Abstract
Background.
The profile of cortical neuroanatomical abnormalities in schizophrenia is not fully understood, despite hundreds of published structural brain imaging studies. This study presents the first meta-analysis of cortical thickness and surface area abnormalities in schizophrenia conducted by the ENIGMA (Enhancing Neuro Imaging Genetics Through Meta Analysis) Schizophrenia Working Group.
Method.
The study included data from 4474 individuals with schizophrenia (mean age=32.3, range: 11–78; 66% male) and 5098 healthy volunteers (mean age=32.8, range: 10–87; 53% male), assessed with standardized methods, at 39 centers worldwide.
Results.
Compared to healthy volunteers, individuals with schizophrenia have widespread thinner cortex (left/right hemisphere: Cohen’s d=−0.530/−0.516) and smaller surface area (left/right hemisphere: d=−0.251/−0.254), with the largest effect sizes for both in frontal and temporal lobe regions. Regional group differences in cortical thickness remained significant when statistically controlling for global cortical thickness, suggesting regional specificity. In contrast, the effects for cortical surface area appear global. Case-control, negative, cortical thickness effect sizes were 2 to 3 times larger in antipsychotic medicated relative to unmedicated individuals. Negative correlations between age and bilateral temporal pole thickness were stronger in individuals with schizophrenia than in healthy volunteers. Regional cortical thickness showed significant negative correlations with normalized medication dose, symptom severity, and duration of illness, and positive correlations with age at onset.
Conclusions.
The findings indicate that the ENIGMA meta-analysis approach can achieve robust findings in clinical neuroscience studies; also, medication effects should be taken into account in future genetic association studies of cortical thickness in schizophrenia.
Keywords: schizophrenia, imaging, cortical, thickness, surface area, meta-analysis
INTRODUCTION
Brain structural abnormalities are widely reported in schizophrenia, but there is no published meta-analysis reporting effect sizes for cortical thickness and surface area abnormalities and their relationships to clinical features of the disease. Several hundred studies have reported on cortical thickness and surface area abnormalities in schizophrenia but it is difficult to meta-analyze published results as they lack a standard format to ease comparisons and are based on atlas (1) or vertex-wise (2) approaches using a variety of methods (3–9). To address these issues, the Schizophrenia Working Group, within the Enhancing Neuro Imaging Genetics through Meta Analysis (ENIGMA (10–12)); http://enigma.ini.usc.edu) consortium, brings together schizophrenia researchers from all over the world to jointly conduct large-scale imaging and imaging-genetics meta-analyses using standardized methods.
This meta-analysis focuses on regional cortical thickness and surface area rather than volume, based on evidence that they are influenced by separate sets of genes (13, 14). Cortical thickness and surface area abnormalities have been reported in individuals with chronic (1, 15–17), short/medium duration (18), first-episode (19–24), child (25, 26) and adolescent onset (27), or antipsychotic naive schizophrenia (28–30), individuals with non-clinical psychotic symptoms (31), and individuals at clinical high risk for psychosis (32–39).
We previously reported effect sizes for deep brain structure volume abnormalities based on 15 samples worldwide, including brain imaging data from 2028 individuals with schizophrenia and 2540 healthy volunteers (40); findings replicated in an independent cohort using similar methods (41). Here we report Cohen’s d effect sizes comparing regional cortical thickness and surface area between 4474 individuals with schizophrenia and 5098 healthy volunteers, and partial correlation effect sizes with continuous clinical measures based on 39 worldwide samples.
Based on prior work, we hypothesized that individuals with schizophrenia, compared to healthy volunteers, show widespread cortical thickness and surface abnormalities, that are most prominent in frontal and temporal lobe regions (15), and that they show significant associations with age at onset or duration of illness (42), symptom severity (43–48), and antipsychotic medication use (49–51).
MATERIALS AND METHODS
Study Samples
Thirty-nine, worldwide, cross-sectional study samples totaling 9572 participants, including 4474 individuals with schizophrenia (SZ) and 5098 healthy volunteers (HV), contributed to the analysis via the ENIGMA Schizophrenia Working Group (Supplementary Table S1a–S1b; Figure S1). Sample-size weighted mean (range) age across samples was 32.3 (21.2–43.6) years for patients and 34.5 (21.8–43.9) years for controls. Patient and control samples were on average 65% (44–100) and 54% (36–100) male. Weighted mean age at onset and duration of illness across the samples were 23.4 (20.0–35.6) and 10.5 (0.6–20.2) years. Weighted mean PANSS (Positive and Negative Syndrome Scale (52)) total, negative, and positive scores across the samples were 68.1 (43.0–90.2), 21.9 (10.0–22.9), and 16.4 (10.6–22.6); weighted mean SANS (Scale for the Assessment of Negative Symptoms (53)) and SAPS (Scale for the Assessment of Positive Symptoms (54)) scores were 20.5 (5.5–33.0) and 19.2 (9.0–32.3). For samples that recorded current antipsychotic type and/or dose, numbers (percentages) of patients on second-generation (atypical), first-generation (typical), both, or none, were 2236 (66%), 447 (13%), 265 (8%), and 425 (13%), respectively, and sample-size weighted mean chlorpromazine dose equivalent (CPZ), based on Woods (2005; www.scottwilliamwoods.com/files/Equivtext.doc), was 399 (167–643). Each study sample was collected with participants’ written informed consent approved by local Institutional Review Boards.
Image acquisition and processing
All sites processed T1-weighted structural brain scans using FreeSurfer (9) (http://surfer.nmr.mgh. harvard. edu) and extracted cortical thickness and surface area for 70 Desikan-Killiany (55) (DK) atlas regions (34 regions per hemisphere + left and right hemisphere mean thickness or total surface area; Table S3). Number of scanners, vendor, strength, sequence, acquisition parameters, and FreeSurfer versions are provided in Table S2. ENIGMA’s quality assurance protocol was performed at each site prior to analysis, and included visual checks of the cortical segmentations and region-by-region removal of values for segmentations found to be incorrect (http://enigma.usc.edu/protocols/imaging-protocols; Table S2). Histograms of all regions’ values for each site were also computed for visual inspection.
Statistical meta-analyses
Group differences for DK atlas regions within each sample were examined using univariate linear regression (R’s linear model function lm) predicting left and right DK atlas region cortical thickness or surface area with group (SZ, HV), sex, and age (model A). To further assess whether group differences in cortical thickness and surface area showed regional specificity, analyses were repeated including global mean cortical thickness or total cortical surface area as covariates, respectively (model B). To test for differential sex or age effects between groups, we also included models with group-by-sex (model C) or group-by-age interaction terms (model D). Significant interactions were further explored through within-group analyses. Medication effects were examined through between-group comparisons of individuals with schizophrenia on second-generation (atypical), first-generation (typical), both, or no (unmedicated) antipsychotic medications and healthy volunteers with sex and age included as covariates; only contrasts with a minimum of 5 subjects per group within site were included in these analyses to enable variance estimation. In patients, relationships were examined between regional cortical measures and several continuous variables, including age at onset, duration of illness, chlorpromazine equivalent antipsychotic medication dose, and total, positive, and negative symptom severity. These partial correlation analyses included age and sex as covariates. Analysis of multi-scanner studies (ASRB, FBIRN, MCIC, Osaka, UPENN) included binary dummy covariates for n-1 scanners. Sites conducted analyses of their sample’s individual subject data using R code created within the ENIGMA collaboration. Random-effects meta-analyses of Cohen’s d and partial correlation effect sizes for each of the DK atlas regions were performed using R’s (version 3.2.2) metafor package (version 1.9–7) (56). False Discovery Rate (pFDR<0.05) (57) was used to control for multiple comparisons. Cortical maps depict significant effect sizes (pFDR<0.05) overlaid on (metallic gray) cortical surface models (brainder.org/research/brain-for-blender). Possible confounding effects of differences in parental socioeconomic status on group differences were examined using subsample analyses (Supplement 1, Supplementary Results, Figures, and Tables SR3, S8a–S9b, S52a–S53b). Effects of FreeSurfer version and scanner field strength were examined using meta-regressions (Supplement 1).
RESULTS
Widespread thinner cortex with regional specificity in schizophrenia
Individuals with schizophrenia, compared to healthy individuals, showed widespread significantly thinner cortex in all DK atlas regions, except the bilateral pericalcarine region (Model A), with effect sizes between d=−0.536 (right fusiform gyrus) and −0.077 (left pericalcarine fissure) and marginal (least square) mean (LSM) thickness differences between − 3.33 (left parahippocampal gyrus) and −0.45 percent (left pericalcarine fissure; Figure 1A and Table S4a). The largest negative effect sizes (d<−0.40) were observed for: left/right hemisphere (d=−0.530/−0.516), bilateral fusiform, temporal (inferior, middle, and superior), and left superior frontal gyri, right pars opercularis, and bilateral insula.
Figure 1.
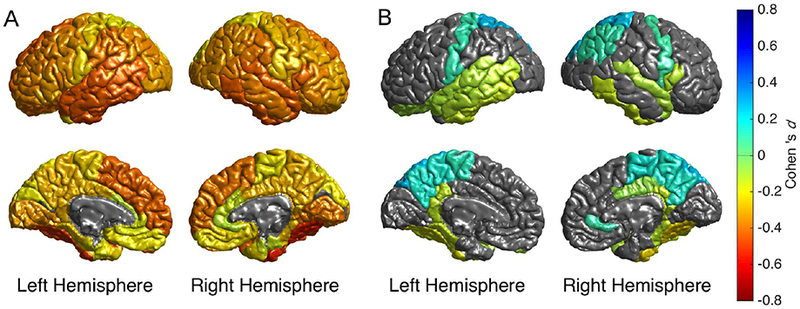
Cortical map of regional Cohen’s d effect sizes for schizophrenia versus healthy group cortical thickness contrast statistically controlling for A) age and sex, and B) age, sex, and global cortical thickness. Only regions with pFDR<0.05 are depicted in color. In figure 1B, warm colors (yellow-red) reflect regions in which the effect of schizophrenia is more than than the mean global cortical thinning, and cool colors (green-blue) reflect regions where the effect of schizophrenia is less than the mean global thinning compared to controls.
In the context of widespread thinner cortex in schizophrenia, we assessed regional specificity of these cortical thickness differences. When controlling for individual differences in global mean cortical thickness, several regions showed significantly thinner cortex (e.g., fusiform, parahippocampal, inferior temporal gyri) while other regions showed significantly thicker cortex (e.g., superior parietal cortex, precuneus, paracentral lobule) in individuals with schizophrenia compared to healthy volunteers (Model B; Figure 1B; Figure 2; Table S4b). These findings suggest regional specificity of thinner cortex in schizophrenia.
Figure 2.
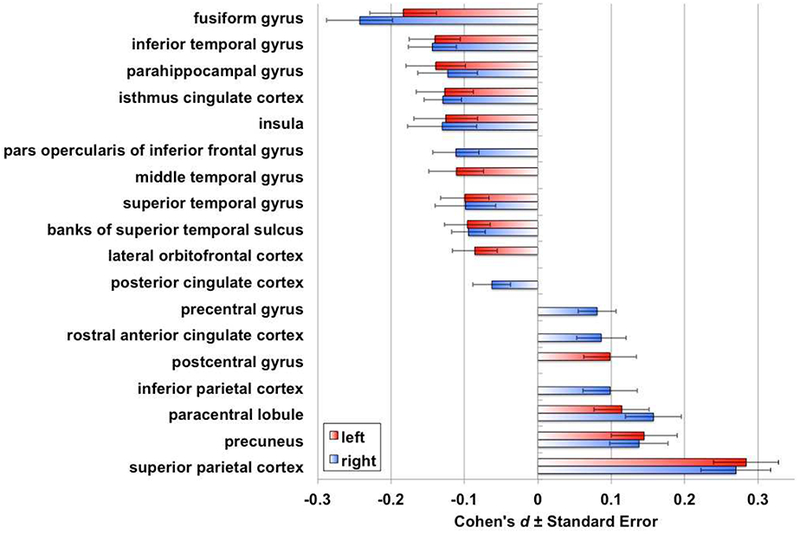
Cohen’s d effect sizes for schizophrenia versus healthy group cortical thickness contrast statistically controlling for age, sex, and global mean cortical thickness. Only regions with pFDR<0.05 are depicted in color.
Widespread smaller cortical surface area without regional specificity in schizophrenia
Individuals with schizophrenia, compared to healthy individuals, showed widespread significantly smaller cortical surface area in all DK atlas regions, except the bilateral isthmus cingulate region (Model A), with effect sizes between d=−0.254 (mean right hemisphere) and −0.040 (right isthmus cingulate) and marginal (least square) mean surface area differences between −3.39 (left rostral anterior cingulate) and −0.55 percent (right isthmus cingulate; Figure 3A; Table S5a). The largest effect sizes (d<−0.20) were observed for: left (d=−0.251) and right (d=−0.254) hemisphere, bilateral superior frontal, fusiform, inferior and middle temporal, and right precentral gyri.
Figure 3.
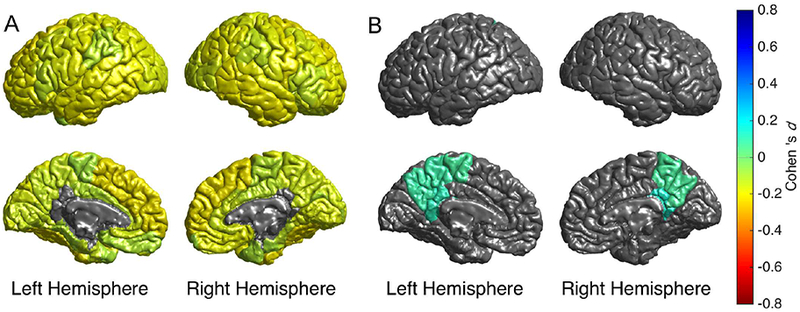
Cortical map of regional Cohen’s d effect sizes for schizophrenia versus healthy group cortical surface area contrast statistically controlling for A) age and sex, and B) age, sex, and total cortical surface area. Only regions with pFDR<0.05 are depicted in color. In figure 3B, warm colors (yellow-red) would reflect regions in which the effect of schizophrenia is more than the mean lower lower surface area, and cool colors (green-blue) reflect regions where the effect of schizophrenia is less than the mean lower global surface area compared to controls.
In the context of widespread smaller cortical surface area in schizophrenia, we assessed regional specificity of these cortical surface area differences. When controlling for individual differences in total cortical surface area, no regions showed significantly smaller surface area, while three regions showed significantly larger cortical surface area (bilateral isthmus cingulate, precuneus, and left paracentral) in individuals with schizophrenia compared to healthy volunteers (Model B; Figure 3B; Table S5b). These findings suggest that smaller cortical surface area is predominantly global in schizophrenia, with exception of the three regions noted which appear less affected.
Group-by-sex interactions
No significant group-by-sex interactions were detected for either cortical thickness or surface area for any of the DK atlas regions (Tables S6–S7).
Group-by-age interactions
There were significant group-by-age interactions for both left (pFDR=0.007) and right temporal pole thickness (pFDR=0.01), with schizophrenia showing stronger negative correlations with age (left: r=−0.13, pFDR=1.51E-13; right r=−0.12=, pFDR=1.55E-07) than healthy subjects (left r=−0.05, pFDR=0.02; right r=−0.04, pFDR=0.03). These interactions remained significant even when controlling for global mean cortical thickness (Figure S2; Tables S8a–S8b, and S10–S11). There were no significant group-by-age interactions for cortical surface area for any of the DK atlas regions (Table S9).
Partial correlations with age of onset and duration of illness
Earlier age of onset (r=0.063, pFDR=0.03) and longer duration of illness (r=−0.061; pFDR=0.04) were significantly correlated with thinner right insula cortical thickness (Tables S33–S34, and Figure S3). There were no significant correlations between age of onset or duration of illness and cortical surface area for any of the DK atlas regions (Tables S43–S44).
Effects of antipsychotic medications on cortical thickness
Effect sizes comparing left and right hemisphere cortical thickness from individuals with schizophrenia on no (unmedicated; left/right d=−0.275/−0.278), second-generation (left/right d=− 0.536/−0.516), first-generation (left/right d=−0.765/−0.648), or both (left/right d=−0.770/−0.704) antipsychotic medications to healthy volunteers were significant for all but the unmedicated group (pFDR>0.05; Figure 4; Tables S12–15).
Figure 4.
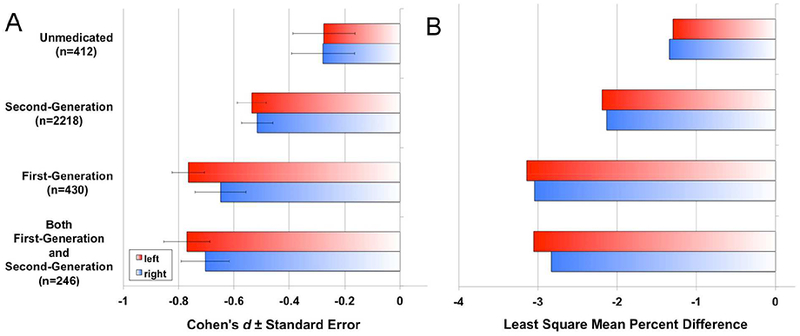
A) Cohen’s d effect sizes, and B) least square mean percent difference for schizophrenia versus healthy group contrasts in global cortical thickness, statistically controlling for age, and sex, by medication group and hemisphere. Nominal one-tailed p-values for left (L) and right (R) hemisphere thickness group comparisons, statistically controlling for age and sex, were: Second-Generation vs. Unmedicated [p(L)<0.05; p(R)<0.06]; First-Generation vs. Unmedicated [p(L)<0.01; p(R)<0.002]; Both First-Generation and Second-Generation vs. Unmedicated [p(L)<0.02; p<0.05]; First-Generation vs. Second-Generation [p(L)<0.03; p(R)<0.03]; Both First-Generation and Second-Generation vs. Second-Generation [p(L)<0.02; p(R)<0.05]; Both First-Generation and Second-Generation vs. First-Generation [p(L)=0.50; p(R)=0.48]; Supplementary Tables S16–S21).
Groupwise comparisons of left and right hemisphere thickness found nominally significant effects for all medicated vs. unmedicated groups (Figure 4, Tables S16–S18). Similarly, nominally significant effects were found for first-generation vs. second-generation, and both vs. second-generation, but not both vs. first generation medication groups (Figure 4; Tables S19–S21). No significant regional effects were observed for the last four group contrasts (pFDR>0.05; Tables S18–S21).
For detailed regional effects of antipsychotic medications on cortical thickness and surface area see Supplementary Results SR1.
Partial correlations with medication dose
Higher chlorpromazine dose equivalents were significantly correlated with thinner cortex in almost all the DK atlas regions, except bilateral entorhinal and pericalcarine cortex, bilateral lingual and transverse temporal gyri, and left postcentral, cuneus, and parahippocampal gyri and caudal anterior cingulate cortex, and right superior parietal and rostral anterior cingulate cortex, and right frontal pole (Figure S6A; Table S32). The correlations were significant for both left (r=−0.126) and right hemisphere thickness (r=−0.126), and were strongest (partial r<−0.10) for left (r=−0.166) and right superior frontal (r=−0.148), left (r=−0.113) and right middle temporal (r=− 0.108), left (r=−0.112) and right superior temporal (r=−0.106), right inferior temporal (r=−0.113), right pars triangularis of inferior frontal (r=−0.113), left (r=−0.102) and right caudal middle frontal (r=−0.108), and left supramarginal gyri (r=−0.103).
Importantly, post-hoc analysis showed that higher chlorpromazine dose equivalents were significantly correlated with thinner cortex even when controlling for negative symptom severity (Table S41; Figure S7).
There were no detectable correlations between chlorpromazine dose equivalents and cortical surface area for any of the DK atlas regions (Table S42).
Partial correlations with symptom severity scores
Higher PANSS total and positive symptom severity scores were significantly correlated with regional thinner cortex (Figure S6B; Table S35, Figure S6D; Table S36), while higher PANSS negative symptom scores were significantly correlated with widespread thinner cortex in left (r=−0.085) and right (r=−0.089) hemispheres (Figure S6C; Table S37; see SR2 for details).
Neither PANSS total, positive, or negative symptom severity scores were significantly correlated with regional cortical surface area for any of the DK atlas regions (Tables S45–S47).
DISCUSSION
The main findings of this study are that individuals with schizophrenia, compared to healthy volunteers, show: (1) widespread thinner cortex (left/right d=−0.530/−0.516); (2) widespread smaller cortical surface area; about half the size of the effect observed for cortical thickness (left/right d=−0.251/−0.254); (3) the largest effect sizes in frontal and temporal lobe regions for both measures, with regional specificity for cortical thickness but not cortical surface area (based on the analyses controlling for global thickness and surface area); (4) approximately two times larger negative cortical thickness effect size when on second-generation antipsychotic medications (left/right d=−0.536/−0.516), and approximately three times larger cortical thickness effect size when on first-generation (left/right d=−0.765/−0.648) or both first- and second-generation antipsychotic medications (left/right d=−0.770/−0.704) relative to unmedicated individuals with schizophrenia (left/right d=−0.275/−0.278), and (5) a stronger negative correlation between age and bilateral temporal pole cortical thickness (left: r=−0.13 vs. −0.05, and right: r=−0.12 vs. −0.04). With regard to partial correlations with clinical variables, (6) earlier age at onset and longer duration of illness were associated with thinner insula cortex, (7) standardized medication dose (CPZ) and (8) negative symptom severity were associated with widespread thinner cortex, while (9) total and (10) positive symptom severity were associated with regional thinner cortex. Most observed correlations were small (r<0.2). Moreover, despite the high power to detect small effects, medication use and other clinical variables were not significantly associated with cortical surface area.
These findings are consistent with the interpretation that the thinner cortex observed in individuals with schizophrenia shows regional specificity and is associated with the disease (28–30), its severity (43–48), and with antipsychotic medication treatment (49–51), with a larger effect for first-compared to second-generation antipsychotic medications (16, 58–60). We cannot fully exclude the possibility that observed medication effects on cortical thickness are partially due to group differences in age or duration of illness (61), which also show patterns of increase across the groups. However, the fact that 1) age was statistically controlled for in the medication type analyses, 2) duration of illness, which is highly collinear with age, only showed effects, above-and-beyond age, on right insula thickness, 3) there was only a group-by-age interaction on temporal pole thickness (while medication effects were widespread), and 4) metaregressions showed no effects of age or duration of illness on group contrast effect sizes, render such an interpretation unlikely (see Supplementary Results SR1). Further, dissociating medication effects from other potentially confounding variables requires well-powered, first-episode longitudinal studies, preferably with random assignment to first- or second-generation antipsychotics. Two longitudinal imaging studies, that randomly assigned individuals to medication treatments, found significant gray matter reductions for haloperidol but not olanzapine (58, 62); findings consistent with our meta-analysis and with reported medication effects on cortical thickness in rodents (63). None of the other potential confounding variables, including sex distribution, age at onset, medication dose, global, negative, or positive symptoms showed a pattern consistent with the observed medication effects. These variables are therefore unlikely to explain the differences in cortical thickness effect sizes across the antipsychotic medication groups on their own; though more complex interactions could exist.
In contrast to thinner cortex, smaller cortical surface area in individuals with schizophrenia appears to be a more global phenomenon associated with the disease but not with its severity or its treatment. It is possible that more focal cortical surface area effects are obfuscated through the averaging of measurements within DK atlas regions; vertex-wise analyses may have higher power for detecting and localizing such effects.
This study found significant group-by-age interactions on cortical thickness in the bilateral temporal pole regions only, with a stronger negative correlation between age and cortical thickness in schizophrenia than in healthy volunteers. In addition, this study found that earlier age at onset and longer duration of illness were associated with thinner cortical thickness in the insula only. These findings corroborate reported longitudinal findings of lower cortical volumes at illness onset as well as progressive volume decline in the temporal pole and insula in schizophrenia (64, 65) and individuals at ultra high risk for psychosis (66). Given our results, these volume declines may reflect cortical thinning rather than cortical surface area reduction. While our findings may suggest that there are few differential effects of age on cortical thickness between individuals with schizophrenia and healthy volunteers, we must keep in mind that age effects on thickness across a large age range are non-linear (67) and that this meta-analysis combines linear age effects across multiple independent cross-sectional cohorts of various ages. Longitudinal studies are better poised to address the question of differential effects of age and duration of illness on cortical thickness in schizophrenia and some have observed steeper rates of cortical thinning in multiple regions in individuals with schizophrenia and their non-ill co-twins (61). ENIGMA Schizophrenia Working Group members are actively working on pooling longitudinal studies for a meta-analysis to further address these questions.
Taken together, these findings may suggest that cortical surface area developmental trajectories in psychosis may be predominantly influenced by early neurodevelopmental, perhaps predominantly genetic, factors. In contrast, cortical thickness, in addition to likely being influenced by different genes (13, 14), may be more plastic and also influenced by additional environmental and neurodegenerative factors (e.g., treatment, cannabis, age) (68).
This study found significant widespread associations between standardized medication doses (chlorpromazine equivalents) and cortical thickness but not cortical surface area. This finding is consistent with and extends a prior meta-regression analysis, which reported that higher medication doses are associated with smaller gray matter volume (51). Given our results, the association with volume is likely due to cortical thickness rather than surface area. The finding is also consistent with the larger effect sizes for individuals with schizophrenia who were on antipsychotic medications compared to those who were not. An alternative interpretation may be that more severely ill patients receive higher doses of medication given the observed significant associations between symptom severity and regional cortical thickness. However, consistent with medication dose effects on cortical thickness, we found that significant associations between CPZ and cortical thickness were still observed in post-hoc partial correlation analyses that statistically controlled for negative symptom severity. In this analysis, we opted to control for negative rather than positive symptom severity as negative symptoms tend to be less influenced by medication dose than positive symptoms.
We caution that the likelihood that antipsychotic medications are associated with thinner cortex in individuals with schizophrenia should by no means be interpreted as a contraindication for their use in treating severe mental illnesses including schizophrenia. In fact, a recent study found that medication treatment was associated with thinner cortex and better behavioral performance on a cognitive control task (26% higher d’-Context score) (24). Most importantly, antipsychotic medications tend to successfully treat severely debilitating psychotic symptoms, reduce relapse risk following a first-episode break (69), and reduce suicide risk (70). As such, they play a critical role in the treatment of psychosis.
Similar published meta-analyses in bipolar disorder (BPD) and major depressive disorder (MDD), with the same study design and analytical methods, found thinner bilateral frontal, temporal, and parietal lobe cortex in BPD with evidence for divergent effects of medication treatments (71), and thinner regional cortex in adult MDD, and smaller total and regional cortical surface area in adolescent MDD (72). Taken together, these very large-scale studies suggest both similarities and differences in cortical abnormalities observed among these three major psychiatric illnesses.
To our knowledge, this is the first meta-analysis of cortical thickness and surface area abnormalities in schizophrenia. Only one other schizophrenia study has provided a comprehensive listing of Cohen’s d effect sizes for regional cortical thickness abnormalities comparing individuals with schizophrenia, non-ill first-degree relatives, and healthy volunteers (1).
The major strength of the study is its large sample size, which provides sufficient power to detect even small effects (e.g., symptom associations). Weaknesses include that (1) the group of unmedicated individuals with schizophrenia does not distinguish never-medicated from unmedicated at time-of-scan, leaving effect sizes for medication-naive subjects to be determined; (2) despite the large total sample size, many regional thickness differences between medication subgroups did not survive multiple comparison correction; (3) this study does not examine possible group differences in brain lateralization, though such analyses will be reported on separately; (4) the analysis of chlorpromazine equivalents did not dissociate first-generation and second-generation antipsychotic medications which may have dissociable effects on cortical thickness (51, 72). Finally, while this meta-analysis is unique in that it standardized image analysis methods across sites, any meta-analysis, including this one, is limited by sources of variation inherent to the analysis of retrospectively collected samples that cannot be fully controlled for. Sample differences include the use of different scanners, different assessments or processes to arrive at diagnosis, age at onset, duration of illness, medication dose and adherence, etc. Meta-analyses control for these differences by summing within-site effects across sites, providing generalized mean effect sizes. Like other meta-analyses, this meta-analysis does not control for all variance in assessments that can lower power to detect effects.
Taken together, the findings from this meta-analysis suggest that thinner cortex in schizophrenia shows regional specificity and is affected by the illness, its severity, and by treatments with antipsychotic medications, while smaller cortical surface area is mainly influenced by widespread effects of the illness possibly mainly influenced by developmental processes. In the context of ENIGMA, these findings suggest that schizophrenia genetic association studies employing cortical thickness as a quantitative trait may need to control for medication effects while those that employ cortical surface area as a quantitative trait may not need to.
Supplementary Material
ACKNOWLEDGMENTS
The ENIGMA project is in part supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health under Award Number U54EB020403. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Acknowledgments for the various participating data contributors are listed in Supplement 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COLLABORATORS
Members of the Karolinska Schizophrenia Project (KaSP): Lars Farde1, Lena Flyckt1, Goran Engberg2, Sophie Erhardt2, Helena Fatouros-Bergman1, Simon Cervenka1, Lilly Schwieler2, Fredrik Piehl3, Ingrid Agartz1,4,5, Karin Collste1, Pauliina Victorsson1, Anna Malmqvist2, Mikael Hedberg2, Funda Orhan2
1Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet, & Stockholm County Council, Stockholm, Sweden; 2Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden; 3Neuroimmunology Unit, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; 4NORMENT, KG Jebsen Centre for Psychosis Research, Division of Mental Health and Addiction, University of Oslo, Oslo, Norway; 5Department of Psychiatry Research, Diakonhjemmet Hospital, Oslo, Norway.
CONFLICTS OF INTEREST
Dr. Van Erp has had a research contract with Otsuka Pharmaceuticals, Inc. Adrian Preda has served as a consultant for Boehringer Ingelheim. The remaining authors report no biomedical financial interests or potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Protocol design, quality testing, and meta-analysis: TGM.v.E., E.W., D.P.H., L.S., and W.J. Data collection, processing, analysis and funding: TGM.v.E., E.W., DP.H., L.S., W.J., DC.G., GD.P., N. Y., M.F., R.H., N.O., H.Y., JR.B., VP.C., I.A., BA.M., W.C., SMC.d.Z., HE.H.P., RS.K., RA.O., NEM.v.H., OA.A., AM.D., NT.D., TP.G., CB.H., UK.H., KN.J., TV.L., I.M., LT.W., O.G., B.K., A.R., D.Z., VD.C., B.C., R.R., MJ C., VJ.C., S.C., J.R., VL.C., JM.F., MJ.G., F.H., A.J., RK.L., C.L., BJ.M., PT.M., C.P., Y.Q., PE.R., G.C., U.S.., RJ.S., M.S., PA.T., CS.W., TW.W., DW.M., E.H., P.K., LM.B., RE.G., RC.G., TD.S., DH.W., A.B., GG.B., JM.F., F.M., DH.M., DS.O., SG.P., A.P., J.V., KO.L., S.M., F.Y., Y.T., S.T., Z.W., F.F., J.C., H.X., S.T., G., P.W., D.W., HJ.B., S.E., RPF.W., J.H., MD.K., JM.S., SR.S., L.D.H., L.K., MW.M., T.v.A., DJ.V., F.A., N.B., P.d.R., M.I., F.P., G.S., PJ.M., E.P., J.R., R.S., A.C., G.D., S.K., CD.W., EW.D., D.R., A.V., S.C., P.D., R.M., T.R.M., A.S., S.B., L.E., F.H., A.R., R.S., KI.A., L.W., EG.J., S.K., IEC.S., A.B., A.B., A.D.G., E.N., AR.M., JM.S., JS.K., JY.Y., DM.C., C.M., L., AS.T., T.A., V.K., H.F.B., L.F., GF.B., PGP.R., MH.S., MV.Z., C.H., A.S., F.S., D.T., SP.H., AM.M., HC.W., SM.L., C.K., V.O., M.S., FM.H., DJ.S., H.T., A.U., C.LJ., D.D., A.M., FI.F, BA.G, N.J., PM.T., JA.T. Manuscript preparation: TGM.v.E., J.A.T, and P.M.T. All authors contributed edits and approved the contents of the manuscript.
REFERENCES
- 1.Goghari VM, Truong W, Spilka MJ (2015): A magnetic resonance imaging family study of cortical thickness in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 168: 660–668. [DOI] [PubMed] [Google Scholar]
- 2.Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. (2003): Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 60: 878–888. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PM, Schwartz C, Toga AW (1996): High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 3: 19–34. [DOI] [PubMed] [Google Scholar]
- 4.Miller MI, Massie AB, Ratnanather JT, Botteron KN, Csernansky JG (2000): Bayesian construction of geometrically based cortical thickness metrics. Neuroimage. 12: 676–687. [DOI] [PubMed] [Google Scholar]
- 5.Kabani N, Le Goualher G, MacDonald D, Evans AC (2001): Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 13: 375–380. [DOI] [PubMed] [Google Scholar]
- 6.Zijdenbos AP, Forghani R, Evans AC (2002): Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 21: 1280–1291. [DOI] [PubMed] [Google Scholar]
- 7.Lerch JP, Evans AC (2005): Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 24: 163–173. [DOI] [PubMed] [Google Scholar]
- 8.Ad-Dab’bagh Y Einarson D Lyttelton O Muehlboeck J-S Mok K, Ivanov O Vincent R Lpage C Lerch J Fombonne E Evans A (2006): The CIVET Image-Processing Environment: A Fully Automated Comprehensive Pipeline for Anatomical Neuroimaging Research. In: Corbetta M, editor. Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping. Presented at the Human Brain Mapping, Florance, Italy: NeuroImage. [Google Scholar]
- 9.Fischl B (2012): FreeSurfer. Neuroimage. 62: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, et al. (2012): Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 44:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, et al. (2014): The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 8: 153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson PM, Andreassen OA, Arias-Vasquez A, Bearden CE, Boedhoe PS, Brouwer RM, et al. (2017): ENIGMA and the individual: Predicting factors that affect the brain in 35 countries worldwide. Neuroimage. 145: 389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. (2009): Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19: 2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. (2010): Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 53: 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ Jr, Pung CJ, et al. (2010): Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 68: 41–50. [DOI] [PubMed] [Google Scholar]
- 16.van Haren NEM, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, et al. (2011): Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 68: 871–880. [DOI] [PubMed] [Google Scholar]
- 17.Sugihara G, Oishi N, Son S, Kubota M, Takahashi H, Murai T (2017): Distinct Patterns of Cerebral Cortical Thinning in Schizophrenia: A Neuroimaging Data-Driven Approach. Schizophr Bull. 43: 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz CC, Koch K, Wagner G, Roebel M, Nenadic I, Schachtzabel C, et al. (2010): Complex pattern of cortical thinning in schizophrenia: results from an automated surface based analysis of cortical thickness. Psychiatry Res. 182: 134–140. [DOI] [PubMed] [Google Scholar]
- 19.Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, et al. (2005): Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 58: 32–40. [DOI] [PubMed] [Google Scholar]
- 20.Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, et al. (2005): Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 15: 708–719. [DOI] [PubMed] [Google Scholar]
- 21.Fornito A, Yucel M, Wood SJ, Adamson C, Velakoulis D, Saling MM, et al. (2008): Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp. 29: 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun D, Stuart GW, Jenkinson M, Wood SJ, McGorry PD, Velakoulis D, et al. (2009): Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol Psychiatry. 14: 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crespo-Facorro B, Roiz-Santianez R, Perez-Iglesias R, Rodriguez-Sanchez JM, Mata I, Tordesillas-Gutierrez D, et al. (2011): Global and regional cortical thinning in first-episode psychosis patients: relationships with clinical and cognitive features. Psychol Med. 41: 1449–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ, et al. (2015): A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 72: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baribeau DA, Anagnostou E (2013): A comparison of neuroimaging findings in childhood onset schizophrenia and autism spectrum disorder: a review of the literature. Front Psychiatry. 4: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ordóñz AE, Luscher ZI, Gogtay N (2016): Neuroimaging findings from childhood onset schizophrenia patients and their non-psychotic siblings. Schizophr Res. 173: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voets NL, Hough MG, Douaud G, Matthews PM, James A, Winmill L, et al. (2008): Evidence for abnormalities of cortical development in adolescent-onset schizophrenia. Neuroimage. 43: 665–675. [DOI] [PubMed] [Google Scholar]
- 28.Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS (2008): Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand. 117: 420–431. [DOI] [PubMed] [Google Scholar]
- 29.Rais M, Cahn W, Schnack HG, Hulshoff Pol HE, Kahn RS, van Haren NEM (2012): Brain volume reductions in medication-naive patients with schizophrenia in relation to intelligence quotient. Psychol Med. 42: 1847–1856. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Lai Y, Wang X, Hao C, Chen L, Zhou Z, et al. (2014): A combined DTI and structural MRI study in medicated-nai’ve chronic schizophrenia. Magn Reson Imaging. 32: 1–8. [DOI] [PubMed] [Google Scholar]
- 31.van Lutterveld R, van den Heuvel MP, Diederen KMJ, de Weijer AD, Begemann MJH, Brouwer RM, et al. (2014): Cortical thickness in individuals with non-clinical and clinical psychotic symptoms. Brain. 137: 2664–2669. [DOI] [PubMed] [Google Scholar]
- 32.Haller S, Borgwardt SJ, Schindler C, Aston J, Radue EW, Riecher-Rossler A (2009): Can cortical thickness asymmetry analysis contribute to detection of at-risk mental state and first-episode psychosis? A pilot study. Radiology. 250: 212–221. [DOI] [PubMed] [Google Scholar]
- 33.Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, et al. (2009): Progressive brain structural changes mapped as psychosis develops in “at risk” individuals. Schizophr Res. 108: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung WH, Kim JS, Jang JH, Choi J-S, Jung MH, Park J-Y, et al. (2011): Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr Bull. 37: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin KS, Jung WH, Kim JS, Jang JH, Hwang JY, Chung CK, Kwon JS (2012): Neuromagnetic auditory response and its relation to cortical thickness in ultra-high-risk for psychosis. Schizophr Res. 140: 93–98. [DOI] [PubMed] [Google Scholar]
- 36.Tognin S, Pettersson-Yeo W, Valli I, Hutton C, Woolley J, Allen P, et al. (2013): Using structural neuroimaging to make quantitative predictions of symptom progression in individuals at ultra-high risk for psychosis. Front Psychiatry. 4: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tognin S, Riecher-Rössler A, Meisenzahl EM, Wood SJ, Hutton C, Borgwardt SJ, et al. (2014): Reduced parahippocampal cortical thickness in subjects at ultra-high risk for psychosis. Psychol Med. 44: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TGM, et al. (2015): Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 77: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchy L, Barbato M, Makowski C, Bray S, MacMaster FP, Deighton S, Addington J (2017): Mapping structural covariance networks of facial emotion recognition in early psychosis: A pilot study. Schizophr Res. 189: 146–152. [DOI] [PubMed] [Google Scholar]
- 40.van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 21: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada N, Fukunaga M, Yamashita F, Koshiyama D, Yamamori H, Ohi K, et al. (2016): Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 21: 1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cahn W, Rais M, Stigter FP, van Haren NEM, Caspers E, Hulshoff Pol HE, et al. (2009): Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 19: 147–151. [DOI] [PubMed] [Google Scholar]
- 43.Gogtay N, Weisinger B, Bakalar JL, Stidd R, Fernandez de la Vega O, Miller R, et al. (2012): Psychotic symptoms and gray matter deficits in clinical pediatric populations. Schizophr Res. 140: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oertel-Knöchel V, Knochel C, Rotarska-Jagiela A, Reinke B, Prvulovic D, Haenschel C, et al. (2013): Association between psychotic symptoms and cortical thickness reduction across the schizophrenia spectrum. Cereb Cortex. 23: 61–70. [DOI] [PubMed] [Google Scholar]
- 45.Padmanabhan JL, Tandon N, Haller CS, Mathew IT, Eack SM, Clementz BA, et al. (2015): Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders. Schizophr Bull. 41: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Y, Lui S, Deng W, Yao L, Zhang W, Li S, et al. (2013): Altered Cortical Thickness Related to Clinical Severity But Not the Untreated Disease Duration in Schizophrenia. Schizophr Bull. 41: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walton E, Hibar DP, van Erp TGM, Potkin SG, Roiz-Santianez R, Crespo-Facorro B, et al. (2017):Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium. Acta Psychiatr Scand. 135: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walton E, Hibar DP, van Erp TGM, Potkin SG, Roiz-Santiañez R, Crespo-Facorro B, et al. (2018): Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol Med. 48: 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navari S, Dazzan P (2009): Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 39: 1763–1777. [DOI] [PubMed] [Google Scholar]
- 50.Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S (2013): Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 37: 1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vita A, De Peri L, Deste G, Barlati S, Sacchetti E (2015): The Effect of Antipsychotic Treatment on Cortical Gray Matter Changes in Schizophrenia: Does the Class Matter? A Meta-analysis and Meta-regression of Longitudinal Magnetic Resonance Imaging Studies. Biol Psychiatry. 78: 403–412. [DOI] [PubMed] [Google Scholar]
- 52.Kay SR, Fiszbein A, Opler LA (1987): The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr Bull. 13: 261–276. [DOI] [PubMed] [Google Scholar]
- 53.Andreasen NC (1984): Scale for the Assessment of Negative Symptoms: SANS. Iowa City: University of Iowa. [Google Scholar]
- 54.Andreasen N (1984): The scale for the assessment of positive symptoms (SAPS). Iowa City: University of Iowa. [Google Scholar]
- 55.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 56.Viechtbauer W (2010): Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 36. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 57.Benjamini Y H H (1995): Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. JR Stat Soc Series B Stat Methodol. 57: 289–300. [Google Scholar]
- 58.Thompson PM, Bartzokis G, Hayashi KM, Klunder AD, Lu PH, Edwards N, et al. (2009): Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb Cortex. 19: 1107–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011): Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 68: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ansell BRE, Dwyer DB, Wood SJ, Bora E, Brewer WJ, Proffitt TM, et al. (2015): Divergent effects of first-generation and second-generation antipsychotics on cortical thickness in first-episode psychosis. Psychol Med. 45: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hedman AM, van Haren NEM, van Baal GCM, Brouwer RM, Brans RGH, Schnack HG, et al. (2016): Heritability of cortical thickness changes over time in twin pairs discordant for schizophrenia. Schizophr Res. 173: 192–199. [DOI] [PubMed] [Google Scholar]
- 62.Lieberman JA (2005): Antipsychotic Drug Effects on Brain Morphology in First-Episode Psychosis. Arch Gen Psychiatry. 62: 361. [DOI] [PubMed] [Google Scholar]
- 63.Vernon AC, Crum WR, Lerch JP, Chege W, Natesan S, Modo M, et al. (2014): Reduced cortical volume and elevated astrocyte density in rats chronically treated with antipsychotic drugs-linking magnetic resonance imaging findings to cellular pathology. Biol Psychiatry. 75: 982–990. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi T, Wood SJ, Soulsby B, McGorry PD, Tanino R, Suzuki M, et al. (2009): Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr Res. 108: 49–56. [DOI] [PubMed] [Google Scholar]
- 65.Lee S-H, Niznikiewicz M, Asami T, Otsuka T, Salisbury DF, Shenton ME, McCarley RW (2016): Initial and Progressive Gray Matter Abnormalities in Insular Gyrus and Temporal Pole in First-Episode Schizophrenia Contrasted With First-Episode Affective Psychosis. Schizophr Bull. 42: 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi T, Wood SJ, Yung AR, Phillips LJ, Soulsby B, McGorry PD, et al. (2009): Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 111: 94–102. [DOI] [PubMed] [Google Scholar]
- 67.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci. 6: 309–315. [DOI] [PubMed] [Google Scholar]
- 68.Birnbaum R, Weinberger DR (2017): Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 18: 727–740. [DOI] [PubMed] [Google Scholar]
- 69.Chen EYH, Hui CLM, Lam MML, Chiu CPY, Law CW, Chung DWS, et al. (2010): Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ. 341: c4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tiihonen J, Walhbeck K, Lönnqvist J, Klaukka T, Ioannidis JPA, Volavka J, Haukka J (2006): Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 333: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hibar DP Westlye LT Doan NT Jahanshad N Cheung JW Ching CRK Versace A Bilderbeck AC Uhlmann A Mwangi B Kramer B Overs B Hartberg CB Abe C Dima D Grotegerd D Sprooten E Boen E Jimenez E Howells FM (2017): Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmaal L Hibar DP Samann PG Hall GB Baune BT Jahanshad N Cheung JW van Erp TGM (2017): Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


