Abstract
Purpose
Although 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is a standard imaging modality for response evaluation in FDG-avid lymphoma, there is a controversy using FDG PET in indolent lymphoma. The purpose of this study was to investigate the effectiveness of quantitative indexes on FDG PET in response evaluation of the indolent lymphoma.
Methods
Fifty-seven indolent lymphoma patients who completed chemotherapy were retrospectively enrolled. FDG PET/computed tomography (CT) scans were performed at baseline, interim, and end of treatment (EOT). Response was determined by Lugano classification, and progression-free survival (PFS) by follow-up data. Maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were measured in the single hottest lesion (target A) or five hottest lesions (target B). Their efficacies regarding response evaluation and PFS prediction were evaluated.
Results
On EOT PET, SUVmax, and MTV of both targets were well associated with visual analysis. Changes between initial and EOT PET were not significantly different between CR and non-CR groups. On interim PET, SUVmax, and %ΔSUVmax in both targets were significantly different between CR and non-CR groups. For prediction of PFS, most tested indexes were significant on EOT and interim PET, with SUVmax being the most significant prognostic factor.
Conclusion
Quantitative indexes of FDG PET are well associated with Lugano classification in indolent lymphoma. SUVmax measured in the single hottest lesion can be effective in response evaluation and prognosis prediction on interim and EOT PET.
Keywords: Indolent lymphoma, 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET), Response evaluation
Introduction
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) is currently a standard imaging modality for staging and response evaluation in FDG-avid lymphoma such as diffuse large B cell lymphoma (DLBCL). According to the Deauville criteria and the Lugano classification, response evaluation of FDG-avid lymphoma is based on visual grading with a 5-point scale. In this classification, a score of 1–3 (uptake lower than or similar to that of the liver) without any new lesion is classified as complete response (CR), and a score of 4–5 (uptake higher than that of the liver) or any new lesion is classified as partial or no response [1–3].
In contrast with aggressive lymphoma such as DLBCL, indolent lymphoma including follicular lymphoma (FL) and marginal zone B cell lymphoma (MZBCL) exhibits relatively slow growth and variable FDG avidity [4–11]. Mean overall survival times are 9–11 years for FL and 8–13 years for MZBCL. Although some indolent lymphoma may transform into aggressive types and eventually leads to poor prognosis, most of indolent lymphoma exhibits slow progression. Thus, it is questioned whether the method of response evaluation for the FDG-avid lymphoma may also be applied to these types of lymphoma.
Quantitation ability is an essential strength of PET imaging. With quantitative analysis, small changes can be detected with high accuracy. Additionally, quantitative analysis is more observer independent than visual qualitative analysis. However, there are still several suggestions for response evaluation method using FDG PET, in terms of target lesions and quantitative indexes. In PERCIST system [12], it is recommended to measure standardized uptake value (SUV) of a single representative lesion, whereas RECIST1.1 recommends to measure tumor diameters of maximum five lesions with no more than two lesions of a single organ [13]. Thus, methods for treatment response evaluation using quantitative indexes of FDG PET need more refinement and validation.
In this study, we aimed to investigate the feasibility and effectiveness of quantitative indexes on FDG PET in treatment response evaluation of the indolent lymphoma for effective use of quantitative analysis in such clinical conditions. Various quantitative indexes on interim and end-of-treatment (EOT) PET were tested in terms of association with response determined by Lugano classification and patients’ final outcome.
Patients and Methods
Patients
From the image archive of our institution, patients who were diagnosed with indolent lymphoma and underwent initial FDG PET/CT scan between 2012 and 2016 were retrospectively enrolled. The inclusion criteria were (1) patient’s age ≥ 20 years, (2) pathologically proven FL or MZBCL treated with the standard regimen of chemotherapy, and (3) available FDG PET/CT scans obtained at baseline, interim (after 2–3 cycles) and EOT phases. The study design and waiver of informed consent were approved by our institutional review board (H-1703-108-840).
FDG PET/CT and Image Analysis
Patients fasted for at least 6 h, and PET/CT was performed at 1 h after intravenous injection of FDG (5.18 MBq/kg) using dedicated PET/CT scanners (Biograph mCT40 or mCT64, Siemens Healthcare, Germany). CT scan for attenuation correction and lesion localization was performed, followed by emission scan from the skull base to the proximal thigh. PET images were reconstructed on 128 × 128 matrices using an iterative algorithm.
Images were analyzed using an analysis software package (Syngo.via, Siemens Healthcare, Knoxville, TN, USA). For quantitative analysis of FDG uptake, maximum SUV (SUVmax; g/mL) was obtained for a lesion. For volumetric analysis, metabolic tumor volume (MTV; cm3) and total lesion glycolysis (TLG; g) of a lesion were measured, for which a spherical volume of interest (VOI) was manually drawn to encompass whole target lesion and a tumor contour was automatically drawn with the margin threshold of SUV 3.0. The volume of the isocontour VOI was defined as MTV, and TLG was calculated by multiplying mean SUV and the MTV. The cutoff value of SUV 3.0 was chosen based on our preliminary analysis, in which various margin thresholds of SUVmax-based relative values (30–70% of SUVmax with increment of 10%), fixed values (SUV 3.0 and 4.0), and a reference tissue-based value (twice the mean SUV of mediastinal blood pool) were tested. Among them, SUV 3.0 showed the highest statistical significances. Additionally, it was considered that SUV 3.0 is usual mean SUV of the liver, which is used as the reference tissue in Deauville criteria and Lugano classification.
Two target lesion sets were defined for analysis; (1) a single hottest lesion (target A), like the target lesion definition of PERCIST, and (2) a maximum of five hottest lesions, like the target lesion definition of RECIST1.1 (target B). In case of target B, quantitative PET indexes of all lesions were summed into a single value. Quantitative indexes at initial, interim, and EOT PET images were measured, and their percent differences (%Δ) between initial and interim, or between initial and EOT PET images were calculated.
Response Evaluation and Follow-up for Survival Analysis
EOT PET was visually analyzed by consensus of two experienced nuclear medicine physicians, and response was determined according to the Lugano classification; CR was defined as scores 1–3 without new lesion, and non-CR was defined as scores 4–5 or any new lesion [2]. SUVmax, MTV, and TLG and their %Δ from initial PET were measured with each of the two target lesion sets (targets A and B). On EOT PET, quantitative indexes were compared with visually determined response. On interim PET, quantitative indexes were tested as the early marker for the response on EOT PET.
For survival analysis, progression-free survival (PFS) was evaluated. Progression of disease was defined by PET/CT performed during follow-up. On follow-up PET/CT, progression was defined by the PERCIST criteria, as any new lesion or > 50% increase of SUVmax in previous lesions [12]. PFS was calculated from the date of baseline scan to progression of disease or death.
Statistical Analysis
Quantitative indexes were compared between groups using Student’s t test. For survival analysis, the optimal cutoff value of each index was determined by the receiver-operating characteristic curve analysis to maximize diagnostic performance. Survival analysis was performed using the Kaplan-Meier curve and Cox regression analysis. All statistical analyses were performed using a commercial statistical software package (MedCalc Ver. 18.2.1, MedCalc Software bvba, Ostend, Belgium), and p values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
A total of 57 patients (27 men and 30 women; mean age, 57 years; range, 25–79 years) were included in the analysis; 39 with FL and 18 with MZBCL. Most of the patients were in the advanced stage, and aggressive treatment was performed; all patients received a combination chemotherapy regimen of rituximab, cyclophosphamide, vincristine, and prednisolone. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Characteristics | Value |
|---|---|
| Number of patients | 57 |
| Age (median (range)) | 57 (25–79) |
| Sex | |
| Male | 27 (47.4%) |
| Female | 30 (52.6%) |
| Pathologic type | |
| Follicular lymphoma | 39 (68.4%) |
| Marginal zone B cell lymphoma | 18 (31.6%) |
| Involved sites | |
| Lymph node | 52 (91.2%) |
| Bone marrow | 31 (54.4%) |
| Spleen | 11 (19.3%) |
| Others | 20 (35.1%) |
| Stage | |
| II | 1 (1.8%) |
| III | 18 (31.6%) |
| IV | 38 (66.7%) |
| Follow-up time (months) | 22.3 ± 11.6 |
During the follow-up period of 22.3 ± 11.6 months (range, 8.2–62.0 months), 14 patients (24.6%) showed disease progression at 25.8 ± 13.5 months (range, 9.1–56.4 months).
Comparison of Quantitative Indexes and Response by Visual Analysis
Most of the cases showed high FDG uptake on initial PET (SUVmax, 12.1 ± 7.9, Table 2). In visual analysis of EOT PET, 37 patients were classified as CR and 20 patients were classified as non-CR according to Lugano classification. SUVmax and MTV on EOT PET were well associated with visual analysis and were significantly different between CR and non-CR groups with both targets A and B, whereas TLG was not. The indexes for changes between initial and EOT PET (%ΔSUVmax, %ΔMTV, and %ΔTLG) were not significantly different between CR and non-CR groups with both targets A and B (Table 2).
Table 2.
Initial values and end-of-treatment values of quantitative indexes according to response by visual analysis
| Index | Initial PET | End-of-treatment PET | %Δ between initial and end-of-treatment PET | ||||
|---|---|---|---|---|---|---|---|
| CR | Non-CR | P | CR | Non-CR | P | ||
| Target A | |||||||
| SUVmax | 12.1 ± 7.9 | 3.0 ± 2.7 | 4.6 ± 3.1 | 0.0454* | 79.2 ± 17.8 | 66.0 ± 31.7 | 0.0971 |
| MTV | 63.5 ± 160.2 | 1.0 ± 3.4 | 3.8 ± 7.1 | 0.0489* | 91.2 ± 32.3 | 39.9 ± 193.2 | 0.1186 |
| TLG | 626.1 ± 1603.5 | 4.2 ± 13.5 | 16.0 ± 36.8 | 0.1796 | 99.0 ± 3.5 | 31.2 ± 279.0 | 0.2906 |
| Target B | |||||||
| SUVmax | 40.4 ± 30.9 | 7.5 ± 4.1 | 12.3 ± 7.5 | 0.0154* | 73.8 ± 14.8 | 63.7 ± 24.1 | 0.1000 |
| MTV | 144.2 ± 332.5 | 1.2 ± 3.4 | 4.8 ± 7.3 | 0.0160* | 95.0 ± 14.9 | 66.9 ± 111.5 | 0.1350 |
| TLG | 57,798 ± 428,539 | 4.4 ± 13.6 | 20.0 ± 38.2 | 0.0916 | 97.3 ± 9.1 | 58.6 ± 158.1 | 0.2881 |
Units: SUVmax (g/mL), MTV (cm3), TLG (g)
CR, complete remission
*Statistically significant
On interim PET, SUVmax and %ΔSUVmax were well associated with final response on EOT PET; SUVmax and %ΔSUVmax with both targets A and B were significantly different between CR and non-CR groups (Table 3). However, %ΔTLG of target A and MTV and TLG of target B did not exhibit significant difference between CR and non-CR groups, mostly due to wide variation in non-CR group.
Table 3.
Interim values of quantitative indexes according to response by visual analysis
| Index | Interim PET | %Δ between initial and interim PET | ||||
|---|---|---|---|---|---|---|
| CR | Non-CR | P | CR | Non-CR | P | |
| Target A | ||||||
| SUVmax | 3.7 ± 3.2 | 6.2 ± 3.7 | 0.0143* | 62.3 ± 26.3 | 43.8 ± 30.2 | 0.0235* |
| MTV | 2.4 ± 5.7 | 12.3 ± 19.5 | 0.0429* | 90.0 ± 30.2 | 52.9 ± 71.1 | 0.0408* |
| TLG | 12.9 ± 35.4 | 65.1 ± 104.7 | 0.0481* | 91.4 ± 36.7 | 62.4 ± 59.3 | 0.0633 |
| Target B | ||||||
| SUVmax | 9.1 ± 6.0 | 16.6 ± 10.3 | 0.0063* | 68.3 ± 18.2 | 52.0 ± 25.1 | 0.0082* |
| MTV | 2.8 ± 5.9 | 38.4 ± 111.5 | 0.1699 | 93.9 ± 15.7 | 75.5 ± 24.6 | 0.0069* |
| TLG | 13.7 ± 35.8 | 215.2 ± 658.5 | 0.1876 | 94.9 ± 20.2 | 79.8 ± 24.7 | 0.0192* |
Units: SUVmax (g/mL), MTV (cm3), TLG (g)
CR, complete remission
*Statistically significant
Prognosis by Quantitative PET Indexes
In visual analysis, non-CR by Lugano classification on EOT PET was a significant prognostic factor (p < 0.0001; HR, 20.07 (95% CI, 6.87–58.66)). Most of the tested indexes were also significant factors for predicting PFS (Table 4). On EOT PET, all of the SUVmax, MTV, and TLG were significant prognostic factors with both targets A and B (Fig. 1). Among them, SUVmax presented the highest HR (6.76 with target A and 8.62 with target B). Regarding the indexes for changes between initial and EOT PET, %ΔMTV of target A and %ΔSUVmax of target B were not significant prognostic factors.
Table 4.
Prognostic values of quantitative PET indexes in predicting progression-free survival
| Index | Interim PET | End-of-treatment PET | ||||
|---|---|---|---|---|---|---|
| Cutoff value | HR (95% CI) | P | Cutoff value | HR (95% CI) | P | |
| Target A | ||||||
| SUVmax | 3.32 | 3.12 (1.06–9.69) | 0.0365* | 2.41 | 6.76 (2.37–19.31) | 0.0033* |
| MTV | 0.12 | 2.56 (0.86–7.62) | 0.1005 | 0.05 | 4.39 (1.39–13.92) | 0.0033* |
| TLG | 0.21 | 3.79 (1.28–11.27) | 0.0276* | 0.12 | 3.78 (1.23–11.58) | 0.0093* |
| %ΔSUVmax | 46.0 | 4.56 (1.18–17.59) | 0.0021* | 86.7 | 7.79 (2.64–22.99) | 0.0173* |
| %ΔMTV | 74.1 | 4.45 (0.85–23.25) | 0.0039* | 97.0 | 5.91 (1.71–20.4) | 0.0002* |
| %ΔTLG | 84.9 | 4.61 (1.03–20.59) | 0.0022* | 99.7 | 2.73 (0.89–8.33) | 0.0501 |
| Target B | ||||||
| SUVmax | 10.95 | 3.03 (1.06–8.69) | 0.0460* | 7.85 | 8.62 (2.97–24.99) | 0.0005* |
| MTV | 0.25 | 2.06 (0.72–5.90) | 0.2064 | 0.30 | 4.53 (1.42–14.48) | 0.0026* |
| TLG | 0.31 | 4.93 (1.71–14.23) | 0.0050* | 0.34 | 5.78 (1.97–16.92) | 0.0018* |
| %ΔSUVmax | 75.5 | 2.38 (0.79–7.16) | 0.1705 | 75.1 | 2.24 (0.78–6.43) | 0.1337 |
| %ΔMTV | 90.1 | 4.79 (1.22–18.85) | 0.0014* | 98.3 | 4.47 (1.40–14.23) | 0.0022* |
| %ΔTLG | 96.5 | 4.67 (1.34–16.26) | 0.0023* | 99.6 | 5.38 (1.74–16.57) | 0.0008* |
Units: SUVmax (g/mL), MTV (cm3), TLG (g)
HR, complete remission
*Statistically significant
Fig. 1.
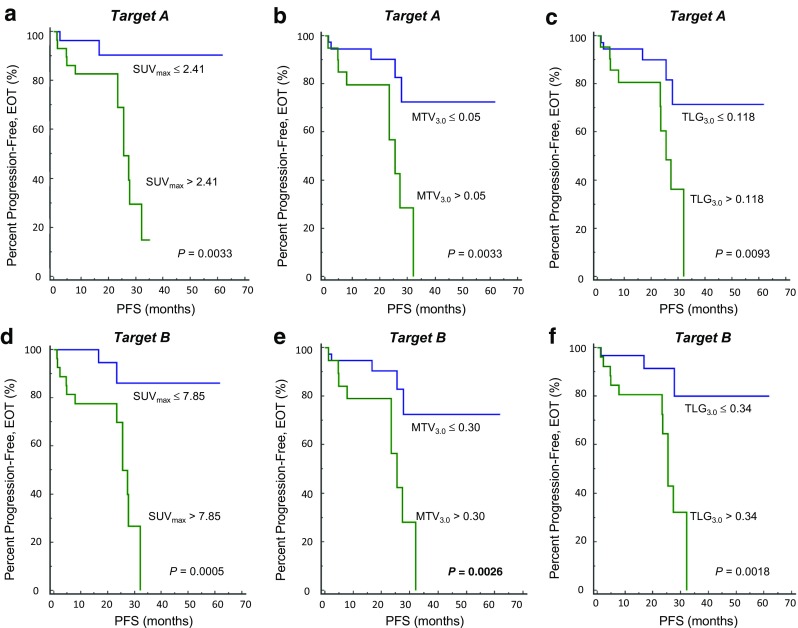
Progression-free survival according to quantitative indexes on EOT PET; with target A (a–c) and target B (d–f). All the indexes were significant prognostic factors, with highest hazard ratio presented by SUVmax (a, d)
On interim PET, most of the tested indexes were also significant prognostic factors, whereas MTV of target A and MTV and %ΔSUVmax of target B were not (Table 4). In accordance with EOT PET, SUVmax was a significant factor with both targets A and B, whereas the highest hazard ratio was presented by %ΔTLG in target A (HR, 4.61 (95% CI, 1.03–20.59)) and TLG in target B (HR, 4.93 (95% CI, 1.71–14.23)) (Fig. 2).
Fig. 2.
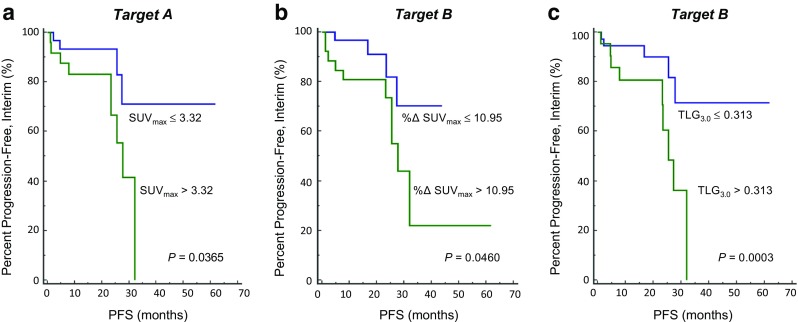
Progression-free survival according to quantitative indexes on interim PET. SUVmax was significantly associated with PFS, both with target A (a) and target B (b). The highest hazard ratio was presented with TLG of target B (c)
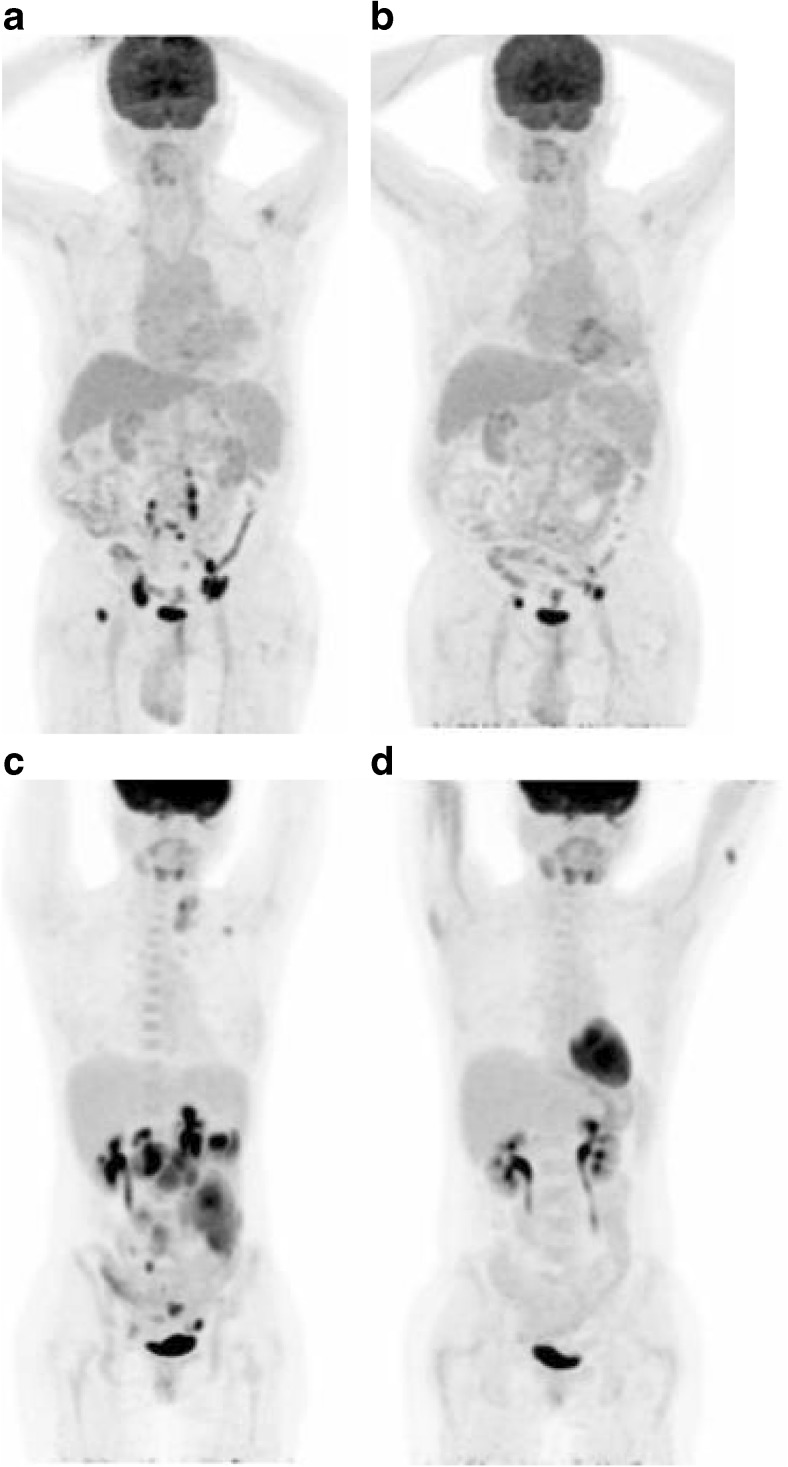
Representative cases regarding prognostic role of FDG PET are shown in Fig. 3.
Fig. 3.
Whole body FDG PET images of representative cases. A 57-year-old male patient with follicular lymphoma exhibited high SUVmax on both baseline (a) and EOT PET (b) (SUVmax 17.22 and 12.46, respectively). The patient experienced disease progression 11.9 months after completion of chemotherapy. A 32-year-old female patient with follicular lymphoma exhibited high SUVmax (SUVmax 18.24) on baseline (c), but low SUVmax (SUVmax 1.56) on EOT PET (d). The patient had been in CR state for 19.5 months after completion of chemotherapy
Discussion
In this study, quantitative indexes from FDG PET/CT have been evaluated for their feasibility and effectiveness in response evaluation and prognosis prediction in indolent lymphoma. On EOT PET, SUVmax and MTV of both targets A and B were well associated with Lugano classification. Because Lugano classification and Deauville score are based on only the tumor uptake at EOT, it is not surprising that SUVmax on EOT PET is well associated with the Lugano classification. MTV may be an additional effective index for response evaluation. It appears that simple measurement of SUVmax for the single hottest lesion (target A) can be enough, while the measurement from multiple samples (target B) would show differences more definitely. Any of %Δ indexes was not significantly associated with Lugano classification, although %ΔSUVmax is the recommended index in PERCIST. On interim PET, SUVmax and its %Δ from initial PET were also well associated with response at EOT.
Despite wide use, Lugano classification is a surrogate marker for response. Treatment response should be finally associated with the efficacy of treatment or outcome of a patient who receives the treatment. Thus, PFS was evaluated in this study as the outcome. In this study, SUVmax at EOT exhibited the highest HR although most of the tested indexes were significant prognostic factor. Similarly to response evaluation, SUVmax of target A was an effective prognostic marker, while SUVmax of target B exhibited a slightly higher HR. The results suggest that simple measurement of SUVmax of the single hottest lesion can be used as an effective index for both response evaluation and PFS prediction.
FDG PET/CT has been reported to be effective for response evaluation in FDG-avid lymphoma [14–19]. Usually, FDG avidity of lesions is determined, and the response is assessed by using the 5-point scale of visual assessment [15, 18, 20]. However, there have not been much evidences for effectiveness of FDG PET-based response evaluation in indolent lymphoma, especially in low FDG-avid tumors. Indolent lymphoma shows slow disease progression with variable metabolic features. Although FL is the most common type of indolent lymphoma, it usually shows moderate to high FDG avidity, and previous studies have shown the effectiveness of FDG PET in FL [4, 6]. These studies reported high performance of FDG PET in lesion detection, which results in more accurate staging and more adequate treatment planning. In contrast, MZBCL shows a wide variety of FDG avidity, with a tendency toward low FDG uptake [11, 20–22], and thus, follow-up using FDG PET/CT is generally not recommended in this type of lymphoma [20, 21]. The present study demonstrated the effectiveness of FDG PET in response evaluation of indolent lymphoma, by using quantitative indexes.
In analyzing FDG PET, SUVmax is the most widely used index for various purposes. In treatment-response evaluation, the use of SUVmax or SUVpeak is recommended in PERCIST. MTV and TLG are volume-based indexes that reflect tumor burden, and they are expected to be effective in prognosis prediction and response evaluation. However, in the present study, MTV and TLG did not surpass SUVmax in terms of response evaluation and prognosis prediction. Particularly, TLG did not show significant differences between CR and non-CR groups, probably due to a wide variation. Although further studies are required for validating the results, it can be asserted that SUVmax is still a simple and effective index.
Recently, the role of interim PET for response evaluation has been emphasized [7, 23–27]. It is generally accepted that preferable response such as metabolic CR on interim PET is well associated with a greater chance of achieving CR at EOT with a lesser chance of relapse [28–30]. On the contrary, if interim PET result does not show response, a patient is more likely to result in poor outcome. If poor response is observed on interim PET, treatment regimen may be changed for better outcome. In DLBCL, recent studies have suggested the usefulness of interim PET assessment using quantitative indexes as well as visual scale [29, 31, 32]. These studies attempted to enhance the utility of interim PET using the quantitative index of SUVmax, which exhibited promising predictive values [29, 31, 32]. In this study, prognostic role of interim FDG PET was tested in a group of indolent lymphoma. In accordance with these previous studies, the results showed that SUVmax and %ΔSUVmax are well associated with final response on EOT PET. Additionally, other factors such as TLG, %Δ TLG, and %Δ MTV on interim PET were also significant in prediction of prognosis.
There are some limitations in this study. First, a small number of patients were included due to relatively low incidence rate of indolent lymphoma. Further studies are required with a larger group of patients. Second, due to the inclusion criteria that required all baseline, interim, and EOT PET scans in a single patient, almost all the enrolled patients were in advanced stage. Accordingly, disease characteristics and treatment regimen of the present study were somewhat different from those of low-stage lymphoma. Additionally, although FL is the most common type of indolent lymphoma, most of FL cases show moderate to high FDG uptake, which resulted in a large variation of FDG uptake in our study cohort. The comparison of PET indexes for response evaluation may have been affected by heterogeneous FDG avidity of lymphoma. With a larger cohort, a homogeneous group of low FDG-avid lymphoma needs to be analyzed in the future regarding the effectiveness of FDG PET in response evaluation.
Conclusion
In indolent lymphoma, quantitative indexes of FDG PET are well associated with Lugano classification results. A simple measurement of SUVmax of the single hottest lesion can be an effective index for response evaluation and prognosis prediction of the indolent lymphoma.
Conflict of Interest
Hyun Joo Kim, Reeree Lee, Hongyoon Choi, Jin Chul Paeng, Gi Jeong Cheon, Dong Soo Lee, June-Key Chung, and Keon Wook Kang declare that they have no conflict of interest.
Ethical Approval
All procedures followed were performed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
The study design of the retrospective analysis and exemption of informed consent were approved by the Institutional Review Board of the Seoul National University Hospital (H-1703-108-840). This manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
References
- 1.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheson BD. Staging and response assessment in lymphomas: the new Lugano classification. Chin Clin Oncol. 2015;4:5. doi: 10.3978/j.issn.2304-3865.2014.11.03. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–2496. doi: 10.1182/blood-2016-05-718528. [DOI] [PubMed] [Google Scholar]
- 4.Gallamini A, Borra A. FDG-PET scan: a new paradigm for follicular lymphoma management. Mediterr J Hematol Infect Dis. 2017;9:e2017029. doi: 10.4084/mjhid.2017.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metser U, Dudebout J, Baetz T, Hodgson DC, Langer DL, MacCrostie P, et al. [(18) F]-FDG PET/CT in the staging and management of indolent lymphoma: a prospective multicenter PET registry study. Cancer. 2017;123:2860–2866. doi: 10.1002/cncr.30672. [DOI] [PubMed] [Google Scholar]
- 6.Smith SD, Redman M, Dunleavy KFDG. PET-CT in follicular lymphoma: a case-based evidence review. Blood. 2015;125:1078–1082. doi: 10.1182/blood-2014-11-537522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metser U, Hussey D, Murphy G. Impact of (18)F-FDG PET/CT on the staging and management of follicular lymphoma. Br J Radiol. 2014;87:20140360. doi: 10.1259/bjr.20140360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciobanu A, Stanca O, Triantafyllidis I, Lupu A. Indolent lymphoma: diagnosis and prognosis in medical practice. Maedica (Buchar) 2013;8:338–342. [PMC free article] [PubMed] [Google Scholar]
- 9.Bodet-Milin C, Eugene T, Gastinne T, Frampas E, Le Gouill S, Kraeber-Bodere F. FDG-PET in follicular lymphoma management. J Oncol. 2012;2012:370272. doi: 10.1155/2012/370272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Dortz L, De Guibert S, Bayat S, Devillers A, Houot R, Rolland Y, et al. Diagnostic and prognostic impact of 18F-FDG PET/CT in follicular lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:2307–2314. doi: 10.1007/s00259-010-1539-5. [DOI] [PubMed] [Google Scholar]
- 11.Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51:25–30. doi: 10.2967/jnumed.109.067892. [DOI] [PubMed] [Google Scholar]
- 12.JH O, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280:576–584. doi: 10.1148/radiol.2016142043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Persijn van Meerten EL, Gelderblom H, Bloem JL. RECIST revised: implications for the radiologist. A review article on the modified RECIST guideline. Eur Radiol. 2010;20:1456–1467. doi: 10.1007/s00330-009-1685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchings M, Barrington SF. PET/CT for therapy response assessment in lymphoma. J Nucl Med. 2009;50(Suppl 1):21S–30S. doi: 10.2967/jnumed.108.057190. [DOI] [PubMed] [Google Scholar]
- 15.Hosein PJ, Lossos IS. The evolving role of F-FDG PET scans in patients with aggressive non-Hodgkin’s lymphoma. Eur J Clin Med Oncol. 2010;2:131–138. [PMC free article] [PubMed] [Google Scholar]
- 16.Metser U, Mohan R, Beckley V, Moshonov H, Hodgson D, Murphy G. FDG PET/CT response assessment criteria for patients with Hodgkin's and non-Hodgkin's lymphoma at end of therapy: a multiparametric approach. Nucl Med Mol Imaging. 2016;50:46–53. doi: 10.1007/s13139-015-0368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson SA, Kumar A, Matasar MJ, Schoder H, Rademaker J. Imaging for staging and response assessment in lymphoma. Radiology. 2015;276:323–338. doi: 10.1148/radiol.2015142088. [DOI] [PubMed] [Google Scholar]
- 18.Ngeow JY, Quek RH, Ng DC, Hee SW, Tao M, Lim LC, et al. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol. 2009;20:1543–1547. doi: 10.1093/annonc/mdp030. [DOI] [PubMed] [Google Scholar]
- 19.Kobe C, Dietlein M, Hellwig D. PET/CT for lymphoma post-therapy response assessment in Hodgkin lymphoma and diffuse large B-cell lymphoma. Semin Nucl Med. 2018;48:28–36. doi: 10.1053/j.semnuclmed.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Karls S, Shah H, Jacene H. PET/CT for lymphoma post-therapy response assessment in other lymphomas, response assessment for autologous stem cell transplant, and lymphoma follow-up. Semin Nucl Med. 2018;48:37–49. doi: 10.1053/j.semnuclmed.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Carrillo-Cruz E, Marin-Oyaga VA, de la Cruz Vicente F, Borrego-Dorado I, Ruiz Mercado M, Acevedo Banez I, et al. Role of 18F-FDG-PET/CT in the management of marginal zone B cell lymphoma. Hematol Oncol. 2015;33:151–158. doi: 10.1002/hon.2181. [DOI] [PubMed] [Google Scholar]
- 22.Albano Domenico, Giubbini Raffaele, Bertagna Francesco. 18F-FDG PET/CT in splenic marginal zone lymphoma. Abdominal Radiology. 2018;43(10):2721–2727. doi: 10.1007/s00261-018-1542-z. [DOI] [PubMed] [Google Scholar]
- 23.Khong PL, Huang B, Lee EY, Chan WK, Kwong YL. Midtreatment (1)(8)F-FDG PET/CT scan for early response assessment of SMILE therapy in natural killer/T-cell lymphoma: a prospective study from a single center. J Nucl Med. 2014;55:911–916. doi: 10.2967/jnumed.113.131946. [DOI] [PubMed] [Google Scholar]
- 24.Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood. 2007;110:3507–3516. doi: 10.1182/blood-2007-06-097238. [DOI] [PubMed] [Google Scholar]
- 25.Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with (18)F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging. 2013;3:272–281. [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Y, Fu X, Sun Z, Xie X, Wang R, Li Z, et al. Utility of baseline, interim and end-of-treatment (18)F-FDG PET/CT in extranodal natural killer/T-cell lymphoma patients treated with L-asparaginase/pegaspargase. Sci Rep. 2017;7:41057. doi: 10.1038/srep41057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheson BD, Kostakoglu L. FDG-PET for early response assessment in lymphomas. Part 1—Hodgkin lymphoma. Oncology (Williston Park) 2017;31:45–49. [PubMed] [Google Scholar]
- 28.Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, et al. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30:184–190. doi: 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- 29.Casasnovas RO, Meignan M, Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118:37–43. doi: 10.1182/blood-2010-12-327767. [DOI] [PubMed] [Google Scholar]
- 30.Liao CC, Qin YY, Tan XH, Hu JJ, Tang Q, Rong Y, et al. Predictive value of interim PET/CT visual interpretation in the prognosis of patients with aggressive non-Hodgkin’s lymphoma. Onco Targets Ther. 2017;10:5727–5738. doi: 10.2147/OTT.S154995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheson BD, Kostakoglu L. FDG-PET for early response assessment in lymphomas. Part 2—diffuse large B-cell lymphoma, Use of Quantitative PET Evaluation. Oncology (Williston Park) 2017;31:71–76. [PubMed] [Google Scholar]
- 32.Lin C, Itti E, Haioun C, Petegnief Y, Luciani A, Dupuis J, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48:1626–1632. doi: 10.2967/jnumed.107.042093. [DOI] [PubMed] [Google Scholar]