are locally available, affordable, and easily accessible to either the consumers and/or patients (Fig. 1). There is a large body of scientific reports documenting that Vernonia amygdalina has anticancer activity [9, 10, 11]; antihepatotoxic activity [12]; antibacterial activity [13]; as well as antioxidant property [14]. Recent reports from our laboratory have demonstrated that in vitro Vernonia amygdalina treatment reduces cellular viability; induces DNA damage leading to apoptosis accompanied by secondary necrosis in breast cancer cells [8, 9]. Although published studies indicate that Vernonia amygdalina has medicinal properties that are effective against many diseases, the molecular mechanisms under which Vernonia amygdalina Delile (a species of Vernonia amygdalina) exerts its therapeutic efficacy in leukemia cells remain largely unknown. Therefore, the aim of the present study was to test the efficacy of VAD as a new targeted therapy for the management of APL, using HL-60 Promyelocytic leukemia cells as a test model.
Figure 1:

Photo of VAD leaves taken in Bangou, West Cameroon on May 10, 2012 by Clement G. Yedjou. This photo shows how VAD leaves are processed for food: (1) Whole leaves; (2) Removal of stem and leaf veins; (3) Leaves cut in small pieces, ready to be washed several times to remove the bitter taste. After washing, it is cooked using different recipes. The production of bitter-leaves from VAD contributes to the food security and economic development in Cameroon, and helps to sustain the environment.
RESULTS
1. Vernonia amygdalina Delile Inhibits Cellular viability of HL-60 Cells
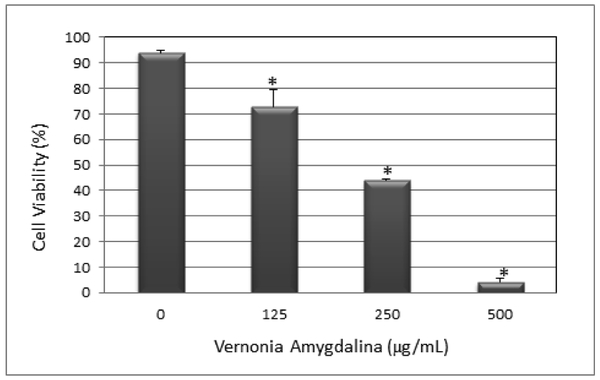
The in vitro cytotoxicity and anti-cancer effect of Vernonia amygdalina Delile (VAD) leaf extracts against HL-60 promyelocytic leukemia cells were determined by acridine orange (AO) and propidium iodide (PI) staining using the Cellometer Vision. We observed a strong dose-response relationship with regard to VAD treatment, showing a significant (p < 0.05) increase in the percentage of dead cells compared to the percentage of live cells in the control (Fig. 2). Acridine orange (OA) is a nuclear dye that is permeable to both live and dead cells. OA stains both live and dead nucleated cells to emit green fluorescence. Propidium iodide (PI) is a nuclear dye that is impermeable to live cells and permeable to dead cells population. PI stains all dead nucleated cells to emit red fluorescence. As seen in Fig. 2, the percentage of dead cells (red) increases with increasing doses of VAD. Fig. 3 shows the percentage of live cells as function of VAD treatment. As seen in Fig. 3, VAD is highly cytotoxic to HL-60 promyelocytic leukemia cells.
Figure 2:
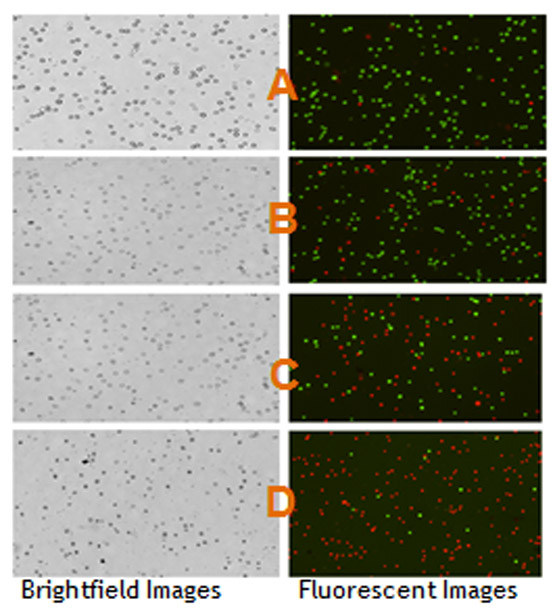
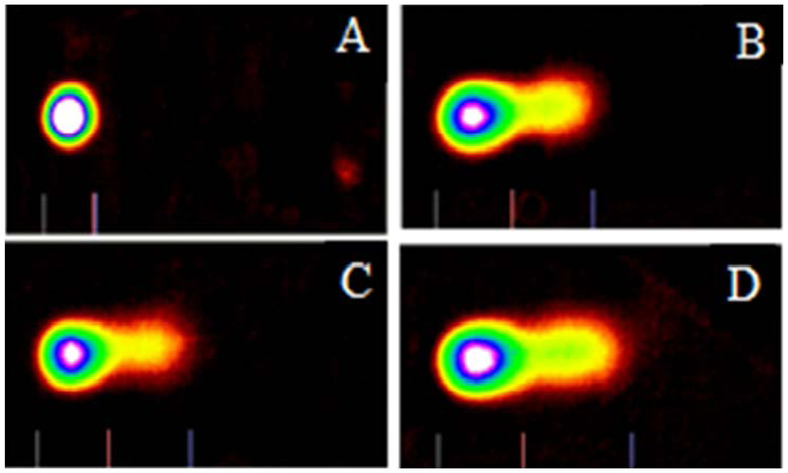
Bright field images (left) and fluorescent images (right) of HL-60 cells exposed to VAD for 24 h. Figure 2 shows HL-60 cells untreated (A-control) and HL-60 cells treated with VAD at 125 µg/mL (B), 250 µg/mL (C), and 500 µg/mL (D). Live cells (green fluorescent) and dead cells (red fluorescent) were determined based on the acridine orange and propidium iodide assay using the Cellometer Vision. The cell diameters vary from 8 to 12 µm with the average of 10 µm.
Figure 3:
Antiproliferative effect of VAD to HL-60 promyelocytic leukemia cells. HL-60 cells were cultured with increasing doses of VAD (0, 125, 250, and 500 µg/mL) for 24 has indicated in the Materials and Methods. Cell viability was determined based on the acridine orange and propidium iodide assay. Each point represents a mean ± SD of 3 experiments with 6 replicates per dose. *Significantly different (p<0.05) from the control, according to the Dunnett’s test.
2. Vernonia amygdalina Delile Induces DNA Damage
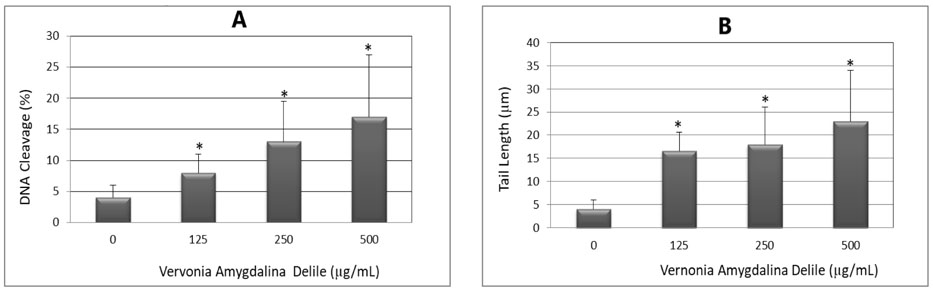
Representative Comet assay images of control and VAD-treated cells stained with SYBR Green are presented in Fig. 4. The percentages of DNA cleavage and tail length are represented in Fig. 5. As denoted in this figure, there is gradual increase in the mean values of comet tail length, and percentages of DNA cleavage in HL-60 cells, with increasing doses of VAD. Overall, the results generated from the comet assay indicated that VAD is highly genotoxic to HL-60 promyelocytic leukemia cells.
Figure 4:
Representative SYBR Green Comet assay images of untreated (A-control) and VAD. HL-60 promyelocytic leukemia cells treated with VAD at 125 µg/mL (B), 250 µg/mL (C), and 500 µg/mL (D). A total volume of 50 µL from 1 × 105 cells/mL was used for each treatment as indicated in the Materials and Methods. Untreated cells (A) showed absence of DNA migration in cultured HL-60 cells while cells treated with VAD (B, C, and D) showed clear migration of DNA from the head to tail regions.
Figure 5:
Bar graph showing the percentage of DNA cleavage (A) and tail length (B) in untreated and VAD-treated HL-60 cells. A total volume of 50 µL from 1 × 105 cells/mL was used for each treatment as indicated in the Materials and Methods. Each point represents mean ± SD of 3 independent experiments. *Significantly different (p < 0.05) from the control, according to the Dunnett’s test.
3. Vernonia amygdalina Delile Induces Apoptosis
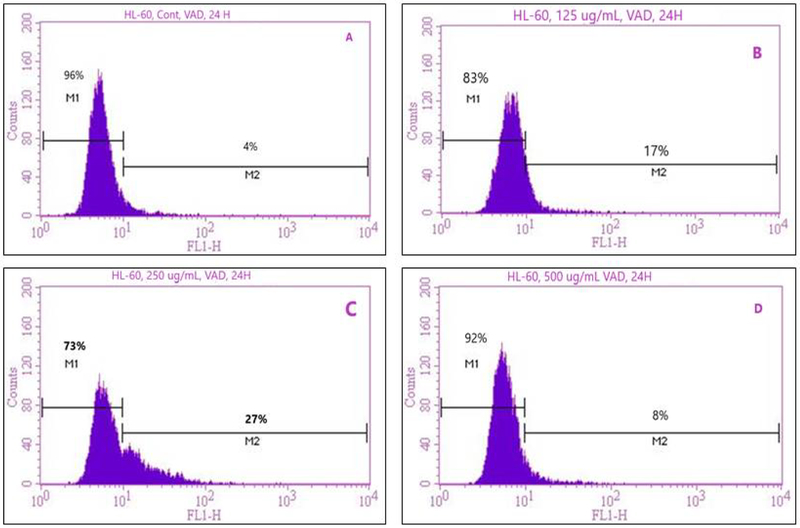
To prove that VAD treatment could induce apoptosis of leukemia cells, we treated HL-60 cells for 24 h. Following treatment, cells were stained with annexin V FITC/PI and analyzed by flow cytometry. We observed a strong dose-response relationship with regard to VAD treatment and annexin V positive cells (apoptotic and/or necrotic cells) (Fig. 6). As seen in Fig. 6, the percentage of apoptotic dead cells increases significantly with increasing doses of VAD compared to the control. However, a markedly decrease in annexin V positive cells was detected at 500 µg/mL VAD, probably due to a high level of cell death. Upon 24 h of exposure, the result of annexin V/PI showed that the percentages of positive annexin V cells (apoptotic cells) were 4.4 ± 4.2%, 17.4 ± 2.6%, 25.5 ± 4.8%, and 7.2 ± 2.5% in 0, 125, 250, and 500μg/mL VAD, respectively (Table 1). VAD-treated cells were significantly different (p < 0.05) at the doses of 125 and 250μg/mL according to ANOVA Dunnett’s test.
Figure 6:
Representative flow cytometry analysis data from Annexin V/PI staining. The histograms show a comparison of the distribution of annexin V/PI negative cells (M1) and annexin V/PI positive cells (M2) of VAD-treated cells for 24 h. A-control; B-125μg/mL VAD; C-250μg/mL VAD; and D-500μg/mL VAD.
Table 1:
Summary data of annexin V/PI assay obtained from the flow cytometry assessment. HL-60 promyelocytic leukemia cells were cultured in the absence or presence of VAD for 24 has indicated in the Materials and Methods. Values are shown as means ± SDs of 3 replicates per experiment. *P < 0.05 versus compared with the control group.
| Concentrations | Annexin V/PI Negative Cells or Viable Cells (Mean ± SD)% |
Annexin V/PI Positive Cells or Apoptotic Cells (Mean ± SD)% |
|---|---|---|
| 0μg/mL | 95.6 ± 4.2 | 4.4 ± 4.2 |
| 125μg/mL | 82.6 ± 2.6* | 17.4 ± 2.6* |
| 250μg/mL | 74.5 ± 4.8* | 25.5 ± 4.8* |
| 500μg/mL | 92.8 ± 2.5 | 7.2 ± 2.5 |
DISCUSSION
In the present study, we first tested the antiproliferative effect of VAD leaf extracts on HL-60 cells by the means of AO/PI assay using the Cellometer Vision. Our results demonstrated that VAD significantly (p<0.05) reduces the percentage of live cells in a dose-dependent manner (Fig. 2 and 3). Based on these observations, we believe that the incorporation of VAD in a person’s diet may help prevent or reduce the risk of acute promyelocytic leukemia (APL) considering the nutritional and therapeutic applications of this natural medicinal plant in many West and East Africa countries. Previous studies indicated that Vernonia amygdalina possesses potent antimalarial and antihelmintic properties [15], and antitumorigenic properties [16]. Vernonia amygdalina contains many active ingredients including Vernonioiside B and Myricetin (flavonol) [15] [17]. Oral administration of the aqueous leaf extract of Vernonia amygdalina has been found to relieve pain and lower body temperature [18]. Many studies have documented the beneficial use of Vernonia amygdalina as a potent botanical agent for the treatment of different diseases [16, 19–21]. VAD leaves (known as bitter-leaves in English) are the most consumed vegetables during special occasions including marriage, baptism, Christmas, birthday, funeral and sometimes on a daily basis in Cameroon. For example, out of 93,600 tons of leafy vegetables harvested in 1998, 21,549 tons were bitter leaf approximately 23% of the total vegetables [22]. It is well-commercialized in Cameroon both for its health promoting benefit as a medicinal plant and for its nutritional value. Scientific data indicated that Vernonia amygdalina contains significant quantities of lipids, proteins with high essential amino acid content, and fiber [23]. It also possesses carbohydrates, high level of vitamin C, and caroteinoids [24]. In 2015, our research group used diverse medicinal plants to treat 328 Cameroonian patients who have been diagnosed at least once by a physician or medical professional with diabetics and/or hypertension for 10 days. At the end of the 10 days treatment, we found that 70% of patients have a complete remission and were free from diabetes and/or hypertension [25].
Next, the comet assay, also known as single cell gel electrophoresis (SCGE) was used to detect DNA damage in VAD-untreated and treated cells. We found that VAD has strong genotoxic damage potential and is able to cause DNA damage in leukemia cells. Our results demonstrated that VAD induces genotoxic effects to HL-60 promyelocytic leukemia cells in a dose-dependent fashion; suggestive clear evidence that VAD is a potent DNA damaging agent against APL. Similarly, other studies in our laboratory showed that the size, shape and distribution of DNA within the comet correlate with the extent of DNA damage in human promyelocytic leukemia cells when treated with arsenic trioxide [26, 27]. There are limited scientific data in the literature explaining how VAD induces DNA damage in leukemia cells. Hence, further studies are needed to establish the genotoxic mechanism on the basis of the genetic damage induced by VAD. Thus, we show in the present study that even at a relatively low dose VAD leaf extracts induce DNA damage in HL-60 promyelocytic leukemia cells. It has been reported that agents that have the ability to cause minimal DNA damage are generally good candidates for cancer therapy [28, 29]. On the other hand, agents that cause cell damage but not cell death, causes sustained DNA damage, and are therefore possible mutagens and/or carcinogens [30].
To explore whether VAD-induces apoptosis of HL-60 promyelocytic leukemia cells, we stained the cells with annexin V FITC/PI antibodies and analyzed by flow cytometry assessment. Annexin V binds to the membrane phospholipid phosphatidylserine that is located within the plasma membrane of apoptotic cells and PI is a nuclear dye that is impermeable to live cells and permeable to dead cells population. PI stains all dead nucleated cells [31]. Data generated from the flow cytometry assessment demonstrated that VAD induced apoptosis of HL-60 promyelocytic leukemia cells in a dose-dependent fashion. The percentage of cells stained with Annexin V (positive cells) and PI (necrotic cells) significantly (P <0.05) increased with the increasing doses of VAD (Fig. 6). By the means of flow cytometry assessment, we were able to show that VAD induced apoptosis and/or necrosis in HL-60 promyelocytic leukemia cells via phosphatidylserine externalization as result of the loss of membrane integrity, a major characteristic of cell death by apoptosis and/or necrosis. These observations are in agreement with a report in which Vernonia amygdalina was shown to alter the cell membrane permeability in MCF-7 cells [32]. Another report indicated that Vernonia amygdalina increased the number of apoptotic cells as demonstrated by the Annexin V-FITC/PI assay [33]. Rupture of the cellular membrane is one of the crucial criteria used to distinguish necrosis from apoptosis [34].
METHODS
Chemicals and Media
Growth medium RPMI 1640 containing 1mmol/L L-glutamine was purchased from Gibco BRL products (Grand Island, NY). Fetal bovine serum (FBS), phosphate buffered saline (PBS), and acridine orange and propidium iodine were obtained from Sigma Chemical Company (St. Louis, MO). Annexin V/PI kit was obtained from BD Biosciences (Pharmingen, Becton Dickinson Co., San Diego, CA, USA).
Vernonia amygdalina Delile Preparation
Vernonia amygdalina Delile leaves (4–5 kg) were collected in Bangou, West Cameroon. They were rinsed with distilled water and dried under the sun. Briefly, 100 g of dried leaves were added to 1200 mL of methanol. The mixture was heated at 500C for 6 h. The mixture was filtered with cheesecloth and later with Whatman No. 1 filter paper to obtain a homogenous filtrate. Excess solvents were trapped, collected and removed from the filtrate using a rotary evaporator. The extracts were then refrigerated at 40C until use. The preparation was done in the Department of Chemistry and Biochemistry at Jackson State University.
Tissue/Cell Culture
HL-60 promyelocytic leukemia cells were purchased from the American Type Culture Collection-ATCC (Manassas, VA). These have been derived from peripheral blood cells of a 36-year old Caucasian female with acute promyelocytic leukemia (APL). In the laboratory, cells were stored in the liquid nitrogen until use. They were next thawed by gentle agitation of their containers (vials) for 2 min in a water bath at 370C. After thawing, the content of each vial of cell was transferred to a 25 cm2 tissue culture flask, diluted with up to 10 mL of RPMI 1640 containing 1 mmol/L L-glutamine (GIBCO/BRL, Gaithersburg, MD) and supplemented with 10% (v/v) fetal bovine serum (FBS), 1% (w/v) penicillin/streptomycin. The 25 cm2 culture flasks (2 × 106 viable cells) were observed under the microscope, followed by incubation in a humidified 5% CO2 incubator at 370 C. Three times a week, they were diluted under same conditions to maintain a density of 5 × 105/mL, and harvested in the exponential phase of growth. The cell viability was assessed by the trypan blue exclusion test (Life Technologies), and manually counted using a hemocytometer.
Cell treatment and Determination of Cell Viability
To determine the anti-proliferative effects of VAD in vitro, 1 × 106 cells/mL were washed with PBS and treated with different doses of VAD and placed in the humidified 5% CO2 incubator for 24h. The cells incubated in culture medium alone served as a control for cell viability (untreated wells). To determine the anti-proliferative effects of VAD in vitro, 900 µL aliquots in six replicates of the cell suspension (1 × 106 cells/mL) were added to 12-well polystyrene tissue culture plates, 100 µL aliquots of stock solutions were added to each well using distilled water as solvent to make-up final VA doses of 0, 125, 250, and 500 µg/mL. Control cells received 100 µL of distilled water. Cells were placed in a humidified 5% CO2 incubator for 24 h at 370C. After incubation, the live and dead cells were determined by acridine orange and propidium iodide (AO/PI) staining using the Cellometer Vision. To perform this experiment, 25 μL of AO/PI dye was added to 25 μL of cell suspension taken out from each sample. Samples were gently mixed and 20μL of cell suspension was loaded into the cellometer counting chamber. The cellometer counting chamber was placed into the Cellometer Vision and both cell concentration and viability were determined using the Vision software as previously described [35].
Detection of DNA Damage by the Comet Assay
The comet assay was carried out by the method previously described by Collins and his collaborators [36, 37] with some modifications [26]. Briefly, 1 × 106 cells/mL were treated with either media or VAD (0, 125, 250, and 500 µg/mL) respectively and incubated in a 5% CO2 at 37oC for 24 h. After incubation, the cells were centrifuged, washed with PBS, and 1 × 105 cells/mL counted from the pool of untreated and treated cells were used for the assay. In a 2 mL tube, 50 µL of the cells suspension and 500 µL of melted LMAgarose were mixed and 75 µL was pipetted onto a pre-warmed cometslide. The side of the pipette tip was used to spread agarose/cells over the sample area completely. The slides were then placed flat in the dark at 40C for 10 minutes to allow the mixture to solidify and then immersed in prechilled lysis solution at 40C for 40 minutes. Next, the slides were removed from lysis solution, tapped, and immersed in Alkaline Solution for 40 minutes at room temperature in the dark. The slides were washed twice for 5 min with Tris-Borate-EDTA (TBE). Next, the slides were electrophoresed at low voltage (300 mA, 25V, 40C) for 20 minutes. The slides were placed in 70% ethanol for 5 min, removed, tapped, and air-dried for overnight. The slides were stained with SYBR Green designed for the Comet Assay, and allowed to air dry at room temperature for 6 h. SYBR Green stained cometslides were viewed with an Olympus fluorescence microscope and analyzed using LAI’s Comet assay Analysis System software (Loates Associates, Inc. Westminster, MD).
Detection of Apoptosis by the Annexin V FITC/PI Assay
To evaluate apoptosis of HL-60 Promyelocytic leukemia cells exposed to VAD, we performed the Flow Cytometry assessment using annexin V FITC/PI staining kit. Annexin-V binds to cells that express phosphatidylserine on the outer layer of the cell membrane, and PI stains the cellular DNA of those that have a compromised cell membrane. This allows for the discrimination of live cells (unstained with either fluorochrome) from apoptotic cells (stained only with annexin-V) and necrotic cells (stained with both annexin-V and PI) [31]. Briefly, 1 × 106 cells/mL were washed in PBS, re-suspended in binding buffer (10 mm Hepes/NaOH pH 7·4, 140 mm NaCl, 2·5 mm CaCl2), and stained with FITC-conjugated annexin V (Pharmingen, Becton Dickinson Co., San Diego, CA, USA). Then, cells were incubated for 15 min in the dark at room temperature, washed with binding buffer and analyzed by flow cytometry (FACS Calibur; Becton-Dickinson) using CellQuest software.
Statistical Analysis
Experiments were performed in triplicates. Data were presented as means ± SDs. Where appropriate, one-way ANOVA or Student paired t-test was performed using SAS Software available in the Biostatistics Core Laboratory at Jackson State University. P-values less than 0.05 were considered statistically significant.
CONCLUSIONS
Medicinal plants have served as valuable starting materials for drug development in both developing and developed countries. Knowing that many pharmacologically active drugs are derived from medicinal plants [38, 39], our goal in the present work was to a test the therapeutic efficacy of Vernonia amygdalina Delile (VAD) towards the treatment of acute promyelocytic leukemia, using HL-60 promyelocytic leukemia cells as test model. Our results demonstrated that VAD has anti-cancer effects against HL-60 promyelocytic leukemia cells. The effect was mediated through the inhibition of cell proliferation of the HL-60 promyelocytic leukemia cells. A novel finding was that the underlying mechanisms by which VAD induced growth inhibition of HL-60 promyelocytic leukemia cells involved the induction of cell death, DNA damage, and cell apoptosis. These results suggest that VAD can act as a complement to the current treatment for APL patients. Because VAD exerts anticancer activity in vitro, it would be interesting to perform clinical trials to confirm its effectiveness as an anticancer agent towards the treatment of patients with APL.
ACKNOWLEDGMENTS
The research described in this publication was made possible by a grant from the National Institutes of Health (Grant No. NIMHD-G12MD007581) through the RCMI-Center for Environmental Health at Jackson State University.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest
REFERENCES
- 1.Tallman MS; Altman JK How I treat acute Promyelocytic Leukemia. Blood 2009, 114, 5126–35. [DOI] [PubMed] [Google Scholar]
- 2.Jurcic JG; Soignet SL; Maslak PG (2007) Diagnosis and treatment of acute promyelocytic leukemia. Current Oncology Reports 2007, 9(5), 337–344. [DOI] [PubMed] [Google Scholar]
- 3.Ghavamzadeh A; Alimoghaddam K; Shahrbano, Ghaffari SH; Jahani M; Iravani M; Mousavi SA; Bahar B; Jalili M Phase II study of single agent arsenic trioxide for the frontline therapy of acute promyelocytic leukemia. J Clin Oncol 2011, 29, 2753–2757. [DOI] [PubMed] [Google Scholar]
- 4.Shen ZX; Chen GQ; Ni JH; Li XS; Xiong SM; Qin QY; Zhu J; Tang W; Sun GL; Yang KQ; Chen Y; Zhou C; Fang ZW; Wang YT; Ma J; Zhang P; Zhang TD; Chen SJ; Chen Z; Wang ZY Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II clinical efficacy and pharmacokinetics in relapsed patients. Blood 1997, 89, 3354–3360. [PubMed] [Google Scholar]
- 5.WCRF/AICR. Food, nutrition and the prevention of cancer: a global perspective: World Cancer Research Fund / American Institute for Cancer Research 1997. [DOI] [PubMed] [Google Scholar]
- 6.Ainslie JR List of plants used in native medicine in Nigeria Imperial Forestry Institute: Oxford, UK: 1973, 42. [Google Scholar]
- 7.Burkill HM The useful plants of West Tropical Africa, 2d ed; Kew. England: Royal Botanical Gardens 1985, 1. [Google Scholar]
- 8.Yedjou CG; Izevbigie E; Tchounwou PB Preclinical assessment of Vernonia amygdalina leaf extracts as DNA damaging anti-cancer agent in the management of breast cancer. Int J Environ Res Public Health 2008, 5, 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yedjou CG; Izevbigie E; Tchounwou PB Vernonia amygdalina-Induced Growth Arrest and Apoptosis of Breast Cancer (MCF-7) Cells. Pharmacology & Pharmacy 2013, 4(1), 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izevbigie EB Discovery of water-soluble anticancer agents (edotides) from a vegetable found in Benin City, Nigeria. Exp. Biol. Med 2003, 228, 293–298. [DOI] [PubMed] [Google Scholar]
- 11.Khalafalla MM; Abdellatef E; Daffalla HD; Nassrallah AA; Aboul-Enein KM; Lightfoot DA; Cocchettoand A; El-Shemy HA Antileukemia activity from root culturesof Vernonia amygdalina. The Journal of International Medical Research 2009. [Google Scholar]
- 12.Arhoghro EM; Ekpo KE; Anosike EO; and Ibeh GO Effect of Aqueous extract of bitter leaf (Vernonia amygdalina Del.) oncarbontetrachloride induced liverdamage in albino wistar rats. European Journal of Scientific Research 2009, 26, 122–130. [Google Scholar]
- 13.Ibrahim TA; Lola A; Adetuyiand FO; Jude-Ojei B Assessmentof the Antibacterial activity of Vernonia amygdalina and Occimum gratissimum leaves on selected food borne pathogens. Journal of Environmental, Agricultural and Food Chemistry 2009, 8(11): 1212–1218. [Google Scholar]
- 14.Adaramoye OA; Akintayo O; Achem J.; Fafunso MA. (2008). Lipid-lowering effects of ethanolic extracts of Vernonia amygdalina leaves in rats fed on high cholesterol diet. Vascular Health and Risk Management 2008, 4, 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abosi O; Raseroka BH In vivo antimalarial activity of Vernonia amygdalina. Br. J. Biomedical Sci 2003, 60(2), 89–91. [DOI] [PubMed] [Google Scholar]
- 16.Izevbige EB; Bryant TL; Walker A A novel natural inhibitor of extracellular signal regulated kinases and human breast cancer cell growth. Experimental Biol. Med 2004, 229(2), 163–169. [DOI] [PubMed] [Google Scholar]
- 17.Manach C; Scalbert A; Morand C; Remesy C; Jimenez H Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr 2004, 79, 727–747. [DOI] [PubMed] [Google Scholar]
- 18.Tekobo AM; Onabanjo AO; Amole OO; Emeka PM Analgesic and antipyretic effects of the aqueous extract of Vernonia amygdalina. West Afri. J. Pharm 2002, 16, 68–74. [Google Scholar]
- 19.Mbinglo SB Survey on the production of bitterleaf Vernonia spp. in Bamenda, N.W. Cameroon. Student project report for Natural Resource Institute, Chatham, United Kingdom/Dschang University Cameroon: 1998. [Google Scholar]
- 20.Onwuka CFL; Akinsoyinu AO; Tewe OO Feed value of some Nigerian browse plants: chemical composition and in vitro digestibility. East African Agriculture and Forestry J 1989, 54, 157–163. [Google Scholar]
- 21.Aregheore EMK; Makkar HPS; Becker K Feed value of some browse plants from the central zone of Delta State. Nig. Trop. Sci 1998, 38, 97–104. [Google Scholar]
- 22.Smith IF; Eyzaguirre P African leafy vegetables: Their role in the world health organization’s global fruit and vegetables initiative. African Journal of Food, Agriculture, Nutrition and Development 2007, 7, 1–17. [Google Scholar]
- 23.Eleyinmi AF; Sporns P; Bressler DC Nutritional composition of Gongronema latifolium and Vernonia amygdalina. Nutrition and Food Science 2008, 38, 99–109. [Google Scholar]
- 24.Ejoh RA; Nkonga DV ; Inocentand G ; Moses MC Nutritionalcomponents of some non-conventionalleafy vegetables consumed in Cameroon. Pakistan Journal of Nutrition 2007, 6, 712–717. [Google Scholar]
- 25.Tsabang N; Yedjou CG; Tsambang LWD; Tchinda AT; Donfagsiteli N; Agbor GA; Tchounwou PB; Nkongmeneck BA Treatment of Diabetes and/or Hypertension Using Medicinal Plants in Cameroon. Med Aromat Plants 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yedjou CG; Tchounwou PB In-vitro genotoxic effect of arsenic trioxide to human leukemia (HL-60) cells using the comet assay. Molecular and Cellular Biochemistry 2007, 301(1–2), 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yedjou CG; Sutton L; Tchounwou PB Genotoxic mechanisms of arsenic trioxide effect in human Jurkat T-lymphoma cells. Metal Ions Biol. Med 2008, 10, 495–499. [PMC free article] [PubMed] [Google Scholar]
- 28.Hsiang YH; Lihou MG; Liu LF Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res 1989, 49, 5077–5082. [PubMed] [Google Scholar]
- 29.Zhang H; D’Arpa P; Liu LF A model for tumor cell killing by topoisomerase poisons. Cancer Cells. 1990, 2, 23–27. [PubMed] [Google Scholar]
- 30.Wei M; Wanibuchi H; Yamamoto S; Li W; Fukushima S Urinary bladder carcinogenicity of dimethylarsinic acid in male F344 rats. Carcinogenesis. 1999, 20, 1873–1876. [DOI] [PubMed] [Google Scholar]
- 31.Koopman G; Reutelingsperger CPM; Kuijten GAM; Keehnen RMJ; Pals ST; van Oers MHJ Annexin-V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415. [PubMed] [Google Scholar]
- 32.Opata MM; Izevbigie EB Aqueous Vernomia amygdalina extracts alter MCF-7 cell membrane permeability and efflux. Int J Environ Res Public Health 2006, 3, 174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong FC; Woo CC; Hsu A; Tan BKH The anti-cancer activities of Vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLOS. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H; You S; Kong B; Foster L; Farris J; Foster D Necrotic cell death by hydrogen peroxide in immortal DF-1 chicken embryo fibroblast cells expressing deregulated MnSOD and catalase. Biochimca et Biophysica Acta 2001, 1540, 137–146. [DOI] [PubMed] [Google Scholar]
- 35.Yedjou CG; Saeed MA; Hossain A; Dorsey W; Yu H; Tchounwou PB Basic apoptotic and necrotic cell death in human liver carcinoma (HepG2) cells induced by synthetic azamacrocycle. Environ Toxicol. 2012. [DOI] [PubMed] [Google Scholar]
- 36.Collins AR; Dusinská M; Horská A Detection of alkylation damage in human lymphocyte DNA with the comet assay. Acta Biochim. Pol 2001, 48, 611–614. [PubMed] [Google Scholar]
- 37.Collins AR Comet Assay for DNA damage and repair: principles, applications and limitations. Mol. Biotechnol 2001, 26, 249–261. [DOI] [PubMed] [Google Scholar]
- 38.Zong A; Cao H; Wang F Anticancer polysaccharides from natural resources: a review of recent research. Carbohydrate Polymers 2012, 90(4), 1395–1410. [DOI] [PubMed] [Google Scholar]
- 39.Efferth T; Koch E Complex interactions between Phytochemicals. The Multi-Target Therapeutic concept of Phytotherapy. Current Drug Targets 2011, 12(1), 122–132. [DOI] [PubMed] [Google Scholar]