Abstract
Objectives
To determine whether a panel of blood-based biomarkers can discriminate between patients with suspected mild traumatic brain injury (mTBI) with and without neuroimaging findings (CT and MRI).
Methods
Study participants presented to the emergency department with suspected mTBI (n = 277) with a CT and MRI scan and healthy controls (n = 49). Plasma concentrations of tau, glial fibrillary acidic protein (GFAP), ubiquitin carboxyl-terminal hydrolase L1, and neurofilament light chain (NFL) were measured using the single-molecule array technology.
Results
Concentrations of GFAP, tau, and NFL were higher in patients with mTBI, compared with those of controls (p's < 0.01). GFAP yielded an area under the curve (AUC) of 0.93 (95% confidence interval [CI] 0.90–0.96), confirming its discriminatory power for distinguishing mTBI from controls. Levels of GFAP, tau, and NFL were higher in patients with trauma-related intracranial findings on CT compared with those with normal CT, with the only significant predictor being GFAP (AUC 0.77, 95% CI 0.70–0.84). Among patients with mTBI, tau, NFL, and GFAP differentiated subjects with and without MRI abnormalities with an AUC of 0.83, with GFAP being the strongest predictor. Combining tau, NFL, and GFAP showed a good discriminatory power (AUC 0.80, 95% CI 0.69–0.90) for detecting MRI abnormalities, even in patients with mTBI with a normal CT.
Conclusion
Our study confirms GFAP as a promising marker of brain injury in patients with acute mTBI. A combination of various biomarkers linked to different pathophysiologic mechanisms increases diagnostic subgroup accuracy. This multimarker strategy may guide medical decision making, facilitate the use of MRI scanning, and prove valuable in the stratification of patients with brain injuries in future clinical trials.
Classification of evidence
Class I evidence that blood concentrations of GFAP, tau, and NFL discriminate patients with mTBI with and without neuroimaging findings.
Millions of patients seek care for traumatic brain injuries (TBIs) every year,1 with over 90% of injuries classified as mild TBI (mTBI), as determined by the Glascow Coma Scale (GCS) scores between 13 and 15, reporting short or no loss of consciousness and brief periods of post-traumatic amnesia.2 CT is used in routine clinical care for detecting more severe intracranial injury and to aid in acute management decisions. MRI may allow for the detection of more subtle injuries not seen on CT, including diffuse axonal injury, which increase risks for neurologic symptoms3,4; however, is not the standard of care for TBI. Thus, having blood-based biomarkers that improve the assessment and characterization of injury and guide the diagnostic pathway would be of immense clinical benefit.
Biomarkers including glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1) have been associated with TBI and related to findings on CT in cohorts with moderate to severe TBIs.5-10 Tau, an axonal injury marker, has been shown to relate to CT findings in patients with TBI with all severites,11 and neurofilament light chain (NFL) relates to the degree of axonal injury in patients with severe TBI.12 Fewer studies have compared these biomarker candidates in patients with mTBI13 and imaging findings. We used ultrasensitive assays to test peripheral biomarkers of axonal and astroglial injury in patients with mTBI and examined their relationship to neuroimaging studies. The purpose was to determine whether these biomarkers can discriminate patients with subtle injuries, determined through MRI, who may be at risk of poor recovery.
Methods
This analysis is part of the Traumatic Head Injury Neuroimaging Classification study (NCT01132937), a large and ongoing natural history study of TBI. Patients seeking care for a suspected brain injury, 18–85 years of age, and a GCS of 13–15 were included in this analysis. Enrollment, blood collection, and imaging (clinical CT and research MRI) were performed within 48 hours of injury. A standardized MRI protocol was used, which included diffusion-tensor imaging, T2*-weighted imaging, T2-fluid-attenuated inversion recovery (FLAIR), high-resolution 3D-T1, dynamic susceptibility contrast perfusion-weighted imaging, and post-contrast T1 and T2-FLAIR. Healthy controls (n = 49) without a history of TBI or neurologic disease were recruited from the National Institute of Health protocols: NCT01762475 and 09-NR-0131. All protocols were approved by their respective institutional review boards, and informed consent was obtained before any data collection.
Blood samples were collected into ethylenediaminetetraacetic acid tubes, centrifuged, aliquoted for plasma, and stored at −80°C. Plasma samples were analyzed using the Simoa (Single Molecule Array) Neurology 4-plex assay kit (Quanterix, Lexington, MA) for simultaneous measurement of NFL, tau, GFAP, and UCH-L1 on the HD-1 Analyzer. The laboratory was blinded to the clinical and imaging data. The average coefficient of variation of measurement of NFL, tau, GFAP, and UCH-L1 in all tested samples was 5%, 9%, 4%, and 29%. Here we show data for UCLH-1 but do not report it as a finding, as approximately one-third did not meet the quality control specifications.
The Kruskal-Wallis and the Mann-Whitney tests were used to compare biomarkers between groups, and correlation analyses were performed using the Spearman rank test, with p-values adjusted for multiple comparisons with the Benjamini-Hochberg procedure. As biomarkers were significantly associated with age, age-adjusted receiver operating curves (ROCs) were used to calculate the biomarker diagnostic accuracy. All tests were 2-tailed; p < 0.05 was considered significant. Statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC).
Data availability
Any data not published within the article are available by request within the Center for Neuroscience and Regenerative Medicine Informatics Core. The dataset will be shared on request from any qualified investigator.
Results
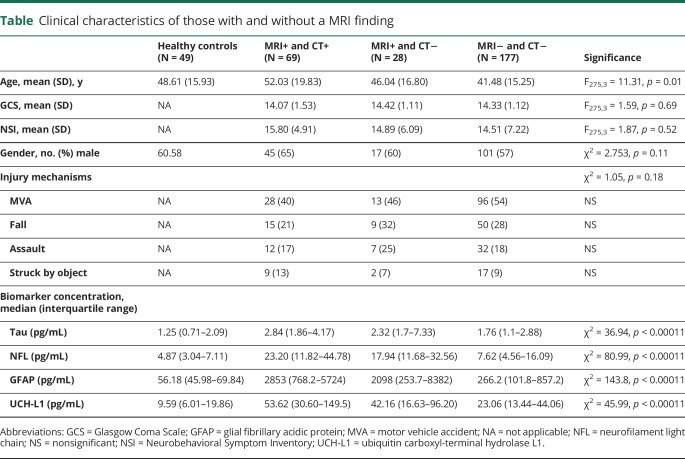
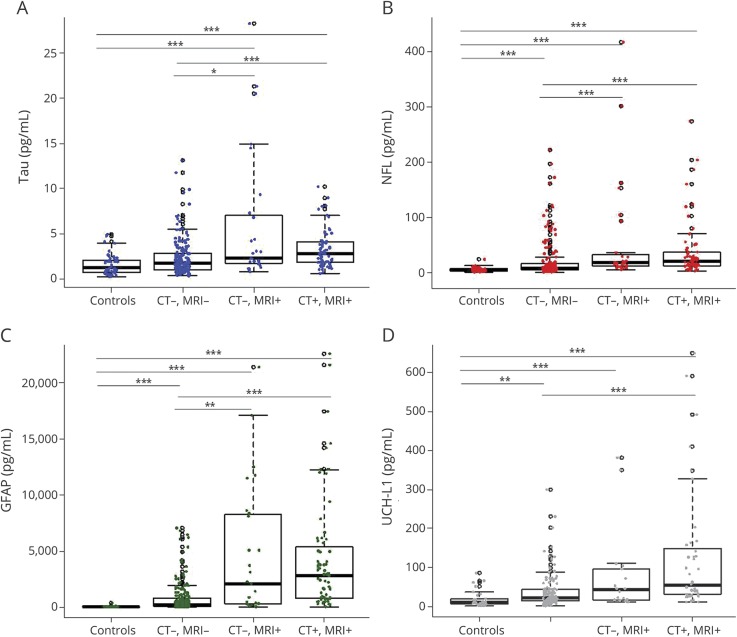
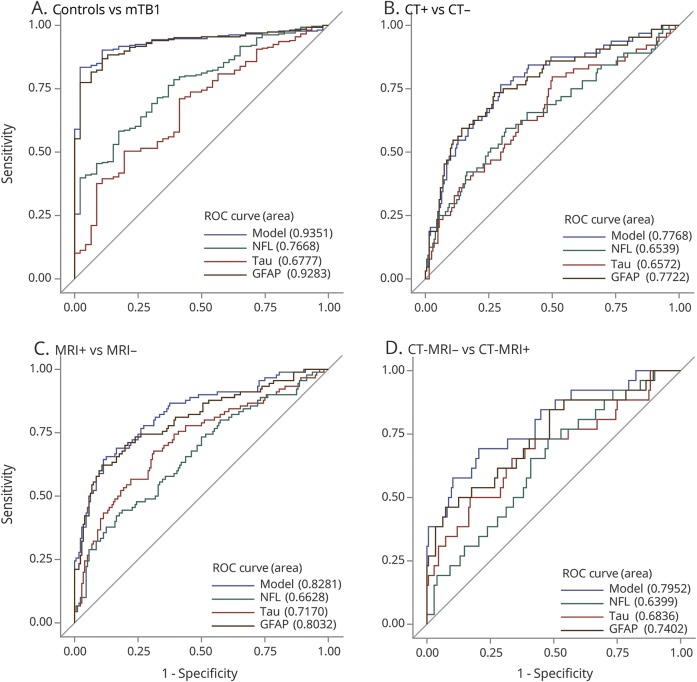
Patients (n = 277) and controls (n = 49) were well-matched with regard to demographic characteristics (table). Patients with imaging findings were older than those without. In addition, biomarker levels were associated with age; thus, age was controlled for in all comparisons. Compared with those of healthy controls, the mTBI cohort had higher concentrations of tau, NFL, and GFAP (figure 1, A–D and table). Age-adjusted ROCs comparing the overall mTBI cohort to controls resulted in an area under the curve (AUC) value for GFAP that was excellent (0.93, 95% confidence interval [CI] 0.90–0.96), and none of the other biomarkers improved the diagnostic accuracy (figure 2A, figure e-1, links.lww.com/WNL/A694).
Table.
Clinical characteristics of those with and without a MRI finding
Figure 1. Box-and-whisker plots of tau (A), NFL (B), GFAP (C) and UCH-L1 (D) concentrations in the different diagnostic groups.
The black horizontal line in each box represents the median, with the boxes representing the interquartile range. Significant differences are indicated with *p < 0.05, **p < 0.01, and ***p < 0.001. GFAP = glial fibrillary acidic protein; NFL = neurofilament light chain; and UCH-L1 = ubiquitin carboxyl-terminal hydrolase L1.
Figure 2. Receiver operating characteristic curves age-adjusted for NFL, tau, and GFAP and “model” which includes all biomarkers (NFL, tau, and GFAP).
(A) ROC stratifying controls vs patients with mTBI, (B) ROC stratifying patients with mTBI with and without CT imaging findings, (C) ROC stratifying patients with mTBI with and without MRI findings, (D) ROC stratifying patients with mTBI who have CT negative findings, both with and without MRI findings. GFAP = glial fibrillary acidic protein; mTBI = mild traumatic brain injury; NFL = neurofilament light chain; and ROC = receiver operating curve.
In stratifying patients with mTBI with and without CT findings, there were higher concentrations of tau, NFL, and GFAP in patients with findings (p's < 0.01), and a fair AUC value, with the only significant predictor being GFAP (AUC 0.77, 95% CI 0.70–0.84) (figure 2B). Similarly, stratifying based on MRI findings, higher concentrations of all biomarkers (p's < 0.05) were seen in those with findings. The overall model showed good discriminatory power (AUC 0.83, 95% CI 0.77–0.88); GFAP was the strongest predictor (p < 0.001), but tau and NFL also contributed independently to predicting findings on MRI (p = 0.03 and p = 0.02, respectively) (figure 2C). Patients with a normal CT but with findings on MRI had significant elevations of GFAP, tau, and NFL compared with patients with mTBI lacking findings on neuroimaging (p's < 0.05). Consistent with the previous model, the AUC value was good (0.80, 95% CI 0.69–0.90), with GFAP being the main predictor (p = 0.0003) and with tau (p = 0.017) and NFL (p = 0.015) being independent predictors of the presence of MRI abnormalities (figure 2D).
Discussion
The present study confirms that GFAP distinguishes patients with mTBI from controls, as well as distinguishing patients with mTBI with a positive CT from patients with mTBI who are CT negative.5-8 We also report that GFAP distinguishes patients with mTBI who are CT negative but are MRI positive from patients with mTBI who are both CT and MRI negative, and that tau and NFL in combination with GFAP improved diagnostic accuracy. Therefore, GFAP shows promise in identifying patients who may need more intensive monitoring and clinical care, and the addition of tau and NFL may increase prediction ability. This is important, as approximately 25%–40% of CT negative patients have a MRI positive finding, which is linked to more long-term neurocognitive and neuropsychiatric disabilities.14 MRI scanning is not universally available and is costly, but may have value in clinical care of mTBI patients. Therefore, a blood-based biomarker to identify these patients would provide an opportunity to detect more subtle injuries and to ultimately improve care for patients with mTBI by identifying those who warrant increased monitoring and possibly improve future preventative interventions. These findings suggest the need for additional studies that include multiple biomarkers to determine whether combinations of biomarkers may improve identification of patients with mTBI with more subtle injuries detected by MRI.
Our conclusions are limited to having a relatively small sample size, mainly in the subgroups. Additionally, UCH-L1 was undetectable in a significant proportion of samples, limiting the comparability with the other markers and the conclusions that could be drawn. In summary, the findings presented here provide important insights into how GFAP may be used as a diagnostic biomarker, with possible utility in determining patients with mTBI with subtle injuries detected only through MRI, which is not widely available in emergency room settings.
Acknowledgment
We also acknowledge Michelle Wolf, who assisted in the laboratory assays.
Glossary
- AUC
area under the curve
- CI
confidence interval
- FLAIR
fluid-attenuated inversion recovery
- GCS
Glascow Coma Scale
- GFAP
glial fibrillary acidic protein
- mTBI
mild traumatic brain injury
- NFL
neurofilament light chain
- ROC
receiver operating curve
- TBI
traumatic brain injury
- UCH-L1
ubiquitin carboxyl-terminal hydrolase L1
Footnotes
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Author contributions
J. Gill and L. Latour: study supervision, study concept and design, analysis and interpretation of data, drafting or revising the manuscript for intellectual content. R. Diaz-Arrastia: drafting or revising the manuscript for intellectual content. V. Motamedi: analysis and interpretation of data, drafting or revising the manuscript for intellectual content. C. Turtzo, P Shahim, S. Mondello, and C. DeVoto: analysis and interpretation of data, drafting or revising the manuscript for intellectual content. E. Veras, D. Hanlon, and L. Song: lab analysis and interpretation of data, drafting or revising the manuscript for intellectual content. A. Jeromin: study supervision, study concept and design, lab analysis and interpretation of data, drafting or revising the manuscript for intellectual content. All authors contributed to manuscript revision and read and approved the submitted version.
Study funding
National Institute of Nursing Research (NINR) Intramural Research Program, National Institute of Neurological Disease and Stroke (NINDS) Team, Center for Neuroscience and Regenerative Medicine, Acute Studies and Biomarker Core, National Football League and General Electric, Head to Head Grant.
Disclosure
J. Gill, L. Latour, R. Diaz-Arrastia, V. Motamedi, C. Turtzo, P. Shahim, S. Mondello, and C. DeVoto report no disclosures relevant to the manuscript. E. Veras, D. Hanlon, L. Song, and A. Jeromin: employed by Quanterix Corp. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology January 19, 2018. Accepted in final form June 29, 2018.
References
- 1.Korley FK, Kelen GD, Jones CM, Diaz-Arrastia R. Emergency department evaluation of traumatic brain injury in the United States, 2009-2010. J Head Trauma Rehabil 2016;31:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol 2015;14:506–517. [DOI] [PubMed] [Google Scholar]
- 3.Yuh EL, Mukherjee P, Lingsma HF, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 2013;73:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amyot F, Arciniegas DB, Brazaitis MP, et al. A review of the effectiveness of neuroimaging modalities for the detection of traumatic brain injury. J Neurotrauma 2015;32:1693–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondello S, Kobeissy F, Vestri A, Hayes RL, Kochanek PM, Berger RP. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein after pediatric traumatic brain injury. Sci Rep 2016;6:28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Arrastia R, Wang KK, Papa L, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma 2014;31:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol 2016;73:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch RD, Ayaz SI, Lewis LM, et al. Ability of serum glial fibrillary acidic protein, ubiquitin C-terminal hydrolase-L1, and S100B to differentiate normal and abnormal head computed tomography findings in patients with suspected mild or moderate traumatic brain injury. J Neurotrauma 2016;33:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okonkwo DO, Yue JK, Puccio AM, et al. GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J Neurotrauma 2013;30:1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondello S, Sorinola A, Czeiter E, et al. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting with mild head injury to emergency departments: a living systematic review and meta-analysis. J Neurotrauma Epub 2018 Jul 2. [DOI] [PMC free article] [PubMed]
- 11.Bogoslovsky T, Wilson D, Chen Y, et al. Increases of plasma levels of glial fibrillary acidic protein, tau, and amyloid beta up to 90 days after traumatic brain injury. J Neurotrauma 2017;34:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ljungqvist J, Zetterberg H, Mitsis M, Blennow K, Skoglund T. Serum neurofilament light protein as a marker for diffuse axonal injury: results from a case series study. J Neurotrauma 2017;34:1124–1127. [DOI] [PubMed] [Google Scholar]
- 13.Peacock WF, Van Meter TE, Mirshahi N, et al. Derivation of a three biomarker panel to improve diagnosis in patients with mild traumatic brain injury. Front Neurol 2017;8:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metting Z, Rodiger LA, De Keyser J, van der Naalt J. Structural and functional neuroimaging in mild-to-moderate head injury. Lancet Neurol 2007;6:699–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within the article are available by request within the Center for Neuroscience and Regenerative Medicine Informatics Core. The dataset will be shared on request from any qualified investigator.