Abstract
Racemic bis-tetrahydrofuran ligand 6 was efficiently synthesized utilizing catalytic cobaloxime 10 mediated radical cyclization as the key step. Optical resolution of the racemic alcohol with immobilized-Amano lipase, afforded optically pure ligands.
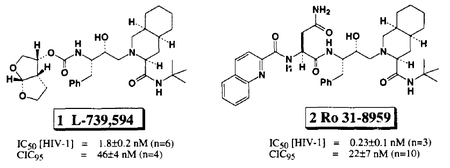
Significant advances have been made in the design and synthesis of non peptidal ligands for the HIV protease substrate binding site.1 We recently reported2 the structure-based design of bistetrahydrofuran ligands that can effectively replace two amide bonds and a 10π-aromatic system of the present clinical candidate 2 (Ro 31–8959).3 These ligands were synthesized in optically pure form utilizing 3(S)- or 3(R)-malic acid as the starting material. Since we required both enantiomers for further structure-activity studies, we became interested in devising a more efficient route to these ligands. In this report, we describe an efficient synthetic route to these ligands in racemic form and their enzymatic resolution, providing optically active ligands with high enantiomeric excess.
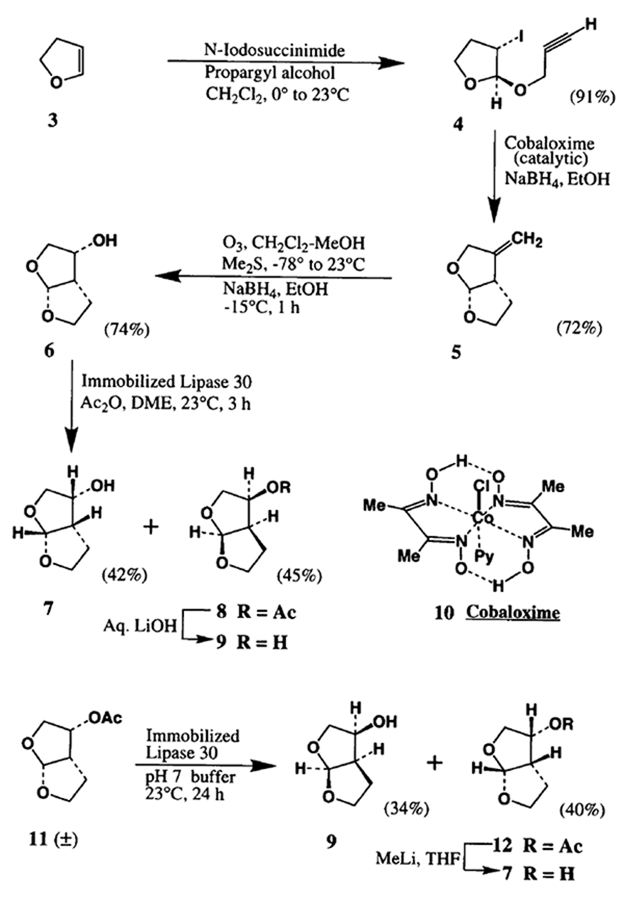
The synthesis of the racemic ligands is illustrated in Scheme I. As shown, treatment of 2,3-dihydrofuran 3 with 1.2 equiv, of N-iodosuccinimide and 2 equiv, of propargyl alcohol in methylene chloride at 0° to 23°C for 3 h, furnished the iodo-ether 4 in high yields (91–95%). Reaction of the iodo-ether 4 with tri-n-butyl tin hydride in refluxing toluene in the presence of a catalytic amount of AIBN provided the corresponding radical cyclization product 5 in 77% yield after silica gel chromatography. Alternatively, radical cyclization utilizing a catalytic amount (10 mole %) cobaloxime 104 in 95% ethanol at 65°C for 3 h in the presence of 1.2 equiv, sodium borohydride afforded 5 in 72% yield (8 g scale) after silica gel chromatography.5 This is an operationally simple and potentially useful procedure for large scale synthesis. Interestingly, cobaloxime mediated reduction of the corresponding bromo-ether of 4 however, provided only 40% yield of 5. The ozonolytic cleavage of the olefin 5 afforded the corresponding ketone which was reduced with sodium borohydride in ethanol at −15°C to furnish the endo alcohol 6 (74–78%) after purification by silica gel chromatography.6 The racemic alcohol 6 was then exposed to Amano lipase7 mediated acylation as well as the hydrolysis of the corresponding acetate 11. Thus, acylation of 6 was accomplished with immobilized8 lipase PS30 (25% by weight with respect to lipase PS30) in the presence of 3 equiv, of acetic anhydride in dimethoxyethane (DME) at 23°C. The reaction was monitored by TLC (50% ethyl acetate/hexane) and 1H NMR (by withdrawal of a small aliquot and workup) until a 50% conversion was reached (about 3 h). After this period, the reaction mixture was simply filtered and the filtrate was evaporated. The resulting residue was chromatographed over silica gel to furnish the unacylated alcohol 7 (42% yield) and the acylated alcohol 8 (45% yield) after further workup with 5% aqueous sodium carbonate to remove acetic anhydride. The control experiment without the enzyme verified that the non-enzymatic acylation reaction is extremely slow (only trace amount of acylation after 48 h). Also, acylation with acetic anhydride and 25% by weight of lipase PS30 at 23°C after 24 h provided only trace amount of acylated product. The optical purity of the alcohol 7 (95% ee, αD23° −11.9°, MeOH) was obtained by formation of Mosher ester and 19FNMR analysis.9 To determine the optical purity of the acylated alcohol 8, the ester was hydrolyzed by treatment with aqueous lithium hydroxide and the resulting alcohol was analyzed as described above (87% ee, αD23° +11.7 °, MeOH).
Alternatively, the enzymatic hydrolysis of the racemic acetate 11, obtained by acetylation with 1.5 equiv, of acetic anhydride and 3 equiv, of triethylamine in methylene chloride (80%), has also resulted in optically active ligands with high enantiomeric excess. Thus, hydrolysis of 11 with immobilized lipase PS30 (25% by weight with respect to lipase PS30) in phosphate buffer (pH=7.0) at 23°C for 24 h furnished the hydrolyzed alcohol 9 (yield 34%, 90% ee) and the acetate 12 (40%). Reaction of 12 with 2 equiv, of methyllithium in THF at 0°to 23°C for 3 h resulted in alcohol 7 (82% yield, 94% ee).10 The represented absolute configuration of the resolved alcohols 7 and 9 were assigned based on comparison of their optical rotation with the ligands synthesized previously with defined absolute configuration utilizing 3(S)- and 3(R)-diethyl malates. Also, the alcohol 7 has been converted to HIV protease inhibitor I as described previously.2
Thus, efficient synthesis of the racemic alcohol 6 and its resolution by immobilized Amano lipase PS 30, provided an easy access to optically active high affinity ligands 7 and 9 in high enantiomeric purity. The resolution process is convenient, economical and simple to carry out. Further application of this protocol is currently underway in our laboratory. The following procedures are illustrative.
Preparation of Immobilized Amano Lipase 30 (modified procedure)8:
Commercially available 4 g of celite 521 (Aldrich) was loaded on a buchner funnel and washed successively with 50 mL of deionized water and 50 mL of 0.05 N phosphate buffer (pH = 7.0; Fisher Scientific). The washed celite was then added to a suspension of I g of Amano lipase 30 in 20 mL of 0.05 N phosphate buffer. The resulting slurry was spread on a glass dish and allowed to dry in the air at 23°C for 48 h (weight 5.4 g; water content about 2% by Fisher method).
Immobilized lipase catalyzed acylation:
To a stirred solution of racemic alcohol 6 (2 g, 15.4 mmol) and acetic anhydride (4 g, 42.4 mmol) in 100 mL of DME, was added 2.7 g (about 25% by weight of lipase PS30) of immobilized Amano lipase and the resulting suspension was stirred at 23°C. The reaction was monitored by TLC and 1H NMR analysis until 50% conversion was reached. The reaction mixture was filtered and the filter cake was washed repeatedly with ethyl acetate. The combined filtrate was carefully concentrated in a rotary evaporator, keeping the bath temperature below 15°C. The residue was chromatographed over silica gel to provide 843 mg (42%) of 7 (95% ee ; αD23° −11.9°, MeOH); 1H-NMR (CDC13) δ 1.85 (m, 2H), 2.3 (m, 1H), 2.9 (m, 1H), 3.65 (dd, J=7.0, 9.1, 1H), 3.85–4.0 (m, 3H), 4.45 (dd, J=6.8, 14.6, 1H), 5.7 (d, J=5.1, 1H) ; also, 1.21 g of 8 after washing with 5% aqueous sodium carbonate (45%, αD23° +31.8°, MeOH ); 1H-NMR (CDC13) δ 1.85–2.1 (m, 2H), 2.1 (s, 3H), 3.1 (m, 1H), 3.75 (dd, I=6.6, 9.2,1H), 3.8–4.1 (m, 3H), 5.2 (dd, J=6.4, 14.5,1H), 5.7 (d, J=5.2, 1H).
Scheme I.
Acknowledgment:
The authors thank the University of Illinois at Chicago for financial support.
References and Notes:
- 1.For a recent review on HIV protease inhibitors, see Thaisrivongs, S.; Annual reports in Med. Chem 1994, 29, 133. [Google Scholar]
- 2.Ghosh AK; Thompson WJ; Fitzgerald PMD; Culberson CJ ; Axel MG; Mckee SP; Huff JR; Anderson PS; J. Med. Chem 1994, 37, 2506. [DOI] [PubMed] [Google Scholar]
- 3.Roberts NA; Martin JA; Kinchington D; Broadhurst AV; Craig JC; Duncan IB; Galpin SA; Handa BK; Kay J; Krohn A; Lambert RW; Merrett JH; Mills JS; Parkes KEB; Redshaw S; Ritchie AJ; Taylor DL; Thomas GJ; Machin PJ Science 1990, 248,358. [DOI] [PubMed] [Google Scholar]
- 4.Schrauzer GN ; Inorg. Synth 1968. 11, 62. [Google Scholar]
- 5.(a) Okabe M ; Abe M ; Tada M ; J. Org. Chem 1982, 47, 1775 [Google Scholar]; (b) Okabe M ; Tada M ; J. Org. Chem 1982, 47, 5382. [Google Scholar]
- 6.The stereochemical assignment of alcohol 7 was made based on extensive 1H-NMR NOE experiments.
- 7.Supplied by Amano International Enzyme Co, Troy, Virginia, USA. [Google Scholar]
- 8.Bianchi D; Cesti P; Battistel E; J. Org. Chem 1988, 53, 5531. [Google Scholar]
- 9.Dale JA; Dull DL; Mosher HS ; J Org. Chem 1969, 34, 2543. [Google Scholar]
- 10.All new compounds gave satisfactory spectroscopic and analytical results.


