Abstract
The lung harbors a complex immune system composed of both innate and adaptive immune cells. Recognition of infection and injury by receptors on lung innate immune cells is crucial for generation of antigen-specific responses by adaptive immune cells. The extracellular matrix of the lung, comprising the interstitium and basement membrane, plays a key role in the regulation of these immune systems. The matrix consists of several hundred assembled proteins that interact to form a bioactive scaffold. This template, modified by enzymes, acts to facilitate cell function and differentiation and changes dynamically with age and lung disease. Herein, we explore relationships between innate and adaptive immunity and the lung extracellular matrix. We discuss the interactions between extracellular matrix proteins, including glycosaminoglycans, with prominent effects on innate immune signaling effectors such as toll-like receptors. We describe the relationship of extracellular matrix proteins with adaptive immunity and leukocyte migration to sites of injury within the lung. Further study of these interactions will lead to greater knowledge of the role of matrix biology in lung immunity. The development of novel therapies for acute and chronic lung disease is dependent on a comprehensive understanding of these complex matrix-immunity interactions.
Introduction
The lungs have a critical primary function in the human body through the exchange of oxygen and carbon dioxide. The maintenance of this physiological function is dependent on lung architecture, elastic recoil and mechanical stability, all related to the pulmonary extracellular matrix (ECM) [1]. However, air not only contains oxygen but also particulate matter, pollutants, and a variety of microbes, all of which may pose a significant threat to human health. Indeed, the human lung is exposed to a high level of exogenous threats through the inhalation of several thousand liters of air everyday [2].
A complex and highly effective lung immune system has evolved with mechanical barriers as well as both an initial first line of defense, termed innate immunity, and a second more specific adaptive immune system. These defenses include several barriers such as mucus, cilia and the mucociliary escalator, as well as proteins (e.g. collectins and defensins) with antimicrobial effects in the airway. Further physical barriers known to play key roles in immunity include the alveolar epithelium and vascular endothelium of the lung. The lung harbors a plethora of innate and adaptive immune cells, including alveolar macrophages, dendritic cells and circulating monocytes and lymphocytes, as well as a respiratory epithelium with major immune functions [3, 4]. The innate system also regulates features of the adaptive system including the activation of antigen specific immune responses in host defense [5]. The ECM of the lung plays a key role in the establishment and regulation of lung immunity. Plants and invertebrates manage solely with innate defense mechanisms [although some invertebrates have adaptive systems [6]]. The divergence of the vertebrate genome lineage from invertebrate heralded an expansion in ECM related genes [7]. The development of a complex adaptive immunity is postulated to have occurred after this expansion of ECM related genes [8]. Therefore, our complex human immune system has evolved through an intricate relationship with our tissue matrix.
In this review, we discuss the interaction between components of the pulmonary ECM and innate and adaptive lung immunity. We highlight known key interactions between innate immune receptors and molecules derived from the ECM, and the influence these interactions have on local inflammation, through cytokine/chemokine expression, and leukocyte recruitment using examples from both studies of systemic and pulmonary disease. We also discuss the role of ECM molecules in modulating the function of the adaptive immune system of the lung. This review emphasizes the important updates in recent years on the ECM’s role in pulmonary immunity but also recognizes the considerable progress still required to decipher these ECM-immunity interactions and their implications for disease mechanisms within the lung.
The ECM of the lung in health and disease
Our knowledge of ECM-immunity interactions is evolving. The composition of the pulmonary ECM had proven difficult to elucidate until recent progress in mass spectrometry and quantification algorithms [9, 10]. We know now that the ECM of the human lung consists of at least 150 different core structural proteins within 2 structures, the basement membrane and interstitial matrix [11]. The ECM proteins also engage with various enzymes, growth factors and other associated proteins which are non-structural.
In health, the interstitial matrix of the lung is a loose connective tissue meshwork comprised of both structural and non-structural content. Collagen constitutes the core protein; the composition of collagen in the lung is abnormally increased in several forms of lung disease [12]. These fibrillar collagens include several types (I, II, III, V, XI) and have considerable tensile strengths. The interstitial matrix also contains elastic fibers formed from a core of crosslinked elastin and an outer layer of microfibrils. Other components include fibrillins, glycoproteins (e.g. fibronectin), proteoglycans (e.g. versican) and glycosaminoglycans or GAGs (e.g. hyaluronan) [13]. A plethora of non-structural proteins, growth factors and cytokines are harbored within the interstitial matrix of the lung. Within the alveoli, the interstitial matrix is composed mainly of type I and type III collagen and elastin, with several other ECM proteins interspersed in the collagen and elastic fibers. The main cell types lining the alveolar spaces include type 1 and type 2 pneumocytes (see Fig. 1). This meshwork of ECM proteins and reservoir of bioactive molecules is built around a range of cell types including fibroblasts, pericytes, epithelial cells and resident leukocytes. The pulmonary blood vessels, capillary network and lymphatics further expose the matrix to large volumes of circulating leukocytes. The meshwork of the interstitial matrix is anchored to the basement membrane layer of the epithelium and endothelium in the alveoli, and is a relatively thin organized layer. Components of the basement membrane include type IV collagen, laminin, nidogen/entactin, and perlecan. While the matrix is minimal within the normal lung parenchyma, disease of the lung often results in gross abnormality with the aberrant deposition of ECM components that leads to significant physiological malfunction (See Fig. 1). In recent years, many interactions between specific components of pulmonary immunity and the lung ECM have been identified [14]. Matrix components such as hyaluronan and versican can act as ligands for innate receptors on resident and recruited leukocytes to regulate injury and inflammation, thereby promoting acute and chronic lung injury. To date most work describing ECM-immunity interactions has focused on interstitial matrix proteins and other components including proteoglycans; thus, this will be our focus. We do not describe in detail the role of the basement membrane components in regulating pulmonary immunity in this review; however, it should be noted that components of the basement membrane can become targets of immune responses to cause important lung diseases. For example, an autoimmune antibody response against type IV collagen α3 causes Goodpasture’s Syndrome[15]. Another example is the ability of type IV collagen and laminin to cause lymphocyte activation in Scleroderma patients[16].
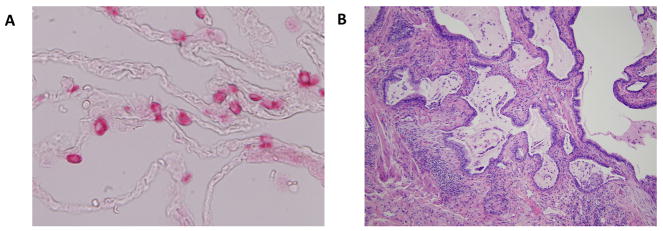
Fig. 1. The pulmonary ECM in health and disease.
Image A depicts the normal alveolar space at high power. The type 2 pneumocytes are stained pink. There is minimal matrix present and the alveolar epithelium is very thin allowing for ease of gas exchange. Image B depicts fibroblastic foci of IPF, with layering down of aberrant ECM and the loss of the normal alveolar epithelium leading to impaired gas exchange.
The pulmonary innate immune system and the ECM of the lung
A family of proteins termed pathogen recognition receptors (PRRs) can be expressed both by hematopoietic and non-hematopoietic cells and are important sensors to trigger innate immunity. These proteins function to sense and initiate a first line host defense response to invading pathogens and initial injury [17]. PRRs are germline encoded and at this time, the family of PRRs includes Toll-like receptors (TLRs), nucleotide-binding oligomerization domain receptors (NOD-like receptors or NLRs), C-type lectin receptors, retinoic acid inducible gene 1(RIG-1)-like receptors (RLRs) and cytosolic DNA receptors [18]. PRRs recognize highly conserved molecular motifs derived from microbes (pathogen associated molecular patterns – PAMPs). PAMPs include a broad range of microorganism-derived molecular patterns that are invariant, difficult to alter and vital for microbial physiology and lifecycle. They include lipopolysaccharide (LPS), peptidoglycan, flagellin and nucleic acids such as ssRNA, dsRNA, and CpG DNA. The full scope of engagement between PRRs and microbial ligands has not yet been fully determined. We now know that the innate immune response is not simply limited to the recognition of invasive pathogens but also acts in the sensing of danger signals derived from endogenous molecules. These molecules arise from cell death, stress and/or injury, and are so called damage associated molecular patterns (DAMPs). One of the first studies to denote immunostimulatory properties for endogenous mammalian derived products examined the effect of heat shock protein 60 (hsp60), a chaperone protein involved in protein synthesis. Treatment of human and murine macrophages with endogenous hsp60 resulted in the generation of pro-inflammatory cytokines [19]. Further studies have confirmed these findings and characterized interactions between DAMPs and innate immune receptors in disease (and are reviewed elsewhere [20, 21]). The ECM provides a reservoir of potential DAMPs for the innate immune system. These molecules are sequestered in the ECM in normal physiological conditions where interactions with PRR expressing innate immune cells are limited. However, changes to normal homeostasis that occur in disease, injury and inflammation alter these conditions, promote the recruitment of leukocytes into the matrix and facilitate engagements between ECM DAMPs and PRR-expressing leukocytes. In the next section, we describe relevant components of innate immunity in the lung.
Innate Immunity and the key role of PRRs
TLRs are a family of transmembrane proteins that play a key role in shaping the innate immune response to acute lung injury and inflammation. The receptor family includes TLR1-13 in mammals. First discovered in 1994 by Nomura et al, the name “toll” was derived from the protein’s similarity to the “toll” protein in the fruit fly (Drosophila melanogaster) [22, 23]. TLRs1-11 are conserved in humans and mice [24]. TLR3, TLR7, TLR8, TLR9, TLR11, TLR12 and TLR13 are expressed in intracellular compartments. TLR1, TLR2, TLR4, TLR5, TLR6 are all expressed at the cellular surface where they interact with different categories of ligands compared to intracellular receptors (see Fig. 2). TLR4 can demonstrate both cell surface and intracellular expression which allows alterations in receptor-ligand interactions and downstream signaling targets [25, 26]. There is an extensive range of TLR-PAMP/DAMP interactions studied to date (Fig. 2). Adaptor molecules, co-receptors and co-factors that interact with or modulate a specific subset of TLRs are reviewed elsewhere [27, 28]. TLR ligation ultimately results in translocation of transcription factors to the nucleus with subsequent changes in inflammatory profiles at cellular and tissue levels.
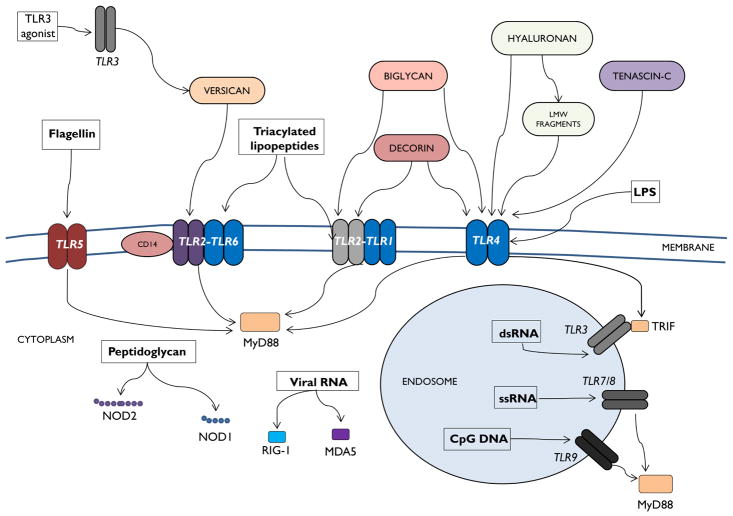
Fig. 2. Innate immune receptors and ECM molecule interactions.
Canonical ligand-pathogen recognition receptor (PRR) interactions in the lung are depicted in this schematic. TLRs, NLRs, cytosolic sensors and their canonical ligands are shown. In addition, known interactions between several ECM molecules and PRRs are also displayed.
In addition, several other PRRs exist including NLRs and the RNA-helicase-family of proteins which includes retinoic acid inducible gene 1 (RIG-1) and melanoma differentiation-associated gene 5 (MDA5) [29, 30]. NOD signaling is implicated in a number of pulmonary disorders including asthma and the systemic granulomatous disease, early onset sarcoidosis [31–33]. The cytokines elicited by innate receptor activation are also known to tune the resultant adaptive immune response.
As a biologically active scaffold the ECM has a key role to play in a wide variety of biological processes including host defense and tissue repair. Small leucine-rich proteoglycans (SLRPs) can exist in a sequestered form bound within the tissue matrix serving as master regulators of ECM assembly in general by binding collagen type I, II, III [34] and type VI [35]. They act to inactivate or in some cases localize certain biological processes by binding and sequestering molecules such as growth factors (e.g. biglycan, decorin and fibromodulin can all bind transforming growth factor (TGF)β within the matrix), thus limiting the abilities of these sequestered growth factors to interact with molecular targets [36, 37]. SLRPs may also exist in a soluble form in which they may display affinities for a wide variety of receptors [38–41]. SLRPs consist of a small protein core and tandem leucine-rich repeats (LRRs) flanked by cysteine rich clusters [42, 43]. Importantly, alterations in the composition of the ECM proteins including SLRPs but also other proteoglycans may arise during injury and inflammation and can result in activation of PRRs. ECM proteins have been implicated in the pathogenesis of pulmonary disorders including asthma, chronic pulmonary obstructive disease, pulmonary fibrosis, sepsis and acute lung injury [44–48]. Next, we discuss the current evidence supporting the key role innate immunity occupies in this context using examples of matrix components including proteoglycans, glycoproteins and GAGs as innate “danger” signals (see Fig. 2).
PRRs and Proteoglycans
Proteoglycans are composed of GAGs covalently linked to specific core proteins, and are major ECM components of most tissues including the lung. They form the hydrated gel in which other core components of the ECM (i.e collagen, elastin fibers) are embedded [49]. Proteoglycans are involved in the maintenance of the mechanical stability of the collagen-elastin network. Examples of proteoglycans include versican, biglycan and decorin but there are many proteoglycans within the lung and other tissues.
Versican is a chondroitin sulfate proteoglycan that exists in several different isoforms and plays a role in matrix assembly, tissue hydration and collagen fibrillation. While other proteoglycans contain LRRs, and interactions with LRR-containing TLRs may be expected, versican does not possess LRR motifs. Studies have shown that versican may act through ligation with TLR2/TLR6 heterodimers and the CD14 co-receptor on myeloid cells with downstream TNF-α and IL-6 production [50]. Versican is synthesized and released in inflammatory disorders by activated macrophages, where it may act as an endogenous ligand for TLRs expressed on the surface of innate immune cells [51]. Versican is also known to bind and interact with hyaluronan, and since hyaluronan can bind to the cell surface of leukocytes and smooth muscle cells, this interaction can lead to cellular adhesion during inflammation (e.g. in vascular walls) [52]. Furthermore, treatment with Poly(I:C), a synthetic TLR3 ligand, has previously been shown in vivo to stimulate hyaluronan and versican expression in lung fibroblasts[53]. Subsequent studies determined that versican was responsible for mediating inflammatory responses through the generation of hyaluronan-containing ECM cable structures that provided a substrate for monocytic cell infiltration into the inflamed lung [54]. Versican−/− mice had markedly reduced inflammatory cell numbers in bronchoalveolar fluid (BALF), reduced inflammatory cytokine expression and an absence of these hyaluronan-enriched ECM cable structures. Therefore, the evidence supports a key role for versican in regulating inflammatory signaling within the lung interstitial matrix through interactions with PRRs. Versican has been implicated in several forms of human lung disease. Versican deposition has been reported in hallmark fibrogenic lesions of idiopathic pulmonary fibrosis (IPF) patients [55]. Several studies have demonstrated a role for versican in pulmonary disorders and have been reviewed elsewhere [56].
Biglycan is a widely expressed proteoglycan that maps to the X chromosome [57]. It has an assembly role in the ECM, and disruption of the biglycan gene results in abnormal collagen fibril morphology [58]. Degradation of the ECM by proteolytic enzymes occurs in injury and stress, releasing soluble biglycan which is then capable of engaging TLRs with resultant inflammatory signaling. When bound to ECM, biglycan is unable to ligate TLRs. Schaeffer et al. described a novel role for biglycan as an endogenous ligand for TLR2 and TLR4 when expressed on the surface of macrophages leading to activation of transcription factors and TNF-α release [48]. Biglycan−/− (null) mice demonstrate a survival benefit after LPS challenge as a result of reduced inflammatory cytokine production including TNF-α and the stimulatory properties of biglycan are abolished in TLR4−/−/TLR2−/− murine macrophages [48]. Biglycan has an important role in IL-1β generation during the innate immune response as well [59]. In summary, biglycan plays a crucial role in orchestrating TLR2 and TLR4 innate responses to injury and the release of biglycan from the ECM and its de novo synthesis are key steps in this process. Biglycan has recently been identified as a potential biomarker of mortality in chronic obstructive pulmonary disease [60]. Higher levels of biglycan, as well as collagen and elastin were associated with a greater risk of mortality. The authors concluded that ECM turnover in the airways and lung parenchyma may be an important marker of disease activity in COPD. Greater biglycan deposition has also been reported in the smooth muscle layer of moderate and severe asthma patients over healthy control biopsy samples, suggesting that biglycan may play a role in the severity of airway hyper-responsiveness and asthma phenotype[44].
Decorin is a SLRP with a protein core consisting of 12 LRRs and one GAG side chain, similar in structure to biglycan[37]. As an ECM protein it fulfills two roles, one as a contributor to ECM structure and the other as a signaling molecule. Decorin displays multiple functions as a signaling molecule including its ability to interact with various tyrosine receptor kinases and low density lipoprotein receptor related protein [39, 61, 62]. Decorin has reported association with innate immune receptors, namely its ability to ligate TLR2 and TLR4 as an endogenous activator [63]. Decorin binds to TLR2 and TLR4 on macrophages with increased production of programed cell death 4 (PDCD4) with subsequent attenuated release of the anti-inflammatory cytokine IL-10 [63]. Thus, decorin may have important roles to play in regulating TLR-mediated inflammation in the lung, however specific lung decorin and innate immune receptor interactions have not been extensively studied. Decorin is present at attenuated levels in the peri-bronchiolar airways in patients with severe emphysema [64]. Fluticasone, an inhaled corticosteroid employed in the treatment of emphysema has been reported to induce decorin expression in airway fibroblasts from obstructive airway disease patients [65].
PRRs and Glycoproteins
Tenascin-C is an ECM glycoprotein which is upregulated in injury and repair, and persistently upregulated in the chronic inflammatory environment [66]. In studies of transgenic mice, tenascin C−/− mice were protected from persistent erosive joint inflammation, through reduced TLR4-mediated pro-inflammatory cytokine signaling. The ligation of tenascin-C to TLR4 was independent of CD14, a TLR4 co-receptor [67]. This innate immune effect was postulated to occupy a key role in the persistent inflammation that is observed in Rheumatoid Arthritis, which can have pulmonary disease manifestations [67]. Furthermore, the activation of TLR4 by endogenous tenascin-C promotes TLR4-dependent collagen gene expression and myofibroblast transformation, both of which are central components of progressive pulmonary fibrosis [68]. Transgenic mice lacking tenascin-C demonstrated attenuated skin and lung fibrosis in models of Systemic Sclerosis [68]. Estany et al. reported that primary pulmonary fibroblasts from idiopathic pulmonary fibrosis (IPF) patients produced higher levels of tenascin C [55]. In addition, tenascin C was found in fibroblastic foci in congregation with versican and fibronectin. Finally, in a deep proteomic study of fibrotic tissue from interstitial lung disease (ILD) patients, tenascin C was reported as significantly upregulated [69]. Although, not studied conclusively, tenascin C may play a key role as a danger signal within the lung, promoting chronic inflammation.
Fibronectin is a glycoprotein involved in cell matrix adhesions. It binds ECM proteins within the matrix such as collagen and others as well as binding to integrins. As a result of alternative splicing of its transcript, there are some 20 different variants of fibronectin in humans [70]. Fibronectin variants can initiate TLR4-dependent NFκB-dependent release of multiple inflammatory cytokines from fibroblasts [71]. The potential impact of TLR4-mediated signaling by danger signals derived from matrix, including fibronectin in pulmonary fibrosis and systemic sclerosis has been reviewed elsewhere [72].
PRRs and GAGs
Hyaluronan is a high molecular weight non-sulfated GAG with a multitude of effects including the ability to ligate several different receptors, promoting various features of cell growth, adhesion and inflammation [73]. Interestingly, alterations in the molecular weight of hyaluronan regulate the anti- vs. pro-inflammatory innate immune signaling outcomes[74]. Hyaluronan is generated in lung injury, correlates with extent of injury and its clearance is crucial for resolution of inflammation [73]. Hyaluronan can be dismantled into low molecular weight (LMW) fragments during the inflammatory process by reactive oxygen species and hyaluronidase, subsequently engaging TLR2 to cause activation of the downstream NF-κB pathway [75]. Hyaluronan oligosaccharides, also generated during inflammation, have reported associations with TLR4 signaling in dendritic cells with downstream activation of transcription factors [76]. However, high molecular weight (HMW) hyaluronan may occupy a key anti-inflammatory role within the matrix. TLR2 signaling by LMW hyaluronan fragments can be inhibited by intact HMW hyaluronan [75]. HMW hyaluronan can serve to actively suppress inflammation through activation of regulatory CD4(+) CD25(+) T cells which in turn suppress effector T cells [77]. LMW hyaluronan molecules are unable to achieve this anti-inflammatory effect. Hyaluronan is an important mediator of the initial innate immune response and has known roles in acute and chronic lung disease. Higher levels of hyaluronan in sputum from COPD patients correlated with lower pulmonary function, higher neutrophil influx and inflammatory cytokine production [78]. Hyaluronan levels are elevated in patients with persistent asthma and primary airway fibroblasts from asthma patients generate higher baseline levels of hyaluronan than normal [79]. Furthermore, TNF-stimulated gene 6 (TSG-6) facilitates transfer of inter-α-inhibitor heavy chains to hyaluronan to facilitate hyaluronan receptor binding, and this pathologic hyaluronan-heavy chain matrix is necessary for the development of allergic airway responsiveness. In the presence of TSG-6, soluble hyaluronan or environmental agents such as ozone can induce Rho A, PI3-K/AKT and ERK signaling in smooth muscle cells to promote airway hyper-responsiveness and TSG6−/− mice are protected from these outcomes[80].
In IPF, elevated levels of hyaluronan have been found in BAL fluid and lung tissue [81, 82]. Hyaluronan was found to be highly sulfated in areas of fibrotic lung bordering normal lung parenchyma. Furthermore, recent work has proposed a pivotal role for hyaluronan in the maintenance of alveolar homeostasis through the promotion of alveolar stem cell renewal [83]. Surfactant-protein-C (SPC) positive type 2 alveolar epithelial cell (AEC) renewal is regulated by interactions between TLR4 and hyaluronan. This results in the renewal of type 2 AECs and the prevention of severe pulmonary fibrosis in animal models [83]. Taken together, hyaluronan in its variable forms is an important regulator of the innate immune response to injury in the lung.
ECM interactions in leukocyte migration
The elaboration of an effective innate and adaptive host defense is mediated in part via the release of chemotactic cytokines known as chemokines. With regards to the subject matter of this review, the composition of the ECM is crucially intertwined with the function of chemokines. Chemokines bind to GAGs present within the ECM to generate chemotactic gradients which leukocytes sense via their expression of corresponding chemokine receptors to navigate to sites of insult. In order to extravasate from vascular beds into most inflamed tissues, leukocytes use cell surface receptors including CD44, P-selectin glycoprotein ligand-1 (PSGL-1) and E selectin ligand-1 (ESL-1) to first bind P-selectin and/or E-selectin to initiate rolling or adherence on activated endothelium [84]. This leucocyte adhesion cascade, mediated by selectin and ligand interactions, results in progressive slowing of leukocytes, facilitating leukocytes ability to derive signals from chemokines at the endothelial surface. This chemokine signal activates integrins and firm adhesion of leukocytes occurs. Although not extensively studied, this may be the case for leukocyte migration within the bronchial vasculature, an extension of the systemic vasculature supplying a small proportion of the lung, in particular the large airways [85]. However, in contrast to the systemic circulation, neutrophil trans-endothelial migration in the lung occurs in the pulmonary capillaries, an enormous complex network of branching vessels. The spatial limitation within the capillary vessels obviates the need for rolling and here neutrophil accumulation is regarded as selectin independent [86]. Rolling may occur in the pulmonary venules which is L-selectin dependent. Neutrophils undergo morphological changes to migrate into the lung [87]. While the sequestration of neutrophils in lung injury has been extensively studied, the migration mechanisms of other leukocytes is less clear, but studies have identified a role for L-selectin and endothelial intercellular adhesion molecule 1 (ICAM-1) in promoting T-cell migration to the inflamed lung [88].
Chemokines, Cytokines, Growth Factors and ECM
As mentioned, chemokines are responsible for the activation of leukocyte firm adhesion to allow transmigration to areas of injury or infection within the body, and they do this in part by binding to GAGs found within the ECM to create a chemotactic gradient [89]. For instance, interleukin-8 (IL-8, CXCL8) is an important recruitment molecule for neutrophils and IL-8 binds to heparan sulfate and chondroitin sulfate in the ECM, a feature which helps to localize and facilitate the dimerization, retention and compartmentalization of this chemokine in the lung [90, 91]. For many chemokines and cytokines, binding to the GAGs in the ECM can protect the chemokine or cytokine from degradation by proteases in the tissue environment thus prolonging the biological action of the molecule to recruit leukocytes or in some cases inducing a confirmation that alters receptor binding (reviewed in [89, 92]). Sulfation status of the ECM has also been suggested to impact the ability of the ECM to sequester growth factors in the lung to mediate cellular responses. In vitro studies of isolated type II AECs have shown that the degree of sulfation of the basement membrane substrata the AECs are grown on can impact their response to heparin-binding growth factors. For example, AECs grown in the presence of de-sulfated chondroitin sulfate or de-sulfated heparin along with laminin proliferate in response to FGF-2 whereas sulfated heparin can inhibit this proliferation [93, 94] suggesting that the sulfation status of the basement membrane proteoglycans may be an important determinant of AEC responses to injury, repair and differentiation. Not surprisingly, the well-known anti-inflammatory effects of heparin have recently been determined to be mediated by the sulfation status of the molecule [95].
Migration of cells can also be influenced by the expression of proteoglycans which can encourage the binding of chemokines to cells to promote cellular movement. An example of this is binding of the somewhat misnamed, macrophage migration inhibitory factor (MIF) to syndecan-1 on lung epithelial tumors to promote cellular migration [96]. Alternatively, the binding of CXCL10, an anti-fibrotic chemokine to syndecan-4 can inhibit lung fibroblast migration [97]. The ECM can also serve as a reservoir to regulate the function of cytokines produced by the recruited immune cells. For instance, binding of interferon-gamma to heparin sulfate can limit its degradation and increase its bioavailability significantly (up to 600-fold) [98].
Not only can the ECM sequester chemokines and cytokines to activate leukocytes, but activated leukocytes can also remodel the ECM through elaboration of reactive oxygen species and proteases which can serve to stimulate, perpetuate or limit inflammation. Examples of this include degradation of collagen XVIII by proteases which can produce endostatin, a potent anti-angiogenic factor formed from cleavage of the C-terminal end of the protein [99]. In contrast, neutrophil-mediated release of matrix metalloproteinase (MMP)-9 can degrade type I collagen to produce N-acetyl Pro-Gly-Pro (Ac-PGP), a peptide which serves as an important neutrophil chemoattractant in chronic inflammation in diseases such as COPD [100] [101]. The role of MMPs in general and MMP-9 in particular however are controversial and likely context-dependent as overexpression of human MMP-9 in murine macrophages reduced inflammatory cell recruitment in a bleomycin model of lung fibrosis, an effect attributed to diminished cleavage of insulin-like growth factor binding protein-3 [102]. The varied impact that MMPs have on leukocyte activation has recently been reviewed [103]. Likewise, previous reviews have also explored the impacts that neutrophil elastases, which can be released from activated neutrophils during inflammation, can have in both lung destruction and repair [104].
Pulmonary inflammation and matricellular proteins
Another way in which the inflammatory response can impact the ECM is through the secretion of matricellular proteins. Matricellular proteins serve as bridges to mediate interactions between ECM and cell surface integrin receptors. There are many matricellular proteins known to impact lung physiology including periostin, osteopontin, thrombospondin and secreted protein acidic and rich in cysteine (SPARC)[105–108]. Matricellular proteins are known to be upregulated in activated cells including leukocytes that are recruited to sites of injury or infection and includes molecules previously discussed e.g Tenascin-C. In our own laboratory, we have shown that CC chemokine receptor (CCR)2-binding chemokines can recruit fibrocytes to the lung in response to fibrotic insults or viral infections [109, 110]. Fibrocytes are circulating inflammatory cells defined by having characteristics of both leukocytes (e.g. expression of CD45 and chemokine receptors) but also mesenchymal cells (e.g. expression of type I and type II collagen)[111]. Many studies have shown that fibrocytes augment or correlate with the development of fibrotic lung diseases [112, 113]. Recently, we suggested that the ability of fibrocytes to increase lung fibrosis in the murine bleomycin model was due to their paracrine functions such as secretion of pro-fibrotic cytokines [114], but also by their ability to secrete periostin [115], rather than their ability to secrete type I collagen [116] or to differentiate into myofibroblasts in vivo [115], a result also seen by Madala et al. using a transgenic TGFα fibrosis model [117]. This was attributed to the ability of periostin to interact with bone morphogenic protein (BMP)1 to localize its ability to activate lysl-oxidase to stimulate collagen type I crosslinking[118]. It should also be noted that fibrocytes themselves are potent secretors of MMPs [119] which may also serve to remodel the ECM as noted above.
Periostin can upregulate expression of TGFβ1 [115] and this cytokine is the best-described regulator of fibrosis. The activation of TGFβ1 is very complex and highly orchestrated by the ECM. This cytokine is held in a latent state complexed with the latency associated protein (LAP) and sequestered to the ECM by latent TGFβ binding protein (LTBP). In this conformation, the growth factor is not bioactive until acted on by proteases, pH or mechanical stress to facilitate the dissociation of active TGFβ as recently reviewed [120–122]. Once TGFβ1 promotes the deposition and stiffening of the lung ECM, this stiffened matrix can serve to activate fibroblasts [123, 124], potentiating the fibrotic reaction. Importantly, this thickening of the ECM, especially in the context of lung cancer can serve to impede the ability of activated T cells to migrate through the stiffened matrix, lessening the chance of an effective anti-tumor CTL response [125]. Similar impediment of CTLs is likely to occur in other granulomatous lung disease as well. When faced with a pathogen that cannot be easily cleared (e.g. mycobacteria or parasites), activated T cell cytokines are believed to modulate granuloma formation [126], with Th2 cytokines such as IL-13 being particularly noted for the ability to activate fibroblasts to promote the development of thickened granuloma walls of ECM by stimulating production of type I collagen [127]. Thus, immune responses can serve to initiate secretion of ECM, ECM can modulate leukocyte inflammation and ultimately, thickened ECM can exclude leukocytes from performing their effector functions at the needed site of infection or tumor niches.
Conclusion
In this review, we have summarized some of the important interactions that have been characterized between the pulmonary ECM and the immune system within the lung. We know the ECM plays an important role in the regulation of innate and adaptive immunity. Recognition of ECM components by PRRs on innate immune cells may be the earliest indication of injury and infection within the lung. The ECM acts as a reservoir where components can interact with a variety of cytokines, chemokine and growth factors thereby modulating inflammation and repair. The release of ECM-bound cytokines can act to shape the effector T cell responses to promote development of variable T cell responses and ultimately disease pathogenesis. However, considerable work remains to understand these pulmonary ECM-immunity interactions in more detail. Furthermore, little is known about how immune cells may sense stiffened lung ECM and if so, whether such mechanosensing alters immune cell functions. A greater understanding of how the matrix promotes innate and adaptive immunity may lead to tailored therapies in both infectious and non-infectious lung disease. These therapies may limit the leukocyte and inflammatory cytokine cascade, may attenuate cell and tissue damage or may be tailored to provoke immune-mediated responses needed for appropriate repair. The next decade should herald a greater understanding of how to harness ECM-immune interactions in these ways.
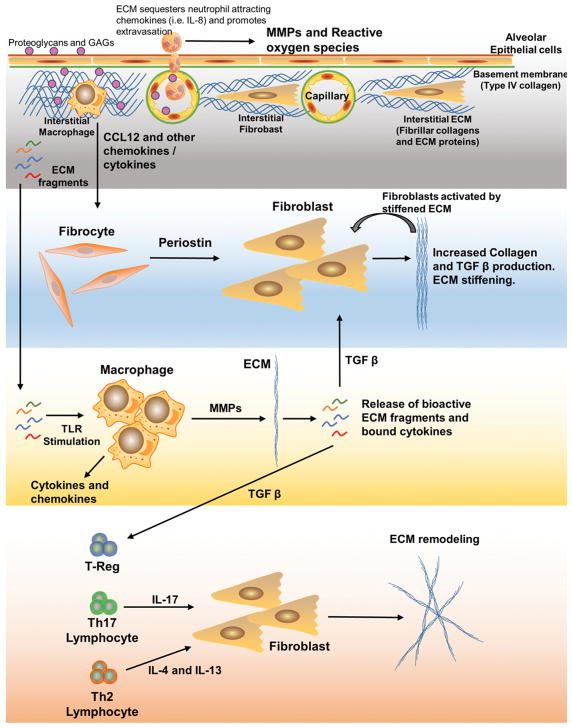
Fig. 3. Features of ECM and adaptive pulmonary immunity.
ECM fragments can stimulate TLRs to generate inflammatory cytokines and matrix metalloproteinases that may alter the ECM resulting in the release of TGF-β which in turn results in ECM remodeling through direct effects on pulmonary fibroblasts. TGF-β also modulates T cell populations to generate cytokines that influence the ECM. Finally, fibrocyte recruitment to the inflamed lung can lead to increased expression of periostin, which alters fibroblasts leading to increased collagen deposition and ECM stiffening.
Highlights.
Pulmonary immunity includes physical barriers including mucus and the mucocilary escalator of the airway, the alveolar epithelium and the capillary endothelium
These physical barriers work in tandem with a well-developed innate and adaptive immune system to protect the lung from injurious agents and pathogens
The lung extracellular matrix is a bioactive scaffold lying beneath these physical barriers that has important roles in the regulation of pulmonary innate and adaptive immunity
Lung pathology promotes abnormalities within the composition and structure of the extracellular matrix and generates matrix molecules that act as “danger signals” for innate immune receptors
The release of extracellular matrix bound cytokines can affect effector T cell responses and disease pathogenesis
Leukocyte recruitment to inflamed pulmonary sites relies on morphological alterations of cells to pass through the confined spaces of the pulmonary capillary network
A greater understanding of these important extracellular-immunity interactions will lead to improved therapies for acute and chronic lung disease
Acknowledgments
Grant support: NIH grants AI117229, HL127805, HL115618, HL119682 (BBM) and K99HL139996 (DOD)
We would like to thank Dr. Rachel Zemans (University of Michigan) for her image of the type 2 human pneumocytes and Dr. Jeffrey Myers (University of Michigan) for his image of the interstitial matrix in IPF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faffe DS, Zin WA. Lung parenchymal mechanics in health and disease. Physiol Rev. 2009;89(3):759–75. doi: 10.1152/physrev.00019.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winslow CE. A New Method of Enumerating Bacteria in Air. Science. 1908;28(705):28–31. doi: 10.1126/science.28.705.28. [DOI] [PubMed] [Google Scholar]
- 3.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16(1):27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16(1):36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler B, Eidenschenk C, Crozat K, Imler JL, Takeuchi O, Hoffmann JA, Akira S. Genetic analysis of resistance to viral infection. Nat Rev Immunol. 2007;7(10):753–66. doi: 10.1038/nri2174. [DOI] [PubMed] [Google Scholar]
- 7.Huxley-Jones J, Robertson DL, Boot-Handford RP. On the origins of the extracellular matrix in vertebrates. Matrix Biol. 2007;26(1):2–11. doi: 10.1016/j.matbio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11(1):47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11(4):M111 014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiller HB, Fernandez IE, Burgstaller G, Schaab C, Scheltema RA, Schwarzmayr T, Strom TM, Eickelberg O, Mann M. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol. 2015;11(7):819. doi: 10.15252/msb.20156123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. 2017;50(1) doi: 10.1183/13993003.01805-2016. [DOI] [PubMed] [Google Scholar]
- 12.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol (1985) 2005;98(5):1892–9. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- 13.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186(9):866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wight TN, Frevert CW, Debley JS, Reeves SR, Parks WC, Ziegler SF. Interplay of extracellular matrix and leukocytes in lung inflammation. Cell Immunol. 2017;312:1–14. doi: 10.1016/j.cellimm.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abreu-Velez AM, Howard MS. Collagen IV in Normal Skin and in Pathological Processes. N Am J Med Sci. 2012;4(1):1–8. doi: 10.4103/1947-2714.92892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huffstutter JE, DeLustro FA, LeRoy EC. Cellular immunity to collagen and laminin in scleroderma. Arthritis Rheum. 1985;28(7):775–80. doi: 10.1002/art.1780280708. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442(7098):39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162(6):3212–9. [PubMed] [Google Scholar]
- 20.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellson CD, Dunmore R, Hogaboam CM, Sleeman MA, Murray LA. Danger-associated molecular patterns and danger signals in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;51(2):163–8. doi: 10.1165/rcmb.2013-0366TR. [DOI] [PubMed] [Google Scholar]
- 22.Nomura N, Miyajima N, Sazuka T, Tanaka A, Kawarabayasi Y, Sato S, Nagase T, Seki N, Ishikawa K, Tabata S. Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001-KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG-1. DNA Res. 1994;1(1):27–35. doi: 10.1093/dnares/1.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42(3):779–89. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 25.Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, Pearce WP, Berenjeno IM, Nock G, Filloux A, Beyaert R, Flamand V, Vanhaesebroeck B. The p110delta isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol. 2012;13(11):1045–1054. doi: 10.1038/ni.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198(8):1225–35. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24(6):286–90. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 28.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12(3):168–79. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–7. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 30.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14(1):9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 31.Duan W, Mehta AK, Magalhaes JG, Ziegler SF, Dong C, Philpott DJ, Croft M. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J Allergy Clin Immunol. 2010;126(6):1284–93 e10. doi: 10.1016/j.jaci.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hysi P, Kabesch M, Moffatt MF, Schedel M, Carr D, Zhang Y, Boardman B, von Mutius E, Weiland SK, Leupold W, Fritzsch C, Klopp N, Musk AW, James A, Nunez G, Inohara N, Cookson WO. NOD1 variation, immunoglobulin E and asthma. Hum Mol Genet. 2005;14(7):935–41. doi: 10.1093/hmg/ddi087. [DOI] [PubMed] [Google Scholar]
- 33.Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, Fuji A, Yuasa T, Manki A, Sakurai Y, Nakajima M, Kobayashi H, Fujiwara I, Tsutsumi H, Utani A, Nishigori C, Heike T, Nakahata T, Miyachi Y. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood. 2005;105(3):1195–7. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- 34.Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules. 2006;7(8):2388–93. doi: 10.1021/bm0603746. [DOI] [PubMed] [Google Scholar]
- 35.Wiberg C, Hedbom E, Khairullina A, Lamande SR, Oldberg A, Timpl R, Morgelin M, Heinegard D. Biglycan and decorin bind close to the n-terminal region of the collagen VI triple helix. J Biol Chem. 2001;276(22):18947–52. doi: 10.1074/jbc.M100625200. [DOI] [PubMed] [Google Scholar]
- 36.Markmann A, Hausser H, Schonherr E, Kresse H. Influence of decorin expression on transforming growth factor-beta-mediated collagen gel retraction and biglycan induction. Matrix Biol. 2000;19(7):631–6. doi: 10.1016/s0945-053x(00)00097-4. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283(31):21305–9. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci U S A. 2013;110(28):E2582–91. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274(8):4489–92. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 40.Zeltz C, Brezillon S, Kapyla J, Eble JA, Bobichon H, Terryn C, Perreau C, Franz CM, Heino J, Maquart FX, Wegrowski Y. Lumican inhibits cell migration through alpha2beta1 integrin. Exp Cell Res. 2010;316(17):2922–31. doi: 10.1016/j.yexcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185(4):743–54. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17(1):1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 43.Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277(19):3864–75. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pini L, Hamid Q, Shannon J, Lemelin L, Olivenstein R, Ernst P, Lemiere C, Martin JG, Ludwig MS. Differences in proteoglycan deposition in the airways of moderate and severe asthmatics. Eur Respir J. 2007;29(1):71–7. doi: 10.1183/09031936.00047905. [DOI] [PubMed] [Google Scholar]
- 45.Noordhoek JA, Postma DS, Chong LL, Menkema L, Kauffman HF, Timens W, van Straaten JF, van der Geld YM. Different modulation of decorin production by lung fibroblasts from patients with mild and severe emphysema. COPD. 2005;2(1):17–25. doi: 10.1081/copd-200050678. [DOI] [PubMed] [Google Scholar]
- 46.Venkatesan N, Ebihara T, Roughley PJ, Ludwig MS. Alterations in large and small proteoglycans in bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med. 2000;161(6):2066–73. doi: 10.1164/ajrccm.161.6.9909098. [DOI] [PubMed] [Google Scholar]
- 47.Shao H, Lee S, Gae-Scott S, Nakata C, Chen S, Hamad AR, Chakravarti S. Extracellular matrix lumican promotes bacterial phagocytosis, and Lum−/− mice show increased Pseudomonas aeruginosa lung infection severity. J Biol Chem. 2012;287(43):35860–72. doi: 10.1074/jbc.M112.380550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115(8):2223–33. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavalcante FS, Ito S, Brewer K, Sakai H, Alencar AM, Almeida MP, Andrade JS, Jr, Majumdar A, Ingenito EP, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol (1985) 2005;98(2):672–9. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- 50.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toeda K, Nakamura K, Hirohata S, Hatipoglu OF, Demircan K, Yamawaki H, Ogawa H, Kusachi S, Shiratori Y, Ninomiya Y. Versican is induced in infiltrating monocytes in myocardial infarction. Mol Cell Biochem. 2005;280(1–2):47–56. doi: 10.1007/s11010-005-8051-4. [DOI] [PubMed] [Google Scholar]
- 52.Wight TN. Arterial remodeling in vascular disease: a key role for hyaluronan and versican. Front Biosci. 2008;13:4933–7. doi: 10.2741/3052. [DOI] [PubMed] [Google Scholar]
- 53.Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion. Am J Respir Cell Mol Biol. 2010;43(1):109–20. doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang I, Harten IA, Chang MY, Braun KR, Sheih A, Nivison MP, Johnson PY, Workman G, Kaber G, Evanko SP, Chan CK, Merrilees MJ, Ziegler SF, Kinsella MG, Frevert CW, Wight TN. Versican Deficiency Significantly Reduces Lung Inflammatory Response Induced by Polyinosine-Polycytidylic Acid Stimulation. J Biol Chem. 2017;292(1):51–63. doi: 10.1074/jbc.M116.753186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Estany S, Vicens-Zygmunt V, Llatjos R, Montes A, Penin R, Escobar I, Xaubet A, Santos S, Manresa F, Dorca J, Molina-Molina M. Lung fibrotic tenascin-C upregulation is associated with other extracellular matrix proteins and induced by TGFbeta1. BMC Pulm Med. 2014;14:120. doi: 10.1186/1471-2466-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersson-Sjoland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, Rydell-Tormanen K, Bjermer L, Malmstrom A, Karlsson JC, Westergren-Thorsson G. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology. 2015;25(3):243–51. doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McBride OW, Fisher LW, Young MF. Localization of PGI (biglycan, BGN) and PGII (decorin, DCN, PG-40) genes on human chromosomes Xq13-qter and 12q, respectively. Genomics. 1990;6(2):219–25. doi: 10.1016/0888-7543(90)90560-h. [DOI] [PubMed] [Google Scholar]
- 58.Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16(7):673–80. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 59.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284(36):24035–48. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sand JM, Leeming DJ, Byrjalsen I, Bihlet AR, Lange P, Tal-Singer R, Miller BE, Karsdal MA, Vestbo J. High levels of biomarkers of collagen remodeling are associated with increased mortality in COPD - results from the ECLIPSE study. Respir Res. 2016;17(1):125. doi: 10.1186/s12931-016-0440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011;25(8):1431–43. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brandan E, Retamal C, Cabello-Verrugio C, Marzolo MP. The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J Biol Chem. 2006;281(42):31562–71. doi: 10.1074/jbc.M602919200. [DOI] [PubMed] [Google Scholar]
- 63.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4(199):ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Straaten JF, Coers W, Noordhoek JA, Huitema S, Flipsen JT, Kauffman HF, Timens W, Postma DS. Proteoglycan changes in the extracellular matrix of lung tissue from patients with pulmonary emphysema. Mod Pathol. 1999;12(7):697–705. [PubMed] [Google Scholar]
- 65.Brandsma CA, Timens W, Jonker MR, Rutgers B, Noordhoek JA, Postma DS. Differential effects of fluticasone on extracellular matrix production by airway and parenchymal fibroblasts in severe COPD. Am J Physiol Lung Cell Mol Physiol. 2013;305(8):L582–9. doi: 10.1152/ajplung.00152.2013. [DOI] [PubMed] [Google Scholar]
- 66.Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol. 2003;200(4):488–99. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- 67.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–80. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 68.Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, Lafyatis R, Lee J, Hinchcliff M, Feghali-Bostwick C, Lakota K, Budinger GR, Raparia K, Tamaki Z, Varga J. Tenascin-C drives persistence of organ fibrosis. Nat Commun. 2016;7:11703. doi: 10.1038/ncomms11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schiller HB, Mayr CH, Leuschner G, Strunz M, Staab-Weijnitz C, Preisendorfer S, Eckes B, Moinzadeh P, Krieg T, Schwartz DA, Hatz RA, Behr J, Mann M, Eickelberg O. Deep Proteome Profiling Reveals Common Prevalence of MZB1-Positive Plasma B Cells in Human Lung and Skin Fibrosis. Am J Respir Crit Care Med. 2017;196(10):1298–1310. doi: 10.1164/rccm.201611-2263OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162(1):149–60. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelsh R, You R, Horzempa C, Zheng M, McKeown-Longo PJ. Regulation of the innate immune response by fibronectin: synergism between the III-1 and EDA domains. PLoS One. 2014;9(7):e102974. doi: 10.1371/journal.pone.0102974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhattacharyya S, Varga J. Endogenous ligands of TLR4 promote unresolving tissue fibrosis: Implications for systemic sclerosis and its targeted therapy. Immunol Lett. 2017 doi: 10.1016/j.imlet.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221–64. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cyphert JM, Trempus CS, Garantziotis S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int J Cell Biol. 2015;2015:563818. doi: 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177(2):1272–81. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 76.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179(2):744–7. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 78.Dentener MA, Vernooy JH, Hendriks S, Wouters EF. Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax. 2005;60(2):114–9. doi: 10.1136/thx.2003.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, Bousquet J. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med. 1998;157(2):403–9. doi: 10.1164/ajrccm.157.2.96-08040. [DOI] [PubMed] [Google Scholar]
- 80.Stober VP, Johnson CG, Majors A, Lauer ME, Cali V, Midura RJ, Wisniewski HG, Aronica MA, Garantziotis S. TNF-stimulated gene 6 promotes formation of hyaluronan-inter-alpha-inhibitor heavy chain complexes necessary for ozone-induced airway hyperresponsiveness. J Biol Chem. 2017;292(51):20845–20858. doi: 10.1074/jbc.M116.756627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bjermer L, Lundgren R, Hallgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax. 1989;44(2):126–31. doi: 10.1136/thx.44.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Westergren-Thorsson G, Hedstrom U, Nybom A, Tykesson E, Ahrman E, Hornfelt M, Maccarana M, van Kuppevelt TH, Dellgren G, Wildt M, Zhou XH, Eriksson L, Bjermer L, Hallgren O. Increased deposition of glycosaminoglycans and altered structure of heparan sulfate in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2017;83:27–38. doi: 10.1016/j.biocel.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 83.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, Kurkciyan A, Mena JM, Stripp BR, Jiang D, Noble PW. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. 2016;22(11):1285–1293. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118(26):6743–51. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doerschuk CM. Leukocyte trafficking in alveoli and airway passages. Respir Res. 2000;1(3):136–40. doi: 10.1186/rr24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gebb SA, Graham JA, Hanger CC, Godbey PS, Capen RL, Doerschuk CM, Wagner WW., Jr Sites of leukocyte sequestration in the pulmonary microcirculation. J Appl Physiol (1985) 1995;79(2):493–7. doi: 10.1152/jappl.1995.79.2.493. [DOI] [PubMed] [Google Scholar]
- 87.Motosugi H, Graham L, Noblitt TW, Doyle NA, Quinlan WM, Li Y, Doerschuk CM. Changes in neutrophil actin and shape during sequestration induced by complement fragments in rabbits. Am J Pathol. 1996;149(3):963–73. [PMC free article] [PubMed] [Google Scholar]
- 88.Keramidaris E, Merson TD, Steeber DA, Tedder TF, Tang ML. L-selectin and intercellular adhesion molecule 1 mediate lymphocyte migration to the inflamed airway/lung during an allergic inflammatory response in an animal model of asthma. J Allergy Clin Immunol. 2001;107(4):734–8. doi: 10.1067/mai.2001.114050. [DOI] [PubMed] [Google Scholar]
- 89.Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec (Hoboken) 2010;293(6):968–81. doi: 10.1002/ar.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frevert CW, Goodman RB, Kinsella MG, Kajikawa O, Ballman K, Clark-Lewis I, Proudfoot AE, Wells TN, Martin TR. Tissue-specific mechanisms control the retention of IL-8 in lungs and skin. J Immunol. 2002;168(7):3550–6. doi: 10.4049/jimmunol.168.7.3550. [DOI] [PubMed] [Google Scholar]
- 91.Frevert CW, Kinsella MG, Vathanaprida C, Goodman RB, Baskin DG, Proudfoot AE, Wells TN, Wright TN, Martin TR. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol. 2003;28(4):464–72. doi: 10.1165/rcmb.2002-0084OC. [DOI] [PubMed] [Google Scholar]
- 92.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 93.Sannes PL, Khosla J, Cheng PW. Sulfation of extracellular matrices modifies responses of alveolar type II cells to fibroblast growth factors. Am J Physiol. 1996;271:L688–97. doi: 10.1152/ajplung.1996.271.5.L688. [DOI] [PubMed] [Google Scholar]
- 94.Sannes PL, Khosla J, Li CM, Pagan I. Sulfation of extracellular matrices modifies growth factor effects on type II cells on laminin substrata. Am J Physiol. 1998;275(4 Pt 1):L701–8. doi: 10.1152/ajplung.1998.275.4.L701. [DOI] [PubMed] [Google Scholar]
- 95.Yi NY, Newman DR, Zhang H, Morales Johansson H, Sannes PL. Heparin and LPS-induced COX-2 expression in airway cells: a link between its anti-inflammatory effects and GAG sulfation. Exp Lung Res. 2015;41(9):499–513. doi: 10.3109/01902148.2015.1091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pasqualon T, Lue H, Groening S, Pruessmeyer J, Jahr H, Denecke B, Bernhagen J, Ludwig A. Cell surface syndecan-1 contributes to binding and function of macrophage migration inhibitory factor (MIF) on epithelial tumor cells. Biochim Biophys Acta. 2016;1863(4):717–26. doi: 10.1016/j.bbamcr.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 97.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, Li Y, Tager AM, Goetinck PF, Luster AD, Noble PW. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120(6):2049–57. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lortat-Jacob H. Interferon and heparan sulphate. Biochem Soc Trans. 2006;34(Pt 3):461–4. doi: 10.1042/BST0340461. [DOI] [PubMed] [Google Scholar]
- 99.Seppinen L, Pihlajaniemi T. The multiple functions of collagen XVIII in development and disease. Matrix Biol. 2011;30(2):83–92. doi: 10.1016/j.matbio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Xu X, Jackson PL, Tanner S, Hardison MT, Abdul Roda M, Blalock JE, Gaggar A. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS One. 2011;6(1):e15781. doi: 10.1371/journal.pone.0015781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdul Roda M, Fernstrand AM, Redegeld FA, Blalock JE, Gaggar A, Folkerts G. The matrikine PGP as a potential biomarker in COPD. Am J Physiol Lung Cell Mol Physiol. 2015;308(11):L1095–101. doi: 10.1152/ajplung.00040.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cabrera S, Gaxiola M, Arreola JL, Ramirez R, Jara P, D’Armiento J, Richards T, Selman M, Pardo A. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. Int J Biochem Cell Biol. 2007;39(12):2324–38. doi: 10.1016/j.biocel.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 103.Smigiel KS, Parks WC. Matrix Metalloproteinases and Leukocyte Activation. Prog Mol Biol Transl Sci. 2017;147:167–195. doi: 10.1016/bs.pmbts.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Lungarella G, Cavarra E, Lucattelli M, Martorana PA. The dual role of neutrophil elastase in lung destruction and repair. Int J Biochem Cell Biol. 2008;40(6–7):1287–96. doi: 10.1016/j.biocel.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CT, Matthews JG, Arron JR. Roles of Periostin in Respiratory Disorders. Am J Respir Crit Care Med. 2016;193(9):949–56. doi: 10.1164/rccm.201510-2032PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O’Regan A. The role of osteopontin in lung disease. Cytokine Growth Factor Rev. 2003;14(6):479–88. doi: 10.1016/s1359-6101(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 107.Rogers NM, Ghimire K, Calzada MJ, Isenberg JS. Matricellular protein thrombospondin-1 in pulmonary hypertension: multiple pathways to disease. Cardiovasc Res. 2017;113(8):858–868. doi: 10.1093/cvr/cvx094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trombetta-Esilva J, Bradshaw AD. The Function of SPARC as a Mediator of Fibrosis. Open Rheumatol J. 2012;6:146–55. doi: 10.2174/1874312901206010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166(3):675–84. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(2):175–81. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loomis-King H, Moore BB. Fibrocytes in the Pathogenesis of Chronic Fibrotic Lung Disease. Curr Respir Med Rev. 2013;9(1):34–41. doi: 10.2174/1573398x11309010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40(10):2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 113.Aono Y, Kishi M, Yokota Y, Azuma M, Kinoshita K, Takezaki A, Sato S, Kawano H, Kishi J, Goto H, Uehara H, Izumi K, Nishioka Y. Role of platelet-derived growth factor/platelet-derived growth factor receptor axis in the trafficking of circulating fibrocytes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;51(6):793–801. doi: 10.1165/rcmb.2013-0455OC. [DOI] [PubMed] [Google Scholar]
- 114.Kleaveland KR, Moore BB, Kim KK. Paracrine functions of fibrocytes to promote lung fibrosis. Expert Rev Respir Med. 2014;8(2):163–72. doi: 10.1586/17476348.2014.862154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ashley SL, Wilke CA, Kim KK, Moore BB. Periostin regulates fibrocyte function to promote myofibroblast differentiation and lung fibrosis. Mucosal Immunol. 2017;10(2):341–351. doi: 10.1038/mi.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kleaveland KR, Velikoff M, Yang J, Agarwal M, Rippe RA, Moore BB, Kim KK. Fibrocytes are not an essential source of type I collagen during lung fibrosis. J Immunol. 2014;193(10):5229–39. doi: 10.4049/jimmunol.1400753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Madala SK, Edukulla R, Schmidt S, Davidson C, Ikegami M, Hardie WD. Bone marrow-derived stromal cells are invasive and hyperproliferative and alter transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50(4):777–86. doi: 10.1165/rcmb.2013-0042OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285(17):13294–303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garcia-de-Alba C, Becerril C, Ruiz V, Gonzalez Y, Reyes S, Garcia-Alvarez J, Selman M, Pardo A. Expression of matrix metalloproteases by fibrocytes: possible role in migration and homing. Am J Respir Crit Care Med. 2010;182(9):1144–52. doi: 10.1164/rccm.201001-0028OC. [DOI] [PubMed] [Google Scholar]
- 120.Hinz B. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 121.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–38. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 122.Sheppard D. Epithelial-mesenchymal interactions in fibrosis and repair. Transforming growth factor-beta activation by epithelial cells and fibroblasts. Ann Am Thorac Soc. 2015;12(Suppl 1):S21–3. doi: 10.1513/AnnalsATS.201406-245MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen H, Qu J, Huang X, Kurundkar A, Zhu L, Yang N, Venado A, Ding Q, Liu G, Antony VB, Thannickal VJ, Zhou Y. Mechanosensing by the alpha6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat Commun. 2016;7:12564. doi: 10.1038/ncomms12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190(4):693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122(3):899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chiu BC, Freeman CM, Stolberg VR, Komuniecki E, Lincoln PM, Kunkel SL, Chensue SW. Cytokine-chemokine networks in experimental mycobacterial and schistosomal pulmonary granuloma formation. Am J Respir Cell Mol Biol. 2003;29(1):106–16. doi: 10.1165/rcmb.2002-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jakubzick C, Choi ES, Kunkel SL, Joshi BH, Puri RK, Hogaboam CM. Impact of interleukin-13 responsiveness on the synthetic and proliferative properties of Th1- and Th2-type pulmonary granuloma fibroblasts. Am J Pathol. 2003;162(5):1475–86. doi: 10.1016/S0002-9440(10)64280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]