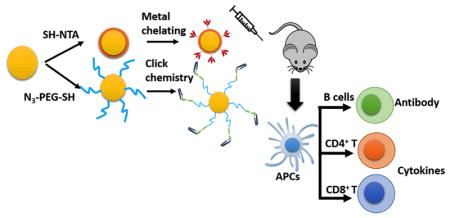
Abstract
The immunogenicity of subunit vaccines can be augmented by formulating them into nanoparticles. We conjugated recombinant trimetric influenza A/Aichi/2/68(H3N2) hemagglutinin (HA) onto functionalized gold nanoparticles (AuNPs) surfaces in a repetitive, oriented configuration. To further improve the immunogenicity, we generated Toll- like receptor 5 (TLR5) agonist flagellin (FliC)-coupled AuNPs as particulate adjuvants.
Intranasal immunizations with an AuNP-HA and AuNP-FliC particle mixture elicited strong mucosal and systemic immune responses that protected hosts against lethal influenza challenges. Compared with the AuNP-HA alone group, the addition of AuNP-FliC improved mucosal B cell responses as characterized by elevated influenza specific IgA and IgG levels in nasal, tracheal, and lung washes. AuNP-HA/AuNP-FliC also stimulated antigen-specific interferon-γ (IFN-γ)-secreting CD4+cell proliferation and induced strong effector CD8+T cell activation.
Our results indicate that intranasal co-delivery of antigen and adjuvant-displaying AuNPs enhanced vaccine efficacy by inducing potent cellular immune responses.
Keywords: adjuvant, cellular immunity, co-delivery, gold nanoparticle, mucosal vaccine
Graphical Abstract
We screened two facile gold nanoparticles (AuNPs) surface conjugation strategies specific to recombinant trimetric A/Aichi/2/68(H3N2) hemagglutinin (HA) and TLR5 agonist flagellin (FliC): metal chelating method shows potentially high conjugation efficacy of HA; click chemistry method shows high stability and could appropriate FliC protein conformations, thus promises better immunostimulation ability of adjuvants. Intranasal co-administration of AuNPs-HA and AuNPs-FliC can elicit stronger systemic and mucosal IgG and IgA due to the enhanced crosslinking of multiple immunoglobulins on B cell surface. Additionally, particular adjuvant could also effectively activate antigen presenting cells (APCs) for better antigen presentation and cross-presentation to improve cellular immunity response.
Background
Ideal vaccines should elicit potent and long- lasting immune responses, have minimum side effects, and require a short production period. Compared with conventional vaccines, recombinant protein-based subunit vaccines offer many advantages: lower levels of toxicity, reduced off-target immune responses associated with live vaccine (e.g. viral) vectors, and shorter production times.1
However, subunit vaccines lack immunogenicity, resulting in short-term immunity and Th2-biased immune responses.2 The dominant responses to subunit vaccines are humoral or allergic but not cytotoxic T lymphocyte (CTL) responses. CTLs clear viral- infected cells if viruses escape antibody-mediated effector mechanisms and infect host cells.3 A good influenza vaccine should be capable of recruiting these CTLs to offer protection against infection.
Particulate vaccines (but not subunit vaccines) are immunogenic because they resemble the natural pathogens that the host immune system evolved to combat. Subunit vaccines lack orientation and context. Nanoscale particles can improve the immunogenicity of subunit vaccines by presenting the antigens more naturally orientated and in a context more readily recognized by the immune system (i.e. a particle that resembles a virion in size and shape).4
Gold nanoparticles (AuNPs) have many uses and can act as vaccine platforms to improve antigen presentation5–7. Compared with large (>100nm) polymeric particles or liposomes, AuNPs can be synthesized at more immunogenic sizes (1–100 nm) and shapes (sphere, rod, star, and cube) for different applications in immunotherapies8. Compared with conventional vaccine formulations, appropriately sized nanoparticles (~100 nm) can drain into lymph nodes (LNs) and elicit stronger antigen-specific T-cell immunity.9 The chemical and optical properties of AuNPs allow for the conjugation and characterization of target proteins/vaccines/adjuvants at high densities onto the AuNPs’ surfaces10,11. Furthermore, as non- living synthetic material, AuNPs will not induce a carrier-specific immunity following repeated administration.12,13
The route of vaccination can also improve the immunogenicity of subunit and nanoparticle vaccines. Immunization via the mucous membranes of the respiratory tract effectively induces protective immunity to viral pathogens which transmit through respiratory mucosa.14 Nasal-associated lymphoid tissues (NALTs) contain abundant antigen-presenting cells (APCs), T cells, and B cells. The delivery of antigen and/or adjuvant nanoparticles to mucosal APCs has many effects: activation of APC maturation, amplification of the capacity of APCs for antigen presentation and cross-presentation, and induction of strong cellular responses. Through intranasal delivery, vaccines can trigger mucosal immunoglobulin A (IgA) and immunoglobulin G (IgG) responses capable of blocking pathogens in the early infection period.15
Adjuvants benefit subunit vaccines by stimulating dendritic cells (DCs) to activate T cells, initiating innate immune responses.16 Flagellin (FliC) —a potential mucosal adjuvant— is recognized by Toll- like receptor 5 (TLR5) and can induce innate immune responses by triggering the release of proinflammatory cytokines17. TLR5 is expressed in various cells located in mucosa, such as DCs, macrophages, and epithelial cells.18 Use of FliC as an adjuvant in an intranasal vaccination strategy can increase mucosal IgA and IgG titers in immunized mice.19 FliC has been used as an antigen carrier in fusion proteins to boost the efficacy of vaccines.20 However, high dose exposure of FliC could induce systemic inflammation and side effect to hosts. Therefore, co-expression of FliC with antigens as fusion proteins may lose the flexibility to adjust antigen/adjuvant ratio to better improve the vaccination efficacy without inducing a systemic inflammatory state. Various nanoparticles carriers and surface modification methods have been developed to customize surface antigen and/or adjuvant (FliC) conjugation.21–23 However, a systematic study of AuNP-based, influenza vaccine-induced, humoral and cellular immune responses is lacking.
We have previously designed and tested a AuNP-based protein vaccine/adjuvant delivery platform; Our in vitro studies showed that AuNP carriers increased cellular uptake of antigen, dendritic cell maturation, and T cell activation.24 In our current study, we investigated the immunopotentiation of this AuNP delivery system using an in vivo mice model.
Methods
Materials
Azido Polyethylene Glycol Thiol (N3-PEG-SH) was purchased from NANOCS. Gold(III) chloride trihydrate (HAuCl4.3H2O), ≥99.9%;N-[Nα,Nα-bis(carboxymethyl)-L- lysine]-12-mercaptododecanamide (SH-NTA), ≥90.0%; Dimethyl sulfoxide (DMSO), ≥99.9%; Copper (II) sulfate Pentahydrate, ≥98.0%; 4-Aminophenyl propargyl ether, 95.0%; Tetrahydrofuran (THF), ≥99.9%; N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), ≥99.0%; 11-mercapto-1-undecanol, 99.0%, were purchased from Sigma-Aldrich. E-pure water (18.2 MΩ.cm) was used for all regents’ preparation.
Preparation of Alkyne-FliC
FliC from Salmonella was purified as previously described.19 Alkyne-FliC was prepared according to a modified EDC method.25 Briefly: 1 mL of 2.4 mg/mL FliC (Phosphate Buffered Saline, PBS, pH=7.4); 12 μL of 4-Aminophenyl propargyl ether (100 mM in Tetrahydrofuran, THF); and 11 μL of EDC (100 mM in PBS) were mixed in 10% v/v THF/PBS overnight at 4°C. The crude alkyne-FliC was purified using an EMD Millipore Microcon centrifugal filter unit (MWCO 10 kDa) and washed with PBS.
Preparation of AuNPs-HA and AuNPs-FliC
18 nm diameter AuNPs were synthesized as previously described.26 Briefly, 36 mL of chloroauric acid (1 mM) was kept well- mixed under boiling conditions for 10 min. Then, 4 mL of sodium citrate (90 mM) was added to the solution for 20 min. 1mL (0.005 nmol) AuNPs solution was mixed with 10 μL of Tween 20 (10 mM) for 30 min to stabilize the particles. After adding 10 μL of SH-NTA (1mg/mL in DMSO) or 100 μL of N3-PEG-SH (100 μM) solution, 2 more hours mixing was required to allow for the complete exchange of citrate with thiol. The mixture was purified by centrifugation (14000 rpm for 30 min at 4 °C).
Recombinant trimeric hemagglutinin (HA) from A/Aichi/2/68 (Aichi, H3N2) was purified as previously described.27
HA (1.8 mg/mL) or alkyne-FliC (1.5 mg/mL) were separately added into copper sulfate pentahydrate catalyzed SH-NTA AuNPs or azide-PEG-SH AuNPs. The solution was mixed overnight at 4°C. The mixture was purified by centrifugation (13500 rpm for 20 min at 4 °C) twice. The amounts of HA and FliC on AuNPs were determined with a Bradford protein assay kit (ThermoFisher).
TLR-5-specific bioactivity assay
The bioactivity of conjugated FliC on AuNPs was assayed using human embryonic kidney cells (HEK 293T, ATCC: CRL-1573) transiently transfected with a human TLR5 and NF- κB/luciferase expression vector.14 In brief, 90%-confluent 293T cells in a 75-cm2 flask were transfected with 10 μg of plasmid pUNO1-hTLR5 (InvivoGen) and reporter plasmid pGL4.3 (Promega) by using Lipofectamine® 2000 (Invitrogen) following the manufacturer’s instructions. The ratio between pTLR5 and pGL4.3 was 5:1 to 10:1. After 24 hours of transfection, cells were split into a 96-well plate with 5×104 cells/well. Serially diluted soluble FliC and AuNP-FliC —from 2 μg/mL to 1.6 ng/mL— were prepared with 1% FBS culture medium and used to treat cells for stimulation. After 5 h incubation, 100 μL of the Luciferase Assay Reagent (Promega) was added to each well. Luciferase activity was read with the GloMax®-Multi Detection System (Promega).
Hemagglutination (HA) and Hemagglutination inhibition (HAI) assays
HA tests were performed based on the WHO protocol with modifications.28 Soluble HA and AuNP-HA (40 μg/mL) were serially 2- fold-diluted in a total volume of 50 μL 13. HAI assays were carried out as described previously.29 Briefly, sera were treated with receptor destroying enzyme (RDE, Denka Seiken) overnight at 37 °C followed by 30 min at 56°C. Then, two- fold serial diluted sera were mixed with 4 HA units of inactivated A/Aichi virus for 15 min. 0.5% Turkey red blood cells (Lampire Biological Laboratories) were added and agglutination was observed after 30 min.
Confocal laser scanning micrograph (CLSM)
Fluorescence imaging samples were prepared as previously described. BMDCs were seeded on a small coverslip in 6-well plates and treated with Alexa fluor 488 (TermoFisher Scientific) conjugated AuNP-HA/AuNP-FliC through primary amines of proteins and N-Hydroxysuccinimide (NHS) ester moiety of Alexa Fluor 488 dye. After incubation for 2 h at 37°C, the cells were washed with PBS three times and then slides were mounted in mounting media containing DAPI. Images were obtained with a Zeiss (LSM 700) confocal laser scanning microscope.
Immunization and challenge
Groups of 6–8-week-old female BALB/c mice (Envigo, n=5) were intranasally (i.n.) immunized twice at an interval of 4 weeks with: AuNPs, AuNP-HA/AuNP-FliC (5 μg HA and 0.25 μg FliC), AuNP-HA, or soluble HA/FliC. Four weeks after the boosting immunization, mice were challenged with 50 times the 50% lethal dose (50× LD50) of mouse-adapted A/Aichi/2/68 (H3N2) virus in 50 μL of PBS per mouse. Mice were monitored daily for body weight changes and survival over 14 days. A weight loss exceeding 20% was used as the experimental endpoint. All animal studies were approved by Georgia State University’s IACUC.
Sample collection
Cellular immune responses were assessed by isolation of splenocytes from immunized mice 4 weeks post the boosting immunization. The spleens were processed into single cell suspensions and seeded into round-bottom 96-well plates (106 cells/well in triplicate) and stimulated with 4 μg/mL inactivated Aichi virus for 36 h. IFN-γ, IL2, IL-4, IL-5, and IL-17A in the culture supernatants were measured by using cytokine kits (Biolegend) following the manufacturer’s instruction.
Bone marrow (BM) DCs obtained from femoral and tibial bones of naive mice were plated at 2 ×105 cells/mL and incubated for six days in RPMI-1640 supplemented with: 10% FBS, 10 ng/mL recombinant murine GM-CSF (R&D Systems), 400 mM L- glutamine, and 100 U of penicillin/streptomycin. The culture medium was replaced with fresh medium every 2 days. Nasal washes, tracheal washes and bronchoalveolar lavage fluid (BALF) were collected by flushing the respective cavities with 1 ml PBS. The upper palates of nasal cavity were removed and the NALTs were isolated under the microscope. NALT cells were suspended and recovered by centrifugation30. The Blood samples were collected on day 21 and day 49 by facial vein bleeding. After clotting and centrifugation, serum samples were collected and stored at −80°C prior to use for assays.
Cytokines in BALF and lung viral titers
Five days post infection, mice were euthanized for BALF and lung collection. Inflammatory cytokines levels (IL-6, IL-12, TNF-α) in BALF were measured by using cytokine kits (Biolegend) following the manufacturer’s instruction. Lung homogenates were prepared in RPMI-1640 medium and viral titers were evaluated using Madin-Darby canine kidney (MDCK, ATCC: PTA-6500) cell-based plaque assay and calculated with the Reed-Muench method as previously described.31
Antibody titration
Antigen-specific antibody endpoint titers in serum, nasal wash, tracheal wash, and BALF, including IgA, IgG and subtypes (IgG1, IgG2a), were determined by ELISA.17 In brief, plates were: coated overnight at 4°C with inactivated A/Aichi virus (100 μL per well, concentration is 4 μg/mL), washed with PBST, loaded with serial dilutions of samples (as the primary antibodies), and incubated for 2 h at 37°C. Horseradish peroxidase (HRP)-conjugated anti- mouse IgG/IgA was added as the secondary antibody and incubated for 1h at 37°C. 3, 3′, 5, 5′-tetramethylbenzidine (TMB) was used as the substrate. The reaction was stopped with 2 M hydrochloric acid and the absorbance was measured at 470 nm using a microplate reader.
Flow cytometry
Single-cell suspensions were obtained by gentle mechanical disruption of lymph nodes or spleens. Cells were stained in fluorescence-activated cell sorting buffer with the appropriate combination of the following anti- mouse antibodies: PE/Cy7-CD4, FITC-CD8α, PerCP-Cy5.5-CD3ε, APC-IFN-γ, APC/Cy7-CD107a, and mAb isotype controls (Biolegend). For intracellular staining, cells were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Bioscience). Samples were analyzed with a BD Fortessa flow cytometer (BD biosciences). Data were analyzed with the FlowJo software suite (FlowJo LLC).
ELISPOT assay
ELISPOT assays were carried out as described previously.17 In brief, multiscreen 96-well filtration plates (Millipore) were coated with inactivated Aichi virus overnight at 4°C, then incubated overnight at 37 °C with 100 μL of splenocytes (106 cells/mL) added to each well, and then incubated with HRP-conjugated anti- mouse IgG/IgA antibody for 1 h at room temperature. 3, 3-Diaminobenzidine substrate was used to develop spots.32 Results were counted using a Bioreader-6000-E (BIOSYS).
Statistical Analysis
Statistical analysis was carried out using Student’s independent t-test. A value of P<0.05 was significant. * p<0.05, **p<0.01, ***p<0.005.
Results
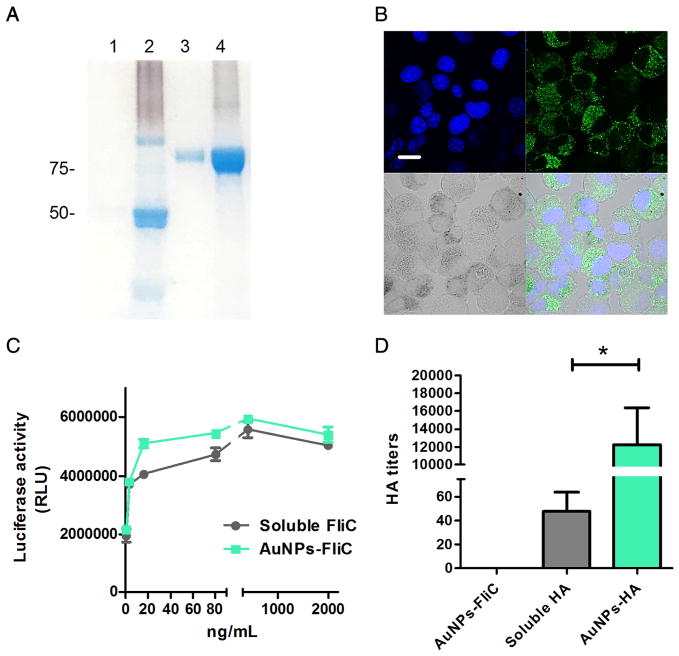
We conjugated His-tagged trimeric HA onto NTA-SH-AuNPs via a metal chelating reaction. Alkyne-FliC was attached to azido-PEG-SH-AuNPs through the click chemistry reaction, as described above. Particles were washed and re-suspended in PBS. To evaluate the stability, protein conjugated nanoparticles were centrifuged after 3-month storage at 4 °C. Supernatants and pellets (conjugated proteins were separated from AuNPs by cleaving the linkers using 11- mercapto-1-undecanol) using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and Coomassie Blue staining (Figure 1A). Little to zero soluble FliC (50 kDa, lane 1) or HA (72 kDa, lane 3) was detected in supernatant, demonstrating that both reactions resulted in strong and stable conjugation events.
Figure 1.
Characterization of HA and FliC- functionalized AuNPs. (A) SDS-PAGE of AuNP-HA and AuNP-FliC after 3- month storage. Lane 1: supernatants of AuNP-FliC; lane 2: pellet of AuNP-FliC; lane 3: supernatants of AuNP-HA; lane 4: pellet of AuNP-HA. (B) CLSM image. BMDCs were incubated with Alexa 488-labeled AuNP-HA/AuNP-FliC for 2 h. Nanoparticles were recognized as black dots in the bright field. The nucleus was stained blue with DAPI. The scale bar represents 20 μm. (C) TLR-5 specific bioactivity of AuNP-FliC and soluble FliC with increased FliC concentration. (D) HA titers of soluble HA, AuNP-HA and AuNP-FliC. Significant difference: *p<0.05.
To assess the internalization efficiency, we incubated fluorophore (Alexa Fluor 488, AF488)- loaded AuNP-HA/AuNP-FliC with bone- marrow-derived DCs in vitro for 2 h and analyzed the cells with confocal laser scanning micrograph. Figure 1B shows that the AuNP-HA/AuNP-FliC treatment had fluorescence signal in DCs. We also investigated the functionality and integrity of the two proteins after conjugation to AuNPs. Similar to soluble FliC, AuNP-FliC induced dose-dependent increases of luciferase activity triggered by the TLR5 signaling pathway in HEK 293 T cells (Figure 1C). HA assay results (Figure 1D) indicated that the AuNP-HA formulation led to higher HA titers compared with soluble HA (213 vs. 28 per 12 μg HA in 50 μL).
After conjugation, the hydrodynamic diameters of AuNPs increased from 18 nm (AuNPs) to 47 nm (AuNP-FliC) and 106 nm (AuNP-HA), as determined by dynamic light scattering (DLS, Figure S3 and Table S1). The particles morphology was confirmed by transmission electron microscopy (TEM, Figure S3). Conjugated protein quantification was determined by using two methods (Table S1). Overall, these characterization data revealed that resulting nanoparticles were functional in remaining features of the conjugated molecules, and the conjugating events were successful.
Our previous studies have shown chimeric virus- like particles containing 10 μg HA and/or 0.5–0.8 μg FliC could elicit robust immune responses.17,33 Although a different nanoparticle carrier platform was used in this study, we decided the dosing here based on this experience. We reduced the FliC dose to 0.25 μg to minimize systemic inflammation and test whether the AuNPs carrier could enhance the delivery efficacy of FliC adjuvants.
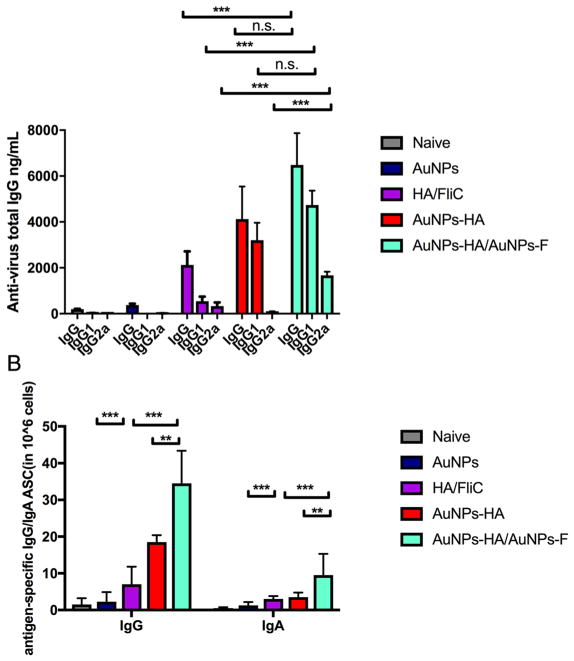
To determine the immunogenic improvement of AuNP formulations versus soluble HA, groups of BALB/c mice were immunized i.n. on days 0 and 28 with resulting experimental nanoparticle vaccines: AuNP-HA/AuNP-FliC, AuNP-HA, or a soluble mixture of HA and FliC. AuNP-HA/AuNP-FliC elicited higher levels of antigen-specific serum IgG antibodies (Figure 2A) compared with the other formulations. Our results indicated sole components (AuNPs, AuNP-FliC, AuNP-HA) could not induce humoral/cellular immunity nor fully protect hosts against lethal dose virus challenge; only AuNP-HA/AuNP-FliC co-delivery had synergistic, protective immune responses.
Figure 2.
Systemic immune responses. Mice were i.n. immunized with different formulations containing 5 μg of HA and 0.25 μg of FliC on day 1 and day 28. Blood was collected on day 49. ELISA plates were coated with 100 μL 4 μg/mL of inactivated A/Aichi/2/68 virus. HRP-conjugated goat anti- mouse IgG was used for detection. (A) serum IgG, IgG1, and IgG2a levels (means±SEM, n=5). (B) antigen specific IgG and IgA ASCs in the spleen measured on day 56. Results shown are the mean ASC frequency per 1×106 cells±SEM of quadruplicate wells. Data represent means ±SEM from triplicate cultures. Significant difference: *p<0.05; **<0.01, ***p<0.005, n.s. no significant difference.
Well-orientated, high-density antigen displaying particles are benefit to improve antibody level and activate T cells.34,35. Our AuNP-HA formulation, with high antigen conjugation efficiency, resulted in a marked increase of antibody response compared to the soluble mixture. However, without adjuvants, AuNP-HA alone elicited a typical Th2 type immune response characterized by the ratio of IgG1 to IgG2a (IgG1/IgG2a=35).2 The AuNP-HA/AuNP-FliC immunization resulted in a more comprehensive humoral response, as indicated by the increase of IgG2a subclass responses. The isotype switching revealed that co-delivery of AuNP-FliC and AuNP-HA enhanced the Th1 and Th2 responses compared with the soluble HA/FliC (p < 0.005 for IgG1 and IgG2a) and AuNP-HA groups (p < 0.005 for IgG2a). When both HA and FliC were conjugated onto the same nanoparticle, it induced a comparable level of antibody response compared with HA and FliC in separate nanoparticles (Figure S1).
Figure 2 shows the presence of antigen-specific antibody-secreting cells (ASCs) in the spleen. We collected splenocytes 1 week following the boosting immunization, stimulated them with inactivated Aichi virus, and analyzed the reactions with ELISPOT assays (Figure 2B). AuNP-HA/AuNP-FliC elevated IgG (p < 0.005) and IgA (p < 0.05) antigen-specific ASC counts in mice compared with AuNP-HA alone or the soluble antigen-adjuvant mixture.
Figure 3 shows the IgG and IgA production in various mucosal sites, including nasal, tracheal, and BALF. AuNP-HA/AuNP-FliC induced greater mucosal IgA and IgG in the nasal (p < 0.005 for IgG, p < 0.05 for IgA), tracheal (p < 0.005 for IgG), and lung (p < 0.005 for both IgG and IgA) samples compared with the soluble mixture of HA and FliC.
Figure 3.
Mucosal immune responses. Antigen-specific IgG and IgA endpoint titers in nasal washes, tracheal washes, and BALF were measured on day 56. Data represent means ±SEM from triplicate cultures. Significant difference: *p<0.05; **<0.01, ***p<0.005, n.s. no significant difference.
Intranasal co-delivery of AuNP-HA and AuNP-FliC increased levels of systemic IgG and mucosal IgA compared with the mixture of soluble HA and FliC, suggesting that particulate HA and FliC work in an additive fashion to induce antibody responses. AuNP-HA alone enhanced serum IgG and induced a Th2-type immune response versus soluble HA/FliC. The data shows antigen coated AuNPs contain inherent adjuvant activity, which can synergize with TLR-stimulating adjuvants such as FliC when delivered through an intranasal route.
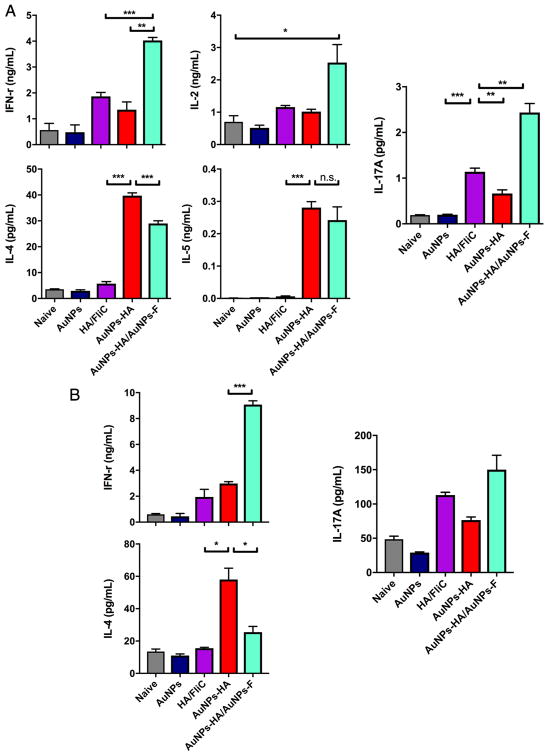
After the two immunizations, we isolated splenocytes and then stimulated them with inactivated virus. the cytokine levels of Th1-type (IFN-γ and IL2), Th2-type (IL-4 and IL-5), and Th17-type (IL-17A) in the culture supernatants. AuNP-HA immunization increased Th2 cytokine production compared with the soluble HA/FliC mixture (Figure 4A, p < 0.005), but not the IFN-γ level. AuNP-HA/AuNP-FliC vaccination enhanced both Th1 and Th2 cytokine production, which is important because Th1 type responses —such as CTL— protect the hosts against intracellular pathogens. Figure 4A shows our measurements of IL-17A; the soluble HA and FliC mixture increased IL-17A production (p < 0.005 vs naïve or AuNPs) and co-delivery of AuNP-HA and AuNP-FliC further enhanced the Th-17 cytokine response (p < 0.01 vs soluble mixture).
Figure 4.
Cytokine profile. Splenocytes (A) and lymphocytes from NALTs (B) isolated 4 weeks after the boosting immunization, and were re-stimulated inactivated Aichi virus. Culture media were collected after 18 h incubation and analyzed for Th1-type cytokines (IFN-γ, IL-2), Th2-type cytokines (IL-4, IL-5), and Th17-type cytokine (IL-17A). Significant difference: *p<0.05, **<0.01, ***p <0.005, n.s. no significant difference (means±SEM, n=2–3).
We also saw nasal vaccination with AuNPs elicited strong, sustained systemic and local adaptive immune responses. We isolated lymphocytes from NALTs and then stimulated them with inactivated virus overnight for cytokine measurement. Figure 4B shows AuNP-HA/AuNP-FliC immunization induced higher levels of IFN-γ and IL-17A but the AuNP-HA immunization induced more IL-4. Th-2 type immune responses and IL-4 production are induced when APCs recognize repetitive antigens —such as the multivalent display of HA on our nanoparticles— through pattern recognition receptors.36 The higher levels of IL-4 induced by the AuNP-HA vaccination may be the result of strong pattern recognition receptor activation in the absence of the counterbalancing Th1 responses induced by AuNP-FliC.
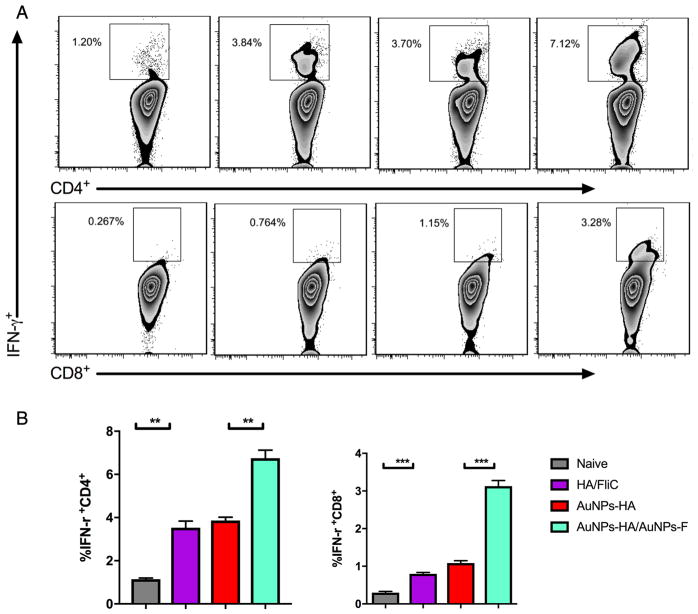
Having established AuNP-HA/AuNP-FliC enhanced antigen-specific antibody levels, we assessed (by flow cytometry) intracellular IFN-γ expression in vaccinated mice. AuNP-HA/AuNP-FliC immunization increased the number of IFN-γ-secreting CD4+ T and CD8+ T cells from the cervical lymph nodes (Figure 5, p < 0.005 vs naive). The soluble mixture of HA and FliC increased the IFN-γ secreting immune cell population compared with the naïve group (p < 0.01). AuNP-HA/AuNP-FliC also induced a higher population of IFN-γ secreting cells in the spleen (Figure S2).
Figure 5.
Antigen specific CD4+ and CD8+ T cell responses. Cervical lymph nodes were collected after the boosting immunization and re-stimulated with inactivated virus. Representative flow cytometry plots (A) and percentages (B) of IFN-γ+CD4+ and IFN-γ+CD8+ T cells in cervical lymph nodes after different vaccination strategies. Significant difference: **p<0.01; ***p<0.005 (means±SEM, n=2–3).
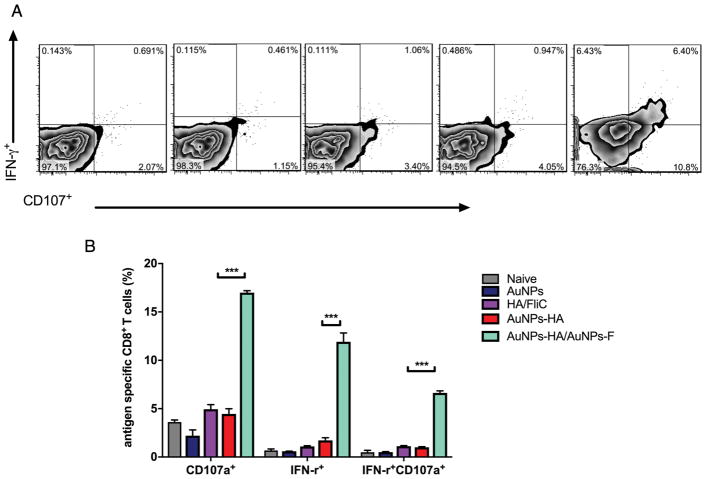
Next, we tested whether the antigen-specific CD8+ T cells exhibited comprehensive cytotoxicity functions by measuring CD107a.37 After re-stimulation with inactivated virus for 6 h, we stained splenocytes for CD8, IFN-γ, and CD107a. The soluble mixture and the AuNP-HA alone did not change T cell populations but AuNP-HA/AuNP-FliC immunization increased the percentage of IFN-γ+CD107a+ cells (Figure 6, 6.40%±1.6 %) among the total CD8+ T cells. AuNP-FliC was the difference that stimulated the proliferation of CD8+ CTLs.
Figure 6.
Antigen-specific CD8+ T cells function. Splenocytes were isolated after the boosting immunization and were re-stimulated ex vivo with inactivated virus for 6 h. Representative flow cytometry plots (A) and percentages (B) of IFN-γ+CD107+ cells among CD8+ T cells after different vaccination strategies. Significant difference: ***p<0.005 (means±SEM, n=2–3).
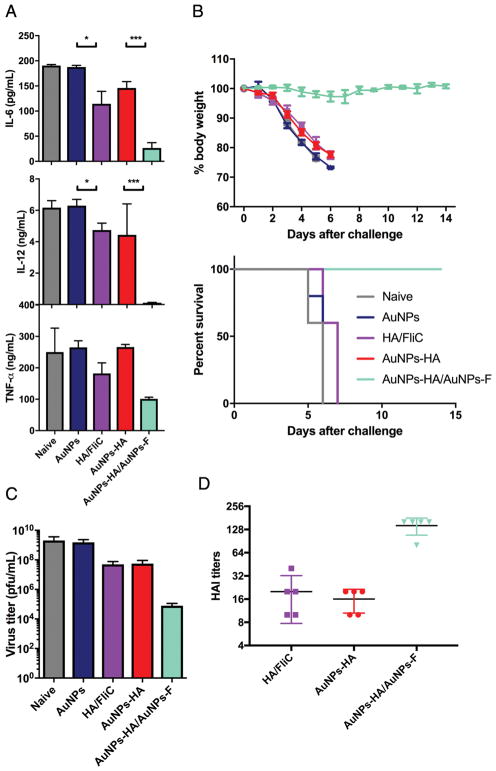
We challenged immunized mice with 50x LD50 of mouse-adapted Aichi virus to investigate whether the above immunity induced by our AuNPs confers protection. Mice in naïve, AuNPs only, soluble mixture, and AuNP-HA groups showed high weight loss and died by day 5 post-challenge but mice immunized with AuNP-HA/AuNP-FliC were fully protected (Figure 7B). AuNP-HA/AuNP-FliC vaccination also enhanced the HAI antibody responses in serum (Figure 7D) and reduced the lung virus titers (Figure 7C) —two parameters correlated with immune protection from influenza vaccines.
Figure 7.
A/Aichi/2/68 viral challenge. (A) the level of inflammatory cytokines in the BALF at 5 dpi. (B) the average body weight loss and the survival rates from the time of infection to 14 days (means±SEM, n=5). (C) virus titers in total lung homogenates at 5 dpi. (D) serum HAI titers against the A/Aichi/2/68 virus. Significant difference: *p<0.05; ***p<0.005 (means±SEM, n=2–3).
We also evaluated the pulmonary immunopathology induced by infection by measuring IL-6, IL-12, and TNF-α in the BALF of immunized mice. Soluble HA/FliC immunization reduced IL-6, IL-12, and TNF-α compared with the naïve or AuNP-HA-immunizations but AuNP-HA/AuNP-FliC decreased these cytokines even more (Figure 7A). This shows that AuNP-FliC is adjuvanting for AuNP-based vaccination against influenza virus infection.
Discussion
Influenza vaccination must augment antigen-specific humoral and cellular immunity at mucosal sites of entry because influenza infiltrates the body and initiates infection via mucosal surfaces.38 Intranasal vaccination with AuNP-HA/AuNP-FliC elicited stronger systemic and mucosal humoral response, compared with soluble HA/FliC. This is partially attributed to the enhanced crosslinking of multiple immunoglobulins on naïve B cell surface by using particulate formulations.39
Compared with other treatment groups, AuNP-HA/AuNP-FliC vaccination established a higher IgG2a/IgG1 ratio. It is known that effective antibody responses to protein antigens (such as appropriate Ig isotype-switching) require helper CD4+ T cells.40 The uptake of AuNP-HA/AuNP-FliC by APCs enhanced the Th1 response.41 As an adjuvant, FliC upregulated the expression of costimulatory molecules and cytokine secretion by activating TLR5 innate signaling pathways. This improved the maturation of APCs and initiated robust activation of subsequent immune responses, including helper CD4+ T cells proliferation.
The increased number of IFN-γ-producing CD4+ T cells also confirms the Th1-dominant cell responses to AuNP-HA/AuNP-FliC vaccination. Without AuNP-FliC, AuNP-HA induced a comparable antigen-specific antibody titer but not a favorable IgG1/IgG2a ratio nor an elevated promotion of cellular immunity —highlighting the essential role of FliC adjuvant and TLR signaling in antigen presentation enhancement and polarizing adaptive immune responses.42
Cytotoxic T lymphocyte (CTL) responses are essential for controlling intracellular infections but infected cells can not initiate CTL response by themselves.43 APCs transport these antigens from infected sites to LNs, where they present the antigens on their surfaces to activate CD8+ T cells.44 Nanoparticle carriers help DCs (typical APCs) phagocytose and translocate antigens into phago-endosomal compartments, inducing efficient cross-presentation and CTL responses.45 Co-delivery of antigen and adjuvant using AuNPs enabled the potentiation of more CTL response. We hypothesize that the reducible coupling between AuNPs surfaces and antigens allows for the cleavage of the bonds within the intracellularly reductive environment, leading to more efficient endosomal escape of antigens to the cytoplasm of DCs.46 Particulate delivery of FliC via AuNPs activated TLR5 signaling and resulted in enhancement of DC maturation and migration to draining LNs.
For nanoparticle vaccine carriers, particle size plays a significant role in antigen localization and subsequent adaptive immune responses.47 70 and 100 nm silica particles enhanced exogenous antigen entry into the cytosol from endosomes and resulted in antigen cross-presentation but 300nm and 1000 nm particles did not.48 For gold nanoparticles, a smaller size promoted endosomal escape of antigens and cytoplasm localization but larger sized AuNPs remained in the endocytic compartments.49
We encapsulated HA and FliC on a single nanoparticle (through metal chelating and click chemistry reactions), tested the relative immune response of the larger resultant particles, and compared the results to co-delivery of separate antigen and adjuvant nanoparticles. Although we detected similar IgG antibody levels in both delivery strategies (Figure S1), co-encapsulation on a single nanoparticle did not stimulate comparable CD8+ T-cell response (data not shown). The high aggregation potential and size increase after two different protein conjugations accounts for this difference (Figure S3 and Table S1).50
Various fundamental studies have reported the nontoxicity of AuNPs.8,51 Although we did not conduct the cell cytotoxicity assays of AuNPs in current study, we did not see any cytotoxicity from AuNP during cell sample preparation in cellular uptake imaging nor in flow cytometry assays —indicating that AuNPs do not exhibit apparent cytotoxicity.
AuNP-FliC enabled a focused delivery of adjuvant, enhanced the immunogenicity of co-administered vaccines, and decreased the formation of systemic inflammation. Our ex vivo data shows AuNP-HA/AuNP-FliC immunization promoted not only Th1- and Th2-, but also Th17-type cellular immunity. A growing body of evidence generated from in vitro studies has indicated that IL-17 has a pro-inflammatory function for inducing IL-6, GM-CSF, and other related chemokines by fibroblasts and epithelial cells.52 However, it has also be reported that overexpression of IL-17 in the lung may be functionally associated with hepatic granulomatous inflammation.53 Our results indicate that AuN-FliC enable a focused delivery of adjuvant and hence enhance the immunogenicity of co-administered vaccines while preventing the overexpression of IL-17 and decreasing the formation of systemic inflammation.
The development of potent and safe subunit vaccines that promote cellular immunity remains of great importance. We explored if conjugation of adjuvant molecules to AuNPs enhanced adaptive immunity while minimizing the adjuvant dose. Our vaccine with AuNP-HA/AuNP-FliC enhanced activation of DCs, leading to a higher frequency of presenting and cross-presenting DCs in draining LNs and spleens. We saw a comprehensive immune response that utilized both the antibody and cellular branches of immunity and provided complete protection against high dose influenza virus challenges.
Supplementary Material
Acknowledgments
Role of funding source
The study was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R01AI101047 and R01AI116835 to BZW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
There are no conflicts of interests identified by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moon JJ, Suh H, Bershteyn A, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–51. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proietti E, Bracci L, Puzelli S, et al. Type I IFN as a Natural Adjuvant for a Protective Immune Response: Lessons from the Influenza Vaccine Model. The Journal of Immunology. 2002;169:375. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- 3.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proceedings of the National Academy of Sciences. 2012;109:1080–5. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical Reviews. 2015;115:11109–46. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhesingh RS, Thambiraj S, Hema S. An overview on applications of gold nanoparticle for early diagnosis and targeted drug delivery to prostate cancer. Recent Pat Nanotechnol. 2017 doi: 10.2174/1872210511666171101120157. [DOI] [PubMed] [Google Scholar]
- 6.Cao-Milan R, Liz-Marzan LM. Gold nanoparticle conjugates: recent advances toward clinical applications. Expert Opinion on Drug Delivery. 2014;11:741–52. doi: 10.1517/17425247.2014.891582. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, McMahon SJ, Paganetti H, Schuemann J. Biological modeling of gold nanoparticle enhanced radiotherapy for proton therapy. Phys Med Biol. 2015;60:4149–68. doi: 10.1088/0031-9155/60/10/4149. [DOI] [PubMed] [Google Scholar]
- 8.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 9.De M, Ghosh PS, Rotello VM. Applications of Nanoparticles in Biology. Advanced Materials. 2008;20:4225–41. [Google Scholar]
- 10.Rasheed PA, Sandhyarani N. Femtomolar level detection of BRCA1 gene using a gold nanoparticle labeled sandwich type DNA sensor. Colloids Surf B Biointerfaces. 2014;117:7–13. doi: 10.1016/j.colsurfb.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Hamme AT., 2nd Gold Nanoparticle Labeling Based ICP-MS Detection/Measurement of Bacteria, and Their Quantitative Photothermal Destruction. J Mater Chem B. 2015;3:3573–82. doi: 10.1039/C5TB00223K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao W, Ziemer KS, Gill HS. Gold nanoparticle–M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine (London, England) 2014;9:237–51. doi: 10.2217/nnm.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Hess A, Chang TZ, et al. Nanoclusters self-assembled from conformation-stabilized influenza M2e as broadly cross-protective influenza vaccines. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:473–82. doi: 10.1016/j.nano.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai C-H, Tang N, Jan J-T, et al. Use of recombinant flagellin in oil- in-water emulsions enhances hemagglutinin-specific mucosal IgA production and IL-17 secreting T cells against H5N1 avian influenza virus infection. Vaccine. 2015;33:4321–9. doi: 10.1016/j.vaccine.2015.03.082. [DOI] [PubMed] [Google Scholar]
- 15.Kiyono H, Fukuyama S. NALT- versus PEYER’S-patch- mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox CB, Kramer RM, Barnes VL, Dowling QM, Vedvick TS. Working together: interactions between vaccine antigens and adjuvants. Therapeutic Advances in Vaccines. 2013;1:7–20. doi: 10.1177/2051013613480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B-Z, Xu R, Quan F-S, Kang S-M, Wang L, Compans RW. Intranasal Immunization with Influenza VLPs Incorporating Membrane-Anchored Flagellin Induces Strong Heterosubtypic Protection. PLoS ONE. 2010;5:e13972. doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong C. Diversification of T- helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–34. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 19.Skountzou I, Martin MdP, Wang B, et al. Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine. 2010;28:4103–12. doi: 10.1016/j.vaccine.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song L, Xiong D, Hu M, Kang X, Pan Z, Jiao X. Enhanced humoural and cellular immune responses to influenza H7N9 antigen HA1-2 fused with flagellin in chickens. BMC Vet Res. 2017;13:190. doi: 10.1186/s12917-017-1106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dakterzada F, Mohabati Mobarez A, Habibi Roudkenar M, Mohsenifar A. Induction of humoral immune response against Pseudomonas aeruginosa flagellin(1–161) using gold nanoparticles as an adjuvant. Vaccine. 2016;34:1472–9. doi: 10.1016/j.vaccine.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Xiong D, Hu M, Kang X, Pan Z, Jiao X. Immunopotentiation of Different Adjuvants on Humoral and Cellular Immune Responses Induced by HA1-2 Subunit Vaccines of H7N9 Influenza in Mice. PLoS One. 2016;11:e0150678. doi: 10.1371/journal.pone.0150678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng L, Kim JR, Chang TZ, et al. Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge. Virology. 2017;509:82–9. doi: 10.1016/j.virol.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Zhu W, Wang BZ. Dual- linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. Int J Nanomedicine. 2017;12:4747–62. doi: 10.2147/IJN.S137222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M-X, Huang B-H, Sun X-Y, Pang D-W. Clickable Gold Nanoparticles as the Building Block of Nanobioprobes. Langmuir. 2010;26:10171–6. doi: 10.1021/la100315u. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y-H, Jeon J, Hong SH, et al. Tumor Targeting and Imaging Using Cyclic RGD-PEGylated Gold Nanoparticle Probes with Directly Conjugated Iodine-125. Small. 2011;7:2052–60. doi: 10.1002/smll.201100927. [DOI] [PubMed] [Google Scholar]
- 27.Weldon WC, Wang B-Z, Martin MP, Koutsonanos DG, Skountzou I, Compans RW. Enhanced Immunogenicity of Stabilized Trimeric Soluble Influenza Hemagglutinin. PLOS ONE. 2010;5:e12466. doi: 10.1371/journal.pone.0012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Manual on Animal Influenza Diagnosis and Surveillance. http://www.wpro.who.int/emerging_diseases/documents/manual_on_animal_ai_diagnosis_and_surveillance/en/2002.
- 29.Compans RW. Hemagglutination-Inhibition: Rapid Assay for Neuraminic Acid-Containing Viruses. Journal of Virology. 1974;14:1307–9. doi: 10.1128/jvi.14.5.1307-1309.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagatomo D, Taniai M, Ariyasu H, et al. Cholesteryl Pullulan Encapsulated TNF-alpha Nanoparticles Are an Effective Mucosal Vaccine Adjuvant against Influenza Virus. Biomed Res Int. 2015;2015:471468. doi: 10.1155/2015/471468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haredy AM, Takenaka N, Yamada H, et al. An MDCK Cell Culture-Derived Formalin-Inactivated Influenza Virus Whole-Virion Vaccine from an Influenza Virus Library Confers Cross-Protective Immunity by Intranasal Administration in Mice. Clinical and Vaccine Immunology: CVI. 2013;20:998–1007. doi: 10.1128/CVI.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo L, Lu X, Kang S-M, Chen C, Compans RW, Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313:502–13. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- 33.Wang B-Z, Quan F-S, Kang S-M, Bozja J, Skountzou I, Compans RW. Incorporation of Membrane-Anchored Flagellin into Influenza Virus-Like Particles Enhances the Breadth of Immune Responses. Journal of Virology. 2008;82:11813–23. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus- like particles. J Immunol. 2002;169:6120–6. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- 35.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachmann MF, Zinkernagel RM. The influence of virus structure on antibody responses and virus serotype formation. Immunol Today. 1996;17:553–8. doi: 10.1016/s0167-5699(96)10066-9. [DOI] [PubMed] [Google Scholar]
- 37.Nembrini C, Stano A, Dane KY, et al. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proceedings of the National Academy of Sciences. 2011;108:E989–E97. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodewes R, Fraaij PLA, Kreijtz JHCM, et al. Annual influenza vaccination affects the development of heterosubtypic immunity. Vaccine. 2012;30:7407–10. doi: 10.1016/j.vaccine.2012.04.086. [DOI] [PubMed] [Google Scholar]
- 39.Gregory A, Williamson ED, Prior JL, Butcher WA, Thompson IJ, Shaw AM, Titball RW. Conjugation of Y. pestis F1-antigen to gold nanoparticles improves immunogenicity. Vaccine. 2012;30:6777–82. doi: 10.1016/j.vaccine.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu YJ, Malisan F, de Bouteiller O, et al. Within Germinal Centers, Isotype Switching of Immunoglobulin Genes Occurs after the Onset of Somatic Mutation. Immunity. 1996;4:241–50. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 41.Mizel SB, Bates JT. Flagellin as an Adjuvant: Cellular Mechanisms and Potential. Journal of immunology (Baltimore, Md: 1950) 2010;185:5677–82. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atif SM, Uematsu S, Akira S, McSorley SJ. CD103-CD11b+ dendritic cells regulate the sensitivity of CD4 T-cell responses to bacterial flagellin. Mucosal Immunol. 2014;7:68–77. doi: 10.1038/mi.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang HK, Kim HI, Kim SH, et al. Prognostic impact of the tumor- infiltrating regulatory T-cell (Foxp3+)/activated cytotoxic T lymphocyte (granzyme B+) ratio on resected left-sided pancreatic cancer. Oncol Lett. 2016;12:4477–84. doi: 10.3892/ol.2016.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rock KL. The ins and outs of cross-presentation. Nat Immunol. 2003;4:941–3. doi: 10.1038/ni1003-941. [DOI] [PubMed] [Google Scholar]
- 45.Heffernan MJ, Kasturi SP, Yang SC, Pulendran B, Murthy N. The stimulation of CD8+ T cells by dendritic cells pulsed with polyketal microparticles containing ion-paired protein antigen and poly(inosinic acid)–poly(cytidylic acid) Biomaterials. 2009;30:910–8. doi: 10.1016/j.biomaterials.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster S, Duvall CL, Crownover EF, Hoffman AS, Stayton PS. Intracellular Delivery of a Protein Antigen with an Endosomal- Releasing Polymer Enhances CD8 T-Cell Production and Prophylactic Vaccine Efficacy. Bioconjugate Chemistry. 2010;21:2205–12. doi: 10.1021/bc100204m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang W, KimBetty YS, Rutka JT, ChanWarren CW. Nanoparticle- mediated cellular response is size-dependent. Nat Nano. 2008;3:145–50. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 48.Hirai T, Yoshioka Y, Takahashi H, et al. Amorphous silica nanoparticles enhance cross-presentation in murine dendritic cells. Biochemical and Biophysical Research Communications. 2012;427:553–6. doi: 10.1016/j.bbrc.2012.09.095. [DOI] [PubMed] [Google Scholar]
- 49.Niikura K, Matsunaga T, Suzuki T, et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano. 2013;7:3926–38. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 50.Ilyinskii PO, Roy CJ, O’Neil CP, et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine. 2014;32:2882–95. doi: 10.1016/j.vaccine.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albanese A, Chan WC. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011;5:5478–89. doi: 10.1021/nn2007496. [DOI] [PubMed] [Google Scholar]
- 52.Stark MA. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T Cell-Mediated Immunopathology in Murine Schistosomiasis Is Dependent on IL-12p40 and Correlates with High Levels of IL-17. The Journal of Immunology. 2005;175:3920. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.