Abstract
Purpose
In malignant melanoma, recurrence is often observed in distant areas from the primary site. While FDG PET is a sensitive imaging for detecting malignant lesions, the role of FDG PET in posttreatment surveillance period has not been investigated sufficiently. The aim of this study was to evaluate the value of PET during posttreatment surveillance in melanoma.
Methods
A total of 76 melanoma patients who underwent FDG PET during surveillance period after completion of the first treatment were retrospectively enrolled. PET scans were grouped according to the purpose and clinical situations, routine surveillance, or evaluating clinical suspicion. Final diagnosis of recurrence was determined by complete clinical evaluation or long-term follow-up. In each situation, the diagnostic role of FDG PET was assessed.
Results
A total of 143 scans of 76 patients were analyzed: 51 for clinical suspicion and 92 for routine surveillance. In the clinical suspicion group, PET correctly diagnosed non-recurrence in 10 cases (20%). In routine surveillance group, 16 cases (17%) presented recurrence, all of which was correctly diagnosed on PET. NPV and PPV were 100% and 76%, respectively. In subgroup analysis, sensitivity and NPV were higher in the low-risk group (stages I–IIA) than in the high-risk group (stages IIB–IV), while specificity and PPV were higher in the high-risk group.
Conclusion
In conclusion, FDG PET is an effective diagnostic tool in posttreatment surveillance of melanoma. Even in cases without clinical suspicion, melanoma recurs in a considerable proportion of patients, which can be sensitively diagnosed on PET.
Keywords: FDG, PET-CT, Melanoma, Surveillance
Introduction
The incidence of melanoma continues to increase worldwide. Although it is a relatively rare malignancy, the incidence of malignant melanoma in most developed countries has risen faster than any other cancers since the mid-1950s [1, 2]. In malignant melanoma, late recurrence after successful initial treatment is not rare. Faries et al. reported that late recurrences are more common in distant areas, whereas early recurrence are predominantly loco-regional [3]. Thus, long-term monitoring is required in survivors of melanoma, which should include evaluation of distant areas. Although local or nodal recurrences can be easily discovered by patients themselves, systemic recurrences are less likely to be detected early [4]. Thus, laboratory examinations and imaging studies are required for appropriate monitoring of asymptomatic patients for early detection of recurrence.
Despite many previous studies, no consensus has been reached on the optimal follow-up method or the incremental value of diagnostic tests, in posttreatment surveillance of melanoma [5–7]. According to the National Comprehensive Cancer Network (NCCN) guidelines for surveillance of melanoma survivors, imaging study is not routinely recommended for stage I–IIA diseases. In stage IIB–IV diseases, it is considered to monitor patients for 5 years by using routine imaging studies, including chest x-ray, computed tomography (CT), and/or 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) scans every 3 to 12 months, as well as annual magnetic resonance imaging (MRI) [7, 8].
Whole-body FDG PET is a non-invasive, high-resolution molecular imaging technique that can detect recurrences or metastases. Many studies have reported high sensitivity and specificity of FDG PET for detecting recurrence or metastases [9–15], which are superior to those of conventional imaging methods. However, FDG PET has not been included in recommendations or guidelines for routine surveillance of cancers [15, 16], probably because of unconfirmed comparative cost-effectiveness. Additionally, surveillance using FDG PET is sometimes considered to be an “unwise” choice in some cancers.
In the present study, we retrospectively surveyed and followed up melanoma patients of a tertiary cancer center who underwent FDG PET/CT for the purpose of posttreatment surveillance. The aim of this study was to assess diagnostic performance and role of FDG PET in posttreatment surveillance of melanoma.
Materials and Methods
Patients
From January 2005 to December 2014, patients with biopsy-proven melanoma who underwent FDG PET/CT scan in Seoul National University Hospital were retrospectively retrieved from the healthcare information system. Among them, those who had no residual malignancy after completion of initial treatment were selected. Repeated PET/CT scans of a patient were all included in the analysis, but scans were excluded in cases where PET/CT was performed for restaging of confirmed recurrence or for second primary cancer. A PET/CT scan was classified as routine surveillance group if it was performed for routine checkup without any suspicion of recurrence. If a PET/CT was performed for any symptom, abnormal physical examination and abnormal laboratory results or radiological findings, it was classified as suspicious recurrence group. For subgroup analysis according to risk of recurrence, patients were divided into two subgroups of low (stages I–IIA) and high risk (stages IIB–IV).
Image Acquisition and Analysis
After fasting for at least 6 h, FDG (5.18 MBq/kg) was injected intravenously, and images were acquired 1 h later using hybrid PET/CT scanners (Biograph mCT40 or mCT64; Siemens Healthcare). CT images were acquired for the whole body (from the vertex to the toe) for attenuation mapping and lesion localization (50 mA, 120 kVp, 5-mm section width, 4-mm collimation). After CT scan, PET images were acquired in three-dimensional mode for 6–7 bed positions (1 min per bed position). Images were reconstructed on 128 × 128 matrices using an iterative algorithm. The images were analyzed using a vendor-supplied analysis software package (Syngo.via, Siemens Healthcare).
PET/CT images were retrospectively interpreted by consensus of two nuclear medicine specialists who were unaware of the final clinical outcome. Definitely abnormal lesions of FDG uptake (with excluding physiological or inflammatory uptake) were classified as positive for recurrence and, otherwise, classified as negative. Indeterminate lesions with borderline uptake increase were classified as negative.
Clinical Outcome and Statistical Analysis
Patients’ information was obtained from the healthcare information system. Final diagnosis of a patient was determined by histologic confirmation of detected lesions and/or follow-up results based on image or clinical findings; if a patient without treatment did not exhibit disease progression for more than 6 months, the patient was deemed to be negative for recurrence. Based on the final diagnosis, PET/CT findings were classified as true positive (TP), false positive (FP), true negative (TN), or false negative (FN). Diagnostic performance of PET/CT was expressed in terms of positive predictive value (PPV) and negative predictive value (NPV).
Descriptive statistical values were expressed as mean ± standard deviation. To evaluate diagnostic performance of imaging tools, McNemar’s test and chi-square test were carried out using a statistical software package (MedCalc version 17.6, MedCalc Software bvba, Ostend, Belgium).
Results
Patients
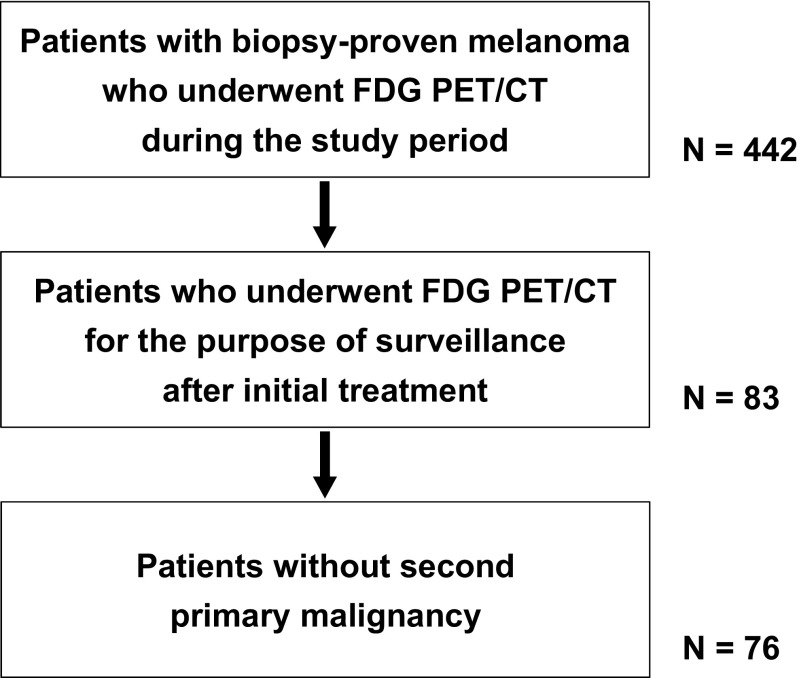
During the study period, 76 patients (M:F = 43:33, age 62 ± 15 years, range 15–88 years) met the inclusion criteria (Fig. 1). Patient characteristics are summarized in Table 1. From the patients, 143 PET/CT scans were included in the final analysis because of repeated surveillance scans: 1 scan in 39 patients, 2 scans in 23, 3 scans in 5, 4 scans in 5, 5 scans in 2, 6 scans in 1, and 7 scans in 1. In cases of repeated scans, the interval between repeated scans was 26.1 ± 20.6 months (range 4–122 months). Among 143 scans, 92 (64%) of 44 patients were performed for routine surveillance; the other 51 (36%) of 32 patients were performed for clinical suspicion of recurrence.
Fig. 1.
Flowchart of patient selection
Table 1.
Characteristics of patients
| Characteristics | Value |
|---|---|
| Total number of patients | 76 |
| Sex | |
| Male | 43 |
| Female | 33 |
| Location of lesions | |
| Extremities | 45 |
| Head and neck | 18 |
| Trunk | 8 |
| Eye | 5 |
| Initial treatment | |
| Surgery | 39 |
| Chemotherapy | 26 |
| RT or CCRT | 11 |
| Stage | |
| I–IIA | 46 |
| IIB–IV | 30 |
Diagnostic Performance of PET/CT
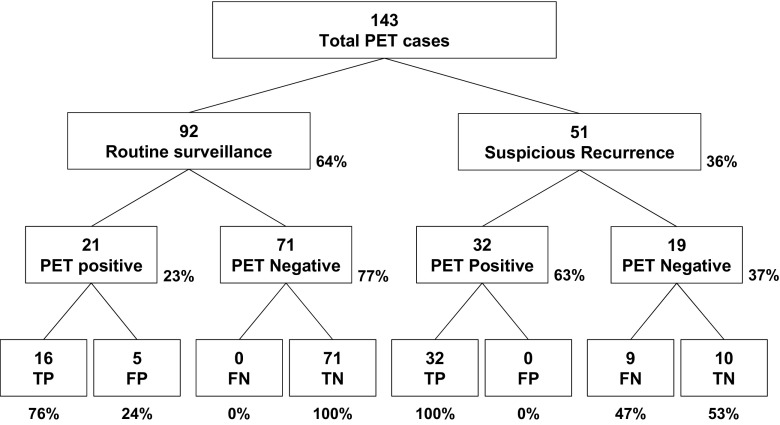
In 92 routine surveillance group, positive findings were detected in 21 cases (21/92, 23%), of which 16 were confirmed as recurrence, or TP (PPV = 16/21, 76%; Fig. 2). FP findings included skin lesions, schwannoma, lymphadenopathy, lung nodule, and artifact in the bladder. Recurrence detection rate of FDG PET was 17% (16/92), and the time interval between completion of initial treatment and recurrence was 32.1 ± 25.3 months (range 8.0–92.0 months). PET findings were negative in 71 cases, of which no one exhibited recurrence during 6 months thereafter (NPV = 71/71, 100%). In 16 cases of TP, treatment including surgical resection and/or chemotherapy was started. In five cases with FP, biopsy was performed in two cases and patients were followed up without treatment in three cases.
Fig. 2.
PET findings and final results in overall patient group
In the suspicious recurrence group, positive findings were detected in 32 cases (32/51, 63%), all of which were confirmed as recurrence, or TP (PPV = 32/32, 100%). PET findings were negative in 19 cases, of which 10 were confirmed as no recurrence, or TN (NPV = 10/19, 53%), whereas 9 cases (47%) were FN (Fig. 2). Thus, sensitivity and specificity were 78% and 100%, respectively. In the suspicious recurrence group, 15 cases were referred to PET/CT due to abnormal findings on preceding image studies: magnetic resonance image (MRI) in seven patients, ultrasonography (US) in four patients, CT in three patients, and bone scan in one patient. Among the 15 lesions (one lesion in each patient), 14 were TP for recurrence, except 1 lesion detected on MRI. On PET/CT, 12 of the 15 lesions were detected and additional 6 lesions were detected in four patients. Among them, 17 lesions were TP (lesion-based PPV = 17/18, 94%). In cases of TP lesions, respective treatments were started. In ten cases with TN, seven cases were followed up with observation based on PET/CT results, whereas excisional biopsy was performed in three cases. In nine cases with FN, a diagnosis was made and treatment was started based on the results from other studies.
Detection of recurrence by PET/CT according to recurrence site is summarized in Table 2.
Table 2.
Detection of recurrence according to recurrence site
| Recurrence site | PET/CT-positive (n) | PET/CT-negative (n) | Total |
|---|---|---|---|
| Lymph node | 23 (100%) | 0 (0%) | 23 |
| Skin | 13 (81%) | 3 (19%) | 16 |
| Lung | 10 (83%) | 2 (17%) | 12 |
| Bone | 6 (100%) | 0 (0%) | 6 |
| Brain | 4 (67%) | 2 (33%) | 6 |
| Liver | 4 (80%) | 1 (20%) | 5 |
| Others* | 4 (67%) | 2 (33%) | 6 |
*Stomach, colon, spleen, hard palate, nasal cavity
Subgroup Analysis According to Risk
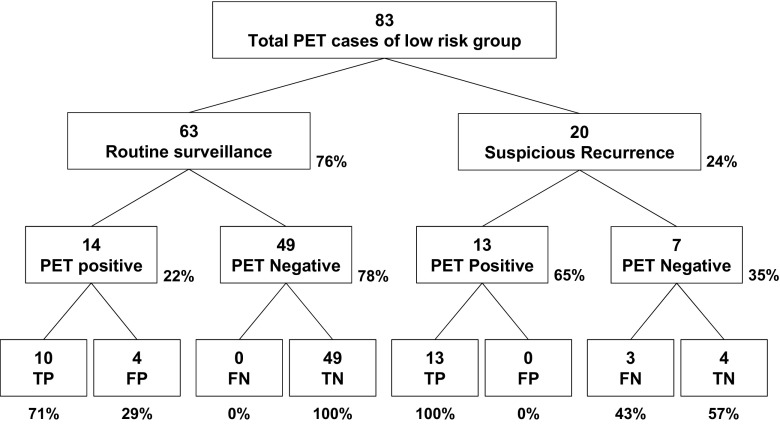
According to the initial stage, patients were divided into two subgroups of low risk (stages I–IIA) and high risk (stages IIB–IV). The low-risk group included 83 PET/CT scans of 46 patients, and the high-risk group included 60 scans of 30 patients. In the low-risk group, 63 scans were performed for routine surveillance (Fig. 3). In this subgroup, 14 were positive and 10 were TP (PPV = 10/14, 71%). Thus, recurrence detection rate of FDG PET in routine surveillance was 16% (10/63). Treatment was initiated in all ten patients. In 20 cases of the suspicious recurrence group, 13 were positive and all of them were TP (PPV = 100%); the other 7 were negative, of which 4 were TN (NPV = 4/7, 57%, Fig. 3).
Fig. 3.
PET findings and final results in low-risk (stage I–IIA) patient group
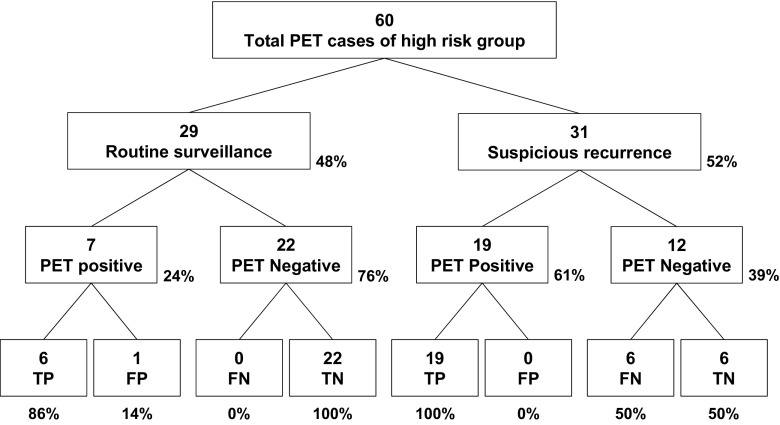
In the high-risk group, 29 scans were performed for routine surveillance (Fig. 4), of which 7 were positive and 6 were TP (PPV = 6/7, 86%). Thus, recurrence detection rate of FDG PET in routine surveillance was 21% (6/29). In 31 cases of the suspicious recurrence group, 19 were positive and all of them were TP (PPV = 100%); the other 12 were negative, of which 6 were TN (NPV = 6/12, 50%, Fig. 4). There was no statistical difference between the diagnostic performances of low-risk and high-risk groups.
Fig. 4.
PET findings and final results in high-risk (stage IIB–IV) patient group
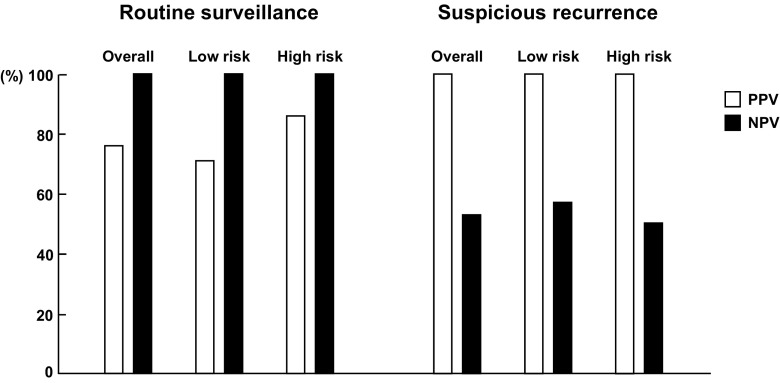
PPV and NPV in overall and each subgroup are shown in Fig. 5.
Fig. 5.
Diagnostic performance of FDG PET/CT in overall group and each subgroup
Discussion
In the present study, diagnostic role of FDG PET was investigated in melanoma survivors. A considerable number of recurrences were detected by FDG PET even in asymptomatic patients, with a high NPV. In patients with suspicious recurrence, PET exhibited a high PPV.
FDG PET is highly sensitive for a malignant lesion and it can cover the whole body in a single scan. Thus, FDG PET is an effective imaging tool not only for initial staging and response evaluation but also for detecting cancer recurrence. Beasley et al. conducted a multicenter prospective study that evaluated clinical utility of FDG PET in patients with stage IIIB–IIIC extremity melanoma. FDG PET was useful for response evaluation and prognosis prediction; 3-year disease-free rate was 62.2% in patients who were determined as complete response by both clinical/pathologic examinations and FDG PET, whereas it was only 29.4% in patients who had residual lesion on PET. In this study, FDG PET was used for surveillance of patients, and 52% of patients developed disease outside the extremity at a median time of 212 days from baseline PET. Because unexpected distant metastasis is frequently detected in melanoma patients, FDG PET may be effective in posttreatment surveillance [9].
There have been a few studies that evaluated diagnostic value of FDG PET in posttreatment surveillance of melanoma. Koskivuo et al. assessed the role of FDG PET in detecting clinically silent metastases during follow-up of 30 asymptomatic patients with stage IIB–IIIC melanoma [13]. In this study, FDG PET was performed at average 11 months (range, 7–24 months) after initial surgery, and seven cases (23%) of recurrence was detected, of which six were detected on FDG PET. This resulted in PPV 86% and NPV 96% of FDG PET for melanoma recurrence. In another study, 8 (6.5%) of 123 PET scans identified malignancy in melanoma patients with no clinical suspicion [17]. In the present study, 92 PET cases of 44 asymptomatic patients were analyzed and recurrence was detected in 17% of patients (16% of the low-risk group, 21% of the high-risk group). It is speculated that recurrence rate is varied between 6.5 and 23% in asymptomatic patients, due to different patient characteristics regarding recurrence risk and follow-up schedule. Despite the variation in recurrence rate, PET has been reported consistently to have high sensitivity. In the present study, NPV of FDG PET was as high as 100%.
Usually, FDG PET is not recommended as a routine surveillance modality in cancer patients. In Korea, FDG PET for recurrence surveillance used to be covered limitedly by the National Health Insurance until 2014, which is currently not allowed by the National Guidance. In the USA, three posttreatment FDG PET scans are covered by Medicare and Medicaid Services, and Taghipour et al. [15] had conducted a retrospective institutional review of 433 cancer patients, including a total of 1659 fourth and subsequent follow-up PET/CT scans after completion of primary treatment. In this study, fourth and subsequent follow-up FDG PET/CT scans led to management change in 31.6% of cases when scans were obtained for clear needs. However, management was changed only in 5.6% of cases when scans were obtained in remission state, or as a part of clinical trial protocols. They did not recommend routine follow-up PET in remission state. In contrast, Choi et al. reported that surveillance using FDG PET in disease-free colorectal cancer patients is valuable, in comparison with other conventional imaging studies [16].
Other imaging modalities as well as FDG PET are also not recommended in routine surveillance of stage I–IIA melanoma by many authoritative guidelines [7]. The National Comprehensive Cancer Network (NCCN) recommends considering every 3–12 months routine imaging to screen recurrence or metastatic disease, which is restricted to stage IIB–IV melanoma patients after 3–5 years. Thus, routine imaging surveillance is not recommended to stage I–IIA melanoma patients [18]. Hengge et al. analyzed follow-up results of melanoma patients on an as-treated basis, in which treatment was based on recommendations of the American Joint Committee on Cancer 2002 and the German Dermatologic Society [12]. This analysis revealed that physical examination and ultrasonography for lymph nodes were cost-effective in melanoma of all stages. In contrast, chest x-ray and abdominal ultrasonography were reported to be cost-effective only in stage III, where only 0.83% of all performed examinations revealed metastases. Lewin et al. also reported high NPV (96%) of surveillance PET/CT in stage III melanoma [17], although Vensby et al. reported that routine surveillance PET may cause unnecessary anxiety and further diagnostic procedures due to relatively high frequency of false positive findings [19]. Considering the results of the present study, FDG PET appears to be effective and probably cost-effective in at least high-risk melanoma patients. Additionally, it is required to evaluate cost-effectiveness of FDG PET for routine surveillance in low-risk group, because the recurrence rate (10/63, 16%) was not ignorable in the present study.
In contrast to routine surveillance, the role of FDG PET in the suspicious recurrence group is very clear. Mena et al. evaluated the added value of fourth and subsequent follow-up FDG PET scans in patients with melanoma [20], and they concluded that patients with clinical signs suggestive of recurrence or metastases can benefit from fourth or subsequent FDG PET, because PET excluded recurrence in 28.5% of patients. In the present study, although NPV of PET was somewhat low (53%) in the suspicious recurrence group, PPV was as high as 100% and recurrence was clearly confirmed by PET.
The present study has some limitations due to its retrospective nature. First, this is a retrospective study in a single tertiary cancer center and the surveillance using FDG PET was not performed systematically. Although the purpose and indication of each PET scan was retrospectively reviewed and determined, there may have been a bias in selecting patients, particularly for routine surveillance. Second, histopathological confirmation was not performed in all PET-positive cases, but clinical diagnosis was made based on follow-up results. However, it is expected that these limitations did not critically affect the results.
Conclusion
In conclusion, FDG PET is an effective diagnostic tool in posttreatment surveillance of melanoma in cases of suspicious recurrence by clinical symptoms, signs, and/or other image findings. Even in cases without clinical suspicion, melanoma recurs in a considerable proportion of patients, which can be sensitively diagnosed on PET. Thus, cost-effectiveness of surveillance PET needs to be evaluated, particularly in high-risk patients.
Conflict of Interest
Hwan Hee Lee, Jin Chul Paeng, Gi Jeong Cheon, Dong Soo Lee, June-Key Chung, and Keon Wook Kang declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
The study design of the retrospective analysis and exemption of informed consent were approved by the Institutional Review Board of the Seoul National University Hospital (H-1804-008-932).
References
- 1.Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170:11–19. doi: 10.1111/bjd.12492. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. PeerJ. 2016;4:e2809. doi: 10.7717/peerj.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faries MB, Steen S, Ye X, Sim M. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27–34. doi: 10.1016/j.jamcollsurg.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol. 2010;28:3042–3047. doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyers MO, Yeh JJ, Frank J, Long P, Deal AM, Amos KD, et al. Method of detection of initial recurrence of stage II/III cutaneous melanoma: analysis of the utility of follow-up staging. Ann Surg Oncol. 2009;16:941–947. doi: 10.1245/s10434-008-0238-y. [DOI] [PubMed] [Google Scholar]
- 6.Bichakjian CK, Halpern AC, Johnson TM, Hood AF, Grichnik JM, Swetter SM, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;65:1032–1047. doi: 10.1016/j.jaad.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Trotter SC, Sroa N, Winkelmann RR, Olencki T, Bechtel M. A global review of melanoma follow-up guidelines. J Clin Aesthet Dermatol. 2013;6:18–26. [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhardt MJ, Joe AY, Jaeger U, Huber A, Matthies A, Bucerius J, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–1187. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 9.Beasley GM, Parsons C, Broadwater G, Selim MA, Marzban S, Abernethy AP, et al. A multicenter prospective evaluation of the clinical utility of F-18 FDG-PET/CT in patients with AJCC stage IIIB or IIIC extremity melanoma. Ann Surg. 2012;256:350–356. doi: 10.1097/SLA.0b013e318256d1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown RE, Stromberg AJ, Hagendoorn LJ, Hulsewede DY, Ross MI, Noyes RD, et al. Surveillance after surgical treatment of melanoma: futility of routine chest radiography. Surgery. 2010;148:711–717. doi: 10.1016/j.surg.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 11.Danielsen M, Hojgaard L, Kjaer A, Fischer BMB, et al. Positron emission tomography in the follow-up of cutaneous malignant melanoma patients: a systematic review. Am J Nucl Med Mol Imaging. 2014;4:17–28. [PMC free article] [PubMed] [Google Scholar]
- 12.Hengge UR, Wallerand A, Stutzki A, Kochel N. Cost-effectiveness of reduced follow-up in malignant melanoma. J Dtsch Dermatol Ges. 2007;5:898–907. doi: 10.1111/j.1610-0387.2007.06454.x. [DOI] [PubMed] [Google Scholar]
- 13.Koskivuo IO, Seppanen MP, Suominen EA, Minn HR. Whole body positron emission tomography in follow-up of high risk melanoma. Acta Oncol. 2007;46:685–690. doi: 10.1080/02841860600972885. [DOI] [PubMed] [Google Scholar]
- 14.Morton RL, Craig JC, Thompson JF. The role of surveillance chest X-rays in the follow-up of high-risk melanoma patients. Ann Surg Oncol. 2009;16:571–577. doi: 10.1245/s10434-008-0207-5. [DOI] [PubMed] [Google Scholar]
- 15.Taghipour M, Marcus C, Sheikhbahaei S, Mena E, Prasad S, Jha AK, et al. Clinical indications and impact on management: fourth and subsequent posttherapy follow-up 18F-FDG PET/CT scans in oncology patients. J Nucl Med. 2017;58:737–743. doi: 10.2967/jnumed.116.183111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi EK, Yoo IR, Park HL, Choi HS, Han EJ, Kim SH, et al. Value of surveillance 18F-FDG PET/CT in colorectal cancer: comparison with conventional imaging studies. Nucl Med Mol Imaging. 2012;46:189–195. doi: 10.1007/s13139-012-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewin J, Sayers L, Kee D, Walpole I, Sanelli A, Marvelde LT, et al. Surveillance imaging with FDG-PET/CT in the post-operative follow-up of stage 3 melanoma. Ann Oncol. 2018; 10.1093/annonc/mdy124. [DOI] [PubMed]
- 18.National Comprehensive Cancer Network (NCCN). NCCN guidelines version 2. 2018 Melanoma. https://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed April 11, 2018.
- 19.Vensby PH, Schmidt G, Kjær A, Fischer BM. The value of FDG PET/CT for follow-up of patients with melanoma: a retrospective analysis. Am J Nucl Med Mol Imaging. 2017;7:255–262. [PMC free article] [PubMed] [Google Scholar]
- 20.Mena E, Taghipour M, Sheikhbahaei S, Mipour S, Xiao J, Shbramaniam RM. 18F-FDG PET/CT and melanoma: value of fourth and subsequent posttherapy follow-up scans for patient management. Clin Nucl Med. 2016;41:e403–e409. doi: 10.1097/RLU.0000000000001275. [DOI] [PubMed] [Google Scholar]