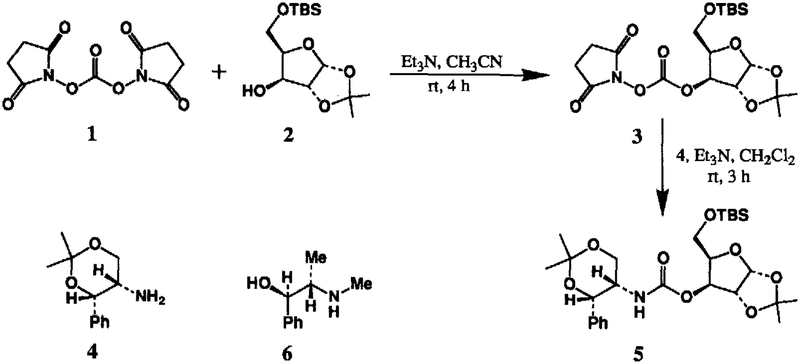
Summary:
An efficient and mild method for alkoxycarbonylation of amines is described, utilizing commercially available N,N’-disuccinimidyl carbonate.
The development of mild and efficient methods for alkoxycarbonylation of amines is of considerable interest in Medicinal Chemistry.1 Consequently, a number of reagents and synthetic procedures were introduced in the literature2 over the past several years. Recently, we have described di(2-pyridyl) carbonate promoted synthesis of variously protected carbamates.3 Continuing our interest in search of other convenient reagents and in view of the synthesis of biologically active polyfunctional molecules for probing enzyme active-sites, we have investigated the scope of N,N’-disuccinimidyl carbonate promoted alkoxycarbonylation of amines with a host of alcohols. N,N’-Disuccinimidyl carbonate has been recognized as a versatile reagent for active ester synthesis.4 However, the reaction of N,N’-disuccinimidyl carbonate and alcohols to form mixed active carbonates and their subsequent utility in the synthesis of functionalized carbamates, is hitherto unknown. In this letter, we report an efficient and more convenient procedure for the synthesis of various carbamate derivatives using commercially available N,N’-disuccinimidyl carbonate (DSC).5
The readily available 1,2-O-isopropylidene-D-xylofuranose was selectively protected6 as the t-butyldimethylsilyl ether 2 by treatment with t-butyldimethylsilyl chloride (1.1 equiv) in pyridine at 23°C for 10 h (90% yield). Reaction of protected xylofuranose 2 with DSC (1.5 equiv) in the presence of triethylamine (3 equiv) in dry acetonitrile at 23°C for 4 h furnished the mixed succinimide carbonate 3 after standard workup with aqueous sodium bicarbonate solution. The structure of 3 was typically identified by lH-NMR spectrum and mass spectral analysis. Among various solvents examined, the formation of mixed carbonate was found to be rapid and proceeded smoothly in acetonitrile. For example, the above reaction at room temperature in acetonitrile was complete within 4 h, whereas in methylene chloride it took about 12 h for completion, Of particular note, the carbonate 3 is quite stable and can be chromatographed7 and stored in the refrigerator for several months. As expected, reaction of mixed carbonate 3 with a slight excess of amine 4 (1.2 equiv) in the presence of triethylamine (2 equiv) in methylene chloride provided the carbamate derivative 5 in very good yield (86%) after flash chromatography over silica gel. Similarly, exposure of mixed carbonate 3 to L-ephedrine 6 in methylene chloride resulted in carbamate 7 in 83% isolated yield. Interestingly, the lH-NMR spectrum of carbamate 5 (CDC13 or DMSO-d6) revealed the presence of a 4:l mixture of rotational isomers at 23°C. When the temperature was raised, the rotation about the N-C bond became appreciable and at coalescence temperature (Tc, ca. 70°C in DMSO-d6), the mixture of peaks merged into one sharp spectrum.
This alkoxycarbonylation procedure was employed to a series of four different types of alcohols8 and the results are summarized in Table I. As is evident, this procedure allows convenient access to the synthesis of a variety of structurally diverse carbamates derived from primary and hindered secondary alcohols (yield 65–89%). In the case of hindered tertiary alcohols, such as 2-methyl-2-adarnantanol, the formation of mixed carbonate with DSC under a variety of reaction conditions was unsuccessful. Further studies are currently in progress to extend the application for tertiary alcohols.
Table I :
Synthesis of carbamates with DSCa
| Entry | Alcohols | Amines | Carbamates | Yields(%)b |
|---|---|---|---|---|
| l. |  |
4 |  |
86 |
| 2. | 2 | 6 |  |
83 |
| 3. | 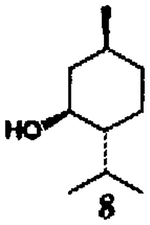 |
4 | 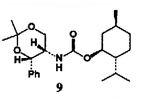 |
77 |
| 4. | 8 | 6 |  |
65 |
| 5. | 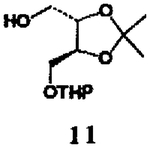 |
4 | 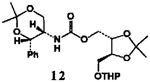 |
89 |
| 6. | 11 | 6 | 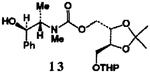 |
78 |
| 7. | 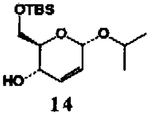 |
4 |  |
72 |
| 8. | 14 | 6 | 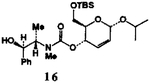 |
75 |
All reactions were carried out under N2
Yield of pure products after silica gel chromatography (0.5–2 mmol scale)
In summary, commercially available N,N’-disuccinimidyl carbonate has been found to be a highly effective alkoxycarbonylating reagent for a variety of primary and sterically hindered secondary alcohols. The present methodology is advantageous because of the ready availability of DSC, stability of the mixed carbonates, and the mildness of the reaction procedure. The following example is representative of this procedure.9
Preparation of Carbamate 5 : To a stirred solution of alcohol 2 (1.0 mmol, 0.31 g) in dry CH3CN (5 ml) at 23°C were added DSC (1.5 mmol, 0.38 g) and Et3N (3 mmol, 0.42 ml). The resulting mixture was stirred at 23°C until no starting alcohol remained by TLC (4 h). The mixture was concentrated under reduced pressure and the residue was diluted with aqueous NaHCO3 solution (10 ml) and extracted thoroughly with EtOAc (2 × 25 ml). The combined extracts were washed with brine (10 ml) and dried over Na2SO4. Evaporation of the solvent provided the mixed carbonate 3 which was used directly for the next reaction. IR (neat); 1810, 1750 cm−1; 1H NMR (CDC13) d 5.9 (d, 1H, J=3.5 Hz), 5.12 (d, 1H, J=2.5 Hz), 4.6 (d, 1H, J=3.5 Hz), 4.4 (m, 1H), 3.8 (m, 2H), 2.8 (s, 4H), 1.5 (s, 3H), 1.3 (s, 3H), 0.9 (s, 9H), 0.05 (s, 6H); MS; 446 (m++H).
Above mixed carbonate was dissolved in CH2C12 (2 ml) and added to a stirred solution of amine 4 (1.2 mmol, 0.25 g) containing triethylamine (1.5 mmol, 0.2 ml) in CH2C12 (5 ml). The resulting mixture was stirred at 23°C until no mixed carbonate remained by TLC (3 h). The mixture was then diluted with CH2C12 (20 ml) and washed successively with aqueous NaHCO3 solution (10 ml), brine (10 ml) and dried over Na2SO4. Removal of the solvent, followed by chromatography over silica gel (25% EtOAc-hexanes) afforded the carbamate 5 (462 mg, 86% yield) as a yellow oil. IR (neat); 1740 cm−l; 1H NMR (DMSO-d6,70°C) d 7.2 (m, 5H), 5.8 (br s, 1H), 5.2 (s, 1H), 4.6 (s, 1H), 4.5 (br s, 1H), 4.3 (d, 1H, J=12 Hz), 4.1 (m, 2H), 3.5–3.8 (m, 4H), 1.5 (s, 3H), 1.45 (s, 3H), 1.38 (s, 3H), 1.23 (s, 3H), 0.9 (s, 9H), 0.1 (s, 3H), 0.05 (s, 3H); MS; 538 (m++H).
Scheme 1.
Acknowledgement:
The authors thank Ms. Joan Murphy and Dr. Steve Pitzenberger for high temperature NMR experiments and acknowledge the encouragement and support of Dr, Joel R. Huff and Dr. Paul S. Anderson.
References and Notes:
- 1.(a) Matassa VG, Maduskuie TP, Shapiro HS, Hesp B, Snyder DW, Aharony D, Krell RD and Keith RA; J. Med. Chem, 1990. 33, 1781; [DOI] [PubMed] [Google Scholar]; (b) Firestone RA, Pisano JM, Falck JR, McPhaul MM and Krieger M; J. Med. Chem, 1984, 27, 1037; [DOI] [PubMed] [Google Scholar]; (c) Khur RJ and Dorough HW; “Carbamate Insecticides: Chemistry. Biochemistry and Toxicology” C. R. C. Press, Cleveland, Ohio, 1976. [Google Scholar]
- 2.(a) Eckert H and Forster B; AnPew. Chem. Int. Ed, 1987, 26, 894; [Google Scholar]; (b) Denarie M, Grenouillat D, Malfroot T, Senet J-P, Sennyey G and Wolf P; Tetrahedron Letters, 1987, 28, 5823; [Google Scholar]; (c) Bruncau C and Dixncuf PH; Tetrahedron. Letters, 1987, 28, 2005; [Google Scholar]; (d) Takeda K, Tsuboyama K, Yamaguchi K and Ogura H; J. Org. Chem, 1985, 50, 273; [Google Scholar]; (e) Shute RE and Rich DH; Synthesis, 1987, 346. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh AK, Duong TT and McKee SP; Tetrahedron Letters, 1991, 32, 4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogura H, Kobayashi T, Kawabe K and Takeda K; Tetrahedron Letters, 1979, 4745. [Google Scholar]
- 5.N,N’-Disuccinimidyl carbonate was obtained from Aldrich Chemical Company, Inc., Milwaukee. DSC was also prepared conveniently from N-hydroxysuccinimide (6 equiv) and triphosgene (1 equiv) in CH3CN in the presence of txiethylamine (71%, m.p 178°C); for isolation and recrystallization, see ref 3 (DPC procedure).
- 6.Ireland RE and Daub JP; J. Org. Chem, 1981, 46, 479. [Google Scholar]
- 7.Crude mixed carbonates obtained after standard workup were used for the reaction with amines.
- 8.Alcohol 11 was obtained by reaction of (−)-2,3-O-isopropylidene-D-threitol (1 equiv) and dihydropyran (1 equiv) in dry methylene chloride in the presence of pyridinum p-toluenesulfonate (65%); for preparation of alcohol 14, see ref 3.
- 9.All new compounds gave satisfactory spectroscopic and analytical results.