Abstract
Syzygium is a large genus of flowering plants, with several species, including the clove tree, used as important resources in the food and pharmaceutical industries. In our continuing search for anticancer agents from higher plants, a chloroform extract of thee leaves and twigs of Syzygium corticosum collected in Vietnam was found to be active toward the ^ HT-29 human colon cancer cell line. Separation of this extract guided by HT-29 cells and nuclear factor-kappa B (NF-κB) inhibition yielded 19 known natural products, including seven triterpenoids, three ellagic acid derivatives, two methylated flavonoids, a cyclohexanone, four megastigmanes, a small lactone, and an aromatic aldehyde. The full stereochemistry of (+)-fouquierol (2) was defined for the first time. Biological investigations showed that (+)-ursolic acid (1) is the major cytotoxic component of S. corticosum, which exhibited also potent activities in the NF-κB and mitochondrial transmembrane potential (MTP) inhibition assays conducted, with IC50 values of 31 nM and 3.5 μM, respectively. Several analogues of (+)-ursolic acid (1) were synthesized, and a preliminary structure-activity relationship (SAR) study indicated that the C-3 hydroxy and C-28 carboxylic acid groups and 19,20-dimethyl substitution are all essential in the mediation of the bioactivities observed for this triterpenoid.
Keywords: Syzygium corticosum, Pentacyclic triterpenoids, Cytotoxicity, NF-κB inhibition, Mitochondrial transmembrane potential inhibition
Graphical abstract
1. Introduction
Syzygium (Myrtaceae) is a large gsrus of flowering plants, containing about 1200–1800 species that are distributed mainly in tropical and subtropical regions.1 Of these, the clove tree (Syzygium aromaticum Merr. & Perry) is used as an important economic plant in the food and pharmaceutical industries with particular use as a spice and a flavoring agent that has antioxidant, antimicrobial. antinociceptive. antiviral. and cytotoxic activities.2 Syzygium cumini (L.) Skeels, known as “jamun” or “jambolan” (black plum) in Hindi, is another important species used widely in several traditional medicine systems for the treatment of diabetes, allergy, arthritis, and other diseases.3 Chemical purification has shown that benzoic acid analogues and flavonoids are the main bioactive components of clove,2 while chalcones and phloroglucinol derivatives have been identified as the major cytotoxic components of S. samarangense Merr. & Perry and S. jambos (L.) Alston, respectively.4–6 Interestingly, the flowers of S. aromaticum were found to contain large quantities of (+)-oleanolic acid [1.6% (w/w)] and eugenol [89% (w/w) of the clove essential oil], and thus this species is regarded as an important natural source of these both compounds.2,7
Pentacyclic triterpenes are common secondary plant metabolites, as represented by ursane-, oleanane-, and lupane-type triterpenes, with (+)-ursolic acid, (+)-oleanolic acid, and (+)-betulinic acid being the representative members. These three natural products are attracting increasing interest for their promising bioactivities, including their anticancer potential as mediated by induction of cancer cell apoptosis and suppression of angiogenesis.7–9 Among these, (+)-ursolic acid was found to induce doxorubicin-resistant HepG2 cell apoptosis through both caspase-dependent and independent pathways.10 Tumor growth was suppressed significantly when 4-week-old athymic BALB/c nude male mice inoculated with DU145 human prostate cancer cells were treated with (+)-ursolic acid (i.p., 200 mg/kg, twice a week) for six weeks,11 and such an antitumor efficacy was found to be mediated through inhibition of NF-κB activation.11,12 Several clinical studies suggested that ursolic acid has potential for the development of an anticancer drug.13 Two other well-known plant triterpenoids, (+)-betulinic acid and (+)-oleanolic acid, have been reported to inhibit proliferation of melanoma and human gallbladder cancer cells, respectively, and in turn, they suppressed tumor growth in representative tumor xenograft models.14,15 Mechanistically, both compounds mediated their anticancer potential by induction of apoptosis through activation of the mitochondrial pathway.15,16
In our continuing search for anticancer agents from higher plants,17 a chloroform partition of the leaves and twigs of Syzygium corticosum (Lour.) Merr. & Perry collected in Vietnam was found to be cytotoxic toward the HT-29 human colon cancer cell line, but the chemical constituents of this plant have not been reported previously. Separation of this extract guided by cytotoxicity for HT-29 cells and NF-κB inhibition yielded 19 known natural products. All compounds isolated were tested for their cytotoxicity against HT-29 cells, and those isolated in sufficient quantities were evaluated further for their cytotoxicity toward other human cancer cell lines and for their NF-κB and mitochondrial transmembrane potential (MTP) inhibitory effects. The functional groups of the major active compound, (+)-ursolic acid (1), have been determined, and a preliminary structure-activity relationship (SAR) study for pentacyclic triterpenes and their cancer cell line cytotoxicity and NF-κB and MTP inhibitory activities are reported.
2. Results and discussion
2.1. Purification and identification of the bioactive compounds from Syzygium corticosum
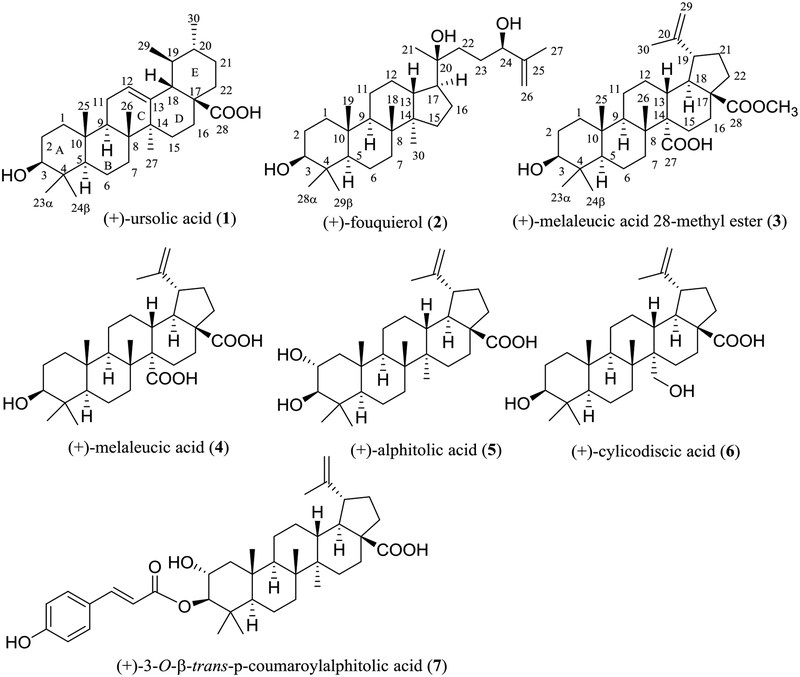
The MeOH extract of the leaves and twigs of S. corticosum was partitioned with n-hexane and CHCl3, and all the dried partitions were evaluated for their cytotoxicity toward HT-29 cells. A major known compound, (+)-ursolic acid (1),18 was purified from this extraction process, and it was found to exhibit cytotoxic and NF-κB inhibitory activities. Following this result, the CHCl3 extract was subjected to chromatographic separation guided by HT-29 cells and NF-κB inhibition, and 18 known compounds, (+)-fouquierol (2),19 (+)-melaleucic acid 28-methyl ester (3),20 (+)-melaleucic acid (4),21 (+)-alphitolic acid (5),22 (+)-cylicodiscic acid (6),23 (+)-3-O−β-trans-p−coumaroylalphitolic acid (7),24 3,4-methylenedioxy-3’-O−methylellagic acid (S1),25 3,4-methylenedioxy-3’,4’,5’-tri-O−methylellagic acid (S2),26 3,3’,4’-tri-O−methylellagic acid (S3),27 sideroxylin (S4),28 (+)-2,3-dihydrosideroxylin (S5),29 (+)-3-hydroxy-6,10-seco−muurol-1-ene-6,10-dione (S6),30 (+)-dehydrovomifoliol (S7),31 (+)-3-oxo-α-ionol (S8),32 (+)-annuionone D (S9),33 (+)-vomifoliol (S10),34 (−)-loliolide (S11),35 and syringaldehyde (S12),36 were isolated from S. corticosum.
(+)-Fouquierol (2) was isolated originally from Fouquiera splendens Engelm. (Fouquieraceae)37, with its 13C NMR data presented in the literature.19 However, detailed information about its configuration and complete assignments of its NMR spectroscopic data have not been reported.38 A C30H52O3 molecular formula deduced from the HRESIMS and NMR spectroscopic data, along with seven tertiary methyl groups at δH 0.77, 0.84, 0.88, 0.96, 0.97, 1.15, and 1.73 (singlets, 3H each) exhibited in the 1H NMR spectrum, indicated a tetracyclic triterpene for 2, which contains three hydroxyl groups and a double bond, as supported by its UV (λmax 201 nm) and IR (νmax 3418 and 1667 cm−1) spectra.39
A dammarane skeleton could be indicated for 2 by comparison of its 1H and 13C NMR data (Table 1)40 with those of lupeol,41 which showed that both compounds possess closely similar NMR spectroscopic data for their A-, B-, and C-ring units. In addition, three hydroxy groups connected to the C-3, C-20, and C-24 positions could be deduced from its two oxygenated methines and one oxygenated quaternary carbon shown in the 13C NMR spectrum and the HMBC correlations between H-28 and H-29 and C-3, H-21 and C-17, C-20, and C-22, and H-26 and H-27 and C-24, respectively (Fig. S29, supplementary data).
Table 1.
1H and 13C NMR spectroscopic data of 2 and 3a
| position | 2b | 2c | 3d | 3e |
|---|---|---|---|---|
| 1 | 39.19 CH2 | α 0.97 m | 39.62 CH2 | α 0.91 m |
| β 1.68 m | β 1.74 m | |||
| 2 | 27.57 CH2 | 1.63 m | 28.25 CH2 | 1.54 m |
| 1.59 m | 1.57 m | |||
| 3 | 79.11 CH | α 3.21 m | 78.54 CH | α 3.06 m |
| 4 | 39.14 C | 39.58 C | ||
| 5 | 56.14 CH | α 0.74 m | 56.55 CH | α 0.65 dd |
| (11.6, 1.6) | ||||
| 6 | 18.42 CH2 | α 1.53 m | 19.04 CH2 | α 1.49 m |
| β 1.44 m | β 1.37 m | |||
| 7 | 35.37 CH2 | α 1.28 m | 38.44 CH2 | α 1.52m |
| β 1.51 m | β 1.61 m | |||
| 8 | 40.52 C | 41.07 C | ||
| 9 | 50.77 CH | α 1.33 m | 52.04 CH | α 1.51 m |
| 10 | 37.28 C | 38.20 C | ||
| 11 | 21.67 CH2 | β 1. 23 m | 21.42 CH2 | 1.48 m |
| α 1. 49 m | 1.32 m | |||
| 12 | 24.99 CH2 | α 1.47 m | 26.91 CH2 | α 2.14 m |
| β 1. 70 m | β 1. 67 m | |||
| 13 | 42.55 CH | β 1.62 m | 40.49 CH | β 2.35 m |
| 14 | 50.48 C | 59.95 C | ||
| 15 | 31.33 CH2 | α 1. 07 m | 28.36 CH2 | α 1.51 m |
| β 1.46 m | β 1.27 m | |||
| 16 | 27.57 CH2 | 1.63 m | 34.99 CH2 | α 2.32 m |
| 1.59 m | β 1.45 m | |||
| 17 | 50.27 CH | α 1. 76 m | 56.96 C | |
| 18 | 15.63 CH3 | β 0.96 s | 52.64 CH | α 1. 70 m |
| 19 | 16.37 CH3 | β 0.84 s | 47.91 CH | β 3.09 m |
| 20 | 75.33 C | 150.95 C | ||
| 21 | 25.54 CH3 | β 1.15 s | 31.06 CH2 | β 1. 87 m |
| α 1.39 m | ||||
| 22 | 36.19 CH2 | 1.46 m | 37.56 CH2 | β 1. 41 m |
| 1.27 m | α 1.91 m | |||
| 23 | 29.52 CH2 | 1.26 m | 28.62 CH3 | α 0. 93 s |
| 24 | 76.13 CH | 4.08 t (5.6) | 16.19 CH3 | β 0.74 s |
| 25 | 147.91 C | 17.09 CH3 | β 0.88 s | |
| 26 | 110.93 CH | 4.85 br s | 17.42 CH3 | β 1.02 s |
| 4.97 br s | ||||
| 27 | 18.17 CH3 | 1.73 s | 177.05 C | |
| 28 | 28.15 CH3 | α 0.97 s | 176.84 C | |
| 29 | 15.51 CH3 | β 0.77 s | 110.50 CH2 | 4.59 br s |
| 4.73 br s | ||||
| 30 | 16.63 CH3 | α 0.88 s | 19.11 CH3 | 1.69 s |
| OH-3 | β 3.67 brs | 3.35 brs | ||
| OCH3−28 | 51.67 CH3 | 3.67 s |
Assignments of chemical shifts are based on the analysis of 1D- and 2D-NMR spectra. The overlapped signals were assigned from 1H −1H COSY, HSQC, and HMBC spectra without designating multiplicity.
13C NMR spectroscopic data (δ) measured in CDCl3 at 100.61 MHz and referenced to the solvent residual peak at δ 77.16.40
1H NMR spectroscopic data (δ) measured in CDCl3 at 400.13 MHz and referenced to the solvent residual peak at δ 7.26.40
The same relative configuration for the A-, B-, and C-ring unit as that of (+)-betulinic acid (9) was assigned for 2 from the consistent 2D NOESY correlations observed for both compounds (Fig. S31, supplementary data). Also, a 3β-hydroxy group of 2 was evident from a large difference observed for the 13C NMR spectroscopic chemical shifts of C-28 and C-2939 and the NOESY correlations between H-3 and H-5 and H-28. A 20 β,24 β-dihydroxy functionality group could be assigned from the NOESY correlations between H-17/H-21, H-17/H-30, H-21/H-24, H-21/H-27, and H-24/H-27 (Fig. S31, supplementary data). This determination has also been supported by a comparison of the 1H NMR spectrum of 2 with reported values. The coupling constant of the 1H NMR signal for H−24 of 2 is similar to that assigned for a 24R−hydroxydammarane at δH 3.33 (dd, J = 7.6 and 4.9 Hz) but different from that for a 24S−hydroxydammarane that appeared at δH 3.28 (dd, J = 11.0, 1.8 Hz).42 Thus, compound 2 could be defined as 3β,20 β,24β-dammar-25-ene-3,20,24-triol, with the full (3S,5R,8R,9R,10R,13R,14R,17S,20S,24R) configuration characterized for the first time. Based on our best knowledge, (+)-fouquierol (2) is the first member of the dammarane-type triterpenes isolated from the genus Syzygium.
All other known compounds, including six pentacyclic triterpenoids, three ellagic acid derivatives, two flavonoids, four megastigmanes, a cyclohexanone, a small lactone, and an aromatic aldehyde isolated from the leaves and twigs of S. corticosum. were identified by comparison of their spectroscopic data with the literature values (Figs. S1–S7 and S14–S25 and Tables S1–S6, supplementary data). The NMR spectroscopic data for (+)-melaleucic acid 28-methyl ester (3) were measured in a deuterated solvent different from that used previously,20 owing to its limited solubility in CDCl3, and these data were assigned completely in the present study (Table 1).
2.2. Evaluation of the cytotoxicity and NF-κB and MTP inhibitory effects fo r compounds isolated from S. corticosum
All chromatographically and spectroscopically pure compounds (Figs. S1–S7 and S14–S25, supplementary data) isolated from S. corticosum were evaluated for their cytotoxicity against the HT-29 human colon cancer cell line, using paclitaxel as the positive control.43,44 The compounds isolated in sufficient quantity were also tested for their cytotoxicity toward the MDA-MB-231 breast, the MDA-MB-435 melanoma, and the OVCAR3 ovarian human cancer cell lines.43,44 Only compounds 1 and (+)-2,3-dihydrosideroxylin (S5) were found to show activity, with IC50 values of 5.9 μM (toward MDA-MB-231 cells) and 6.0 μM (against MDA-MB-435 cells), respectively, and all other compounds tested were inactive, with IC50 values being larger than 10 μM (Table 2 and Table S9, supplementary data), indicating that (+)-ursolic acid (1) is the major cytotoxic component of S. corticosum.
Table 2.
Cytotoxicity and NF-κB and MTP from S. aromaticum *
| compound | Aa | Bb | Cc | Dd | Ee | Ff |
|---|---|---|---|---|---|---|
| 1 | >10*1 | 5.9 | >10*2 | >10*3 | 0.031 | 3.5 |
| 2 | >10 | >10 | >10 | >10 | NT | NT |
| 3 | >10 | >10 | NT | NT | 1.5 | >10 |
| 4 | >10 | >10 | >10 | >10 | >10 | >10 |
| 5 | >10 | >10 | >10 | >10 | 8.7 | >10 |
| 6 | >10 | >10 | >10 | >10 | >10 | >10 |
| 7 | >10 | >10 | >10 | >10 | 4.7 | >10 |
| Paclitaxelg | 9 | 17 | 13 | 13 | ||
| Rocaglamideh | 70 | |||||
| Staurosporinei | 20 |
IC50 values are the concentration (μM) required for 50% inhibition of cell viability for a given test compound and were calculated using nonlinear regression analysis with measurements performed in triplicate and representative of three independent experiments, where the values generally agreed within 10%.
IC50 value (μM) toward the HT-29 human colon cancer cell line with 72 h treatment.
IC50 value (μM) toward the MDA-MB-231 human breast cancer cell line with 72 h treatment.
IC50 value (μM) toward the MDA-MB-435 human melanoma cell line with 72 h treatment.
IC50 value (μM) toward the OVCAR3 human ovarian cancer cell line with 72 h treatment.
IC50 value (μM) for inhibition of nuclear factor kappa B (NF-κB p65) in HeLa cells with 3 h treatment.
IC50 value (μM) for inhibition of mitochondrial transmembrane potential (MTP) in HT-29 cells with 3 h treatment.
NT: Not tested yet.
Positive control for the cytotoxicity assay (IC50 values presented in nM).
Positive control for the NF-κB p65 inhibition assay (IC50 values presented in nM).
Positive control for the mitochondrial potential assay ential assay (IC5 0 values presented in nM).
IC50 values of 11.1, 13.1, and 12.9 μg/mL, respectively.
To further test for their bioactivities, the compounds isolated in a sufficient amount from S. corticosum were also evaluated for their inhibitory effects in standard in vitro NF-κB and MTP bioassays, with rocaglamide and staurosporine used as the positive control, respectively.43,45 Five of these compounds [1, 3, 5, 7, and 3,3’,4’-tri-O−methylellagic acid (S3)] tested were found to be active, among which compound 1 was the most potent in the NF-κB inhibition assay, showing an IC50 value of 31 nM. It also exhibited MTP inhibitory activity, with an IC50 value of 3.5 μM (Table 2), indicating that this compound mediates its cytotoxicity at least in part through mechanisms involving inhibition of NF-κB and activation and mitochondrial transmembrane potential (MTP).
2.3. Structure-activity relationships (SARs) of pentacyclic triterpenoids and their cytotoxicity and NF-κB and MTP inhibition
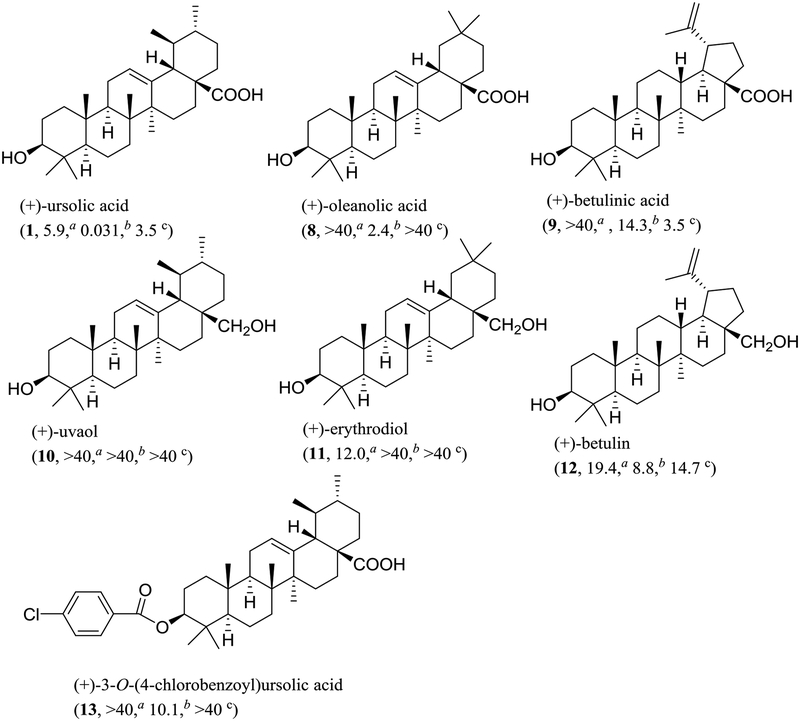
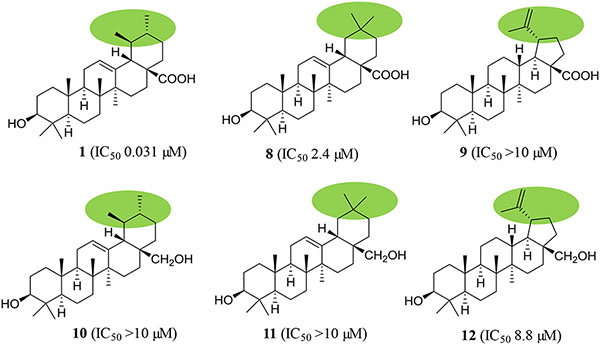
To identify the main functional groups responsible for the activities of (+)-ursolic acid (1), two of its analogues, (+)-oleanolic acid (8, a commercially available sample) and (+)-betulinic acid (9),39 were evaluated for their cytotoxic and NF-κB and MTP inhibitory activities. Neither 8 nor 9 showed activity in any of the cytotoxicity assays. In the NF-κB inhibition assay, compounds 1 and 8 exhibited activity, with the potency of these compounds found to be increased following the sequence 9 (IC50 14.3 μM), 8 (IC50 2.4 μM), and 1 (IC50 0.031 μM). In the MTP assay, compound 9 showed the same potency as 1, but compound 8 was inactive (Chart 1 and Table S9, Supplementary data). These results indicate that the 19,20-dimethyl substitution is required structurally for (+)-ursolic acid (1) to mediate its cytotoxicity and NF-κB inhibitory effect, but replacement of the C-19 methyl group with a prop-1-en-2-yl substituent and removal of C-20 and its methyl group did not change its potency of MTP inhibition. In addition, compound 1 showed activity in all the cytotoxicity against MDA-MB-231 cells and NF-κB and MTP inhibition assays, while, 8 was active only in the NF-κB inhibition assay, and 9 exhibited potency only in the MTP inhibition assay, suggesting that (+)-ursolic acid (1), (+)-oleanolic acid (8), and (+)-betulinic acid (9) mediate their bioactivities probably through a different mechanism of action.
Chart 1.
Structures and bioactivities of (+)-ursolic acid (1), (+)-oleanolic acid (8), (+)-betulinic acid (9) and their semi-synthetic derivatives. a IC50 value (μM) toward the MDA-MB-231 human breast cancer cell line. b IC50 value (μM) for inhibition of NF-κB (p65) in HeLa cells. c IC50 value (μM) for inhibition of mitochondrial transmembrane potential (MTP) in HT-29 cells.
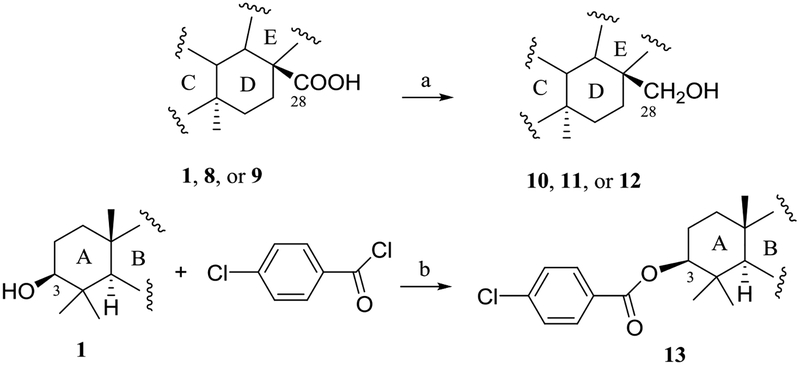
To test the importance of the C-28 carboxylic acid substituent, three pentacyclic triterpene derivatives, (+)-uvaol (10),46 (+)-erythrodiol (11),47 and (+)-betulin (12) (Figs. S10–S12 and Tables S7 and S8, supplementary data),48 were prepared by reduction of 1, 8 or 9 with lithium aluminium hydride (LAH) (Scheme 1),47 with their cytotoxic and NF-κB and MTP inhibitory activities evaluated. Except for compound 12, which exhibited activity in the NF-κB inhibition assay, all these products (10, 11, and 12) did not show any activities in any of the assays utilized (Chart 1 and Table S9, supplementary data). Thus, reduction of the C-28 carboxylic acid group to a primary alcohol led to the loss of the cytotoxic and NF-κB and MTP inhibitory activities of 1 and the NF-κB inhibitory activity of 8, decreased the MTP inhibitory activity of 9, and a slightly increased cytotoxicity of 8 and 9, indicating that this carboxylic acid group is important for 1, 8, and 9 to mediate their bioactivities evaluated. Also, it implies that introduction of an electron-donating hydroxy functional group at the C-28 position results in the bioactivities of these pentacyclic triterpenes changed.
Scheme 1.
Synthesis of compounds 10–13 Reagents and conditions: (a) LAH/THF (dried), reflux, 5 h. (b) pyridine, reflux, 3 h.
To investigate the functionality of the C-3 hydroxy group of (+)-ursolic acid (1), a new ester, (+)-3-O−(4-chlorobenzoyl)ursolic acid (13), was prepared (Scheme 1)43 and evaluated biologically. This product was found to be inactive in all the assays tested (Chart 1), indicating that the free C-3 hydroxy group is important for (+)-ursolic acid (1) to mediate its cytotoxic and NF-κB and MTP inhibitory activities, and also the introduction of a chlorine in the C-3 ester unit did not improve these activities of 1. This means that introduction of a COC6H5Cl or -Cl electron-withdrawing functional group at the C-3 hydroxy group results in the cytotoxic and MTP inhibitory activities of 1 abolished and the NF-κB inhibitory activity decreased (Chart 1).
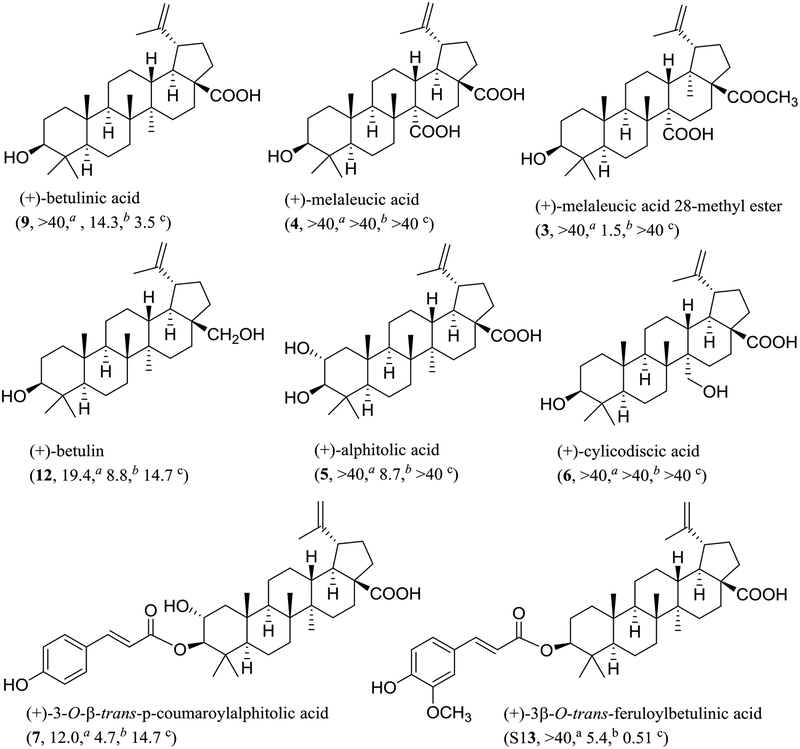
Previously, it has been reported that (+)-betulinic acid (9) exhibited potent and selective antitumor activity targeted at the mitochondria.16 In the present study, compound 9 and several of its analogues [3−7, 12, and (+)-3β-O−tram,-feruloylbetulinic acid (S13)] have been evaluated biologically. All these derivatives did not exhibit any activities in the cytotoxicity assays (IC50 >10 μM), and 3−7 and 12 were also inactive in the MTP inhibition assay, in which compounds 9 and S13 were active (Chart 2 and Table 2 and Table S9, supplementary data). In the NF-κB inhibition assay, analogues 3, 5, 7, 12, and S13 exhibited activity, but 4 and 6 were inactive. These results indicate that changing the C-27 methyl group of 9 to an electron-withdrawing carboxylic acid unit in 4 or to an electron-donating hydroxy group of 6 leads to a loss of NF-κB and MTP inhibition potency, but methyl esterification of C-28 carboxylic acid group of 4 or introducing a hydroxy group at the C-2 position in 5 results in an increased NF-κB inhibition and abolished MTP inhibition potency of 9. In addition, reduction of the C-28 carboxylic J acid group of 9 to a primary alcohol group of 12 or esterification of the C-3 hydroxy group of 5 with 4-p−coumaric acid (7) increased the cytotoxic and NF-κB inhibitory activity but decreased the MTP inhibitory potency of 9. However, esterification of the C-3 hydroxy group of 9 with a trans- feruloyl unit (S13) increased both NF-κB and MTP inhibitory activities of 9. Thus, the C-2 methylene protons, the C-3 hydroxy, the C-27 methyl, and the C-28 carboxylic acid substituents all are important for mediating the NF-κB and MTP inhibitory effect of (+)-betulinic acid (9).
Chart 2.
Structures and bioactivities of (+)-betulinic acid (9) and its analogues. a IC50 value (μM) toward the MDA-MB-231 human breast cancer cell line. b IC50 value (μM) for inhibition of NF-κB (p65) in HeLa cells. c IC50 value (μM) for inhibition of mitochondrial transmembrane potential (MTP) in HT-29 cells.
Inhibition of NF-κB activation can contribute to both induction of cancer cell apoptosis and limit tumor cell resistance to the chemotherapeutic agents.49 Similar to these mechanisms, disruption of mitochondrial function results subsequently in apoptosis through interfering in the release of cytochrome c and several antiapoptotic proteins located in the inner or outer mitochondrial membranes.50 Thus, these molecular targets have been used for discovery of new anticancer agents to overcome the serious reverse effects and the mechanisms of resistance of cancer cells to the cytotoxic drugs.49,50 Several pentacyclic triterpenoids have been investigated in some detail for their potent and promising anticancer activity mediated through the NF-κB and or mitochondrial pathways.11–16 Among these, (+)-ursolic acid (1) was reported to induce DU145 prostate cancer cell apoptosis partially through NF-κB inhibition, and it was found to suppress prostate tumor growth, without significant decrease of mouse body weight.11 (+)-Betulinic acid (9) was demonstrated to inhibit completely melanoma tumor growth in vivo, without any obvious toxicity observed in mice,14 and its molecular target was identified as the mitochondrial apoptosis.16
Recently, a synthetic oleanane-type triterpenoid, bardoxolone methyl (CDDO-Me), which suppressed the activity of NF-κB, has been developed as a promising agent that has been tested in phase I/II trials for the treatment of leukemias and solid tumors.51 In a previous preliminary SAR study, the C-28 carbonyl group was proposed as the critical functional group for (+)-betulinic acid (9) to induce melanoma cell apoptosis.52 Consistent with these previous studies, the present study showed that (+)-ursolic acid (1) exhibits cytotoxic and NF-κB and MTP inhibitory activities, with its C-28 carboxylic acid unit functioned critically. Thus, this pentacyclic triterpene [(+)-ursolic acid (1)] might be a promising lead for design and discovery of new anticancer drugs with decreased multidrug resistance, based on a previous proposal that inhibition of NF-κB activation indicates a potential candidate for new anticancer drug development.53
3. Conclusion
Several triterpenoids, ellagic acid analogues, flavonoids, and megastigmanes have been isolated and identified for the first time from the leaves and twigs of Syzygium corticosum collected in Vietnam, of which (+)-ursolic acid (1) was isolated in a large quantity, with a yield of up to 0.2% (w/w). Thus, S. corticosum can be regarded as a new natural source of (+)-ursolic acid (1), which exhibited cytotoxicity against the MDA-MB-231 human breast cancer cells and potent NF-κB and MTP inhibitory activities, with the 19,20-dimethyl substitution, the C-28 carboxylic acid unit, and the C-3 hydroxy group being important for these biological activities observed.
4. M aterials and methods
4.1. General experimental procedures
Optical rotations were measured at room temperature on an Anton Paar MCP 150 polarimeter. UV spectra were recorded on a Hitachi U2910 ultraviolet spectrophotometer. ECD measurements were performed using a JASCO J-810 spectropolarimeter. IR spectra were recorded on a Nicolet 6700 FT-IR spectrometer. 1H and 13C, DEPT 90, DEPT 135, HSQC, HMBC, NOESY, and COSY NMR spectra were recorded at room temperature on a Bruker Avance DRX 400, a Bruker Avance II 400, a Bruker Avance III HD 700, or a Bruker Avance III HD 800 MHz NMR spectrometer. ESIMS and HRESIMS data were collected on a Bruker Maxis 4G Q-TOF mass spectrometer in the positive-ion mode. Column chromatography was conducted using silica gel (65 × 250 or 230 × 400 mesh, Sorbent Technologies, Atlanta, GA, USA). Analytical thin-layer chromatography (TLC) was performed on precoated silica gel 60 F254 plates (Sorbent Technologies). Sephadex LH-20 was purchased from Amersham Biosciences, Uppsala, Sweden. For visualization of TLC plates, H2SO4 was used as spray reagent. All procedures were carried out using solvents purchased from commercial sources and employed without further purification. Paclitaxel and compounds used for chemical synthesis were purchased from Sigma-Aldrich (St. Louis, MO, USA) (purity ≥ 98%), if not otherwise noted.
4.2. Plant material
A sample of the leaves and twigs (acquisition number AA06822) of Syzygium corticosum was collected in July 2011 by D.D.S. and T.N.N. (voucher specimen: DDS 14648) from a tree (30-meters tall) at Cotuy forest at Vung Olim campsite (11° 43.469’ N; 109° 08.255’ E), Nui Chua National Park, Ninh Hai District, Ninh Thuan Province, Vietnam. A voucher herbarium specimen has been deposited at the John G. Searle Herbarium of the Field Museum of Natural History, Chicago, IL, USA, under the accession number FM2294588.
4.3. Extraction and isolation
The milled air-dried leaves and twigs of S. corticosum (sample AA06822, 6100 g) was extracted with MeOH (6 L × 7) at room temperature. The solvent was evaporated in vacuo, and the dried MeOH extract (299.8 g, 4.9%) was resuspended in 10% H2O in MeOH (2000 mL) and partitioned with n-hexane (1000, 1000, 600, and 600 mL) to yield a n-hexane-soluble residue (35.4 g, 0.58%). Then, 200 mL of H2O were added to the aqueous MeOH layer, and this was partitioned with CHCl3 (1000, 500, and 500 mL). The interfacial layer was filtered, and 12.0 g of precipitate were obtained, which showed cytotoxicity toward HT-29 cells (IC50 < 20 pg/mL). Recrystallization of 60 mg of this filtrate in MeOH yielded 30 mg of (+)-ursolic acid (1), and the material left is the impure form of 1.
The CHCl3 partition was washed with a 1% aqueous solution of NaCl, to partially remove plant polyphenols, and the solvent was evaporated to afford a CHCl3-soluble extract (66.1 g, 1.08%). All the CHCl3-, n-hexane- and water-soluble extracts were inactive (IC50 > 20.0 μg/mL) in the cytotoxicity bioassay used. However, when (+)-ursolic acid (1) was evaluated, it was found to be both cytotoxic against the MDA-MB-231 human breast cancer cell line (IC50 5.9 μM) and potently active in the NF-κB inhibition assay, with an IC50 value of 31 nM. To isolate more analogues of this active compound, a portion of the CHCl3−soluble extract (10.0 g) was subjected to silica gel column chromatography (6.0 × 45 cm), eluted with a gradient of n−hexane-acetone. Eluates were pooled by TLC analysis to give 21 combined fractions (D2F1–D2F21), of w which all were inactive in the cytotoxicity assay, but fractions D2F8 and D2F9 were found to show activity in the NF-κB inhibition assay. Thus, fractions D2F7–D2F9 were selected for further chromatography, based on their TLC analysis and NF-κB inhibitory activity.
Fraction D2F7 was chromatographed over a silica gel column (4.5 × 40 cm), eluted with a gradient of n−hexane-acetone to yield three pooled subfractions, D2F7F1–D2F7F3. Of these, fraction D2F7F1 was further chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n−hexane-acetone, and purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2−MeOH (1:1), affording (+)-melaleucic acid 28-methyl ester (3, 3.0 mg), (+)-3-hydroxy-6,10-seco−muurol-1-ene-6,10-dione (S6, 1.0 mg), and (−)-loliolide (S11, 1.0 mg). Fraction D2F7F2 was further chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n−hexane-acetone, and purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2−MeOH (1:1), affording 3,4-methylenedioxy-3’-O−methylellagic acid (S1, 1.5 mg), 3,4-methylenedioxy-3’,4’,5’-tri-O−methylellagic acid (S2, 5.0 mg), and (+)-annuionone D (S9, 1.0 mg). Fraction D2F7F3 was further chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n−hexane-acetone, and purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2−MeOH (1:1), affording sideroxylin (S4, 3.0 mg), (+)-2,3-dihydrosideroxylin (S5, 1.0 mg), and (+)-3-oxo-a-ionol (S8, 1.0 mg).
Fraction D2F8 was chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n−hexane-acetone to yield two pooled subfractions, D2F8F1 and D2F8F2. Of these, D2F8F1 was further chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n-hexane-acetone, and purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2−MeOH (1:1), affording (+)-melaleucic acid (4, 12.0 mg). Fraction D2F8F2 was as further chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n−hexane-acetone, and purified by separation over a Sephadex LH-20 column, eluted with CH2Cl2−MeOH (1:1), yielding (+)-dehydrovomifoliol (S7, 2.0 mg) and syringaldehyde (S12, 1.0 mg).
Fraction D2F9 was chromatographed over a silica gel column (2.5 × 30 cm), eluted with a gradient of n-hexane-acetone, to yield three pooled subfractions, D2F9F1–D2F9F3. Of these, fraction D2F9F1 was further chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n-hexane-acetone and purified by passage over a column containing Sephadex LH-20, eluted with CH2Cl2−MeOH (1:1), to yield (+)-alphitolic acid (5, 3.0 mg) and (+)-3-O−β-trans-p−coumaroylalphitolic acid (7, 2.0 mg). Fraction D2F9F2 was further chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n-hexane-acetone and purified by passage over a column containing Sephadex LH-20, eluted with CH2Cl2−MeOH (1:1), to yield (+)-cylicodiscic acid (6, 1.5 mg) and 3,3’,4’-tri-O−methylellagic acid (S3, 15.0 mg). Finally, fraction D2F9F3 was chromatographed over a silica gel column (2.5 × 20 cm), eluted with a gradient of n-hexane-acetone and purified by passage over a column containing Sephadex LH-20, eluted w d with CH2Cl2−M C eOH (1:1), to yield (+)-fouquierol (2, 1.5 mg) and (+)-vomifoliol (S10, 1.0 mg).
3β,20β,24β-Dammar-25-ene-3,20,24-triol [(+)-fouquierol (2)]: Amorphous colorless powder; [a]25D +3.3 (c 0.6, MeOH) (c: mg/mL); UV (MeOH) λmax (log ε) 201 (3.96) nm; ECD (MeOH, nm) λmax (Δε) 202.7 (+8.37); IR (dried film) νmax 3417, 1667, 1556, 1463, 1378, 1263, 1088 cm−1; 1H and 13C NMR data, see Table 1; positive-ion HRESIMS m/z 483.3815 (calcd for C30H52O3Na, 483.3809).
4.4. Reduction of C-28 carboxylic acid group of 1, 8, and 9
To a dried 25 mL glass vial equipped with a magnetic stirrer, containing 13.7 mg (0.03 mmol) of (+)-ursolic acid (1), 2 mL of a 0.02 M solution of LiAlH4 in dried THF (0.04 mmol) were added to the vial under argon. The vial was sealed, and the mixture was stirred with reflux for 5h, it was cooled to room temperature. Next, the reaction mixture was diluted with aqueous ether, extracted with CH2Cl2 (DCM), dried over anhydrous Na2SO4, evaporated to dryness and chromatographed over silica gel to obtain 12.0 mg (0.027 mmol) of the product, (+)-uvaol (10), with a reaction yield of 90.9%. Following this same procedure, 12.0 mg (0.03 mmol) of the product (+)-erythrodiol (11, with a yield of 90.9%) were prepared from 13.7 mg (0.03 mmol) of (+)-oleanolic acid (8, purchased from Sigma), and 7.9 mg (0.018 mmol) of the product (+)-betulin (12) (with a yield of 90.0%) were prepared from 9.1 mg (0.02 mmol) of (+)-betulinic acid (9, isolated from our previous study).39
4.5. Esterification of 1
To a dried 25 mL glass vial equipped with a magnetic stirrer, containing 13.7 mg (0.03 mmol) of (+)-ursolic acid (1), 5 μL of 4-chlorobenzoyl chloride and 1 mL of pyridine were added. The vial was sealed, and the mixture was stirred with reflux for 3 h. The vial was cooled to room temperature, and CH2Cl2 (DCM, 5 mL) was added, and the solution was extracted with distilled H2O. The organic layer was washed with distilled H2O, dried over anhydrous Na2SO4, and evaporated at reduced pressure. The residue was purified by silica gel column chromatography, using n-hexane-acetone (5:1 →1:1), to afford 11.0 mg (0.019 mmol) of 13 (with a yield of 61.7%).
(+)-3-O−(4-Chlorobenzoyl)ursolic acid (13): Amorphous colorless powder showing a pink color under UV light at 365 nm; [α]25 D +33.8 (c 0.8, MeOH) (c: mg/mL); UV (MeOH) λmax (log ε) 239 (4.21), 201 (4.54) nm; ECD (MeOH, nm) λmax (Δε) 208.2 (+11.0); IR (dried film) νmax 1721, 1609, 1557, 1455, 1392, 1272, 1116, 1015 cm−1; 1H and 13C NMR data, see Tables S7 and S8 (supplementary data); positive-ion HRESIMS m/z 617.3382 (calcd for C37H51O4ClNa, 617.3368).
4.6. Cell lines
All the HT-29 colon, MDA-MB-231 breast, MDA-MB-435 melanoma, and OVCAR3 ovarian human cancer cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), and they all were cultured in RPMI 1640 medium, supplemented with FBS (10%), penicillin (100 units/mL), and streptomycin (100 μg/mL).
4.7. Cytotoxicity assay
Follollowing a protocol used previously,44 HT-29, MDA-MB-231, MDA-MB-435, or OVCAR3 cells in log phase growth were harvested by trypsinization and seeded in 96-well clear flat-bottomed plates (Microtest 96, Falcon). Cells were incubated at 37 °C in 5% CO2 overnight and then treated with the samples or paclitaxel (the positive control) (both dissolved in DMSO and diluted to different concentrations required) or the vehicle (DMSO) for 72 h. Viability of cells was evaluated by a commercial absorbance assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega Corp, Madison, WI, USA), with the IC50 values calculated from the vehicle control.
4.8. Enzyme-based ELISA assay for NF-κB inhibition
A NF-κB inhibition assay was carried out using a published procedure, with an EZ-Detect Transcription Factor Assay System ELISA kit (Pierce Biotechnology) used.43 The nuclear extracts of HeLa cells treated with the controls and the test samples at four different concentrations for 3 h were used to determine the specific binding ability of the activated p65 subunit of NF-κB to the biotinylated-consensus sequence and was measured by detecting the chemiluminescent signal in a Fluostar Optima plate reader (BMG Labtech, Inc.). Rocaglamide was used as a positive control.
4.9. Mitochondrial transmembrane potential (Δψm) inhibition assay
The mitochondrial transmembrane potential assay kit from Cayman Chemical Company was used to assess the inhibition of mitochondrial transmembrane potential (Δψm) in HT-29 colon cancer cells following a previously published protocol.45 Briefly, cells were seeded in 96-well culture plates at 1 × 106 density. After 24 h incubation at 37 °C in 5% CO2, cells were treated with samples or the controls for 3 h, and then 10 μL of 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazoylcarbocyanine iodide (JC-1 staining solution) were added to each well, followed by 15 min incubation. Cells were washed with the assay buffer, of which 100 μL were added to each well, and the fluorescent intensity was measured using a FLUOstar Optima plate reader. In healthy cells, JC-1 forms J-aggregates, and Δψm displays strong red fluorescence intensity, which was measured with the excitation and emission at 535 and 595 nm, respectively. However, in apoptotic cells, JC-1 remains in the monomeric form that exhibits strong green fluorescence intensity, which was measured with the excitation and emission at 485 and 535 nm, respectively. Staurosporine was used as a positive control in this assay.
4.10. Statistical analysis
The measurements were performed in triplicate and are representative of two three independent experiments, where the values generally agreed within 10%. The dose response curve was calculated for IC50 determinations using non-linear regression analysis (Table Curve2DV4; AISN Software Inc.). Differences among samples were assessed by one-way ANOVA followed by Tukey-Kramer’s test, and the significance level was set atp < 0.05.
Supplementary Material
Fig. 1.
Structures of triterpenoids (1–7) isolated from Syzygium corticosum.
Acknowledgments
This investigation was supported by grant P01 CA125066 funded by the National Cancer Institute, NIH, Bethesda, MD. The plant sample of Syzygium corticosum was collected under a collaborative agreement between the University of Illinois at Chicago (USA) and the Institute of Ecology and Biological Resources of the Vietnam Academy of Science and Technology, Hanoi (Vietnam). We thank Drs. Appaso Jadhav and Nelson Freitas Fernandes, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, The Ohio State University, Colurmbus for assistance for the chemical synthesis and bioassay testing, respectively. Drs. Arpad Somogy and Nanette M. Kleinholz of the Mass Spectrometry and Proteomics of the Campus Chemical Instrument Center, The Ohio State University, are thanked for acquisition of the MS data. Drs. Craig A. McElroy and Deepa Krishnan, College of Pharmacy, The Ohio State University, are thanked for access to some of the NMR spectroscopic and other instrumentation used in this investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors declare no competing financial interest.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tbmc.
References
- 1.Ahmad B, Baider C, Bernardini B, et al. Syzygium (Myrtaceae): Monographing a taxonomic giant via 22 coordinated regional revisions. Peer J Preprints 2016;doi: 10.7287/peerj.preprints.1930v1. [DOI] [Google Scholar]
- 2.Cortes-Rojas DF, Fernandes de Souza CR, Oliveira WP. Clove (Syzygium aromaticum): A precious spice. Asian Pac J Trop Biomed. 2014;4:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava S, Chandra D. Pharmacological potentials of Syzygium cumini: A review. J Sci Food Agric. 2013;93:2084–2093. [DOI] [PubMed] [Google Scholar]
- 4.Amor EC, Villasenor IM, Antemano R, et al. Cytotoxic C-methylated chalcones from Syzygium samarangense. Pharm Biol.2007;45:777–783. [Google Scholar]
- 5.Simirgiotis MJ, Adachi S, To S, et al. Cytotoxic chalcones and antioxidants from the fruits of Syzygium samarangense (Wax Jambu). Food Chem. 2008;107:813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li GQ, Zhang YB, Wu P, et al. New phloroglucinol derivatives from the fruit tree Syzygium jambos and their cytotoxic and antioxidant activities. J Agric Food Chem. 2015;63:10257–10262. [DOI] [PubMed] [Google Scholar]
- 7.Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75:1549–1560. [DOI] [PubMed] [Google Scholar]
- 8.Shanmugam MK, Nguyen AH, Kumar AP, et al. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012;320:158–170. [DOI] [PubMed] [Google Scholar]
- 9.Kamble SM, Goyal SN, Patil CR. Multifunctional pentacyclic triterpenoids as adjuvants in cancer chemotherapy: A review. RSC Adv. 2014;4:33370–33382. [Google Scholar]
- 10.Yang L, Liu X, Lu Z, et al. Ursolic acid induces doxorubicin-resistant HepG2 cell death via the release of apoptosis-inducing factor. Cancer Lett. 2010;298:128–138. [DOI] [PubMed] [Google Scholar]
- 11.Shanmugam MK, Rajendran P, Li F, et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J Mol Med. 2011;89:713–727. [DOI] [PubMed] [Google Scholar]
- 12.Shishodia S, Majumdar S, Banerjee S, et al. Ursolic acid inhibits nuclear factor-KB activation induced by carcinogenic agents through suppression of iKBa kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–4383. [PubMed] [Google Scholar]
- 13.Shanmugam MK, Dai X, Kumar AP, et al. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochemn Pharmacol. 2013;85:1579–1587. [DOI] [PubMed] [Google Scholar]
- 14.Pisha E, Chai H, Lee IS, et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1:1046–1051. [DOI] [PubMed] [Google Scholar]
- 15.Li HF, Wang XA, Xiang SS, et al. Oleanolic acid induces mitochondrial-dependent apoptosis and G0/G1 phase arrest in gallbladder cancer cells. Drug Design Dev Ther. 2015;9:3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulda S, Kroemer G. Targeting mitochondrial; hondri; apoptosis by betulinic acid in human cancers. Drug Discov Today 2009;14:885–890. [DOI] [PubMed] [Google Scholar]
- 17.Kinghorn AD, Carcache de Blanco EJ, Lucas DM, et al. Discovery of anticancer agents of diverse natural origin. Anticancer Res. 2016;36:5623–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriwacki RW, Pitner TP. Current aspects of practical two-dimensional (2D) nuclear magnetic resonance (NMR) spectroscopy: Applications to structure elucidation. Pharm Res. 1989;6:531–554. [DOI] [PubMed] [Google Scholar]
- 19.Pakhathirathien C, Karalai C, Ponglimanont C, et al. Dammarane triterpenes from the hypocotyls and fruits of Ceriops tagal. J Nat Prod. 2005;68:1787–1789. [DOI] [PubMed] [Google Scholar]
- 20.Kaneta Y, Arai MA, Ishikawa N, et al. Identification of BMI1 promoter inhibitors from Beaumontia murtonii and Eugenia operculata. J Nat Prod. 2017;80:1853–1859. [DOI] [PubMed] [Google Scholar]
- 21.Chopra CS, Cole ARH, Theiberg KJL, et al. Triterpene compounds-VII. The constitution of melaleucic acid. Tetrahedron 1965;21: 1529–1536. [Google Scholar]
- 22.Aguirre MC, Delporte C, Backhouse N, et al. Topical anti-inflammatory activity of 2α-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorg Chem. 2006;14:5673–5677. [DOI] [PubMed] [Google Scholar]
- 23.Tchivounda HP, Koudogbo B, Besace Y, et al. Cylicodiscic acid, a dihydroxy pentacyclic triterpene carboxylic acid from Cylicodiscus gabunensis. Phytochemistry 1990;29:3255–3258. [Google Scholar]
- 24.Bai L, Zhang H, Liu Q, et al. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct. 2016;7:2870–2877. [DOI] [PubMed] [Google Scholar]
- 25.Atta-ur-Rahman Ngounou FN, Choudhar y MI, et al. New antioxidant and antimicrobial ellagic acid derivatives from Pteleopsis hylodendron. Plant Med. 2001;67:335–339. [DOI] [PubMed] [Google Scholar]
- 26.Pasqua G, Silvestrini A, Monacelli onacelli B, et al. Triterpenoids and ellagic acid derivatives from in vitro cultures of Camptotheca acuminata Decaisne. Plant Physiol Biochem. 2006;44:220–225. [DOI] [PubMed] [Google Scholar]
- 27.Khac DD, Tran-Van S, Campos AM, et al. Ellagic compounds from Diplopanax stachyanthus. Phytochemistry 1990;29:251–256. [Google Scholar]
- 28.Pagning ALN, Tamokou JDD, Khan ML, et al. Antimicrobial, antioxidant and butyrylcholinesterase inhibition activities of extracts and isolated compounds from Scadoxuspseudocaulus and semi-synthetic farrerol derivatives. S Afr J Bot. 2016;102:166–174. [Google Scholar]
- 29.Wollenweber E, Wehde R, Dorr M, et al. C-Methyl-flavonoids from the leaf waxes of some Myrtaceae. Phytochemistry 2000;55:965–970. [DOI] [PubMed] [Google Scholar]
- 30.Kiem PV, Minh CV, Nhiem NX, et al. Muurolane-type sesquiterpenes from marine sponge Dysidea cinerea. Magn Reson Chem. 2014;52:51–56. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen THT, Pham NKT, Pudhom K, et al. Structure elucidation of four new megastigmanes from Sonneratia ovata Backer. Magn Reson Chem. 2014;52:795–802. [DOI] [PubMed] [Google Scholar]
- 32.Oelschlaegel S, Pieper L, Staufenbiel R, et al. Floral markers of cornflower (Centaurea cyanus) honey and its peroxide antibacterial activity for an alternative treatment of digital dermatitis. J Agric Food Chem. 2012;60:11811–11820. [DOI] [PubMed] [Google Scholar]
- 33.D’Abrosca B, DellaGreca M, Fiorentino A, et al. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui. Phytochemistry 2004;65:497–505. [DOI] [PubMed] [Google Scholar]
- 34.Powell RG, Weisleder D, Smith CR, et al. Drummondones A and B: Unique abscisic acid catabolites incorporating a bicyclo[2.2.2]octane ring system. J Org Chem. 1986;51:1074–1076. [Google Scholar]
- 35.Ren J, Qin JJ, Cheng XR, et al. Five new sesquiterpene lactones from Inula hupehensis. Arch Pharm Res. 2013;36:1319–1325. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Seeram NP. Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. J Agric Food Chem. 2010;58:11673–11679. [DOI] [PubMed] [Google Scholar]
- 37.Butruille D, Dominguez XA. Fouquierol and isofouquierol: Two new triterpenes of the dammarane series. Tetrahedron Lett. 1974;1974:639–642. [Google Scholar]
- 38.Ngo QMT, Lee HS, Nguyen VT, et al. Chemical constituents from the fruits of Ligustrum japonicum and their inhibitory effects on T cell activation. Phytochemistry 2017;141:147–155. [DOI] [PubMed] [Google Scholar]
- 39.Ren Y, VanSchoiack A, Chai HB, et al. Cytotoxic barrigenol-like triterpenoids om an extract of Cyrilla racemiflora housed in a repository. J Nat Prod. 2015;78:2440–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlieb HE, Kotlyar V, Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem. 1997;62:7512–7515. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds WF, McLean S, Poplawski J, et al. Total assignment of 13C and 1H spectra of three isomeric triterpenol derivatives by 2D NMR: An investigation of the potential utility of 1H chemical shifts in structural investigations of complex natural products. Tetrahedron 1986;42:3419–3428. [Google Scholar]
- 42.Khripach VA, Zhabinskii VN, Antonchik AV, et al. High-resolution NMR analysis of the structure of C-24 functionalized steroids. Sery Khim Navuk 2008:64–68. [Google Scholar]
- 43.Ren Y, Matthew S, Lantvit DD, et al. Cytotoxic and NF-κB inhibitory constituents of the stems of Cratoxylum cochinchinense and their semisynthetic analogues. J Nat Prod. 2011;74:1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren Y, Chen WL, Lantvit DD, et al. Cardiac glycoside constituents of Streblus asper with potential antineoplastic activity. J Nat Prod. 2017;80:648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miuñoz Acuña U, Matthew S, Pan L, et al. Apoptosis induction by 13-acetoxyrolandrolide through the mitochondrial intrinsic pathway. Phytother Res. 2014;28:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang HP, Que S, Shi YP, et al. Triterpenoids from Gentiana veitchiorum. J Chem Pharm Res. 2014;6:1986–1990. [Google Scholar]
- 47.García-Granados A, López PE, Melguizo E, et al. Partial synthesis of C-ring derivatives from oleanolic and maslinic acids. Formation of several triene systems by chemical and photochemical isomerization processes. Tetrahedron 2004;60:1491–1503. [Google Scholar]
- 48.Tijjani A, Ndukwe IG, Ayo RG. Isolation and characterization of lup-20(29)-ene-3,28-diol (betulin) from the stem-bark of Adenium obesum (Apocynaceae). Trop J Pharm Res. 2012;11:259–262. [Google Scholar]
- 49.Nakanishi C, Toi M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer 2005;5:297–309. [DOI] [PubMed] [Google Scholar]
- 50.Solary E, Bettaieb A, Dubrez-Daloz L, et al. Mitochondria as a target for inducing death of malignant hematopoietic cells. Leuk Lymphoma 2003;44:563–574. [DOI] [PubMed] [Google Scholar]
- 51.Wang YY, Yang YX, Zhe H, et al. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: An update on its pharmacokinetic and pharmacodynamic properties. Drug Des Dev Ther. 2014;8:2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hata K, Hori K, Takahashi S. Differentiation- and apoptosis-inducing activities by pentacyclic triterpenes on a mouse melanoma cell line. J Nat Prod. 2002;65:645–648. [DOI] [PubMed] [Google Scholar]
- 53.Garg A, Aggarwal BB. Nuclear transcription factor-κB as a target for cancer drug development. Leukemia 2002;16:1053–1068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.