Abstract
Background:
Traumatic Stressful Events (TSE) during childhood and adolescence are associated with increased risk for psychopathology and with cognitive impairment. Aberrations in social cognition may contribute to the psychopathology risk. We examined performance differences on social cognitive measures between youth with high TSE exposure and no TSE exposure, and how these effects vary in females and males.
Methods:
The Philadelphia Neurodevelopmental Cohort (PNC) investigates clinical and cognitive phenotypes in a US youth (aged 8–21) community population. Here we compare performance in social cognition tasks between youth with high exposure (≥3 TSE, N=830) and youth with no exposure (N=5202). Three social cognition tasks were analyzed: (1) Age Differentiation, (2) Emotion Identification (happy, sad, angry, fearful, or neutral) and (3) Emotion Intensity Differentiation (happy, sad, angry, and fearful).
Results:
A significant TSE Group by Sex interaction was observed in all social cognitive tasks. In the Emotion Identification task, males with high traumatic stress exposure out-performed non-exposed males; exposure did not impact performance in females. In the Emotion Intensity Differentiation task, females with high traumatic stress exposure performed worse than non-exposed females, with no difference in males between exposure groups. Exploratory analyses revealed sex differences were driven by improved identification of angry expressions in stress exposed males and poorer performance in differentiating intensity of happy expressions in stress exposed females.
Conclusion:
Exposure to high levels of early life traumatic stress was associated with sex-specific differences in social cognition. These findings might be related to the sex specific patterns of psychopathology emerging during adolescence.
Keywords: Traumatic Stress, Social Cognition, Developmental Psychopathology, Sex Differences, Childhood Trauma, Emotion Processing
Introduction
The association between traumatic or stressful events during childhood and the subsequent development of psychopathology is well established (1, 2). Mounting evidence suggests that, in clinical psychiatric populations, exposure to traumatic stress early in life has detrimental developmental effects that are often associated with a more severe course of illness (3–5) and decreased treatment response (6– 9). Despite the well-established link between trauma and psychopathology, more research is needed to elucidate the mechanisms of this association. If processing of social cues is affected by stress exposure, then social cognitive capacities may in part underpin the association between early life stress and psychopathology (10).
Exposure to stress in childhood is associated with aberrant social cognitive abilities, including identification of emotional expressions (10, 11). Studies have reported that exposure to traumatic stress disrupts a child’s ability to properly identify or process emotional facial expressions (11, 12). For example, compared to their non-exposed peers, children with a history of maltreatment perform worse at recognizing sad expressions (13, 14). Conversely, another line of research supports the notion that a history of traumatic stress may actually facilitate some types of social-emotional processing. Compared to non-exposed youth, those with traumatic stress exposure are reported to require less perceptual information to identify angry expressions (14, 15), detect fearful expressions at lower intensities (16), and preferentially allocate attention to angry (17) or sad expressions (18). Other studies find no link between stress exposure and emotion processing (19). Moreover, while many studies do not examine sex differences, some report that the effects of stress exposure on social cognition differs in males and females (20, 21). More work is needed to understand if there are sex differences in the effects of stress exposure on social cognition (22).
Studies of healthy youth show that social cognitive capacity improves with age throughout development (23, 24) and is better in females compared to males (25, 26). Traumatic stress exposure and Post Traumatic Stress Disorder (PTSD) have also been linked to social-emotional processing abnormalities (16, 27, 28). Given that stress-related disorders are more prevalent in females (29, 30), research is needed to address the possible roles of age, sex and the presence of PTSD to better understand the links between stress exposure and social cognition. Such linkage requires examining cognitive and clinical phenotypes in large samples of youth with substantial traumatic stress exposure. The Philadelphia Neurodevelopmental Cohort (PNC) is a unique resource with genetic, clinical, and neurocognitive data from a large community sample of youth, representative of the US urban population (31). We have recently shown that the PNC sample has substantial traumatic stress exposure that is associated with psychopathology (32). This study showed that traumatic stress exposure was associated with lower executive function and complex reasoning efficiency; however, the association with social cognition was more complex. Impairment in social cognitive tasks was evident only at the highest level of trauma exposure, and the linear association between trauma exposure and social cognitive dysfunction did not survive correction for covariates, suggesting a possible sex-specific pattern. Here, we aim to better understand this association and investigate specific social cognitive abilities and sex differences. We hypothesize that high traumatic stress exposure is associated with altered social cognitive performance across the different social cognitive constructs in a sex divergent manner. We evaluated links between high traumatic stress exposure during childhood and adolescence and social cognitive processing abilities in the presence or absence of PTSD.
Methods
Participants
The PNC is a collaboration between the Children’s Hospital of Philadelphia and the Brain Behavior Laboratory at the University of Pennsylvania (31). The sample (N=9498) is racially diverse (56% Caucasian, 33% African American and 11% other) and economically diverse (33), and about evenly divided between males and females. Notably, participants were recruited from the pediatric network and not from psychiatric clinics, and the sample is not enriched for individuals seeking psychiatric help.Enrollment criteria included (1) stable health; (2) proficiency in English; (3) physically and cognitively capable of completing interview and neurocognitive assessment; and (4) absence of a disorder that significantly impairs motility or cognition (e.g., paresis or palsy, intellectual disability).After complete description of the study, written informed consent was obtained from participants aged ≥ 18, and written assent and parental permission were obtained from children aged<18 and their parents/legal guardian. The University of Pennsylvania and CHOP Institutional Review Boards approved all procedures.
Clinical Assessment including Evaluation of Traumatic Stressful Events (TSE)
Psychopathology symptoms were evaluated using a structured screening interview (GOASSESS), as detailed elsewhere (34), which was based on the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (35). Computerized algorithms used endorsement of symptoms, their frequency and duration, and the presence of distress or impairment to approximate DSM-IV criteria of PTSD, conduct disorder and major depressive episode. Demographics of participants meeting DSM threshold criteria are detailed in supplemental data (Table S1-S3). The GOASSESS TSE screen assessed lifetime exposure to situations in which the participant: (1) experienced a natural disaster or (2) experienced a bad accident; (3) thought that s/he or someone close to him/her was killed or hurt badly; (4) witnessed someone getting killed, badly beaten, or die; (5) saw a dead body; or was ever her/himself a victim of one of the following assaults: (6) attacked or badly beaten, (7) threatened with a weapon, or (8) sexual assault. For 190 participants (2% of PNC), GOASSESS sections including TSE screening were missing and therefore they were excluded from analyses. In the current study, we compared participants with no TSE exposure (N=5204) to participants with high TSE exposure (endorsement of three or more TSE, N=830, Table 1), which is consistent with prior work (32, 36).
Table 1.
Demographic characteristics of study participants.
| No TSE exposure N=5202 |
High TSE exposure N=830 |
Test | t/X2 | P-value | |
|---|---|---|---|---|---|
| Age, years (SD) | 13.4 (3.6) | 16.6 (3) | T-Test | −24.6 | <.001 |
| Sex Male, N (%) | 2544 (48.9%) | 416 (50.1%) | Chi-square | .44 | .509 |
| SES z-score (SD) | .16 | −.53 | T-Test | −19.4 | <.001 |
| Caucasian, N (%) | 3243 (62.3%) | 277 (33.4%) | Chi-square | 272 | <.001 |
TSE= Traumatic Stressful Events.
Evaluation of social cognition-related measures
Cognitive assessment used the Penn Computerized Neurocognitive Battery (CNB), a well-established battery, which includes tasks assessing four cognitive domains: executive function, episodic memory, complex reasoning and social cognition (37, 38). The social cognition domain includes three tasks, two assessing central aspects of social-emotional processing and one non-emotional social processing task. The two social emotion processing tasks were Emotion Identification and Emotion Intensity Differentiation. The non-emotion related social task was Age Differentiation.
The Emotion Identification task evaluated participants’ ability to accurately identify different emotions. Participants were presented with photographs of faces displaying a happy, sad, angry, fearful, or neutral expression and asked to identify the emotion displayed on each face. Eight pictures of each facial expression were presented, balanced for poser’s age, sex, and ethnicity.
The Emotion Intensity Differentiation task measured participants’ accuracy in differentiating the intensity of four facial emotional expressions (happy, sad, angry, and fearful). Two expressions of the same poser appeared simultaneously on the screen, both representing the same emotional expression. For each pair, the level of emotional intensity was identical or one face displayed a higher intensity. Participants were asked to indicate which face had the higher level of emotional intensity (e.g., “happier”) or indicate that the intensities were identical (“same”). Intensity levels were created by morphing a neutral face with an emotionally intense expression; intensity levels varied from 10–60%. Ten images from each of the four emotional expressions were presented.
The Age Differentiation task was administered to measure participants’ ability to discern age differences between two individuals. Two images appeared simultaneously on the screen, both with neutral expressions. Participants were asked to indicate whether the two individuals were the same or different ages, and if different, which was older. The face stimuli were created by morphing young faces into old faces, which resulted in graded levels of age and difficulty. A total of 40 face-pairs were presented. Male and female faces were equally presented.
Statistical Analysis
Given known associations between age and social cognitive performance (39) coupled with the age differences between the high and no TSE exposure groups, linear and non-linear age effects were regressed from all the social cognition outcome measures. As socioeconomic status (SES) is associated with psychopathology (40) and stress exposure also differed between the two groups, SES was also regressed from all outcome variables. This allowed for the direct assessment of traumatic stress exposure association with social cognitive performance. Of note, the two groups also differed in race, with more minority participants in the high trauma stress exposure group. In the current sample, SES and race were highly collinear. Thus, only one of the two variables (i.e., SES) was used as a covariate in the main set of analyses; however, analyses were repeated regressing out race instead of SES and a similar pattern of findings emerged. Additionally, parallel analyses comparing social cognitive performance between high TSE and no TSE groups matched on sex, age, SES, and race are presented in the supplemental material.
To examine sex differences in the relations between traumatic stress exposure and social emotion processing, we conducted separate two-way analyses of variance (ANOVA) for each task (Emotion Identification, Emotion Intensity Differentiation and Age Differentiation). Group (No TSE exposure, High TSE exposure) and Sex (Male, Female) were between-subject factors, and standardized overall task score was the dependent variable. For each of the three tasks, post-hoc analyses were conducted to compare group performance (High vs. No TSE) in each sex separately. We used a Bonferroni correction (p≤.025 for all tasks) for multiple comparisons. In the two emotion related tasks that showed sex specific differences between High vs. No TSE Groups, we conducted exploratory analyses comparing accuracy in each emotion (for males in the Emotion Identification task and for females in the Emotion Intensity Differentiation task). We used a Bonferroni correction (p≤.01 for the Emotion Identification and p≤.013 for the Emotion Intensity Differentiation) to correct for multiple comparisons of the exploratory analyses. To examine differences in social cognition between high TSE youth with and without PTSD, we performed separate two-way ANOVA for each task (Emotion Identification, Emotion Intensity Differentiation) with Group (No PTSD, PTSD) and Sex (Male, Female) as between subjects’ factors, with standardized overall accuracy scores on each task as the dependent variable. Significant Group-by-Sex interactions were probed in planned comparisons that examined group differences on performance for each emotion in males and females. When post-hoc analyses violated the assumption of homogeneity of variance, Welch-Satterthwaite adjustments were used. To better understand the sex-specific findings related to the TSE exposure association with social cognitive performance, analyses were conducted to examine associations between performance in social cognitive tasks with sex specific psychopathology. To do this, we conducted binary logistic regression with psychopathology as the dependent variable (conduct disorder or depressive episode), and the cognitive task performance (accurate identification of angry faces or discrimination between the intensity of happy faces) as the independent variable, controlling for age and SES. A two-tailed p-value less than .05 was considered statistically significant in the omnibus analyses.
Results
Exposure to traumatic stress load
We examined performance on social cognition tasks comparing accuracy between participants with no traumatic stress exposure (No TSE, N=5202) versus high traumatic stress exposure (High TSE, N=830).
Age Differentiation task
Results from the ANOVA for overall accuracy in the Age Differentiation task revealed no main effect of Group, F(1,5897)=<1, but there was a main effect of Sex, F(1,5897)=15.09, p<.001, Cohen’s d=0.23, such that females (M=.10; SD=.94) outperformed males (M=−.12; SD=1.04). This main effect was qualified by a significant Group-by-Sex interaction, F(1,5897)=8.28, p=.004. To probe the nature of this interaction, we examined Group differences in performance for males and females. For males, traumatic stress exposure group (M=−.02; SD=1.00) showed a trend to be more accurate at differentiating between ages compared to the control group (M=−.13; SD=1.04), t(2881)= −2.039, p=.04, Cohen’s d=0.11. For females, the opposite pattern emerged at trend level, such that the traumatic stress exposure group (M=.02; SD=0.97) tended to be less accurate compared to the control group (M=.12; SD=0.94), t(3016)= 2.03, p=.04, Cohen’s d=0.11.
Emotion Identification task
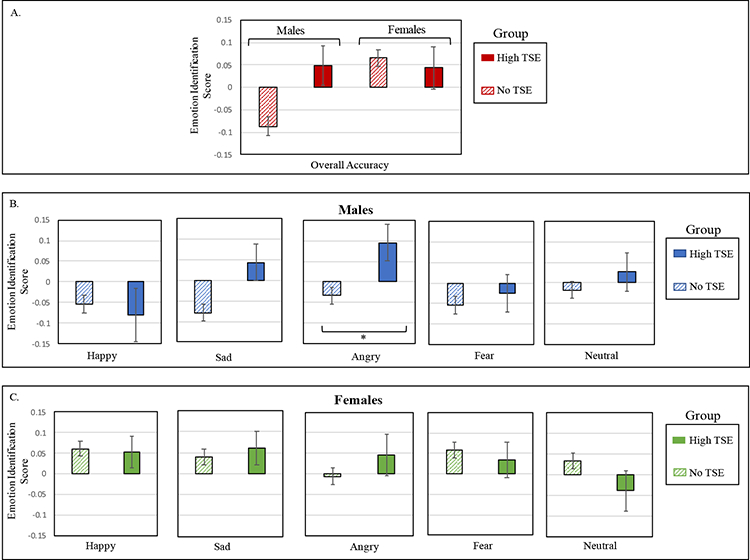
Results for overall accuracy on the Emotion Recognition task revealed no significant main effect of Exposure Group, F(1,5982)=2.27, p=.13, but a main effect of Sex, F(1,5982)=3.93, p=.048, Cohen’s d=0.13, where females had higher scores (M=.06; SD=.98) than males (M= −.06; SD=1.02). This effect was qualified by an Exposure Group-by-Sex interaction, F(1,5982)=4.42, p=.036 (Figure 1). To probe the nature of this interaction, we examined Group differences in performance for males and females. For females, no group difference emerged, t(3051)= 0.43, p=.67. For males, the High TSE group (M=.05; SD=0.89) was more accurate at identifying emotions compared to the No TSE group (M=−.09; SD=1.04), t(2931)= −2.50, p=.013, Cohen’s d=0.14.
Figure 1. Accuracy performance on Emotion Identification task, by Exposure Group.
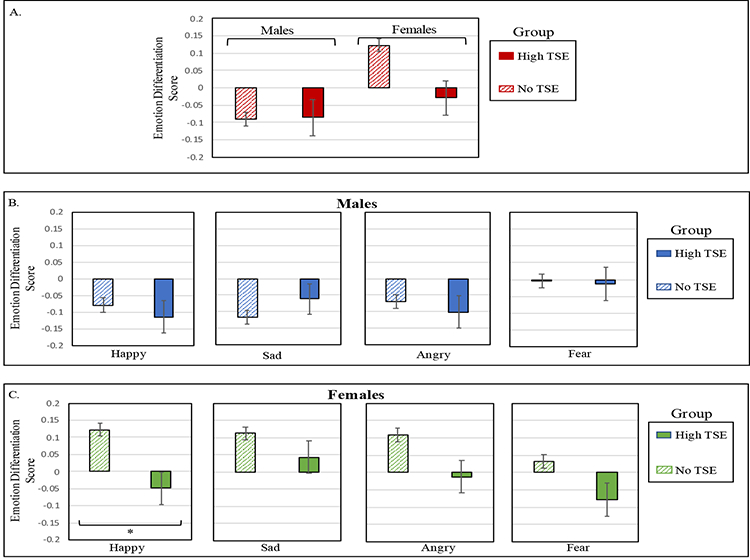
Panel A. shows the means and standard errors for performance on Emotion Identification task accuracy, plotted by Sex and Exposure Group. Performance on each of the separate emotions are displayed for males (panel B) and females (panel C). In all analyses, age and race/socioeconomic Z-score were regressed out. TSE=Traumatic Stressful Events. *p ≤ .01.
To better understand which emotions drive the better performance of males in the Exposure Group, we conducted exploratory post-hoc analyses across the five emotions. Males in the High TSE group significantly outperformed the No TSE group on identifying angry expressions, t(597.43)= 2.63, p=.009, Cohen’s d=0.13, with a trend in the same direction for sad expressions, t(616.24)= 2.50, p=.013, Cohen’s d=0.12. No other group differences emerged for males, all p’s > .42. More accurate identification of angry expressions in stress-exposed males was not associated with a bias to inaccurately identify angry expressions when seeing non-angry expressions (see Supplement). Comparison of emotion identification between Exposure Groups matched on sex, age, SES, and race resulted in similar findings (see Supplement).
Emotion Intensity Differentiation task
Results for overall performance on the Emotion Intensity Differentiation task revealed a main effect of Exposure Group, such that the No TSE group (M=.02; SD=1.00) outperformed the High TSE group (M=−.06; SD=1.03), F(1,5938)=3.91, p=.048, Cohen’s d=0.08. A main effect of Sex also emerged, F(1,5938)=12.94, p<.001, Cohen’s d=0.19, as females (M=.10; SD=.97) outperformed males (M=−.09; SD=1.03). These main effects were qualified by a significant Exposure Group-by-Sex interaction, F(1,5938)=4.36, p=.037 (see Figure 2). To probe the nature of this interaction, we examined Group differences in performance for males and females. For males, no group difference emerged, t(2902)= −0.76, p=.94. For females, the High TSE group (M=.03; SD=1.01) was less accurate discriminating between emotional intensities than the control group (M=.12; SD=0.96), t(3036)= 2.98, p=.003, Cohen’s d=0.15.
Figure 2. Accuracy performance on Emotion Intensity Differentiation task, by Exposure Group.
Panel A. Means and standard errors for accuracy on the Emotion Intensity Differentiation task, plotted by Sex and Exposure Group. Performance on each of the separate emotions are displayed for males (panel B) and females (panel C). In all analyses, age and race/socioeconomic Z-score were regressed out. TSE=Traumatic Stressful Events. *p ≤ .01.
To better understand which emotions drive the poorer performance of females in the Exposure Group, we conducted exploratory post-hoc analyses across the four emotions. Females in the High TSE group, relative to the No TSE group, were less accurate in differentiating between intensities of happy expressions, t(3036)= 3.31, p=.001, Cohen’s d=0.18. There was also a trend for females in the High TSE group to also show reduced accuracy in differentiating between angry expressions, t(3036)= 2.33, p=.02, Cohen’s d=0.13, and fearful expressions, t(3036)= 2.14, p=.03, Cohen’s d=0.11. No differences were found in females in differentiating sad expressions, p=.18. Comparison of Emotion Intensity Differentiation between the Exposure Groups matched on sex, age, SES, and race resulted in similar findings (see Supplement).
Lifetime PTSD diagnosis in association with performance on social cognition tasks
To assess whether the differences in emotion processing observed in the stress exposed population were attributable to PTSD, we conducted separate analyses only on the participants in the High TSE group, grouping them by a lifetime history of PTSD. We found no differences in the Emotion Identification task or in the Emotion Intensity Differentiation task comparing stress exposed participants with and without a lifetime history of PTSD (PTSD main effect and PTSD-by-Sex interaction, all p’s >.05, see supplemental tables S4–5). We found no associations of assaultive traumatic stress exposure (physically, sexually or threatened with a weapon) with altered accuracy social cognitive tasks compared to exposure to non-assaultive stress exposure (main effects for assaultive stress, all p’s >.1, all assault by stress interactions p’s >.5).
Sex specific association of social cognitive tasks with sex specific psychopathology
To assess whether the sex specific associations we observed between traumatic stress exposure and the social cognitive performance were also associated with sex specific psychopathology, we examined each task’s association with psychiatric disorders that have significant sex differences in youth. To do this, we employed binary logistic regressions predicting conduct disorder (more prevalent in males, N= 195, 6.6% of males and N=133, 4.3 % of females) and depressive episode (more prevalent in females, N=379, 12.3% of females and N=235, 7.9% of males).
In males, more accurate identification of angry faces was associated with a diagnosis of conduct disorder (OR 1.175, 95% confidence interval 1.012–1.363, p=.034, corrected for age and SES). No similar association was found for females (OR 1.044, 95% confidence interval 0.878–1.24, p=.562, corrected for age and SES). No association was observed between a diagnosis of a depressive episode and reduced accuracy in discriminating intensity of happy faces in either sex (both p’ s>.05).
Discussion
In the current study, we report sex-specific alterations in youth with a significant history of traumatic stress exposure in three tasks relevant to social cognition - one non-emotion and two emotion-processing tasks: (1) Age Differentiation task, in which for males, exposure to multiple traumatic stressors was associated with enhanced performance, whereas females with stress exposure performed worse than non-exposed; (2) the Emotion Identification task, in which stress exposure in males was associated with enhanced ability to recognize angry cues, whereas females with stress exposure did not perform differently than non-exposed; and (3) the Emotion Intensity Differentiation task, where for females, traumatic stress exposure was associated with diminished ability to differentiate between intensities of happy expressions, whereas for males with a history of traumatic stress this ability was spared. Importantly, for those in the high exposure group, these associated changes in emotion processing performance were not associated with a lifetime diagnosis of PTSD, suggesting that high traumatic stress exposure itself is sufficient for the associated emotion processing aberration, even in the absence of a fuller constellation of PTSD symptoms.
The sex differences found in the current study add to the evidence suggesting that there are sex-specific mechanisms associated with stress exposure. For example, prior work has documented sex differences in the effect of stress exposure on brain structure and function (41, 42). Sex differences are also reported in the physiological response to stress (43), with males showing more pronounced cortisol levels and blood pressure changes compared to females when responding to acute stress (43–45). It has been proposed that sex differences in the physiological response to stress may stem from the traditional “fight or flight” stress response having a greater evolutionary advantage for males, priming them to fight or escape threat (44). Speculatively, such sex differences in normative stress responses and threat-associated behaviors may in turn relate to variation in the processing of anger expressions and to the sex-dependent differences in anger identification we see here in stress-exposed youth.
The differential association of stress exposure with social cognition in males and females may also be linked to sex differences in the emergence of psychopathology during and after adolescence. Early life exposure puts females at greater risk for internalizing symptoms and males at risk for externalizing symptoms (46). Thus, underlying social-emotional processing differences in females and males in association with stress exposure might be related to sex specific psychopathology phenotypes. We observed an association between the ability to identify angry faces and diagnosis of conduct disorder in males, and not in females. This finding may suggest that the improved ability of males with high traumatic stress exposure to identify angry faces may be related to the increased prevalence of conduct in males by reducing the threshold for perceived threat, or alternatively that males with conduct symptoms are more likely to incite angry faces in others and therefore “more experienced” in identifying this specific emotion.
By contrast, depression, that is more common in stress-exposed females, has been associated with more difficulties in differentiating between happy facial expressions (47) and the need for higher levels of emotional intensity to recognize happy facial expressions (48). In our study, reduced accuracy in differentiating the intensity of happy faces was found but it was not associated with increased depression risk in females (nor in males). One possible explanation for the differences between the sex specific findings in conduct and depression diagnoses may be due to the clinical nature of these diagnoses and the fact that we assessed a lifetime history of a depressive episode. While conduct disorder may be viewed as a trait (49), depressive episodes are considered more as states. Therefore, it is possible that participants who endorsed lifetime depressive episodes were not depressed while taking the cognitive tasks, therefore no association was found between the social cognitive performance and the psychopathology measures. Future longitudinal studies are needed to tease apart the temporal relation among stress exposure, social cognitive constructs and various psychopathology phenotypes. In addition, that we found sex by exposure interaction in a non-emotion related social cognitive task raises the possibility that sex specific associations of trauma may be linked more broadly to face processing or social cognition abilities, rather than specifically to emotion processing. This possibility merits further investigation in the context of sex differences in association with trauma exposure.
Traumatic events during brain development are associated with significant cognitive impact (50), most notably described in the field of adult PTSD research in the context of memory and executive function (51–53). The question remains whether the atypical cognitive function is associated with the trauma exposure alone, or with the sequelae of brain changes associated with full blown PTSD diagnosis. A systematic review of studies of adult male veterans suggests that, more significant cognitive deficits are observed in individuals with PTSD compared to individuals who also have traumatic stress exposure but do not have PTSD. This association is less consistent in other clinical populations, with some studies reporting no specific PTSD related cognitive abnormalities beyond the exposure alone (54). There are relatively few studies of the association of trauma with social cognitive capacities. These studies suggest that impaired social cognition may be one mechanism contributing to PTSD risk, for example by reducing the capacity to maintain close social relationships, thus lessening the protective effect of social bonds (55). A recent study conducted in adolescent psychiatric inpatients suggested that social cognition deficits mediate the relationship between insecure attachment and PTSD (56). However, here we did not find differences in social cognitive performance comparing stress-exposed youth with PTSD to those without PTSD. Our results are in line with a study conducted in children reporting an association between maltreatment and atypical processing of emotion that is independent of PTSD diagnosis (16). Taken together, these findings do not support the notion that atypical social cognition in stress-exposed youth is associated with the criterial symptoms of PTSD. Rather, the data suggest that, in non-psychiatric-help-seeking youth, the high traumatic stress exposure itself is associated with specific emotion identification and intensity differentiation patterns - regardless of PTSD. One possible explanation for the inconsistent findings could be that our study population was not clinically ascertained in contrast to the above studies that examined help-seeking populations. Another possibility is an age effect contributing to different findings in adolescents compared to adults with a history of traumatic stress exposure. Finally, the psychopathology screening tool we used may have had reduced sensitivity to detect PTSD compared to studies focused at PTSD that employ more robust tools to assess PTSD diagnosis and severity.
Our study has several limitations. First, the cross sectional nature of the study does not permit causal inference hence we cannot know whether the social cognition abnormalities are due to stress exposure, or if some cognitive traits may put one at increased risk to experience potentially traumatic events, as was suggested previously (57). In addition, we cannot test whether these abnormalities put youth at risk for the development of PTSD or other stress related phenotypes. Second, the exposure to traumatic events was assessed using a list of eight events, without indication of chronicity or specificity regarding the timing of the traumatic events – each of which may affect the formation of social cognition during childhood and adolescence. The relatively narrow scope of traumatic stressors assessed by the tool limits our ability to directly compare our findings to the available literature relating a broader range of life stressors, including child maltreatment, to social cognitive capacities. Thirdly, although we controlled for such variables as SES and age, other factors (e.g., developmental, demographic, social, or emotional factors) may affect the current results. Future work in this area will need to explore how these other factors may interact with sex and traumatic life events to influence social cognition. Lastly, while our findings show significant sex specific alterations in social cognition related tasks, effect sizes were small. Notwithstanding the small effect sizes, sex specificity of the direction of change associated with high trauma exposure sheds new light on potential brain differences between the sexes that emerges during adolescence. More research is needed to better understand the task- and emotion-specificity of stress-exposure effects on social information processing. The current study only examined social cognition in the context of three tasks; different types of social and emotional processing may yield different patterns of findings.
In summary, this study aimed to elucidate the relationship between traumatic life stress and social cognitive abilities in a large youth cohort including a substantial portion with high traumatic stress exposure. Findings revealed sex-specific associations between exposure and social cognitive capacities. Males exposed to traumatic stress showed a selectively enhanced ability to identify angry facial expressions, which was associated with a diagnosis of conduct disorder, without alterations in identifying other emotions or in differentiating emotional intensity. For females, in contrast, stress exposure was associated with reduced ability to differentiate the intensity of happy emotional expressions, without alterations in differentiating intensity for other emotions or in identifying emotions. That more detrimental and widespread effects of stress exposure did not emerge in the current results is notable and suggests that social cognition may be relatively robust to negative environment influences. This is in contrast to the dose-response negative association between traumatic stress exposure and executive function and complex reasoning that we have previously described in the same cohort (32). Future longitudinal studies examining association between early life trauma and social cognition are needed to delineate the causal pathways.
Supplementary Material
Acknowledgements:
This work was supported by NIH grant MH-107235, MH-089983, MH-096891, MH-P50MH06891, the Dowshen Neuroscience fund, and the Lifespan Brain Institute of Children’s Hospital of Philadelphia and Penn Medicine, University of Pennsylvania. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- TSE
Traumatic stressful event
- PNC
Philadelphia Neurodevelopmental Cohort
Footnotes
Additional Contributions: Kathleen Merikangas, PhD, and Marcy Burstein, PhD, Genetic Epidemiology Research Branch, Intramural Research Program, National Institute of Mental Health, assisted in development of GOASSESS; they received no compensation from the funding sponsor. We thank participants and families; staff of the Brain Behavior Laboratory, University of Pennsylvania, Philadelphia, for their contribution to data generation; study assessors and recruiters for their invaluable contributions to data collection; and staff from the Center of Applied Genomics, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, for the genomics interphase with brain behavior measures. Potential conflict of interest disclosure: Dr. Barzilay serves on the scientific board and reports stock ownership in ‘Taliaz Health’, with no conflict of i nterest relevant to this work. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nemeroff CB (2016): Paradise lost: The neurobiological and clinical consequences of child abuse and neglect. Neuron. 89: 892–909. [DOI] [PubMed] [Google Scholar]
- 2.Teicher MH, Samson JA (2013): Childhood Maltreatment and Psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 170: 1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnhofer T, Brennan K, Crane C, Duggan D, Williams JMG (2014): A comparison of vulnerability factors in patients with persistent and remitting lifetime symptom course of depression. J Affect Disord. 152–154: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin JR, Arseneault L, Caspi A, Fisher HL, Moffitt TE, Odgers CL, et al. (2018): Childhood victimization and inflammation in young adulthood: A genetically sensitive cohort study. Brain Behav Immun. 67: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly KM, Mezuk B (2017): Predictors of remission from generalized anxiety disorder and major depressive disorder. J Affect Disord. 208: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everaerd D, Klumpers F, Zwiers M, Guadalupe T, Franke B, van Oostrom I, et al. (2016): Childhood abuse and deprivation are associated with distinct sex-dependent differences in brain morphology. Neuropsychopharmacology. 41: 1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL (2013): The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatr Scand. 128: 434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacPherson HA, Algorta GP, Mendenhall AN, Fields BW, Fristad MA (2014): Predictors and moderators in the randomized trial of multifamily psychoeducational psychotherapy for childhood mood disorders. J Clin Child Adolesc Psychol. 43: 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller S, McTeague LM, Gyurak A, Patenaude B, Williams LM, Grieve SM, et al. (2015): Cognition-childhood maltreatment interactions in the prediction of antidepressant outcomes in major depressive disorder patients: Results from the i-SPOT D trial. Depress Anxiety. 32: 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollak SD (2015): Multilevel developmental approaches to understanding the effects of child maltreatment: Recent advances and future challenges. Dev Psychopathol. 27: 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luke N, Banerjee R (2013): Differentiated associations between childhood maltreatment experiences and social understanding: A meta-analysis and systematic review. Dev Rev. 33: 1–28. [Google Scholar]
- 12.During SM, McMahon RJ (1991): Recognition of emotional facial expressions by abusive mothers and their children. J Clin Child Psychol. 20: 132–139. [Google Scholar]
- 13.Pollak SD, Cicchetti D, Hornung K, Reed A (2000): Recognizing emotion in faces: Developmental effects of child abuse and neglect. Dev Psychol. 36: 679–688. [DOI] [PubMed] [Google Scholar]
- 14.Pollak SD, Sinha P (2002): Effects of early experience on children’s recognition of facial displays of emotion. Dev Psychol. 38: 784–791. [DOI] [PubMed] [Google Scholar]
- 15.Pollak SD, Kistler DJ (2002): Early experience is associated with the development of categorical representations for facial expressions of emotion. Proc Natl Acad Sci U S A. 99: 9072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masten CL, Guyer AE, Hodgdon HB, McClure EB, Charney DS, Ernst M, et al. (2008): Recognition of facial emotions among maltreated children with high rates of post-traumatic stress disorder. Child Abus Negl. 32: 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shackman JE, Shackman AJ, Pollak SD (2007): Physical abuse amplifies attention to threat and increases anxiety in children. Emotion. 7: 838–852. [DOI] [PubMed] [Google Scholar]
- 18.Günther V, Dannlowski U, Kersting A, Suslow T ( 2015): Associations between childhood maltreatment and emotion processing biases in major depression: results from a dot-probe task. BMC Psychiatry. 15: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD (2006): Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 60: 296–301. [DOI] [PubMed] [Google Scholar]
- 20.Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R (2010): Neural responses to masked fear faces: Sex differences and trauma exposure in posttraumatic stress disorder. J Abnorm Psychol. 119: 241–247. [DOI] [PubMed] [Google Scholar]
- 21.Quinlan EB, Cattrell A, Jia T, Artiges E, Banaschewski T, Barker G, et al. (2017): Psychosocial stress and brain function in adolescent psychopathology. Am J Psychiatry. 174: 785–794. [DOI] [PubMed] [Google Scholar]
- 22.Pechtel P, Pizzagalli D (2012): Effects of Early Life Stress on Cognitive and Affective Function. Psychopharmacology (Berl). 214: 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blakemore S-J (2010): The developing social brain: Implications for education. Neuron. 65: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blakemore S-J (2008): The social brain in adolescence. Nat Rev Neurosci. 9: 267–277. [DOI] [PubMed] [Google Scholar]
- 25.McClure EB (2000): A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychol Bull. 126: 424–53. [DOI] [PubMed] [Google Scholar]
- 26.Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker WB, et al. (2014): Within-individual variability in neurocognitive performance: Age- and sex-related differences in children and youths from ages 8 to 21. Neuropsychology. 28: 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Xie H, Cotton AS, Duval ER, Tamburrino MB, Brickman KR, et al. (2016): Preliminary study of acute changes in emotion processing in trauma survivors with PTSD symptoms. PLoS One. 11: e0159065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fertuck EA, Tsoi F, Grinband J, Ruglass L, Melara R, Hien DA (2016): Facial trustworthiness perception bias elevated in individuals with PTSD compared to trauma exposed controls. Psychiatry Res. 237: 43–48. [DOI] [PubMed] [Google Scholar]
- 29.Pratchett LC, Pelcovitz MR, Yehuda R (2010): Trauma and violence: Are women the weaker sex? Psychiatr Clin North Am. 33: 465–474. [DOI] [PubMed] [Google Scholar]
- 30.Olff M, Langeland W, Draijer N, Gersons BPR (2007): Gender differences in posttraumatic stress disorder. Psychol Bull. 133: 183–204. [DOI] [PubMed] [Google Scholar]
- 31.Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. (2015): The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 56: 1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barzilay R, Calkins ME, Moore TM, Wolf DH, Satterthwaite TD, Scott JC, et al. (2018): Association between traumatic stress load, psychopathology and cognition in the Philadelphia Neurodevelopmental Cohort. Psychol Med. In press. [DOI] [PubMed] [Google Scholar]
- 33.Moore TM, Martin IK, Gur OM, Jackson CT, Scott JC, Calkins ME, et al. (2016): Characterizing social environment’s association with neurocognition using census and crime data linked to the Philadelphia Neurodevelopmental Cohort. Psychol Med. 46: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. (2014): The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 13: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 36: 980–988. [DOI] [PubMed] [Google Scholar]
- 36.McCutcheon VV, Heath AC, Nelson EC, Bucholz KK, Madden PAF, Martin NG (2009): Accumulation of trauma over time and risk for depression in a twin sample. Psychol Med. 39: 431– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. (2010): A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods. 187: 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. (2012): Age group and sex differences in performance on a computerized neurocognitive battery in children age 8−21. Neuropsychology. 26: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gur RC, Gur RE (2016): Social cognition as an RDoC domain. Am J Med Genet Part B Neuropsychiatr Genet. 171: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson JG, Cohen P, Dohrenwend BP, Link BG, Brook JS (1999): A longitudinal investigation of social causation and social selection processes involved in the association between socioeconomic status and psychiatric disorders. J Abnorm Psychol. 108: 490–9. [DOI] [PubMed] [Google Scholar]
- 41.Teicher MH, Samson JA (2016): Annual research review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry Allied Discip. 57: 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ (2013): Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci. 110: 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ordaz S, Luna B (2012): Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 37: 1135–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA (2000): Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 107: 411–29. [DOI] [PubMed] [Google Scholar]
- 45.Verma R, Balhara YPS, Gupta CS (2011): Gender differences in stress response: Role of developmental and biological determinants. Ind Psychiatry J. 20: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keyes KM, Eaton NR, Krueger RF, McLaughlin KA, Wall MM, Grant BF, Hasin DS (2012): Childhood maltreatment and the structure of common psychiatric disorders. Br J Psychiatry. 200: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surguladze SA, Young AW, Senior C, Brébion G, Travis MJ, Phillips ML (2004): Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 18: 212–218. [DOI] [PubMed] [Google Scholar]
- 48.LeMoult J, Joormann J, Sherdell L, Wright Y, Gotlib IH (2009): Identification of emotional facial expressions following recovery from depression. J Abnorm Psychol. 118: 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blair RJR, Leibenluft E, Pine DS (2014): Conduct disorder and callous–unemotional traits in youth. N Engl J Med. 371: 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bower GH, Sivers H (1998): Cognitive impact of traumatic events. Dev Psychopathol. 10: 625–53. [DOI] [PubMed] [Google Scholar]
- 51.Sumner JA, Hagan K, Grodstein F, Roberts AL, Harel B, Koenen KC (2017): Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depress Anxiety. 34: 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNally R (2006): Cognitive abnormalities in post-traumatic stress disorder. Trends Cogn Sci. 10: 271–277. [DOI] [PubMed] [Google Scholar]
- 53.Clouston SAP, Kotov R, Pietrzak RH, Luft BJ, Gonzalez A, Richards M, et al. (2016): Cognitive impairment among World Trade Center responders: Long-term implications of re-experiencing the 9/11 terrorist attacks. Alzheimer’s Dement (Amsterdam, Netherlands). 4: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qureshi SU, Long ME, Bradshaw MR, Pyne JM, Magruder KM, Kimbrell T, et al. (2011): Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 23: 16–28. [DOI] [PubMed] [Google Scholar]
- 55.Nietlisbach G, Maercker A (2009): Social cognition and interpersonal impairments in trauma survivors with PTSD. J Aggress Maltreat Trauma. 18: 382–402. [Google Scholar]
- 56.Venta A, Hatkevich C, Mellick W, Vanwoerden S, Sharp C (2017): Social cognition mediates the relation between attachment schemas and posttraumatic stress disorder. Psychol Trauma Theory, Res Pract Policy. 9: 88–95. [DOI] [PubMed] [Google Scholar]
- 57.Lauterbach D, Vrana S (2001): The relationship among personality variables, exposure to traumatic events, and severity of posttraumatic stress symptoms. J Trauma Stress. 14: 29–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


