Abstract
Background
Error-related brain activities are altered in individuals with substance use disorders. Here we examined error-related activities in relation to problem drinking in nondependent alcohol drinkers. In particular, we investigated sex differences and whether altered error responses are related to post-error behavioral control.
Methods
A sample of 145 non-dependent drinkers (77 women) performed the stop signal task during fMRI. Imaging data were processed and modeled using statistical parametric mapping. Independent sample t-test and linear regression were employed to examine sex differences in error response and relationship between error response and problem drinking.
Results
Compared to men, women showed greater error-related (stop error > go success) activations in bilateral thalamus, right middle/superior temporal cortex and bilateral dorsal anterior cingulate cortex. In whole-brain linear regression of error responses against the Alcohol Use Disorder Identification Test (AUDIT) score, a wide swath of cortical and subcortical regions, including the thalamus, showed decreased activation in association with problem drinking in women but not in men. However, men and women were not different in the extent of post-error slowing (PES) and decreased thalamic error response in association with problem drinking was not related to the extent of PES in women.
Conclusions
The results suggest sex differences in error-related activations with heavier drinking associated with reduced error activations in women but not in men. These differences in cerebral activations may reflect higher physiological arousal in response to errors and greater vulnerability of saliency-related arousal response to problem drinking in female as compared to male social drinkers.
Keywords: gender, cognitive control, imaging, thalamus, error detection, drinking problem
1. Introduction
Error-related cerebral and behavioral responses are an important phenotype of various neuropsychiatric conditions including substance use disorders (1). Compared with non-drug users, chronic cannabis users showed diminished error-correcting behavior and error response in the dorsal anterior cingulate cortex and left hippocampus in a paired associative learning task (2). Similar findings were also documented for cocaine addicted individuals in behavioral paradigms of response inhibition (3-5). Blunted left middle frontal cortical activations to commission errors in a go/no-go task predicted early substance use in adolescents (6). Together, these findings associate deficient error monitoring and error-related behavioral adjustment with substance misuse.
Whereas the findings on illicit substance users appear to be largely consistent, the literature is mixed for alcohol misuse. For instance, regional activations to no-go errors distinguished heavy and light non-dependent drinkers, each engaging predominantly visually-driven emotional and task control neural networks (7). Alcohol dependence and higher alcohol consumption is associated with less post-error behavioral adjustment in a stop signal task (8, 9). In contrast, some studies failed to identify differences in error-related brain response or in post-error behavioral adjustment in heavy as compared to light drinkers (10). In fact, alcohol dependent individuals appeared to demonstrate higher error-related responses as compared to controls (11, 12). An earlier review highlighted the complexity in employing interference control as a cognitive phenotype as a severity marker of alcohol misuse, with many studies revealing negative findings (13). However, what exactly accounted for the discrepancy in findings remained unclear. In particular, sex differences were often not considered in these studies.
Men and women demonstrate important differences in their drug and alcohol use behaviors, clinical profiles of substance and alcohol use disorders (14-21) as well as the risk factors and consequences of problem drinking (22). The biological bases of these sex differences have been a focus of active research and studies of brain imaging aimed to understand how men and women respond differently to psychological challenges of importance to habitual drug and alcohol use. For instance, stress may precipitate drug and alcohol use via distinct neural processes in men and women, with women more vulnerable to the influence of negative affect and stress (23-25). In support, stress induced by personalized guided imagery elicited sex-specific cerebral responses (26, 27). More broadly, men and women engaged distinct neural circuits in affective processing (28); women but not men showed correlated activations of bilateral caudate head and left thalamus during experience of social conflicts (29), a potential risk factor for problem drinking. Of direct relevance to the current study, men and women exhibited differences in error-related responses, with women showing higher electrophysiological or blood oxygenation level dependent (BOLD) responses to errors as compared to men (30, 31). On the other hand, men and women did not appear to differ in the extent of post-error behavioral control (32, 33). Thus, error-related responses may relate to problem drinking differently in men and women but whether these differences manifest in altered post-error behavioral control remains an open question.
In the current study, we investigated how problem drinking is associated with error-related cerebral responses and whether men and women differ in error-related response, and in its association with problem drinking and deficits in cognitive control. We combined fMRI and the stop signal task (SST) to address these questions. In the SST participants are required to respond to a frequent go signal and withhold response to an occasional stop signal. Participants showed higher skin conductance response, reflecting higher physiological arousal, to stop error as compared to both go and stop success trials (34, 35). Further, participants typically slowed down in response in go trials following a stop error trial as compared to those following another go trial (36-38). Post-error slowing reflects behavioral adjustment, a critical component process of cognitive control frequently compromised in individuals with substance use disorders (2-5, 9). Thus, the SST would provide an opportunity to investigate whether error-related cerebral responses are associated with problem drinking and deficits in cognitive control.
2. Methods
2.1 Subjects, Informed Consent, and Assessment
Study participants were recruited with newspaper and radio advertisements as well as flyers posted in the greater New Haven area. All participants were free of major medical illness, past or present neurological and psychiatric illnesses including substance use disorders (SCID-I for DSM-IV; (39)), denied current use of illicit substance, and showed negative urine toxicology tests for stimulants, opioids, marijuana, and benzodiazepines at the time of MRI. Individuals who were using any psychotropic medications were not invited to participate in the study. Pregnant or lactating women were also excluded. Participants were further required to be free of MRI-contraindications per Yale Magnetic Resonance Research Center's safety guidelines.
One hundred and forty-five social drinkers (77 women; age 31 ± 13 years; all right-handed) were included in this study. This cohort is a subsample of the 158 (86 women) social drinkers reported in our recent work (40), where 13 subjects participated in fMRI of a different behavioral task. All participants completed questionnaires regarding alcohol use over the past year, including average number of days of drinking and average number of drinks consumed per occasion, framed on a monthly basis, as well as the Alcohol Use Disorders Identification Test (AUDIT) (41). AUDIT score was calculated by summing subscores of ten self-report questions regarding level of alcohol use, alcohol-related problems, and concern expressed by others for one's drinking behavior. Each question receives a score ranging from 0 to 4, with higher numbers corresponding to a greater level of risk for having or developing an alcohol use disorder. The mean (± SD) AUDIT score was 5.1 (± 4.2) across all subjects, in a range typical of non-dependent drinkers and significantly lower than those reported for alcohol dependent individuals (42).
Participants were also assessed with the Barratt Impulsivity Scale (BIS-11; (43)) and Alcohol Expectancy Questionnaire (AEQ-3; (44)). BIS-11 is a 30-item self-report questionnaire designed to measure impulsivity. All items are scored on a 4-point scale (1 = rarely/never; 2 = occasionally; 3 = often; 4 = almost always/always). The total score thus ranges from 30 to 120, with a higher score indicating higher impulsivity. Eleven of the 30 items are reverse scored to avoid response bias. The AEQ-3 consisted of 40 items to address both positive (6 subscales) and negative (2 subscales) alcohol expectancy, confirmed by factor analysis and invariant across sex and race. Each subscale contains 4 to 6 statements that can be endorsed on a six-point scale, from “disagree strongly (1)” to “agree strongly (6)”. Despite the 8 factors identified and confirmed with factor analysis, ratings on the 8 factors are highly correlated (George et al, 1995). Thus, the authors acknowledged that the discriminant validity among the 8 subscores was at best moderate. Here, we used the global positive subscore to represent alcohol expectancy. A summary of demographic and clinical measures is presented in Table 1.
Table 1.
Demographics and drinking measures.
| Age (years) | AUDIT score | Years drinking | #Drinks /month | AE score | BIS score | |
|---|---|---|---|---|---|---|
| All (n = 145) | 31.5 ± 12.5 | 5.1 ± 4.2 | 13.6±11.6 | 16.9 ± 17.9 | 10.9 ± 4.1 | 60.7±9.0 |
| Women (n = 77) | 30.0 ± 11.6 | 4.2 ± 3.6 | 11.8±10.8 | 13.4 ± 15.0 | 10.6 ± 4.1 | 59.1±9.2 |
| Men (n = 68) | 33.1 ± 13.4 | 6.1 ± 4.6 | 15.6±12.3 | 20.9 ± 20.1 | 11.2 ± 4.2 | 62.5±8.4 |
| p-value * | 0.1437 | 0.0061 | 0.0508 | 0.0122 | 0.4348 | 0.0221 |
Note:
two-tailed two-sample t test; AUDIT: Alcohol Use Disorder Identification Test; AE: alcohol expectancy (global positive); BIS: Barratt Impulsivity Scale.
All subjects signed a written informed consent, in accordance to a protocol approved by the Yale Human Investigation Committee, prior to the study.
2.2 Behavioral task
We employed a simple reaction time task in this stop-signal paradigm (36, 45-51). There were two trial types: “go” and “stop,” randomly intermixed in presentation each with a probability of 0.75 and 0.25, and with an inter-trial interval of 2 s. A small dot appeared on the screen to engage attention at the beginning of a go trial. After a randomized time interval between 1 and 5 s, drawn from a uniform distribution, the dot turned into a circle (the “go” signal), prompting the subjects to quickly press a button. The circle vanished at a button press or after 1 s has elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. In a stop trial, an additional “X,” the “stop” signal, appeared after and replaced the go signal, and instructed participants to withhold their response. Similar to go trials, a stop trial terminated at button press or 1 s after the appearance of the stop signal. Failure to withhold the response for the 1 s constituted a stop error. The stop signal delay (SSD) – the time interval between go and stop signals – started at 200 ms and was adjusted according to a staircase procedure, increasing and decreasing by 67 ms each after a successful and failed stop (52). Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up occasionally. The staircase procedure ensures that subjects would succeed in withholding their response in approximately half of the stop trials.
2.3 Analyses of behavioral performance in the stop signal task
We computed a critical SSD that represents the time delay between go and stop signals that a subject would need to succeed in 50% of the stop trials (52). Specifically, SSDs across trials were grouped into runs, with each run defined as a monotonically increasing or decreasing series. We derived a mid-run estimate by taking the median SSD of every second run. The critical SSD was computed by taking the mean of all mid-run SSDs. It was reported that, except for experiments with a small number of trials (less than 30), the mid-run estimate was close to the maximum likelihood estimate of X50 (50% positive response; i.e., 50% stop success in the SST, (53)). The stop signal reaction time (SSRT) was computed by subtracting the critical SSD from the median go trial RT (54).
It is known that in the SST the RT of a correct response is prolonged following a stop-error trial, compared with other correct responses, and this prolonged RT is thought to reflect error-detection and conflict monitoring (Hendrick et al., 2010). We thus compared the RT of the go trials that followed a stop-error trial and those that followed another go trial, and termed the effect size of this RT difference “post-error slowing” (PES) (11).
2.4 Image acquisition, preprocessing and statistical tests
All imaging data were collected in the same 3T Siemens Trio scanner (Erlangen, Germany) while subjects performed the SST, as described in detail in our previous work. Each scan comprised four 10-minute runs of the SST. Caffeine-using subjects were allowed drink coffee or other caffeinated beverages until 30 minutes before MRI. Functional blood oxygenation-level dependent (BOLD) signals were acquired with a single-shot gradient echo echo-planar imaging sequence, with 32 axial slices parallel to the AC-PC line covering the whole brain, using published parameters: TR=2000 ms, TE=25 ms, bandwidth=2004 Hz/pixel, flip angle=85°, FOV=220×220 mm2, matrix=64×64, slice thickness=4 mm and no gap. A high-resolution 3D structural image (MPRAGE; 1 mm resolution) was also obtained for anatomical co-registration.
Functional MRI data was preprocessed with Statistical Parametric Mapping 12 (SPM12) (Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each trial were discarded so only BOLD signals in a steady state were included in analyses. Images of each individual subject were first corrected for slice timing, realigned (motion-corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. The anatomical images (T1-weighted) were co-registered to the mean functional image, and normalized to an MNI (Montreal Neurological Institute) template with affine registration followed by nonlinear transformation using a unified segmentation and registration approach (55). The normalization parameters determined for the anatomical volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum. Images from the first five TRs at the beginning of each session were discarded so only signals with steady-state equilibrium between radio frequency pulsing and relaxation were included in data analyses.
We distinguished four trial outcomes in this event-related study: go success (GS), go error, stop success (SS), and stop error (SE), and modeled BOLD signals by convolving the onsets of the go signals of each trial with a canonical hemodynamic response function (HRF) and the temporal derivative of the canonical HRF. Realignment parameters in all six dimensions were entered in the model. We included the following variables as parametric modulators in the model: RT of GS trials, SSD of SS trials, and SSD of SE trials, in that order. Serial autocorrelation of the time series was corrected by a first degree autoregressive or AR(1) model. The data were high- pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts. Group analyses were performed also using SPM12 on the error contrast maps (SE>GS) using one and two sample t-tests and multiple regressions, with age as covariate (56). Specifically, we employed a whole-brain regression against AUDIT score to examine regional responses to errors in association with problem drinking for men and women separately. All group analyses were examined with a threshold of voxel p<0.001 uncorrected in combination with cluster p<0.05, FWE corrected, according to current reporting standards (57).
3. Results
3.1 Clinical assessment and behavioral performance
As shown in Table 1, men showed higher AUDIT score, BIS score and average monthly number of drinks in the prior year, compared to women. In linear regression, the AUDIT score was correlated with alcohol expectancy score in men and women combined (r=0.52, p=2.39e-11), as well as in men (r=0.45, p=0.0001) and women (r=0.60, p=7.54e-09) separately. The AUDIT score was also correlated with BIS score in men and women combined (r=0.29, p=0.0003) and in men (r=0.40, p=0.0007), but not in women (r=0.13, p=0.2770) alone.
Behavioral performance in the SST is summarized in Table 2. Men and women did not differ in any aspects of task performance, including the extent of post-error slowing (PES). The effect size of the PES was correlated negatively with AUDIT score in men (r=-0.271, p=0.0255), but not in women (r=0.113, p=0.3300) or in men and women combined (r=-0.097, p=0.2440).
Table 2.
Performance in the stop signal task.
| SSRT (ms) | Median go RT (ms) | % GS | % SS | PES (effect size) | |
|---|---|---|---|---|---|
| Women (n = 77) | 217 ± 47 | 629 ± 89 | 91.1 ± 9.4 | 52.3 ± 3.9 | 2.2 ± 1.4 |
| Men (n = 68) | 224 ± 38 | 648 ± 97 | 90.2 ± 9.3 | 52.5 ± 4.0 | 1.9 ± 1.2 |
| p-value * | 0.34 | 0.21 | 0.54 | 0.66 | 0.16 |
Note: All values are mean ± standard deviation; SSRT: stop signal reaction time; RT: reaction time; % GS: percentage of go success trials (RT < 1 s); % SS: percentage of stop success trials; PES: post-error slowing.
p-value based on 2-tailed 2-sample t-test.
3.2 Error-related responses and sex differences
We contrasted stop error and go success trials in a one-sample t-test, which showed higher activations in bilateral frontal and parietal structures, medial frontal cortex, thalamus, bilateral insula and cerebellum (Figure 1A and Table 3). In a two-sample t-test we compared men and women in error-related responses. Compared to men, women showed higher activities in the thalamus, middle/superior temporal gyrus, and dorsal anterior cingulate cortex (Figure 1B and Table 4). In contrast, no brain regions showed higher activations in men as compared to women when examined at the same threshold.
Figure 1.
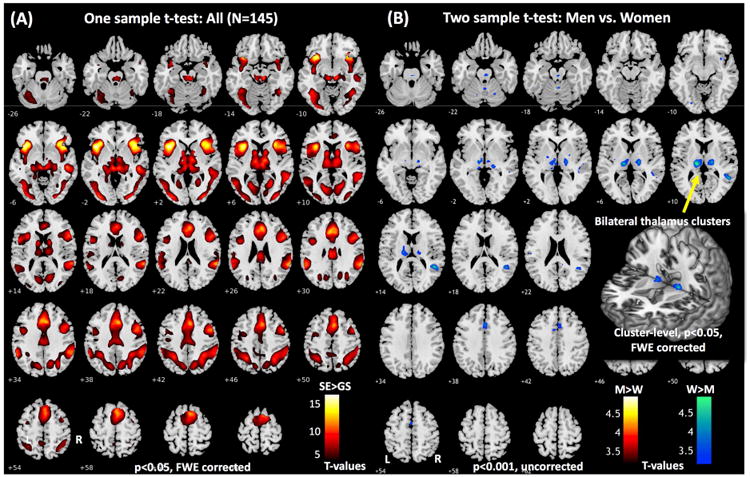
(A) Brain regions responding to error: stop error > go success (p<0.05, FWE corrected). (B) Regions showing greater error-related activations in women as compared to men (p<0.001, uncorrected). The inset highlights the bilateral thalamus clusters. Clusters that met the corrected extent threshold p<0.05 FWE are shown in Tables 3 and 4.
Table 3.
Brain regions showing error-related activations in the stop signal task. One sample t-test, p<0.05, FWE corrected. Cluster k>100. All peak voxels 8 mm apart are identified.
| Volume voxels (k) | Peak voxel Z | MNI coordinate | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 3898 | 17.03 | -40 | 18 | 0 | L | Insula |
| 15.74 | -32 | 20 | -8 | L | Insula | |
| 10.76 | -50 | 10 | 4 | L | Inferior frontal cortex | |
|
| ||||||
| 4786 | 15.46 | 40 | 20 | -2 | R | Insula/ Inferior frontal cortex |
| 15.15 | 32 | 24 | -6 | R | Insula/ Inferior frontal cortex | |
| 11.71 | 48 | 4 | 42 | R | Precentral gyrus | |
|
| ||||||
| 7040 | 13.48 | 60 | -42 | 22 | R | Superior temporal gyrus |
| 11.60 | 52 | -42 | 34 | R | Supramarginal gyrus | |
| 11.60 | 50 | -28 | -4 | R | Middle temporal gyrus | |
|
| ||||||
| 7481 | 13.46 | -4 | 24 | 32 | L | ACC/preSMA |
| 13.19 | 6 | 22 | 36 | R | ACC/preSMA | |
| 13.05 | 4 | 32 | 26 | R | ACC/preSMA | |
|
| ||||||
| 6136 | 11.82 | -6 | -26 | -4 | L | Thalamus |
| 11.30 | 8 | -24 | -12 | R | Thalamus | |
| 11.22 | 10 | -12 | 6 | R | Thalamus | |
|
| ||||||
| 6341 | 11.40 | -56 | -46 | 34 | L | Supramarginal gyrus |
| 11.40 | -32 | -90 | -2 | L | Middle occipital cortex | |
| 10.90 | -28 | -54 | 44 | L | Inferior parietal cortex | |
ACC: anterior cingulate cortex; preSMA: pre-supplementary motor area
Table 4.
Regions with higher error-related responses in women as compared to men (p<0.001, uncorrected, k>100 voxels). All voxel peaks 8mm apart are shown.
| Volume voxels (k) | Peak voxel Z | MNI coordinate | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 951 | 4.72 | -16 | -20 | 10 | L | Thalamus* |
| 4.15 | -10 | -14 | -2 | L | Thalamus* | |
| 4.13 | 18 | -18 | 8 | R | Thalamus* | |
|
| ||||||
| 947 | 4.45 | 48 | -48 | 14 | R | Middle temporal cortex* |
| 3.47 | 44 | -40 | 4 | R | Middle temporal cortex* | |
| 3.27 | 46 | -28 | 2 | R | Superior temporal cortex* | |
|
| ||||||
| 114 | 3.89 | 2 | 18 | 40 | R | dACC |
| 3.36 | -8 | 6 | 44 | L | dACC | |
Note:
indicates clusters with a significant voxel p<0.05, FWE corrected. dACC: dorsal anterior cingulate cortex.
To examine whether error-related activations are related to PES across subjects, we conducted a voxel-wise linear regression against PES with age as a covariate for men and women combined and for men and women separately. The results showed that, at the same threshold, no clusters were significantly correlated with PES in any of the three samples of participants.
3.3 Error-related responses in association with AUDIT score
In a whole-brain linear regression against AUDIT score, with age as a covariate, we examined how error-related responses are related to problem drinking. At the threshold of voxel p<0.001, uncorrected and cluster p<0.05 FWE, no clusters showed significant correlations with AUDIT score in men and women combined. We performed the same analyses for men and women separately. At the same threshold, no clusters were significantly correlated with AUDIT in men; in contrast, a number of cortical and subcortical structures, including the thalamus, showed negative correlation with AUDIT score in women (Figure 2 and Table 5). In a slope test (58), the correlations between error-related response of the thalamus and AUDIT score, as well as average monthly number of drinks were significantly different between men and women (Figure 3A and 3B). Notably, at this threshold, the thalamic cluster showing negative correlation with AUDIT score did not appear to overlap with the cluster showing greater error-related activation in women as compared to men.
Figure 2.
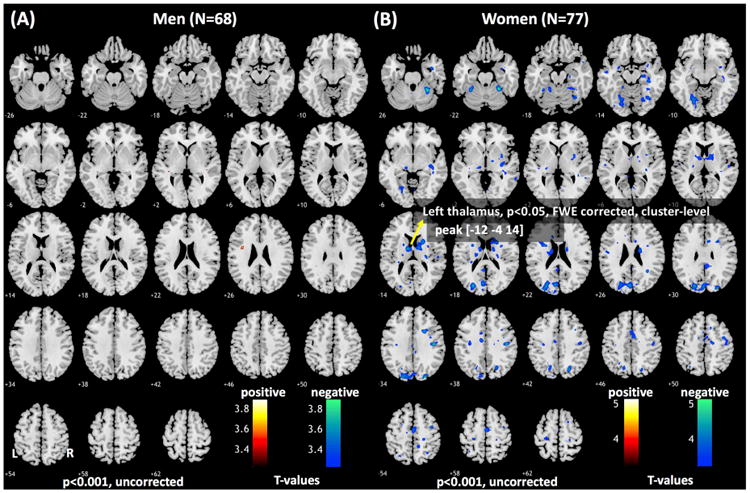
Error-related regional activations in correlation with AUDIT score in (A) men and (B) women (p<0.001, uncorrected). Clusters that met the corrected extent threshold are shown in Table 5.
Table 5.
Brain regions showing error-related response in correlation with AUDIT score in women. Multiple regression, p<0.001, uncorrected in combination with cluster p<0.05 FWE corrected (k>100). All peak voxels 8 mm apart are identified.
| Volume voxels (k) | Peak voxel Z | MNI coordinate | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 192 | 4.73 | 28 | -44 | -24 | R | Fusiform gyrus |
| 3.56 | 40 | -48 | -16 | R | Fusiform gyrus | |
| 3.46 | 20 | -42 | -16 | R | Lingual gyrus | |
|
| ||||||
| 225 | 4.62 | 44 | -18 | 36 | R | Postcentral gyrus |
| 4.07 | 40 | -4 | 52 | R | Precentral gyrus | |
| 3.64 | 46 | -16 | 48 | R | Postcentral/precentral gyrus | |
|
| ||||||
| 889 | 4.41 | -26 | -82 | 20 | L | Middle occipital gyrus |
| 4.31 | -20 | -82 | 32 | L | Superior occipital gyrus | |
| 4.23 | -6 | -78 | 24 | L | Cuneus | |
|
| ||||||
| 650 | 4.41 | 22 | 10 | 16 | R | Caudate, putamen |
| 4.21 | -12 | -4 | 14 | L | Thalamus, caudate | |
| 3.97 | 12 | -2 | 12 | R | Thalamus, caudate | |
|
| ||||||
| 245 | 4.06 | -22 | -66 | -12 | L | Lingual gyrus |
| 3.99 | -20 | -74 | -12 | L | Lingual gyrus | |
| 3.66 | -26 | -58 | -12 | L | Fusiform gyrus | |
|
| ||||||
| 191 | 3.96 | 36 | -28 | -14 | R | Hippocampus, fusiform gyrus |
| 3.83 | 34 | -18 | -4 | R | Insula, putamen | |
| 3.72 | 40 | -32 | -4 | R | Middle temporal gyrus | |
|
| ||||||
| 192 | 3.88 | 2 | -10 | 56 | R | Supplementary motor area |
| 3.62 | 6 | 0 | 46 | R | Anterior cingulate cortex | |
| 3.46 | 0 | 10 | 46 | R/L | Pre-supplementary motor area | |
Figure 3.
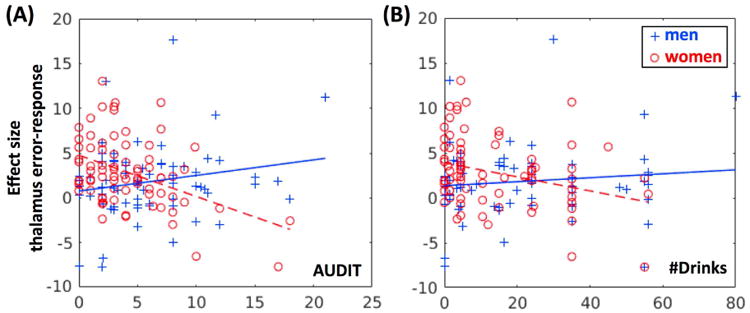
Slope tests confirmed sex differences in the correlation of thalamic error-related response and drinking variables. (A) Effect size vs. AUDIT (men: r=0.20 and p=0.1091, women: r=-0.43, p=0.0001; slope test: t=4.05; p=8.3e-05). (B) Effect size vs. average monthly number of drinks (men: r=0.11 and p=0.3781, women: r=-0.30, p=0.0071; slope test: t=2.64; p=0.0092).
4. Discussion
4.1 Sex differences in error-related activations
Consistent with a recent meta-analytic review (59), women showed higher error-related activations as compared to men in the thalamus, middle/superior temporal gyrus, and dorsal anterior cingulate cortex (dACC). The thalamus and dACC respond to salient stimuli, likely via noradrenergic projections from the midbrain (60, 61). However, women and men did not differ in the extent of post-error slowing and none of the regional activations to errors were related to the extent of post-error slowing either in women or in men, or in women and men combined. These findings suggested that increased error-related activities may not reflect a mechanism in women to support post-error behavioral control but simply higher saliency and/or physiological arousal elicited by outcome discrepancy (59, 62). In support, increased thalamic responses were also reported to uncued electric shock in contrast to cued safe condition in female but not male patients with irritable bowel syndrome (63) and to conditioning cues of painful rectal extension in female but not male healthy participants (64). A rodent study similarly demonstrated greater thalamic and dACC response to visceral pain in female than in male rats (65). In a study of the effects of stress on fear conditioning, administration of hydrocortisone reduced differential thalamic responses to conditioned and unconditioned stimulus in men but enhanced the responses in women (66). In resting state functional connectivity of the anterior insula, a core structure of the salience processing network, women but not men showed right-lateralized connectivity to the thalamus (67). Together, these studies support increased thalamic responses to error-related saliency in women as compared to men.
More broadly, saliency response is supported by the midbrain noradrenergic system (60, 61). Animal studies have documented the cellular and molecular mechanisms involving the locus coeruleus (LC) and noradrenergic signaling that underlie sex differences in hyperarousal and other stress-related physiological responses (68, 69). For instance, there were important sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the LC (70, 71). Further, hyperarousal has been attributed to corticotropin-releasing factor (CRF) regulation of LC activity, with females particularly affected by conditions resulting in elevated CRF receptor trafficking and LC discharge rate (68). Heightened LC activity and saliency response may contribute to the increased incidence of stress-related psychiatric disorders in females. More studies are needed to directly investigate the role of the LC and saliency circuit in error-elicited stress response and sex differences in the saliency circuit functions.
4.2 Error-related activations and problem drinking
In women but not in men, AUDIT score was correlated with decreased thalamic and medial prefrontal cortical activations. In the context of thalamic response to saliency, this finding suggested that problem drinking is associated with less thalamic activation to saliency in women. Alcohol consumption is known to impair detection of performance errors in the dACC (72). Another study examined the effects of emotional distracters on working memory performance (73). Although the study did not focus on sex differences, compared to participants with attention deficit hyperactive disorder, individuals with alcohol dependence were less vulnerable to the intrusion of salient emotional stimuli. In an event-related potential study heavy drinkers demonstrated reduced P3 amplitude to visual oddball stimuli (Chen et al., 2007). A more recent study showed that alcohol dampened functional connectivity between the bilateral anterior insula and the dACC, with greater reduction in right insula-dACC connectivity associated with calmer subjective experience (74). The current findings suggest that, compared to men, women are more vulnerable to the influence of problem drinking in cerebral responses to salient stimuli. An alternative explanation is that diminished saliency response may represent a more significant risk to problem drinking in women than in men.
Thalamic medial prefrontal cortical circuits are critical to performance monitoring (36, 37, 75). However, here, diminished thalamic and dACC responses to errors were not associated with changes in post-error behavioral adjustment. One possibility is that women were able to elicit reserve mechanisms to compensate for the disruption in saliency response in cognitive control. Interestingly, a closer examination showed that problem drinking is associated with decreased thalamic activity in the ventral and posterior lateral nuclei and the pulvinar in women whereas, compared to men, women showed higher response in the area of dorsal medial and ventral anterior nuclei, according to a recent functional parcellation study of the thalamus (76). We speculate that, by engaging the frontal executive thalamic subregions to a greater extent, women may be better able to accommodate behavioral control despite diminished saliency related activities in the perceptual thalamus. Compared to women, men are more prone to risk taking and impulsive behavior. Here, men demonstrated higher BIS score, which was associated with elevated physiological arousal to salient stimuli (Zhang et al., 2015), and a significant correlation between BIS and AUDIT scores. Thus, although men overall showed less cerebral activations to errors, heavy-drinking men may be more impulsive with higher error response masking the influence of alcohol use. These considerations suggest the importance in considering personality traits, including impulsivity, and their interacting effects with alcohol consumption in understanding cerebral responses to cognitive challenges.
4.3 Limitations and conclusions
There are a few limitations to consider. First, we employed a convenience sample in the current study and did not perform a power analysis of the sample size needed to detect a correlation between error-related activities and post-error slowing. Thus, whether sex and drinking related differences in error response relate to post-error control remains to be clarified. Second, it is not clear from the cross-sectional findings whether decreased error-related responses reflect the effects of alcohol use or a neural process that may dispose women to heavier drinking. Further, an earlier study reported diminished error-related negativity in heavy as compared to light or non-drinkers but noted no sex differences (77). Thus, a prospective study involving more heavier-drinking individuals is needed to address these issues. In particular, men showed higher AUDIT and BIS score than women, and studies with more women with higher impulsivity and more severe drinking problems would clarify the sources and significance of the sex differences reported here. Another critical limitation concerns the lack of menstrual cycle information of female participants. Women are known to demonstrate behavioral and cerebral functional differences as hormones fluctuate between follicular and luteal phases (78, 79). More studies are needed to confirm and extend the current findings on sex differences. Finally, the current cohort comprised primarily light, social drinkers and the findings should be considered as specific to this population. Further, we did not assess family history of alcoholism, which is known to increase the risk of dysfunctional impulse control and alcohol drinking (80). Future work is warranted to address these issues and extend the findings.
In conclusion, women as compared to men showed higher thalamic cortical responses to errors and reduction in the error responses in association with problem alcohol use. The findings suggested sex differences in arousal elicited by salient events. More studies are required to determine whether this phenotype reflect sex differences in the influence of alcohol or in the neural processes disposing individuals to problem drinking.
Acknowledgments
This study was supported by NIH grants R01AA021449 (Li), K25DA040032 (Zhang), and P50AA12870 (Krystal). The study was also supported by the VA National Center for PTSD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or VA.
Footnotes
Financial Disclosures: JHK is cosponsor of a patent for the intranasal administration of ketamine for the treatment of depression that was licensed by Janssen Pharmaceuticals, the maker of S-ketamine. JHK has a patent related to the use of riluzole to treat anxiety disorders that was licensed by Biohaven Medical Sciences. JHK has stock or stock options in Biohaven Medical Sciences, ARett Pharmaceuticals, Blackthorn Therapeutics, Spring Health, and Luc Therapeutics. JHK consults broadly to the pharmaceutical industry, but his annual income over the past year did not exceed $5000 for any organization. JHK receives more than $5000 in income from the Society of Biological Psychiatry for editing Biological Psychiatry. The other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manoach DS, Agam Y. Neural markers of errors as endophenotypes in neuropsychiatric disorders. Front Hum Neurosci. 2013;7:350. doi: 10.3389/fnhum.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey SE, Nestor L, Jones J, Garavan H, Hester R. Impaired learning from errors in cannabis users: Dorsal anterior cingulate cortex and hippocampus hypoactivity. Drug Alcohol Depend. 2015;155:175–182. doi: 10.1016/j.drugalcdep.2015.07.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hester R, Bell RP, Foxe JJ, Garavan H. The influence of monetary punishment on cognitive control in abstinent onetary punishment on cognitive control in abstinent cocaine-users. Drug Alcohol Depend. 2013;133:86–93. doi: 10.1016/j.drugalcdep.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ide JS, Hu S, Zhang S, Mujica-Parodi LR, Li CS. Power spectrum scale invariance as a neural marker of cocaine misuse and altered cognitive control. NeuroImage Clinical. 2016;11:349–356. doi: 10.1016/j.nicl.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ide JS, Hu S, Zhang S, Yu AJ, Li CS. Impaired Bayesian learning for cognitive control in cocaine dependence. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, et al. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend. 2014;141:51–57. doi: 10.1016/j.drugalcdep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campanella S, Absil J, Carbia Sinde C, Schroder E, Peigneux P, Bourguignon M, et al. Neural correlates of correct and failed response inhibition in heavy versus light social drinkers: an fMRI study during a go/no-go task by healthy participants. Brain Imaging Behav. 2017;11:1796–1811. doi: 10.1007/s11682-016-9654-y. [DOI] [PubMed] [Google Scholar]
- 8.Bo R, Landro NI. Inhibitory control and response monitoring are not systematically related to weekly alcohol consumption in the general population. Psychopharmacology (Berl) 2017;234:1761–1768. doi: 10.1007/s00213-017-4578-9. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology (Berl) 2009;207:163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JL, Mattick RP, Sufani C. Error detection and behavioural inhibition in young heavy drinkers. Drug Alcohol Depend. 2017;171:20–30. doi: 10.1016/j.drugalcdep.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Li CsR, Luo X, Yan P, Bergquist K, Sinha R. Altered Impulse Control in Alcohol Dependence: Neural Measures of Stop Signal Performance. Alcoholism: Clinical and Experimental Research. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, Franken IH. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. Journal of psychiatry & neuroscience : JPN. 2014;39:149–169. doi: 10.1503/jpn.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA. Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev Neurosci. 2014;25:1–24. doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck KH, Thombs DL, Mahoney CA, Fingar KM. Social context and sensation seeking: gender differences in college student drinking motivations. Int J Addict. 1995;30:1101–1115. doi: 10.3109/10826089509055830. [DOI] [PubMed] [Google Scholar]
- 15.Brady KT, Randall CL. Gender differences in substance use disorders. The Psychiatric clinics of North America. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- 16.Derringer J, Krueger RF, Iacono WG, McGue M. Modeling the impact of age and sex on a dimension of poly-substance use in adolescence: a longitudinal study from 11- to 17-years-old. Drug Alcohol Depend. 2010;110:193–199. doi: 10.1016/j.drugalcdep.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenfield SF, Pettinati HM, O'Malley S, Randall PK, Randall CL. Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcoholism, clinical and experimental research. 2010;34:1803–1812. doi: 10.1111/j.1530-0277.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hensing G, Spak F. Introduction: gendering socio cultural alcohol and drug research. Alcohol Alcohol. 2009;44:602–606. doi: 10.1093/alcalc/agp073. [DOI] [PubMed] [Google Scholar]
- 19.Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcoholism, clinical and experimental research. 2004;28:1291–1298. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- 20.McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use disorders: I. Effects of gender and alcoholism subtype. Alcoholism, clinical and experimental research. 1997;21:513–520. [PubMed] [Google Scholar]
- 21.Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29:535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39:1527–1537. doi: 10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2012;21:210–220. doi: 10.1111/j.1521-0391.2012.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walitzer KS, Dearing RL. Gender differences in alcohol and substance use relapse. Clin Psychol Rev. 2006;26:128–148. doi: 10.1016/j.cpr.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Seo D, Ahluwalia A, Potenza MN, Sinha R. Gender differences in neural correlates of stress-induced anxiety. J Neurosci Res. 2017;95:115–125. doi: 10.1002/jnr.23926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li CS, Kemp K, Milivojevic V, Sinha R. Neuroimaging study of sex differences in the neuropathology of cocaine abuse. Gend Med. 2005;2:174–182. doi: 10.1016/s1550-8579(05)80046-4. [DOI] [PubMed] [Google Scholar]
- 28.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Shirao N, Okamoto Y, Okada G, Ueda K, Yamawaki S. Gender differences in brain activity toward unpleasant linguistic stimuli concerning interpersonal relationships: an fMRI study. Eur Arch Psychiatry Clin Neurosci. 2005;255:327–333. doi: 10.1007/s00406-005-0566-x. [DOI] [PubMed] [Google Scholar]
- 30.Fischer AG, Danielmeier C, Villringer A, Klein TA, Ullsperger M. Gender Influences on Brain Responses to Errors and Post-Error Adjustments. Sci Rep. 2016;6:24435. doi: 10.1038/srep24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moran TP, Taylor D, Moser JS. Sex moderates the relationship between worry and performance monitoring brain activity in undergraduates. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2012;85:188–194. doi: 10.1016/j.ijpsycho.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Li CS, Zhang S, Duann JR, Yan P, Sinha R, Mazure CM. Gender Differences in Cognitive Control: an Extended Investigation of the Stop Signal Task. Brain Imaging Behav. 2009;3:262–276. doi: 10.1007/s11682-009-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansouri FA, Fehring DJ, Gaillard A, Jaberzadeh S, Parkington H. Sex dependency of inhibitory control functions. Biol Sex Differ. 2016;7:11. doi: 10.1186/s13293-016-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, Hu S, Chao HH, Luo X, Farr OM, Li CS. Cerebral correlates of skin conductance responses in a cognitive task. Neuroimage. 2012;62:1489–1498. doi: 10.1016/j.neuroimage.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Hu S, Hu J, Wu PL, Chao HH, Li CS. Barratt Impulsivity and Neural Regulation of Physiological Arousal. PloS one. 2015;10:e0129139. doi: 10.1371/journal.pone.0129139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrick OM, Ide JS, Luo X, Li CS. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PloS one. 2010;5:e13155. doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. NeuroImage. 2011;54:455–464. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Ide JS, Zhang S, Hu S, Valchev NS, Tang X, et al. Distinct neural processes support post-success and post-error slowing in the stop signal task. Neuroscience. 2017;357:273–284. doi: 10.1016/j.neuroscience.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington DC: American Psychiatric Association; 1995. [Google Scholar]
- 40.Ide JS, Zhornitsky S, Hu S, Zhang S, Krystal JH, Li CR. Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. NeuroImage Clinical. 2017;14:750–759. doi: 10.1016/j.nicl.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babor T, Higgins-Biddle J, Saunders J, Monteiro M. AUDIT: The alcohol use disorders identification test. 2nd. Geneva: Department of Mental Health and Substance Dependence; 2001. [Google Scholar]
- 42.Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend. 2010;108:29–36. doi: 10.1016/j.drugalcdep.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 44.George WH, Frone MR, Cooper ML, Russell M, Skinner JB, Windle M. A revised Alcohol Expectancy Questionnaire: factor structure confirmation, and invariance in a general population sample. Journal of studies on alcohol. 1995;56:177–185. doi: 10.15288/jsa.1995.56.177. [DOI] [PubMed] [Google Scholar]
- 45.Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 47.Farr OM, Hu S, Zhang S, Li CS. Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. Neuroimage. 2012;63:1070–1077. doi: 10.1016/j.neuroimage.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ide JS, Li CSR. Error-Related Functional Connectivity of the Habenula in Humans. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li CS, Luo X, Sinha R, Rounsaville BJ, Carroll KM, Malison RT, et al. Increased error-related thalamic activity during early compared to late cocaine abstinence. Drug Alcohol Depend. 2010;109:181–189. doi: 10.1016/j.drugalcdep.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkler AD, Hu S, Li CS. The influence of risky and conservative mental sets on cerebral activations of cognitive control. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2012 doi: 10.1016/j.ijpsycho.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu S, Tseng YC, Winkler AD, Li CS. Neural bases of individual variation in decision time. Hum Brain Mapp. 2014;35:2531–2542. doi: 10.1002/hbm.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(2):467+. [PubMed] [Google Scholar]
- 53.Wetherill GB, Chen H, Vasudeva RB. Sequential estimation of quantal response curves: A new method of estimation. Biometrika. 1966;53:439–454. [Google Scholar]
- 54.Logan GD. On the ability to inhibit thought and action: A user's guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory and Language. San Diego: Academic Press; 1994. pp. 189–239. [Google Scholar]
- 55.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 56.Hu S, Chao HH, Winkler AD, Li CS. The effects of age on cerebral activations: internally versus externally driven processes. Frontiers in aging neuroscience. 2012;4:4. doi: 10.3389/fnagi.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zar JH. Biostatistical Analysis. Forth. New Jersey: Prentice-Hall, Inc; 1999. [Google Scholar]
- 59.Moser JS, Moran TP, Kneip C, Schroder HS, Larson MJ. Sex moderates the association between symptoms of anxiety, but not obsessive compulsive disorder, and error-monitoring brain activity: A meta-analytic review. Psychophysiology. 2016;53:21–29. doi: 10.1111/psyp.12509. [DOI] [PubMed] [Google Scholar]
- 60.Schneider M, Hathway P, Leuchs L, Samann PG, Czisch M, Spoormaker VI. Spontaneous pupil dilations during the resting state are associated with activation of the salience network. Neuroimage. 2016;139:189–201. doi: 10.1016/j.neuroimage.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Venkatraman A, Edlow BL, Immordino-Yang MH. The Brainstem in Emotion: A Review. Front Neuroanat. 2017;11 doi: 10.3389/fnana.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Compton RJ, Carp J, Chaddock L, Fineman SL, Quandt LC, Ratliff JB. Anxiety and error monitoring: increased error sensitivity or altered expectations? Brain Cogn. 2007;64:247–256. doi: 10.1016/j.bandc.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong JY, Naliboff B, Labus JS, Gupta A, Kilpatrick LA, Ashe-McNalley C, et al. Altered brain responses in subjects with irritable bowel syndrome during cued and uncued pain expectation. Neurogastroenterol Motil. 2016;28:127–138. doi: 10.1111/nmo.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benson S, Kattoor J, Kullmann JS, Hofmann S, Engler H, Forsting M, et al. Towards understanding sex differences in visceral pain: enhanced reactivation of classically-conditioned fear in healthy women. Neurobiol Learn Mem. 2014;109:113–121. doi: 10.1016/j.nlm.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Guo Y, Bradesi S, Labus JS, Maarek JI, Lee K, et al. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145:120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, et al. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35:33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Kann S, Zhang S, Manza P, Leung HC, Li CR. Hemispheric Lateralization of Resting-State Functional Connectivity of the Anterior Insula: Association with Age, Gender, and a Novelty-Seeking Trait. Brain Connect. 2016;6:724–734. doi: 10.1089/brain.2016.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, et al. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Molecular psychiatry. 2013;18:166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pendergast JS, Tuesta LM, Bethea JR. Oestrogen receptor beta contributes to the transient sex difference in tyrosine hydroxylase expression in the mouse locus coeruleus. J Neuroendocrinol. 2008;20:1155–1164. doi: 10.1111/j.1365-2826.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 71.Thanky NR, Son JH, Herbison AE. Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the locus coeruleus of TH9-LacZ transgenic mice. Brain Res Mol Brain Res. 2002;104:220–226. doi: 10.1016/s0169-328x(02)00383-2. [DOI] [PubMed] [Google Scholar]
- 72.Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, et al. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- 73.Marx I, Krause J, Berger C, Hassler F. Dissociable patterns in the control of emotional interference in adults with attention-deficit/hyperactivity disorder (ADHD) and in adults with alcohol dependence. PloS one. 2014;9:e107750. doi: 10.1371/journal.pone.0107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorka SM, Phan KL, Childs E. Acute calming effects of alcohol are associated with disruption of the salience network. Addict Biol. 2017 doi: 10.1111/adb.12537. [DOI] [PubMed] [Google Scholar]
- 75.Seifert S, von Cramon DY, Imperati D, Tittgemeyer M, Ullsperger M. Thalamocingulate interactions in performance monitoring. J Neurosci. 2011;31:3375–3383. doi: 10.1523/JNEUROSCI.6242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 77.Smith JL, Iredale JM, Mattick RP. Sex differences in the relationship between heavy alcohol use, inhibition and performance monitoring: Disconnect between behavioural and brain functional measures. Psychiatry research. 2016;254:103–111. doi: 10.1016/j.pscychresns.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 78.Bale TL, Epperson CN. Sex as a Biological Variable: Who, What, When, Why, and How. Neuropsychopharmacology. 2017;42:386–396. doi: 10.1038/npp.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hausmann M. Why sex hormones matter for neuroscience: A very short review on sex, sex hormones, and functional brain asymmetries. J Neurosci Res. 2017;95:40–49. doi: 10.1002/jnr.23857. [DOI] [PubMed] [Google Scholar]
- 80.Kareken DA, Dzemidzic M, Wetherill L, Eiler W, 2nd, Oberlin BG, Harezlak J, et al. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology (Berl) 2013;228:335–345. doi: 10.1007/s00213-013-3038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


