Abstract
Objective:
To determine the relationship between synovitis detected on non-contrast-enhanced (CE) MRI, biochemical markers of inflammation and clinical assessment of effusion in persons with knee OA.
Design:
We examined data from the OA Biomarkers Consortium within the Osteoarthritis Initiative (n=600). Non-CE MRIs were semi-quantitatively scored (grades 0–3) for severity of Hoffa- and effusion-synovitis. Serum (s) matrix metalloproteinase-3 (sMMP-3), hyaluronic acid (sHA) and nitrated epitope of the α-helical region of type II collagen (sColl2–1NO2) were quantified. The bulge and patellar tap clinical tests were performed at baseline and performance characteristics were assessed for the detection of effusion-synovitis on MRI. Multinomial logistic regression adjusted for covariates was used to assess the association between biochemical and imaging markers at baseline and over 12 and 24 months.
Results:
At baseline, sHA and sMMP-3 were associated with moderate to large (score ≥ 2, n=117) effusion-synovitis with OR=1.35 and 1.30 per 1 standard deviation in biochemical markers (95% CI=1.07–1.71 and 1.00–1.69), c-statistics 0.640 and 0.626, respectively. C-statistics for the presence of Hoffa-synovitis (score ≥ 2) were 0.693, 0.694 and 0.694 for sHA, sMMP-3 and sColl2–1NO2, respectively. There was no significant association between biochemical markers (baseline and 12- and 24-month time integrated concentrations) and changes in MRI markers. The bulge and patellar tap signs were 22.0% and 4.3% sensitive and 88.8% and 94.8% specific, respectively, for detecting effusion-synovitis (score ≥ 1) on MRI.
Conclusion:
sHA and sMMP-3 were modestly associated with effusion-synovitis at baseline. Clinical signs of effusion are insensitive but highly specific for the presence of any effusion-synovitis on non-CE MRI.
Keywords: osteoarthritis, knee, synovitis, biomarkers, clinical
INTRODUCTION
There is growing evidence demonstrating the importance of synovial inflammation in early and late stages of osteoarthritis (OA) (1). MRI-detected synovitis has been independently associated with incidence of radiographic OA (2–4), cartilage loss (5) and clinical progression of disease (6). Moreover, change in synovitis over time has been shown to correlate with changes in pain (7), representing a target for drugs aimed at easing symptoms and potentially modifying the course of OA (8, 9).
The gold standard to assess synovitis is histological analysis but other non-invasive methods, particularly ultrasound and MRI, have been widely used to evaluate presence and severity of synovitis [2]. Despite the increasing use of contrast enhanced (CE) MRI more recently in OA research, non-CE MRI remains the most common method of evaluating synovitis, particularly in large epidemiological OA studies (10).
Biochemical markers of synovial inflammation may also be useful as tools for assessing synovitis. However, none of these have been qualified for clinical use as yet. Among these, serum hyaluronic acid levels (sHA) have been associated with effusion on ultrasound in knee OA patients (11), while nitrated epitope of the α-helical region of type II collagen (sColl2–1NO2), a putative marker of oxidative stress, has been correlated with C-reactive protein in the sera of OA and rheumatoid arthritis (RA) patients (12). In addition, immunoreactivity of matrix metalloproteinase-3 (sMMP-3) in synovial tissue was positively correlated with OA severity (13) and, in RA, sMMP-3 correlates with synovial fluid MMP-3 and immunoreactivity of MMP-3 in synovial tissue (14).
The main advantage of establishing an association between biochemical markers and synovitis on MRI is that if they are associated, there would be a serum marker to be used as a surrogate of synovitis in future clinical research. This is likely to be more readily accessible and affordable compared to MRI and is not as operator-dependent as ultrasound (15). Similarly, clinical assessment of effusion has been used in trials to screen for presence of knee joint effusion (16–18) but there is a lack of studies examining the diagnostic performance of these clinical tests against MRI or ultrasound (19).
Therefore, the main objective of our study was to determine the association between sHA, sMMP-3 and sColl2–1NO2, and presence of synovitis on non-CE MRI. Our secondary objective was to examine the diagnostic performance of two commonly used clinical tests of knee effusion, namely bulge and patellar tap signs, in detecting the presence of effusion-synovitis on MRI.
MATERIALS AND METHODS
We conducted an ancillary exploratory analysis of data from the Foundation for the National Institutes of Health (FNIH) OA Biomarkers Consortium study, a nested case-control study within Osteoarthritis Initiative (OAI) whose aim is to investigate biological markers involved in knee OA progression (20). The OAI is an ongoing prospective and observational cohort which included 4,796 participants aged 45–79 years, with publicly accessible clinical, radiologic and other data collected at baseline and at annual follow-up visits (21). The Institutional Review Board for the University of California, San Francisco (UCSF), and its affiliates, approved the OAI study (approval number 10–00532).
Study participants
Six hundred participants from OAI were included in the FNIH OA Biomarkers Consortium study (one index knee per participant) and all 600 participants were included in the present study. Eligible participants were those with at least one knee with a Kellgren-Lawrence grade (KLG) of 1–3 at baseline, assessed by central reading of standardized posterior-anterior radiographs, and availability at baseline and 24 months of knee radiographs, knee MRI, stored biological specimens and clinical data. Only knees with potential to meet criteria for radiographic and pain progression at baseline (i.e. minimum medial joint space width ≥ 1.0 mm and/or WOMAC pain ≤ 91 on 0–100 scale at baseline) were included in the FNIH OA Biomarkers Consortium. The final sample was selected to include 1/4 OA progressors (both clinical and radiographic progression), 1/4 non-progressors and 1/2 either clinical only or radiographic only progressors. Additional exclusion criteria can be found at https://oai.epi-ucsf.org/datarelease/FNIH.asp. The baseline radiographs were acquired at the same time as the baseline MRIs. Details of radiograph reading and MRI acquisition have been previously described (22).
MRI assessment of synovitis
MRIs of all participants in the FNIH OA Biomarkers Consortium study were scored paired and unblinded to time-point using the MOAKS (MRI Osteoarthritis Knee Score) method at baseline, 12 and 24 months (23). According to MOAKS, synovitis is assessed semi-quantitatively using two different markers: Hoffa-synovitis and effusion-synovitis. Hoffa-synovitis has been used as a proxy for synovitis as it was found to be correlated to chronic synovitis on histology (24), although its specificity was low when compared to CE MRI (25). It is defined as diffuse hyperintense signal on T2, proton density, or intermediate-weighted fat-suppressed sequences within the Hoffa’s fat pad and is scored on sagittal images. A score was given for assessment of degree of hyperintensity in Hoffa’s fat pad: 0= normal; 1= mild; 2= moderate; 3= severe. Effusion-synovitis represents a composite of effusion and synovial thickening and was determined by the presence and amount of intra-articular hyperintensity on axially reformatted dual echo at steady state (DESS) and sagittal intermediate-weighted fat suppressed images. Effusion-synovitis was scored based on maximum distension of the synovial capsule due to intraarticular joint fluid: 0= physiologic amount; 1= small – fluid continuous in the retropatellar space; 2= medium – with slight convexity of the suprapatellar bursa; 3= large – evidence of extensive capsular distension. Changes in Hoffa- and effusion-synovitis were calculated as the difference between each synovitis score at 12 and 24 months and the respective score at baseline, and further classified into improvement, no change or worsening.
Biochemical markers assessment
Morning blood and second morning void urine specimens were collected at each visit after an overnight fast using a uniform protocol and sent to a commercial specimen repository where they were stored at −70°C. In this study, we investigated several markers with face validity for synovial inflammation based on previous studies: sHA, sMMP-3 and sColl2–1NO2. Biochemical markers were measured in duplicate by enzyme-linked immunosorbent assays (ELISAs) with low inter-plate coefficients of variation (7.4%, 9.6% and 13.6%, respectively) as previously described (26). We used an interpolated value from the standard curve extended from the lowest standard to zero if the concentration of the biochemical marker was below the lowest reportable value. This method was used in the primary, main FNIH analyses as it was considered superior over random imputation, particularly for biomarkers whose standard curves were evidently linear below the lowest standard, such as sHA (30). More details about the assays can be found at https://oai.epi-ucsf.org/datarelease/.
Clinical assessment of effusion
Physical examination of both knees was performed at baseline, including clinical signs of effusion (i.e. bulge and patellar tap signs). Bulge sign is commonly used in clinical practice to detect small effusions while patellar tap sign is best for large effusions (27, 28). Examiners received central training and performed knee examination under supervision of physician examiners at each site. At least one exam done by each examiner per month was either repeated or observed by the local investigator for quality assurance. Participants were lying supine on an examination table in a relaxed comfortable position with the knees extended to neutral and all muscles relaxed. Signs were scored as present or absent in each knee. Tests were repeated once to confirm findings (whether positive or negative) by the same examiner and were recorded as positive if confirmed in the second examination. Data regarding reliability of these examinations was not available but inter-rater reliability of bulge and patellar tap signs have been previously reported as adequate (prevalence-adjusted bias-adjusted kappa 0.78 for patellar tap and intraclass correlation coefficient 0.97 for bulge sign) (29).
Statistical analysis
We used multinomial logistic regression to determine the following associations: 1) baseline biochemical markers and Hoffa- and effusion-synovitis on MRI (cross-sectional analysis); 2) baseline biochemical markers and changes in Hoffa- and effusion-synovitis over 12 and 24 months (prognostic analysis); 3) time-integrated concentrations (TICs) of biochemical markers (area under the concentration versus time curve) and changes in Hoffa- and effusion-synovitis from baseline to 12 and 24 months (concurrent analysis).
Baseline Hoffa- and effusion-synovitis scores (ranging from 0–3) were further grouped into 0, 1, or ≥2 and biochemical markers concentrations were transposed to z values before the analysis in order to obtain standardized values. For the baseline imaging marker analysis, the reference group is the group without the imaging marker (i.e. score = 0) and the odds are presented as the odds of being in each MRI group (e.g. score of 1 or ≥2) vs. the reference group for a 1 standard deviation (SD) increase in biochemical marker. Linear tests for trend were used to assess the relationship between biochemical markers values and severity of the MRI markers at baseline. Moreover, the performance of biochemical markers in predicting the presence of Hoffa- and effusion-synovitis on MRI at baseline was evaluated using Receiver Operating Characteristic (ROC) curve analysis according to a binary outcome (score ≥ 2) for each imaging marker. The relationship between the two synovitis scores at baseline was assessed using the Spearman’s correlation coefficient.
We categorized change in the MRI markers as improvement, no change or worsening from baseline to 12 and 24 months and further collapsed improvement and no change in a single category for Hoffa-synovitis, due to the low frequency of scores that improved over 12 and 24 months. For the change in imaging marker analysis, the reference group is the group that did not change over time (or improvement/no change for Hoffa-synovitis), and the odds are presented as the odds of either improving or worsening for a 1 standard deviation (SD) increase in a biochemical marker.
Age, gender, BMI and baseline KLG were included as covariates in the regression analysis. In the longitudinal analyses, baseline score of the respective imaging marker was also included as covariates. P values less than 0.05 were considered statistically significant for associations.
The diagnostic value of clinical signs of effusion for presence of effusion-synovitis on MRI (score ≥ 1 and score ≥ 2) was assessed using sensitivity, specificity, positive and negative predictive value, and accuracy of each clinical test. The software used was SPSS for Windows, version 22.0 (SPSS, Chicago, IL, USA).
RESULTS
Baseline characteristics and prevalence of imaging markers of the 600 participants are presented in Table 1. Mean age of participants was 61.6 years, 58.8% were female, mean BMI was 30.7 kg/m2 and most participants had a KLG 2 at baseline (51.0%). Hoffa-synovitis and effusion-synovitis (score ≥ 1) were present in 59.1% and 61.0% of knees, while score ≥ 2 were found in 8.6% and 19.4%, respectively. Grade 3 scores were observed in 0.8% and 3.3% of participants, respectively. The relationship between these two MRI features (0–3) as assessed by the Spearman’s correlation coefficient was weak (rs= 0.284).
Table 1.
Baseline characteristics of study participants (n=600).
| Parameter | Value |
|---|---|
| Mean age (SD) | 61.6 (8.9) |
| Female sex-(%) | 58.8 |
| BMI (Kg/m2); mean (SD) | 30.7 (4.7) |
| Race n (%) | |
| Other Non-white | 11 (1.8) |
| White or Caucasian | 475 (79.2) |
| Black or African American | 109 (18.2) |
| Asian | 5 (0.8) |
| KLG at baseline n, (%) | |
| 1 | 75 (12.5) |
| 2 | 306 (51.0) |
| 3 | 219 (36.5) |
| Hoffa-synovitis n (%) | |
| 0 | 246 (41.0) |
| 1 | 302 (50.3) |
| 2 | 47 (7.8) |
| 3 | 5 (0.8) |
| Effusion-synovitis n (%) | |
| 0 | 233 (38.8) |
| 1 | 250 (41.7) |
| 2 | 97 (16.2) |
| 3 | 20 (3.3) |
KLG=Kellgren Lawrence grade, BMI=body mass index.
Association of biochemical and imaging markers
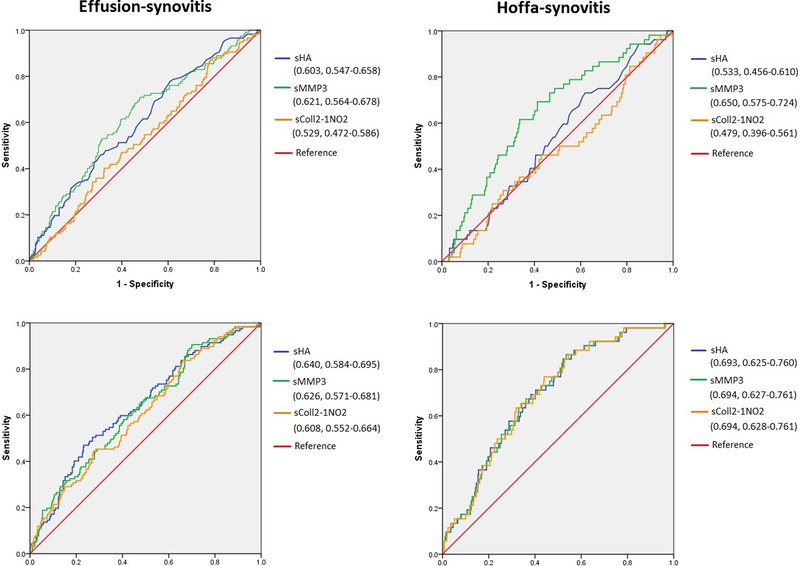
The association between baseline biochemical and imaging markers are displayed in Table 2. There was a direct relationship between sHA and sMMP3 values and increasing effusion-synovitis severity at baseline (p value for trend 0.007 and 0.032, respectively). In the regression analysis, serum HA and sMMP-3 were associated with moderate to large effusion-synovitis (score ≥ 2) compared to those with a score of 0 (OR = 1.35 [95% CI = 1.07, 1.71], and OR = 1.30 [95% CI = 1.00, 1.69], respectively). No statistically significant associations were observed for sColl2–1NO2 or for any of the biochemical markers and Hoffa-synovitis. The c-statistics for presence of effusion-synovitis ≥ 2 were 0.640, 0.626 and 0.608 for sHA, sMMP-3 and sColl2–1NO2, respectively, and 0.693, 0.694 and 0.694 for Hoffa-synovitis grade ≥2, respectively (Figure 1).
Table 2.
Association between baseline biochemical markers and baseline severity of MRI features.
| Biochemical marker* | P valuefor trend | 0 | 1 | 2–3 | |
|---|---|---|---|---|---|
| Hoffa-synovitis | sColl2,1NO2 (nM) | 0.493 | |||
| Mean (SD) | - | 9.31 (5.97) | 9.01 (7.40) | 8.32 (4.03) | |
| OR (95% CI) | - | REF | 0.97 (0.82, 1.15) | 0.91 (0.62, 1.35) | |
| sHA (ng/mL) | 0.132 | ||||
| Mean (SD) | - | 43.93 (45.33) | 50.61 (43.46) | 46.79 (34.15) | |
| OR (95% CI) | - | REF | 1.14 (0.93, 1.39) | 1.15 (0.83, 1.58) | |
| sMMP-3 (ng/mL) | 0.074 | ||||
| Mean (SD) | - | 16.90 (16.75) | 17.98 (14.79) | 20.34 (9.56) | |
| OR (95% CI) | - | REF | 1.07 (0.88, 1.30) | 1.04 (0.76, 1.42) | |
| Effusion-synovitis | sColl2,1NO2 (nM) | 0.641 | |||
| Mean (SD) | - | 8.78 (5.20) | 9.38 (8.40) | 8.46 (3.87) | |
| OR (95% CI) | - | REF | 1.07 (0.87, 1.32) | 1.06 (0.83, 1.34) | |
| sHA (ng/mL) | 0.007 | ||||
| Mean (SD) | - | 44.29 (43.88) | 45.72 (39.08) | 57.86 (45.38) | |
| OR (95% CI) | - | REF | 0.99 (0.79, 1.24) | 1.35 (1.07, 1.71) | |
| sMMP-3 (ng/mL) | 0.032 | ||||
| Mean (SD) | - | 16.98 (17.18) | 16.90 (12.71) | 21.68 (16.64) | |
| OR (95% CI) | - | REF | 1.12 (0.87, 1.45) | 1.30 (1.00, 1.69) | |
Adjusted for age, gender, BMI, baseline KLG.
Mean and standard deviation (SD) of biochemical markers are presented as non-transformed values. Odds ratio were calculated using baseline values of biochemical markers transformed to z values.
Figure 1.
Receiver operating characteristic (ROC) curves of baseline biochemical markers for predicting the presence of effusion- and Hoffa-synovitis (score ≥2 for each parameter) on MRI at baseline. Upper figures: unadjusted. Lower figures: adjusted for covariates. Biochemical markers transposed to z values were used. C-statistic of each test and 95% confidence intervals are presented in the figure.
Five hundred and eighty six participants had biochemical data available for all three time points and were included in the longitudinal analyses. Table 3 displays the changes in the MRI parameters of synovitis and their association with baseline levels of biochemical markers. Over 12 months, Hoffa- and effusion-synovitis scores improved in 1.7% and 14.2% of participants, did not change in 92.3% and 64.5% and worsened in 6.0% and 21.3% of participants, respectively. Over 24 months, Hoffa- and effusion-synovitis scores improved in 1.5% and 13.1%, did not change in 88.7% and 61.4% and worsened in 9.7% and 25.4% of participants, respectively. Overall, baseline biochemical markers were not associated with changes in either parameter of synovitis on MRI during follow-up, except for an inverse association between sHA and worsening in effusion-synovitis over 12 months (OR = 0.70 [95% CI = 0.51, 0.94]) (Table 3). Similarly, TICs of biochemical markers over 12 and 24 months were not associated with concurrent changes in Hoffa- or effusion-synovitis (Table 4). Unadjusted baseline and longitudinal analyses revealed similar results with no differences regarding significance of associations (results not shown).
Table 3.
Association between baseline biochemical markers and change in MRI features over 12 and 24 months.
| Biochemical markers | MRI markers | ||||
|---|---|---|---|---|---|
| Hoffa-synovitis | Effusion-synovitis | ||||
| Improvement/No change | Worsening | Improvement | No change | Worsening | |
| 12 months | n = 10 (1.7%)/541 (92.3%) | n = 35 (6.0%) | n = 83 (14.2%) | n = 378 (64.5%) | n = 125 (21.3%) |
| sHA | REF | 0.83 (0.54, 1.28) | 1.02 (0.80, 1.31) | REF | 0.70 (0.51, 0.94) |
| sMMP-3 | REF | 0.57 (0.27, 1.22) | 1.00 (0.79, 1.28) | REF | 0.78 (0.55, 1.11) |
| sColl2–1NO2 | REF | 0.77 (0.45, 1.31) | 0.89 (0.65, 1.20) | REF | 0.98 (0.79, 1.21) |
| 24 months | n = 9 (1.5%)/520 (88.7%) | n = 57 (9.7%) | n = 77 (13.1%) | n = 360 (61.4%) | n = 149 (25.4%) |
| sHA | REF | 1.01 (0.76, 1.36) | 1.10 (0.86, 1.39) | REF | 0.87 (0.69, 1.11) |
| sMMP-3 | REF | 0.64 (0.35, 1.15) | 1.06 (0.84, 1.33) | REF | 0.84 (0.62, 1.13) |
| sColl2–1NO2 | REF | 1.00 (0.74, 1.34) | 1.03 (0.77, 1.37) | REF | 1.15 (0.94, 1.40) |
Adjusted for age, gender, BMI, baseline KLG and respective baseline feature on MRI. Odds ratio and 95% confidence intervals (CI) are presented in the table.
Table 4.
Association between TICs of biochemical markers and changes in MRI features over 12 and 24 months.
| Biochemical marker | MRI markers | ||||
|---|---|---|---|---|---|
| Hoffa-synovitis | Effusion-synovitis | ||||
| Improvement/No change | Worsening | Improvement | No change | Worsening | |
| 12 months | |||||
| sHA | REF | 0.85 (0.54, 1.33) | 0.92 (0.71, 1.21) | REF | 0.82 (0.61, 1.11) |
| sMMP-3 | REF | 0.79 (0.44, 1.39) | 0.85 (0.59, 1.22) | REF | 0.88 (0.65, 1.18) |
| sColl2–1NO2 | REF | 0.85 (0.51, 1.41) | 0.81 (0.55, 1.29) | REF | 1.04 (0.85, 1.26) |
| 24 months | |||||
| sHA | REF | 1.03 (0.78, 1.35) | 1.01 (0.80, 1.26) | REF | 1.01 (0.78, 1.31) |
| sMMP-3 | REF | 0.92 (0.62, 1.36) | 0.87 (0.59, 1.27) | REF | 0.84 (0.63, 1.10) |
| sColl2–1NO2 | REF | 1.07 (0.87, 1.36) | 0.97 (0.68, 1.38) | REF | 1.17 (0.96, 1.42) |
Adjusted for age, gender, BMI, baseline KLG and respective baseline feature on MRI. Odds ratio and 95% confidence intervals (CI) are presented in the table.
Association of clinical assessment and effusion-synovitis on MRI
The diagnostic performance of the clinical signs of effusion is presented in Table 5. Both bulge and patellar-tap signs had a high specificity but low sensitivity to detect the presence of effusion-synovitis on MRI (88.8% and 22.0% for bulge sign and 94.8% and 4.3% for patellar tap sign, respectively). When restricted to moderate to large effusion-synovitis (score ≥ 2), there was an improvement in the tests’ accuracy and negative predictive values, but their sensitivity remained low (37% and 7% for bulge and patellar tap signs, respectively).
Table 5.
Performance characteristics of physical examination tests for detecting effusion-synovitis on MRI in
| Bulge sign*Present (total) = 107 (17.8%) | Patellar tap sign* Present (total) = 28 (4.7%) |
|||
|---|---|---|---|---|
| Effusion-synovitis ≥ 1 (n=367) | Effusion-synovitis ≥ 2** (n=117) | Effusion-synovitis ≥ 1 (n=367) | Effusion-synovitis ≥ 2 (n=117) | |
| Test positive, n (%) | 81 (22.1) | 44 (37.6) | 16 (4.4) | 9 (7.7) |
| Test negative, n (%) | 286 (77.9) | 73 (62.4) | 351 (95.6) | 108 (92.3) |
| Sensitivity (%) | 22.0 | 37.6 | 4.3 | 7.6 |
| Specificity (%) | 88.8 | 86.9 | 94.8 | 96.0 |
| Positive predictive value (%) |
75.7 | 41.1 | 57.1 | 32.1 |
| Negative predictive value (%) | 41.9 | 85.1 | 38.6 | 81.2 |
| Accuracy (%) | 48.0 | 77.3 | 39.5 | 78.8 |
Assessed on the index knee.
Compared with individuals with scores 0 and 1.
DISCUSSION
We demonstrated that baseline concentrations of sHA and sMMP-3 were associated with presence of moderate to large effusion-synovitis on non-CE MRI, although these associations were weak. The prevalence of moderate to severe MRI markers of synovitis in this study at baseline was relatively low (9% for Hoffa-synovitis and 19% for effusion-synovitis) compared to a previous report of nearly 55% prevalence of moderate to large effusions in persons with symptomatic knee OA recruited from the general population (30). On the other hand, baseline biochemical markers and their TICs over time were, in general, not predictive of changes in Hoffa- and effusion-synovitis on MRI. We observed inverse associations between biochemical markers and change in the MRI parameters in the predictive and concurrent analyses, although most were not statistically significant, which could be explained by regression to the mean. In addition, we showed that both bulge and patellar tap signs on clinical assessment of the knee have high specificity but low sensitivity for detecting effusion-synovitis on non-CE MRI.
To our knowledge, the association between MRI-detected synovitis and biochemical markers of inflammation has not been well established in the field of OA research. However, previous studies have examined the association between biochemical markers and synovitis detected by other methods. Jung et al. used ultrasound to investigate the association between sHA and soft tissue changes in patients with knee OA and found a statistically significant difference between sHA levels in individuals with larger effusion, divided into two groups according to the median value of joint effusion (quantified in centimeters, p = 0.024) (11). In addition, sMMP-3 has been positively correlated with histological synovitis in patients with rheumatoid arthritis (31).
Furthermore, our study showed that clinical signs of effusion lack accuracy to detect the presence of effusion-synovitis on MRI. These same signs have been evaluated in another case-control study of 80 participants (abstract only) (32), also embedded in the OAI, which resulted in findings similar to ours: high specificity and low sensitivity of the bulge sign (100% and 29%, respectively) and low sensitivity of the patellar tap sign (4.8%) for knee effusion. In a further analysis of this same study including the whole OAI cohort (n = 9302), the presence of positive bulge sign was significantly associated with a 2–3 fold increase in total knee replacement within 5 years. Effusion-synovitis on non-CE MRI was shown to have good correlations with effusion volume measured by arthrocentesis (r = 0.6, p < 0.05), and with effusion and synovial thickness assessed by CE-MRI (r = 0.68, p < 0.001, and 0.63, p < 0.05, respectively) (33). The main implication of these findings for observational studies and clinical trials is that the absence of clinical signs of effusion in the clinical examination of the knee should not be used to rule out the presence of any effusion in that joint. These signs have been used in trials that analysed predictors of response to steroid injections and, to date, there is a great disagreement among the results of these studies, potentially as a result of the lack of accuracy of these clinical examination findings (17). On the other hand, accuracy, sensitivity and negative predictive value of these tests were higher when restricted to moderate to large effusions, meaning that there were less false negatives. The combination of inflammation-related symptoms such as night pain and joint stiffness to clinical examination may have a role in detecting presence of knee effusion and synovitis (28); however, to our knowledge, this is not well established in the literature in the context of patients with knee OA.
Limitations of our study include that it is a post-hoc analysis of a nested case-control study in which outcome status (case or control) has not been considered in the analysis. It is not certain whether the same associations would be found in a different non-selected OA population. In addition, although non-CE MRIs remains the most common method to assess synovitis on imaging, gadolinium-enhanced MRI is currently the gold standard for evaluating synovitis on MRI as it has greater accuracy to detect synovial inflammation and ability to differentiate joint effusion from synovial thickening (10, 33). However, CE-MRI is challenging to apply in large OA studies due to costs, practicality and rare but possible side effects of the contrast agent (10, 34). In addition, non-CE MRI was the imaging modality used in most knee OA studies that demonstrated the relevance of synovitis for clinical and research applications (2–5, 7, 10, 35). Nevertheless, the fact that modest associations were found in our study should encourage further studies investigating this association using more reliable methods, such as CE MRI or histological analysis, which may potentially yield more robust associations. Also, the relatively low prevalence of moderate to severe MRI-defined inflammation may also have limited the power of our analysis. Finally, while there is no serum biochemical marker highly specific for synovial inflammation, our analyses were limited to putative biochemical markers of synovitis that were available in the FNIH OA Biomarkers study.
In conclusion, we demonstrated that sHA and sMMP-3 are associated with effusion-synovitis at baseline, although these associations were modest. This seems to limit the utility of these systemic biochemical markers by themselves to detect the presence of synovitis in practice in individual knees, as detected by the non-CE MRI in this study. It is not known, however, whether assessment of synovitis using CE MRI or inclusion of a population with higher prevalence of moderate to larger effusion would reveal different associations. It also remains to be determined whether more comprehensive phenotyping including more joints, would result in stronger associations of synovitis on imaging and systemic concentrations of these biomarkers.
Financial Support and Acknowledgments:
Scientific and financial support for the FNIH OA Biomarkers Consortium and the study are made possible through grants, direct and in-kind contributions provided by: AbbVie; Amgen Inc.; Arthritis Foundation; Bioiberica S.A.; DePuy Mitek, Inc.; Flexion Therapeutics, Inc.; GlaxoSmithKline; Merck Serono; Rottapharm | Madaus; Sanofi; Stryker; The Pivotal OAI MRI Analyses (POMA) Study, NIH HHSN2682010000. We thank the Osteoarthritis Research Society International (OARSI) for their leadership and expertise on the FNIH OA Biomarker Consortium project. The OAI is a public-private partnership comprised of five contracts (N01-AR-2–2258; N01-AR-2–2259; N01-AR-2–2260; N01-AR-2–2261; N01-AR-2–2262) funded by the National Institutes of Health. Funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the Consortium and OAI is managed by the FNIH.
Footnotes
COMPETING INTEREST STATEMENT: Leticia Deveza, David Hunter, Jamie Collins and Virginia Kraus declare they have no conflict of interest. Ali Guermazi has received consulting fees from MerckSerono, Genzyme, OrthoTrophix, TissueGene, AstraZeneca and owns stock or stock options in Boston Imaging Core Lab (BICL), LLC. Frank Roemer owns stock or stock options in Boston Imaging Core Lab (BICL), LLC. Michael Nevitt receives grants from NIH and FNIH.
REFERENCES
- 1.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35. [DOI] [PubMed] [Google Scholar]
- 2.Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Fujii T, et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis Rheumatol. 2015;67:2085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, Roemer F, et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Blizzard L, Halliday A, Han W, Jin X, Cicuttini F, et al. Association between MRI-detected knee joint regional effusion-synovitis and structural changes in older adults: a cohort study. Ann Rheum Dis. 2016;75:519–25. [DOI] [PubMed] [Google Scholar]
- 6.Bastick AN, Runhaar J, Belo JN, Bierma-Zeinstra SM. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis Res Ther. 2015;17:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Neill TW, Parkes MJ, Maricar N, Marjanovic EJ, Hodgson R, Gait AD, et al. Synovial tissue volume: a treatment target in knee osteoarthritis (OA). Ann Rheum Dis. 2016. January;75:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attur M, Samuels J, Krasnokutsky S, Abramson SB. Targeting the synovial tissue for treating osteoarthritis (OA): where is the evidence? Best Pract Res Clin Rheumatol. 2010;24:71–9. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi D, Roemer FW, Katur A, Felson DT, Yang SO, Alomran F, et al. Imaging of synovitis in osteoarthritis: current status and outlook. Semin Arthritis Rheum. 2011;41:116–30. [DOI] [PubMed] [Google Scholar]
- 11.Jung YO, Do JH, Kang HJ, Yoo SA, Yoon CH, Kim HA, et al. Correlation of sonographic severity with biochemical markers of synovium and cartilage in knee osteoarthritis patients. Clin Exp Rheumatol. 2006;24:253–9. [PubMed] [Google Scholar]
- 12.Deberg M, Labasse A, Christgau S, Cloos P, Bang Henriksen D, Chapelle JP, et al. New serum biochemical markers (Coll 2–1 and Coll 2–1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2005;13:258–65. [DOI] [PubMed] [Google Scholar]
- 13.Chen JJ, Huang JF, Du WX, Tong PJ. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients. Asian Pac J Trop Med. 2014;7:297–300. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi A, Naito S, Enomoto H, Shiomoi T, Kimura T, Obata K, et al. Serum levels of matrix metalloproteinase 3 (stromelysin 1) for monitoring synovitis in rheumatoid arthritis. Arch Pathol Lab Med. 2007;131:563–70. [DOI] [PubMed] [Google Scholar]
- 15.Iagnocco A Imaging the joint in osteoarthritis: a place for ultrasound? Best Pract Res Clin Rheumatol. 2010;24:27–38. [DOI] [PubMed] [Google Scholar]
- 16.Cohen SB, Proudman S, Kivitz AJ, Burch FX, Donohue JP, Burstein D, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther. 2011;13:R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maricar N, Callaghan MJ, Felson DT, O’Neill TW. Predictors of response to intra-articular steroid injections in knee osteoarthritis--a systematic review. Rheumatology (Oxford). 2013;52:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arden NK, Reading IC, Jordan KM, Thomas L, Platten H, Hassan A, et al. A randomised controlled trial of tidal irrigation vs corticosteroid injection in knee osteoarthritis: the KIVIS Study. Osteoarthritis Cartilage. 2008;16:733–9. [DOI] [PubMed] [Google Scholar]
- 19.Maricar N, Callaghan MJ, Parkes MJ, Felson DT, TW ON. Clinical assessment of effusion in knee osteoarthritis-A systematic review. Semin Arthritis Rheum. 2016;45:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol. 2014;28:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Osteoarthritis Initiative. Protocol for the cohort study [Internet]. Available from: http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf.
- 22.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13:177–83. [DOI] [PubMed] [Google Scholar]
- 25.Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, et al. Hoffa’s Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. AJR Am J Roentgenol. 2009;192:1696–700. [DOI] [PubMed] [Google Scholar]
- 26.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 2017;76:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolf AD. How to assess musculoskeletal conditions. History and physical examination. Best Pract Res Clin Rheumatol. 2003;17:381–402. [DOI] [PubMed] [Google Scholar]
- 28.Kastelein M, Luijsterburg PA, Wagemakers HP, Bansraj SC, Berger MY, Koes BW, et al. Diagnostic value of history taking and physical examination to assess effusion of the knee in traumatic knee patients in general practice. Arch Phys Med Rehabil. 2009;90:82–6. [DOI] [PubMed] [Google Scholar]
- 29.Cibere J, Bellamy N, Thorne A, Esdaile JM, McGorm KJ, Chalmers A, et al. Reliability of the knee examination in osteoarthritis: effect of standardization. Arthritis Rheum. 2004;50:458–68. [DOI] [PubMed] [Google Scholar]
- 30.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–7. [PubMed] [Google Scholar]
- 31.Ma JD, Zhou JJ, Zheng DH, Chen LF, Mo YQ, Wei XN, et al. Serum matrix metalloproteinase-3 as a noninvasive biomarker of histological synovitis for diagnosis of rheumatoid arthritis. Mediators Inflamm. 2014;2014:179284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith B MD, Lambert RG, Maksymowych W, et al. Implications of a Positive Patellar Bulge Sign of Knee Joint Effusion in Patients with Osteoarthritis: Association with MRI Signs of Inflammation and with Increased Rate of 5-Year Total Knee Arthroplasty. Data from the Osteoarthritis Initiative. Poster session presented at: 2015 American College of Rheumatology Annual Meeting; 2015, November 6–11, San Francisco, CA. [Google Scholar]
- 33.Loeuille D, Sauliere N, Champigneulle J, Rat AC, Blum A, Chary-Valckenaere I. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2011;19:1433–9. [DOI] [PubMed] [Google Scholar]
- 34.Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG. Revisiting the risks of MRI with Gadolinium based contrast agents-review of literature and guidelines. Insights Imaging. 2015;6:553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Jin X, Han W, Cao Y, Halliday A, Blizzard L, et al. Cross-sectional and Longitudinal Associations between Knee Joint Effusion Synovitis and Knee Pain in Older Adults. J Rheumatol. 2016;43:121–30. [DOI] [PubMed] [Google Scholar]