Abstract
Objective
To characterize the functional brain changes involved in δ-9-tetrahydrocannabinol (THC) modulation of chronic neuropathic pain.
Methods
Fifteen patients with chronic radicular neuropathic pain participated in a randomized, double-blind, placebo-controlled trial employing a counterbalanced, within-subjects design. Pain assessments and functional resting state brain scans were performed at baseline and after sublingual THC administration. We examined functional connectivity of the anterior cingulate cortex (ACC) and pain-related network dynamics using graph theory measures.
Results
THC significantly reduced patients' pain compared to placebo. THC-induced analgesia was correlated with a reduction in functional connectivity between the anterior cingulate cortex (ACC) and the sensorimotor cortex. Moreover, the degree of reduction was predictive of the response to THC. Graph theory analyses of local measures demonstrated reduction in network connectivity in areas involved in pain processing, and specifically in the dorsolateral prefrontal cortex (DLPFC), which were correlated with individual pain reduction.
Conclusion
These results suggest that the ACC and DLPFC, 2 major cognitive-emotional modulation areas, and their connections to somatosensory areas, are functionally involved in the analgesic effect of THC in chronic pain. This effect may therefore be mediated through induction of functional disconnection between regulatory high-order affective regions and the sensorimotor cortex. Moreover, baseline functional connectivity between these brain areas may serve as a predictor for the extent of pain relief induced by THC.
The possible therapeutic role of cannabis and of its main psychoactive substance, δ-9-tetrahydrocannabinol (THC), is a subject of intense interest in Western medicine. Currently, the most prevalent medical use of cannabis is for the treatment of chronic pain.1 Increasing evidence shows that cannabis may be safe and effective for refractory chronic pain.2,3 Nevertheless, the brain mechanism underlining this analgesic effect remains unknown.
A recent functional neuroimaging study found that THC reduced acute experimentally induced pain in healthy participants, and its analgesic effect was correlated with reduced activity of the anterior cingulate cortex (ACC).4 This result is compatible with the fact that the ACC has been shown to be densely populated with cannabinoid-1 receptors,5 which are agonistically activated by THC. Interestingly, cannabinoid receptors in the ACC were found to have decreased binding capability in an animal model of chronic neuropathic pain.6 These findings suggest the ACC may have a substantial contribution to cannabinoid analgesia in clinical pain states.
As the neural underpinnings of cannabis-induced analgesia in chronic pain remain poorly understood, we assessed the effect of a single dose of THC vs placebo on chronic pain by evaluating subjective pain ratings and resting-state brain connectivity of the ACC using fMRI. To that aim, we devised a double-blind, placebo-controlled, counterbalanced within-subjects study in patients with chronic neuropathic lower limb radicular pain. We hypothesized that THC will have an analgesic effect on patients' pain ratings and that this effect will correlate with functional changes in the ACC.
Methods
Patients
Seventeen patients with chronic lumbar radicular pain completed the study (27–40 years old; mean age 33.3 ± 3.9; all male). Women were excluded due to evidence that menstruation-related hormonal fluctuations may alter pain sensitivity.7 The inclusion criterion was established neuropathic lower limb radicular pain for over 6 months, with medium to high chronic pain (over 40 on a 100-point visual analog scale [VAS]) with no other known comorbidities. Participants' demographic and clinical data, as well as detailed inclusion and exclusion criteria data, are available in table e-1 (doi.org/10.5061/dryad.df6qd5n).
Standard protocol approvals, registrations, and patient consents
Seventeen participants gave written informed consent approved by the Tel-Aviv Sourasky Medical Center institutional review board (Clinical Trial Registration: clinicaltrials.gov/show/NCT02560545). Patients were recruited from The Institute of Pain Medicine at Tel-Aviv Sourasky Medical Center. Two participants were excluded because on second examination they did not fulfill the inclusion criteria.
CONSORT diagram data are available in figure e-1 (doi.org/10.5061/dryad.df6qd5n).
Study procedure
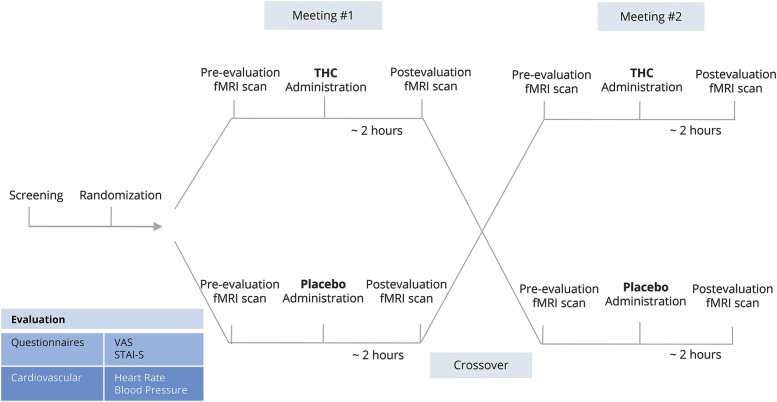
Patients participated in a counterbalanced manner in 2 meetings of a randomized, double-blind, placebo-controlled trial. In each meeting, patients received THC oil or placebo oil sublingually (0.2 mg/kg, average THC dosage = 15.4 ± 2.2 mg; Panaxia Pharmaceutical Industries, Lod, Israel). Randomization was done by a dedicated physician using a sealed envelope website (sealedenvelope.com/). Nine patients received THC in the first meeting and placebo in the second meeting, whereas 6 patients received the reverse treatment order. The experimental design is depicted in figure 1.
Figure 1. Experiment design.
Fifteen patients with chronic lumbar radicular pain participated in 2 meetings of a counterbalanced randomized, double-blind, placebo-controlled trial. Patients underwent clinical evaluation and an fMRI scan pre and post δ-9-tetrahydrocannabinol (THC)/placebo administration. STAI-S = State-Trait Anxiety Inventory–State; VAS = visual analog scale.
In each session, patients underwent baseline clinical evaluation composed of pain rating on a 0–100 VAS, anxiety questionnaire (State-Trait Anxiety Inventory–State [STAI-S]), and heart rate (HR) and blood pressure (BP) measurements (Nexfin BMEYE, Amsterdam, the Netherlands). Subsequently, patients underwent an fMRI scan composed of a non-task resting state, after which they received the treatment (THC/placebo). The resting state scan lasted 6 minutes and patients were instructed to keep their eyes closed, rest, and relax, but not to fall sleep. One hour post drug administration, the same procedure was repeated. The second fMRI scan was thus preformed about 2 hours post drug administration, in accordance with THC sublingual absorption showing maximal plasma concentrations usually after 2 to 3 hours.8 The meetings were separated by at least a week in order to enable a washout period of the possible THC treatment (average weeks interval = 2.9 ± 3.3).
fMRI data acquisition
fMRI data were acquired with a 3T MRI scanner (Magnetom Prisma, Siemens, Munich, Germany), with a 20-channel head coil, located at the Wohl Institute for Advanced Imaging at the Tel-Aviv Sourasky Medical Center. Functional scans were performed with T2*-weighted echoplanar images (44 axial interleaved slices, repetition time [TR] 3,000 ms, echo time [TE] 35 ms, field of view [FOV] 220 mm, in-plane matrix resolution 96 × 96, voxel size 2.3 × 2.3 × 3.0 mm, slice thickness 3 mm, flip angle 90°). Anatomical scan consisted of T1-weighted magnetization-prepared rapid gradient echo structural images (TR 1,860 ms, TE 2.74 ms, FOV 256 mm, in-plane matrix resolution 256 × 256, voxel size 1 × 1 × 1 mm, slice thickness 1 mm, flip angle 8°).
Data analysis
Behavioral and physiologic measurements
Statistical analyses for behavioral and physiologic evaluations were performed using STATISTICA 10 (TIBCO Software Inc., Palo Alto, CA). Within-subjects repeated measures analysis of variance was employed to ascertain significant interaction and simple main effects between the treatment (THC, placebo) and the state (pre, post) for VAS, STAI-S, and cardiovascular measures (HR and BP). For each participant, in each treatment condition, pain reduction was determined as the delta between the VAS score postintervention and the VAS score preintervention. These VAS subtraction values were used in subsequent analyses as indication for individual improvement in pain, that is, the more negative the values, the higher the improvement in pain.
fMRI data analysis
Preprocessing and functional connectivity analysis
Functional analyses were performed using Statistical Parametric Mapping (SPM12) software (fil.ion.ucl.ac.uk/spm/software/spm12/) and the Functional Connectivity toolbox9 (nitrc.org/projects/conn). Preprocessing included the following; first 18 seconds of the functional data were discarded to allow steady-state magnetization. Functional images were slice-time corrected, realigned to the middle scan, motion-corrected, and normalized according to standard Montreal Neurological Institute (MNI) space. Spatial smoothing was performed using a 6-mm full width at half maximum Gaussian kernel. In order to reduce noise, functional volumes were bandpass filtered at 0.008–0.15 and component-based method (CompCor) was used for noise signals such as white matter, CSF, and movement artifact that were taken as confounders. In addition, images that were regarded as movement outliers were regressed out. Outliers were detected using the ART toolbox (nitrc.org/projects/artifact_detect/) and defined as volumes with a movement greater than 2 mm or signal intensity changes greater than 9 SD.
Functional connectivity was performed using a seed-based analysis looking for temporal correlations of the resting-state blood oxygenation level–dependent (BOLD) signal time series between the ACC as the seed region and the rest of the brain. The region of interest (ROI) was defined using the ACC peak coordinates from an activation likelihood estimate (ALE) meta-analysis of chronic neuropathic pain.10 The coordinates were converted from Talairach space to MNI space using Lancaster Transform, and a 3-mm radius sphere was generated (MNI coordinates 2, 30, 29).
For each participant, first-level correlation maps were produced by extracting the residual BOLD time course from the seed and computing the Pearson correlation coefficients between that time course and the time course of all other voxels. Correlation coefficients were converted to normally distributed z scores using the Fisher transformation to allow second-level general linear model analyses.
To examine pain decrease–related changes in connectivity, first-level connectivity maps for each participant, at each state (pre, post), were entered into a whole-brain regression analysis with pain decrease as a covariate. The states were contrasted (post > pre) in order to examine the change in the treatment state (post) compared to baseline state (pre). In this analysis, reported clusters survived a height threshold of uncorrected p < 0.001 and an extent threshold of false discovery rate (FDR)–corrected p < 0.05 at the cluster level.
Anatomical allocation of the connectivity maps was done in reference to probabilistic cytoarchitectonic maps using the SPM Anatomy Toolbox.11 Activations were assigned to the most probable histologic area.
Graph theory analysis
Graph theory analysis was performed for 9 regions extracted from an ALE meta-analysis of chronic neuropathic pain10: operculum (secondary somatosensory cortex [SII]), insula, thalamus, putamen, ACC, middle cingulate cortex (MCC), and dorsolateral prefrontal cortex (DLPFC). The amygdala was added to these regions as a central hub of emotion processing and as it has also been shown to have an important role in persistent and chronic pain states,12 and in particular in our patient cohort. In addition, it has been shown to be involved in cannabinoid analgesia in humans.4 Left and right amygdala clusters were extracted from Neurosynth term-based meta-analyses of chronic pain. In total, 11 ROI were included. Presentation of all ROI data is available in figure e-2 (doi.org/10.5061/dryad.df6qd5n).
For each participant, the BOLD time course was extracted from each ROI and correlation coefficients between its time course and the time course of all other ROIs were computed. The ROI-to-ROI correlation matrix was then thresholded to construct a binary matrix where existing connections were assigned a value of 1, while the absence of a functional connection between network nodes was designated by a value of 0.
The unweighted ROI-to-ROI correlation matrices were thresholded with cost value (K). Cost is a measure of the proportion of connections for each ROI in relation to all connections in the network. A cost threshold allows for roughly the same number of connections across participants by varying the correlation threshold for each participant to achieve the fixed cost threshold.
Observing a range of network costs values between 0 < K < 0.5 over global and local efficiency networks, compared with the same measures estimated in a random graph and a regular lattice, can be an indication for small world network properties,13 where global efficiency is greater than that of a lattice graph and local efficiency is greater than that of a random graph.14 The data showed this behavior at a cost of 0.45 (figure e-3, doi.org/10.5061/dryad.df6qd5n); therefore it was chosen as a threshold.
The following measures for integration (global efficiency) and segregation (local efficiency and clustering coefficient) of the network were examined: global efficiency was calculated as the average of the inverse of the shortest path length between each ROI and all other ROIs. The shortest path length is defined as the fewest number of connections must be traversed to go from one ROI to another. Thus, a network with high global efficiency would be one in which nodes are highly integrated so the path between nodes is consistently short. Local efficiency was calculated as the average global efficiency between only ROIs neighboring a given ROI. Clustering coefficient is the proportion of connected ROIs neighboring a given ROI. Altogether, local efficiency and clustering coefficient measures the extent to which ROIs are part of a local cluster. Within-subjects repeated-measures analysis of variance was used to compute network measures interaction effects between the treatment (THC, placebo) and state (pre, post). Regression analysis was used to examine network change posttreatment (post > pre) covariation with pain decrease posttreatment (post-pre). These analyses were corrected for FDR of p < 0.05.
Data availability
Anonymized grouped data will be shared upon request by any qualified investigator.
Results
Behavioral results
As hypothesized, compared with placebo, THC significantly reduced the subjective perceived ongoing pain rated with the VAS score prior to and immediately after fMRI scanning, about 2 hours following drug administration (interaction effect F1,14 = 5.9, p < 0.05, simple effect p < 0.005, THC post-pre = 18.8 ± 5.6, placebo post-pre = 8.7 ± 5.5; figure 2).
Figure 2. Visual analog scale (VAS) score after δ-9-tetrahydrocannabinol (THC)/placebo administration.
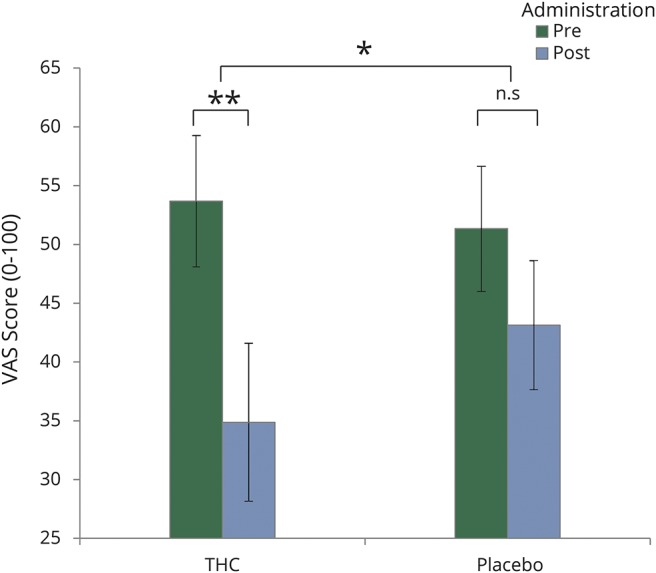
Compared to placebo, THC significantly reduced the subjective perceived ongoing pain. Error bars represent SEM. *p < 0.05, **p < 0.005. n.s = not significant.
The anxiety and the cardiovascular measures did not change significantly post THC administration compared to placebo.
Functional connectivity results
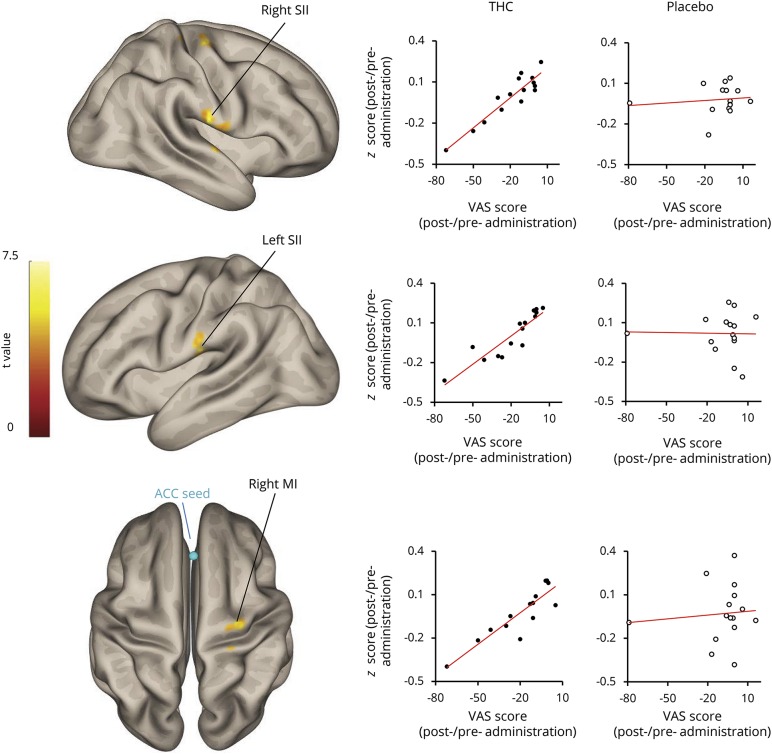
In order to test the association between the analgesic effect of THC and functional changes in ACC, patients' pain ratings were used as a covariate in the seed-to-whole-brain functional connectivity regression analysis. ACC ROI was used as the seed and the change in functional connectivity was examined by the contrast between the pre and post THC administration resting state scans (pre < post). We found a reduction in functional connectivity between the ACC and the sensorimotor cortex that covaried with the reduction in the subjective pain ratings after THC treatment (figure 3). Three clusters within the sensorimotor cortex were found: the right and left secondary somatosensory cortex (SII) and the right motor cortex (Ml) (right SII [areas OP4, OP1: MNI coordinates 64, −16, 20; 121 voxels, T(13) = 8.92, cluster p-FDR = 0.0023]; left SII [areas OP4, OP1: MNI coordinates 66, −20, 22; 67 voxels, T(13) = 7.77, cluster p-FDR = 0.0286]; and right Ml [area 4a: MNI coordinates 30, −16, 64; 95 voxels, T(13) = 7.22, cluster p-FDR = 0.0081]. The MNI coordinates of local maxima for each region are reported).
Figure 3. Seed-to-whole-brain functional connectivity reduction between anterior cingulate cortex (ACC) and sensorimotor cortex covariates with visual analog scale (VAS) score reduction after δ-9-tetrahydrocannabinol (THC) administration.
Sensorimotor cortex composed of ACC, secondary somatosensory cortex (SII), and primary motor cortex (M1). The regions were defined using the SPM toolbox for probabilistic cytoarchitectonic maps.
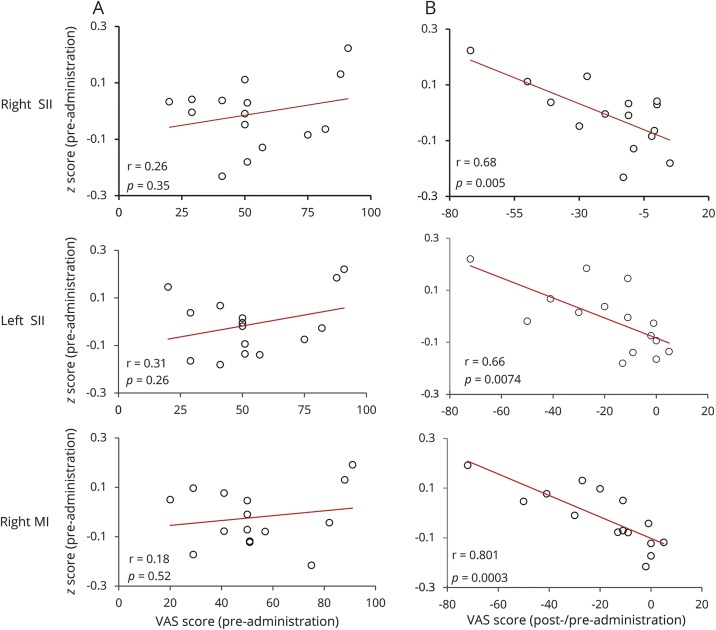
The relation between the reduction in the functional connectivity and the reduction in pain ratings was represented by β coefficient of 0.01. Crucially, there was no relation between the change in pain ratings and the ACC seed-to-whole-brain functional connectivity change in the placebo condition. To further examine the different components of the association observed following THC administration, a post hoc analysis of the functional connectivity brain state pre-THC treatment was tested for correlation with the pain ratings of the pre-THC treatment, as well as with the improvement in pain ratings post-THC treatment (post-pre). There was no correlation between pre-THC treatment ACC functional connectivity and pain ratings (figure 4A). Contrarily, pre-THC treatment ACC functional connectivity with right, left SII, and M1 correlated with the improvement in pain scores following THC administration (Pearson r = 0.679, p < 0.01, Pearson r = 0.66, p < 0.01, and Pearson r = 0.8, p < 0.005. respectively, figure 4B).
Figure 4. Correlation between anterior cingulate cortex (ACC) functional connectivity with right secondary somatosensory cortex (SII), left SII, and right primary motor cortex (M1) and pre and post δ-9-tetrahydrocannabinol (THC) treatment visual analog scale (VAS) scores.
(A) Low correlation between VAS scores and functional connectivity pre-THC treatment. (B) Correlation between the functional connectivity pre-THC treatment and the pain improvement reports post THC treatment. r = Pearson r correlation coefficient.
Graph theory results
The results showing functional connectivity changes within areas that are considered parts of the so-called chronic pain network10 prompted us to further examine functional changes among all chronic pain network components using graph theory measures.
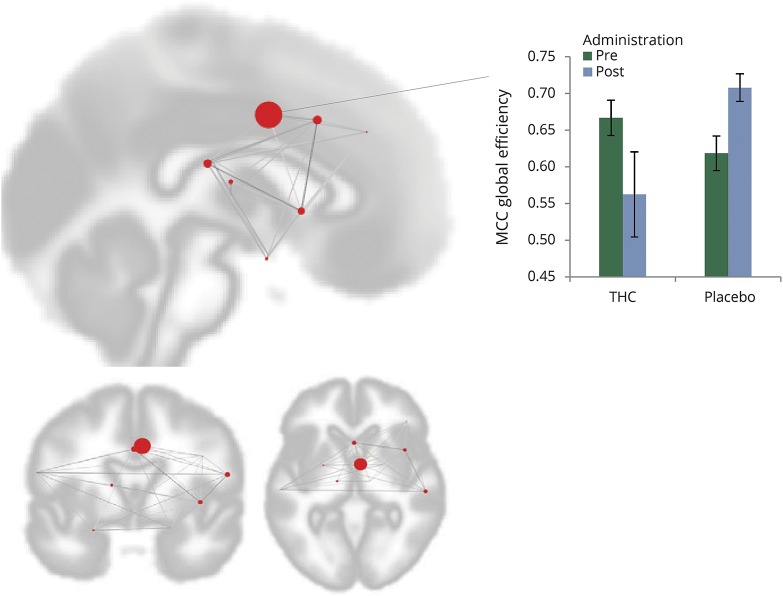
A significant change in global efficiency was found for the MCC when comparing THC and placebo treatment effects (figure 5; interaction effect F1,14 = 3.05, p-FDR = 0.0477).
Figure 5. Global efficiency change in middle cingulate cortex (MCC) after δ-9-tetrahydrocannabinol (THC)/placebo administration.
MCC global efficiency changed significantly after THC administration (interaction effect F1,14 = 3.05; p-FDR = 0.0477). Each red circle represents a cluster within the network and its size represents the effect change. The bar graph demonstrates the interaction effect of THC and placebo. Error bars represent SEM.
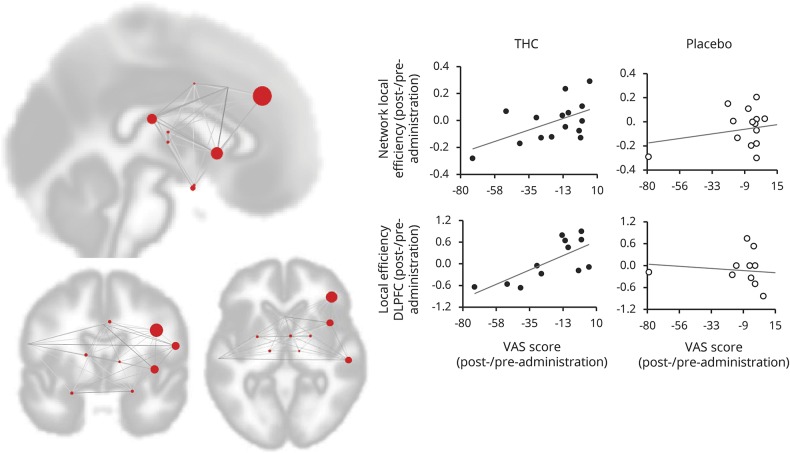
Local efficiency and clustering coefficient measure the extent to which a given ROI is part of a local network. Both measures showed reduction in the whole network (local efficiency network: T13 = 2.33, p = 0.018, clustering coefficient network: T13 = 2.72, p = 0.0087, figure 6), as well as in the right DLPFC cluster, which covaried with the reduction in pain scores after THC administration (local efficiency of DLPFC: T10 = 3.31, p-FDR = 0.0435, clustering coefficient DLPFC: T10 = 3.34, p-FDR = 0.0037, figure 6). Placebo administration did not show such association.
Figure 6. Reduction of local efficiency in structures of the chronic pain network and right dorsolateral prefrontal cortex (DLPFC) covariates with visual analog scale (VAS) score reduction after δ-9-tetrahydrocannabinol (THC) administration.
Reduction of local efficiency covariates with reduction in VAS score after THC administration (network p-FDR = 0.018; DLPFC p-FDR = 0.0435). Each red circle represents a cluster within the network and its size represents the regression effect. The graphs (right side) depict the regression in the THC condition as compared to placebo.
Discussion
The purpose of this study was to explore the poorly understood neural correlates of cannabis-induced analgesia in chronic pain. To this aim, we employed fMRI during single-dose THC vs placebo administration in a cohort of patients with well-documented chronic neuropathic pain and examined its clinical and brain effects.
THC resulted in significant analgesia compared with placebo, in line with previous studies indicating the analgesic properties of THC in chronic pain, and specifically neuropathic pain.2,3,15 The present study critically adds to existing clinical literature, as this has not yet been convincingly shown to our knowledge in chronic radicular pain, an extremely common neuropathic pain state, using a double-blind randomized design. In line with our hypothesis, the extent of individual pain relief was associated with reduced ACC–sensorimotor cortex functional connectivity.
Pain is a complex multimodal subjective experience, encompassing sensory and emotional domains. A traditional view divides the ascending nociceptive information into parallel pathways, in which the ACC is part of the “medial” stream, involved in processing affective aspects of pain, whereas the somatosensory cortex is considered part of the “lateral” stream, involved in the sensory aspects of pain processing.16 Nevertheless, it is clear that these 2 pathways are heavily interconnected, and are thought to eventually converge at the ACC.17 The ACC and SII are anatomically connected and both are main cortical targets of the spinothalamic system, a major pathway of peripheral nociceptive information.18 The ACC and the primary motor cortex have strong anatomical connections as well.19
The fact that our results associate pain relief with ACC–sensorimotor cortex functional connectivity reduction suggests that THC may alleviate subjectively experienced pain by disrupting the synchrony and integration between these pain processing 2 pathways. Functional connectivity reduction between sensorimotor and affective pain-related areas was also reported during THC analgesia in the context of acute experimental pain in healthy participants,4 supporting our suggestion of THC cognitive–affective integration interference.
Accordingly, different treatments for chronic pain aim to modulate the ACC. Cingulotomy has long been used for refractory chronic pain, where analgesia is characterized by altered emotional responses to pain.20 Real-time fMRI neurofeedback targeting the ACC reduced patients' spontaneous pain,21 and deep brain stimulation targeting the ACC similarly relieves the affective component of pain.22 Furthermore, both invasive and noninvasive brain stimulation of the primary motor cortex have been shown to reduce chronic pain,23 and this effect has been suggested to stem from interfering with the activity of the ACC.24 Taken together, this evidence supports the notion that lesioning and modulation of the ACC affects pain perception.
Interestingly, our results also demonstrate that pretreatment functional connectivity between the ACC and the sensorimotor cortex positively correlated with the improvement in pain scores induced by THC, that is to say, the higher the positive functional connectivity at baseline, the more benefit was gained from THC administration. It has been shown before that functional connectivity of the ACC and SII correlated with clinical pain ratings.25 Our results suggest that this regional functional connectivity may also serve to predict the extent of pain relief induced with THC.
Further investigation of the brain effects of cannabis focused on the well-characterized set of regions often referred to as the chronic pain network.26 We used graph theory measures to test for changes in integration measured by global efficiency and segregation measured by local efficiency and clustering coefficient of the network. This analysis first revealed global efficiency changes in the MCC cluster post THC treatment compared to placebo, signifying reduced integration between the cluster and the rest of the network post THC treatment. While this cluster is slightly posterior to the seed region ROI in the ACC used in the previous analysis, both are part of the anterior MCC (aMCC) subdivision, according to a recent proposed division of the ACC into anterior and mid cingulate cortices based on cytoarchitectural studies.27 This finding implicates the aMCC as the component that has functionally changed in comparison to the other regions, and showed less connectivity to the other pain-related regions, as has been shown in 2 different analyses. Our results echo previous findings of reduced activity of the same cingulate subdivision during THC analgesia seen in experimental pain induced in healthy participants,4 and further emphasize the role of the aMCC in the disintegration of multilevel pain processing that delineates THC-induced analgesia in clinical chronic pain.
In addition, we found local efficiency and clustering coefficient decreases in the right DLPFC cluster and in the whole network, which covaried with pain reduction. These measures show that, on average, the whole network has become locally less connected, and in particular, the right DLPFC is less connected to it. Resting functional connectivity of the cortical network consisting of pain-related areas has been shown to increase in chronic neuropathic pain compared to controls.28 Accordingly, the network modulation as expressed by the reduction in local connectivity measures of this network observed after THC in this cohort of patients may represent a trend towards normalization of the network connectivity.
The DLPFC has been shown to be involved in the cognitive–affective aspects of processing painful stimuli, and was proposed to exert active control on pain perception by top-down modulation.29 Accumulating evidence suggests that DLPFC may also have a role in recovery from chronic pain. It has been shown to have thinner gray matter in chronic back pain patients, which reverses following successful surgical treatment.30 Similarly, the DLPFC presented altered functional connectivity with various brain areas, including sensorimotor regions,31 which normalized following alleviation of pain. In addition, patients who transition from acute back pain to chronic back pain show less negative functional connectivity between the insula and DLPFC.32 Moreover, DLPFC stimulation has an analgesic effect on chronic pain, and it has been shown that this stimulation induces functional connectivity changes between the medial thalamus and sensory-affective cortices.33 Our results further corroborate the involvement of this region in pain modulation and specifically suggest its involvement in THC-mediated analgesia in chronic clinical pain.
Importantly, the aMCC and the DLPFC, the 2 main areas that we found changed their connectivity indices after THC administration, have substantial anatomical reciprocal connections, strong positive resting-state functional connectivness,34 and both have been suggested as major components of supraspinal pain modulation in a top-down manner.26,29,35
Limitations
Women were excluded from this study due to concern regarding menstruation-induced fluctuations in pain sensitivity. In addition, larger scale studies should examine whether these results are reproduced. Moreover, future investigations should include other chronic conditions to better understand whether our results represent a pervasive neuronal mechanism of cannabis effects on chronic pain or are unique to neuropathic pain states. Finally, it should be emphasized that the cannabis plant contains a multitude of cannabinoids beyond THC, of which special attention has been directed to cannabidiol. Such compounds may play important roles in observed variability of the clinical properties of the plant; for example, the observation that in cancer pain, the combination of THC:cannabidiol may be superior to THC alone.36 Future studies studying the differential brain-based effects of different cannabinoids or cannabinoid compositions may thus prove of major importance.
In this study, cannabis analgesia was associated with reduced ACC–sensorimotor cortex functional connectivity and reduced connectivity between the DLPFC and chronic pain network. In addition, pretreatment functional connectivity predicted the extent of pain relief. These results suggest that THC analgesia in chronic pain is mediated through brain areas underlying affective processing of pain, as well as supraspinal pain modulation, potentially addressing an imbalance in pain processing dynamics that occurs in chronic pain states.
Acknowledgment
The authors thank Avi Lougassi for his support, Dr. Joumana Espanioly for assistance with clinical evaluation of patients, and PhD candidate Itamar Jalon for assistance with data analysis.
Glossary
- ACC
anterior cingulate cortex
- aMCC
anterior middle cingulate cortex
- ALE
activation likelihood estimate
- BOLD
blood oxygenation level–dependent
- BP
blood pressure
- DLPFC
dorsolateral prefrontal cortex
- FDR
false discovery rate
- FOV
field of view
- HR
heart rate
- MCC
middle cingulate cortex
- MNI
Montreal Neurological Institute
- ROI
region of interest
- SII
secondary somatosensory cortex
- STAI-S
State-Trait Anxiety Inventory–State
- TE
echo time
- THC
δ-9-tetrahydrocannabinol
- TR
repetition time
- VAS
visual analog scale
Author contributions
Libat Weizman: study concept and design, acquisition of data, analysis and interpretation. Lior Dayan: study concept and design, clinical screening and evaluation. Silviu Brill: clinical evaluation. Hadas Nahman-Averbuch: study concept and design. Talma Hendler: study concept and design, revision of the manuscript. Giris Jacob: clinical evaluation, study supervision. Haggai Sharon: study concept and design, clinical evaluation, revision of the manuscript.
Study funding
This project was supported by Yahel Foundation, Recanati, New York, and by the Ministry of Science, Technology and Space (Grant no. 3-11170).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology April 3, 2018. Accepted in final form June 29, 2018.
References
- 1.Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse 2014;40:23–30. [DOI] [PubMed] [Google Scholar]
- 2.Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. J Am Med Assoc 2015;313:2474–2483. [DOI] [PubMed] [Google Scholar]
- 3.Ashton JC, Milligan ED. Cannabinoids for the treatment of neuropathic pain: clinical evidence. Curr Opin Investig Drugs 2008;9:65–75. [PubMed] [Google Scholar]
- 4.Lee MC, Ploner M, Wiech K, et al. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain 2013;154:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex 2006;17:175–191. [DOI] [PubMed] [Google Scholar]
- 6.Hoot MR, Sim-Selley LJ, Poklis JL, et al. Chronic constriction injury reduces cannabinoid receptor 1 activity in the rostral anterior cingulate cortex of mice. Brain Res 2010;1339:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent K, Tracey I. Sex hormones and pain: the evidence from functional imaging. Curr Pain Headache Rep 2010;14:396–403. [DOI] [PubMed] [Google Scholar]
- 8.Guy GW, Robson PJ. A phase I, double blind, three-way crossover study to assess the pharmacokinetic profile of cannabis based medicine extract (CBME) administered sublingually in variant cannabinoid ratios in normal healthy male volunteers (GWPK0215). J Cannabis Ther 2004;3:121–152. [Google Scholar]
- 9.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 10.Friebel U, Eickhoff SB, Lotze M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage 2011;58:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005;25:1325–1335. [DOI] [PubMed] [Google Scholar]
- 12.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neurosci 2004;10:221–234. [DOI] [PubMed] [Google Scholar]
- 13.Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature 1998;393:440–442. [DOI] [PubMed] [Google Scholar]
- 14.Achard S, Bullmore E, Papathanassiou B, Crivello D, Etard F. Efficiency and cost of economical brain functional networks. PLoS Comput Biol 2007;3:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaulieu P, Ware M. Reassessment of the role of cannabinoids in the management of pain. Curr Opin Anaesthesiol 2007;20:473–477. [DOI] [PubMed] [Google Scholar]
- 16.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277:968–971. [DOI] [PubMed] [Google Scholar]
- 17.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000;288:1769–1772. [DOI] [PubMed] [Google Scholar]
- 18.Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 2009;29:14223–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull 2012;87:457–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen CP, Kung SS, Su YF, Lin WC, Howng SL, Kwan AL. Stereotactic bilateral anterior cingulotomy for intractable pain. J Clin Neurosci 2005;12:886–890. [DOI] [PubMed] [Google Scholar]
- 21.deCharms RC, Maeda F, Glover GH, et al. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci USA 2005;102:18626–18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boccard SGJ, Fitzgerald JJ, Pereira EAC, et al. Targeting the affective component of chronic pain. Neurosurgery 2014;74:628–637. [DOI] [PubMed] [Google Scholar]
- 23.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology 2008;70:2329–2337. [DOI] [PubMed] [Google Scholar]
- 24.DosSantos MF, Ferreira N, Toback RL, Carvalho AC, DaSilva AF. Potential mechanisms supporting the value of motor cortex stimulation to Treat chronic pain Syndromes. Front Neurosci 2016;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Cao D, Remeniuk B, Krimmel S, Seminowicz DA, Zhang M. Altered brain structure and function associated with sensory and affective components of classic trigeminal neuralgia. Pain 2017;158:1561–1570. [DOI] [PubMed] [Google Scholar]
- 26.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005;6:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cauda F, Sacco K, Duca S, et al. Altered resting state in diabetic neuropathic pain. PLoS One 2009;4:e4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079–1091. [DOI] [PubMed] [Google Scholar]
- 30.Seminowicz DA, Wideman TH, Naso L, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci 2011;31:7540–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Čeko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp 2015;36:2075–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15:1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankarasubramanian V, Cunningham DA, Potter-Baker KA, et al. Transcranial direct current stimulation targeting primary motor versus dorsolateral prefrontal cortices: proof-of-concept study investigating functional connectivity of thalamocortical networks specific to sensory-affective information processing. Brain Connect 2017;7:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C, Zhou Y, Liu Y, et al. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage 2011;54:2571–2581. [DOI] [PubMed] [Google Scholar]
- 35.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci 2008;12:306–313. [DOI] [PubMed] [Google Scholar]
- 36.Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010;39:167–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized grouped data will be shared upon request by any qualified investigator.