Abstract
Long noncoding RNA (lncRNA) are non-protein-coding transcripts greater than 200 nucleotides that regulate gene expression. The field of transcriptomics is only beginning to understand the role of lncRNA in host defense. Little is known about the role of lncRNA in the response to infection by intracellular pathogens such as Toxoplasma gondii. Using a microarray, we examined the differential expression of 35,923 lncRNAs and 24,881 mRNAs in mouse bone-marrow-derived macrophages during infection with high- and low-virulence T. gondii strains. We found that 1,522 lncRNA molecules were differentially regulated during infection with the high-virulence Type I strain, versus 528 with the less-virulent Type II strain. Of these lncRNAs, 282 were co-regulated with a nearby or overlapping mRNA–including approximately 60 mRNAs with immune-related functions. We validated the microarray for 4 lncRNAs and 4 mRNAs using qRT-PCR. Using deletion strains of T. gondii, we found that the secretory kinase ROP16 controls upregulation of lncRNAs Csf1-lnc and Socs2-lnc, demonstrating that the parasite directly manipulates host lncRNA expression. Given the number of regulated lncRNAs and the magnitude of the expression changes, we hypothesize that these molecules constitute both an additional regulatory layer in the host response to infection and a target for manipulation by T. gondii.
Introduction
It is estimated that less than 3% of the genome codes for proteins, yet recent data indicate that up to 80% of the genome is actively transcribed as non-translated RNA1,2. The largest group of non-translated, non-ribosomal RNA is long noncoding RNA (lncRNA), conventionally defined as non-protein-coding transcripts greater than 200 nucleotides, that together account for up to 68% of the transcriptome3,4. Despite the prevalence of lncRNA, functions have been ascribed for only approximately 1%5. lncRNAs are widely involved in gene regulation at both the transcriptional and post-transcriptional level. Known functions of lncRNA molecules include transcriptional coactivation, recruitment of chromatin modifiers, miRNA sponge function, regulation of splicing, and mRNA stabilization6,7. Very little is understood regarding the role of lncRNA in innate and adaptive immunity. Yet, emerging evidence strongly indicates that this class of regulatory molecules significantly impacts host immunity and the ability to respond to infection1,4,7.
Here, we examined the global lncRNA response to Toxoplasma gondii. This intracellular protozoan is known for its ability to infect a wide range of species and establish latent infection in tissues of the central nervous system and skeletal muscle8. As a primarily asymptomatic pathogen, establishment of latent T. gondii infection requires a balance between immune-induction to prevent host death and immune-evasion to prevent parasite elimination. Failure to maintain this balance may result in toxoplasmic encephalitis, as can occur in AIDS populations, underscoring the opportunistic nature of this parasitic pathogen9. Toxoplasma is well known for its ability to initiate Th1 immunity through induction of IL-12 and to induce the activity of counter-regulatory cytokines such as IL-1010-12. In macrophages, which along with dendritic cells serve as a major target of in vivo infection, Toxoplasma triggers both pro-inflammatory and anti-inflammatory pathways13–16. Parasite secreted effectors such as ROP16, GRA15, and GRA24 are known to directly stimulate signaling responses in macrophages and other host cell types through activation of signal transducer and activator of transcription (STAT), NFκB, and p38 mitogen-activated protein kinase (MAPK) pathways17–22. Maintaining a balance between pro-inflammatory and anti-inflammatory responses is key to the success of Toxoplasma as an intracellular parasite, and we hypothesize that lncRNAs are involved in regulating these responses.
In this study, we report the comprehensive survey of host lncRNAs regulated during Toxoplasma infection. Using a commercial microarray, we examined the differential gene expression of 35,923 putative lncRNA and 24,881 mRNA species in mouse bone-marrow-derived macrophages (BMDM). We found that 1,902 unique lncRNAs and 1,927 unique mRNAs were regulated by infection with either the high-virulence RH or the less-virulent PTG strain. Of these lncRNAs, 282 were co-regulated with an associated (adjacent or overlapping) protein-coding gene, and approximately 60 lncRNA species were co-regulated with an infection- or immune-related protein-coding gene. Employing qRT-PCR, we validated the results of the microarray for several of these co-regulated, potentially immune-related lncRNA and mRNA species. Using a T. gondii strain deleted for the ROP16 gene – which encodes a protein controlling STAT activation – we found that this host-directed kinase controls expression of two lncRNAs. Our new findings show that T. gondii is a strong stimulator of the lncRNA transcriptome in host cells and that the parasite exerts an important influence on this response in a strain-specific manner.
Results
Hundreds of putative lncRNAs are differentially regulated during infection with T. gondii
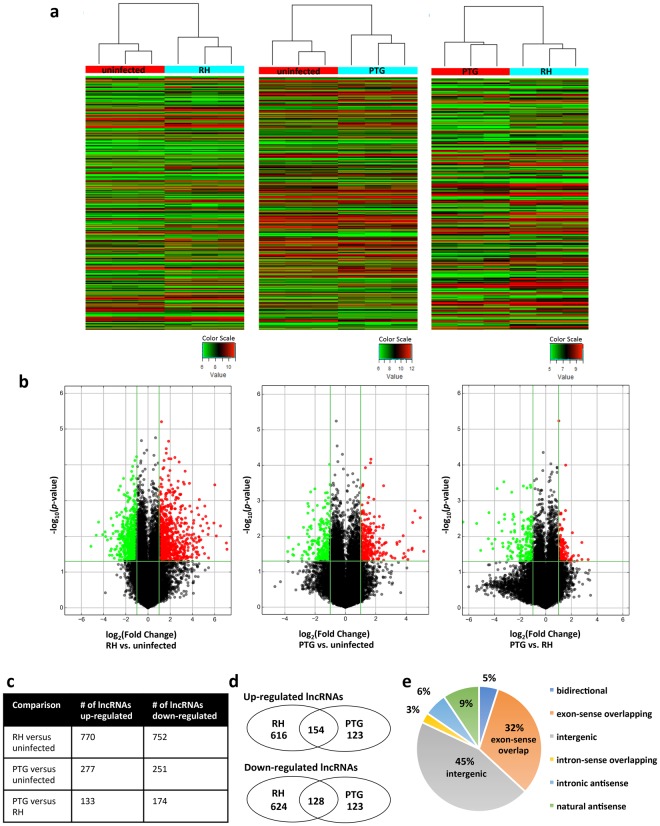
We infected mouse bone marrow-derived macrophages (BMDM) with high (Type I, RH) and low (Type II, PTG) virulence T. gondii at a 4:1 ratio of parasites to cells. This MOI resulted in approximately 80% infection for both strains (Supplementary Fig. S1). Samples of RNA were prepared 6 hr after infection, a time prior to the first parasite mitotic division when on average there was a single parasite per infected cell (Supplementary Fig. S1). Using a commercial microarray (Arraystar), we examined the differential gene expression of 35,923 putative lncRNAs after infection. The array was constructed using public transcriptome databases (Refseq, UCSC knowngenes, Ensembl, etc.), as well as relevant publications23,24. Differentially expressed lncRNAs and mRNAs were identified through p-value (<0.05) and fold change filtering (>2 fold up or down). The complete dataset is provided in Supplementary Data S1. Hierarchical clustering of the differentially regulated lncRNAs in three replicate experiments indicated that both RH and PTG infections regulated lncRNA expression and that the lncRNA landscape differed between the two infections (Fig. 1a). We also used volcano plot analysis to visualize the differentially expressed lncRNAs. This technique reveals significant changes in large sets of replicate data by plotting fold changes versus p-values. We identified substantial populations of lncRNAs that were up- or down-regulated in a statistically significant manner by RH and PTG infection (Fig. 1b). As shown in Fig. 1c, 770 lncRNAs were upregulated during RH infection, and a similar number were down-regulated. Infection with PTG had less extensive effects on lncRNA expression, with a total of 277 species upregulated and 251 down-regulated. 133 lncRNAs were upregulated by PTG relative to RH, while 174 lncRNAs were down-regulated. A total of 282 lncRNAs were either up- or down-regulated during infection with each strain type as depicated by Venn diagrams (Fig. 1d and Supplementary Data S2). However, a substantial number of lncRNAs were regulated in a parasite-strain-specific manner. Taking together all three comparisons (RH vs. uninfected, PTG vs. uninfected, and PTG vs. RH), 1,902 unique lncRNAs were identified in the microarray as significantly differentially regulated. We examined how these lncRNAs were distributed according to protein coding genes (Fig. 1e). The majority of the lncRNAs were either intergenic (>1 kb from the promoter of a protein coding gene) or exon-sense overlapping (overlapping an exon of a protein-coding gene in the sense direction).
Figure 1.
Hundreds of putative lncRNAs are differentially regulated during infection with Toxoplasma, as determined using a microarray for mouse lncRNAs. Mouse BMDM were infected with either the highly-virulent RH or the less-virulent PTG strain, and 6 hr later, RNA was isolated for microarray analysis. (a) Hierarchical clustering of differentially expressed lncRNAs for uninfected vs. RH-infected, uninfected vs. PTG-infected, and PTG vs. RH-infected. Values in the color scale are normalized intensities. Red bands indicate high relative expression, and green bands indicate low relative expression. (b) Volcano plot filtering to visualize fold regulation and statistical significance in lncRNA populations. Statistically significant (p-value < 0.05) up (red) or down (green) regulated expression changes (>2-fold) are shown in pairwise comparisons of uninfected, RH-infected, and PTG-infected samples. (c) Total number of lncRNA species up- or down-regulated by infection. (d) Venn diagrams of up- and down-regulated lncRNA populations reveal shared and unique expression patterns. (e) Classification of lncRNA differentially regulated by Toxoplasma infection. Experiments were performed in triplicate with BMDM from three separate mice.
Not only were many lncRNAs differentially regulated, but also the magnitude of the observed fold changes was large (Table 1). For all three strain comparisons, the top 20 hits had fold changes larger than ±7, and 31 lncRNAs had fold changes larger than ±20. Notably, infection with high-virulence RH had greater impact on the number and magnitude of lncRNA responses than low-virulence PTG. For example, the average magnitiude of upregulated responses shown in Table 1 was 54.8 and 18.7 for RH and PTG, respectively. These effects could not be attributed to differences in replication rate (and therefore antigen load per cell) because the analyses were carried out 6 hr after infection, prior to the first parasite cell division. Also, the difference in the number of lncRNAs regulated between RH and PTG was not due to invasion efficiency, as percent infection was approximately 80% for each strain.
Table 1.
lncRNAs with the 20 largest fold changes for each comparison: RH vs. uninfected, PTG vs. uninfected, and PTG vs. RH.
| Fold Change | Sequence name | Relationship | Associated gene |
|---|---|---|---|
| RH versus uninfected | |||
| 139.03 | humanlincRNA2313+ | intergenic | |
| 133.80 | ENSMUST00000143128 | exon sense | Chia1 |
| 92.56 | uc029scs.1 | exon sense | Fam169a |
| 71.36 | ENSMUST00000176310 | exon sense | Six1 |
| 64.97 | uc007gwf.2 | exon sense | Socs2 |
| 61.44 | AK052777 | intergenic | |
| 44.28 | ENSMUST00000174768 | intergenic | |
| 44.10 | ENSMUST00000138295 | intergenic | |
| 42.63 | TCONS_00036195 | intergenic | |
| 39.92 | uc008nps.1 | exon sense | Tgm2 |
| 36.88 | ENSMUST00000143423 | exon sense | Il1rn |
| −35.35 | AK152734 | intergenic | |
| 34.86 | AK079750 | intergenic | |
| 34.23 | ENSMUST00000149686 | exon sense | Nup62-il4i1 |
| 30.66 | AK018095 | intergenic | |
| 30.60 | AK076251 | natural antisense | Hs3st3b1 |
| 30.55 | AK042611 | natural antisense | Ano4 |
| 27.28 | ENSMUST00000125412 | intergenic | |
| 26.73 | NR_033499 | intergenic | |
| −25.41 | ENSMUST00000060680 | intergenic | |
| PTG versus uninfected | |||
| 36.04 | ENSMUST00000180991 | intergenic | |
| 30.72 | AK018095 | intergenic | |
| 24.10 | AK078961 | intergenic | |
| 21.71 | AK020242 | intergenic | |
| 21.24 | ENSMUST00000176310 | exon sense | Six1 |
| 20.94 | DT905422 | intergenic | |
| 17.81 | uc007pnu.1 | intronic antisense | Inhba |
| 17.40 | ENSMUST00000143423 | exon sense | Il1rn |
| 15.50 | humanlincRNA2313+ | intergenic | |
| 14.58 | ENSMUST00000143128 | exon sense | Chia1 |
| 14.06 | uc008nps.1 | exon sense | Tgm2 |
| −14.02 | uc029skf.1 | intergenic | |
| 13.98 | ENSMUST00000177234 | exon sense | Cav1 |
| −13.44 | AK157381 | intronic antisense | Rapgef6 |
| 11.98 | NR_040343 | intronic antisense | Crtc3 |
| −11.14 | uc009lws.1 | exon sense | Lpl |
| −10.96 | ENSMUST00000060680 | intergenic | |
| 10.63 | ENSMUST00000074412 | intron sense | Agpat4 |
| −10.63 | ENSMUST00000145263 | natural antisense | Ctnnbip1 |
| 10.52 | uc009luk.1 | intergenic | |
| PTG versus RH | |||
| −87.44 | uc029scs.1 | exon sense | Fam169a |
| −41.83 | ENSMUST00000174768 | intergenic | |
| −33.69 | uc007gwf.2 | exon sense | Socs2 |
| −22.23 | AK086192 | intergenic | |
| −21.47 | NR_033499 | intergenic | |
| −18.36 | ENSMUST00000165968 | intergenic | |
| −17.34 | AK076251 | natural antisense | Hs3st3b1 |
| −15.48 | ENSMUST00000161131 | exon sense | Spag16 |
| −13.81 | uc007mlf.1 | natural antisense | Sphk1 |
| −12.66 | ENSMUST00000155850 | exon sense | Hhip |
| −10.66 | AK089320 | intergenic | |
| −10.28 | uc007eyw.1 | intergenic | |
| −10.02 | ENSMUST00000149686 | exon sense | Nup62-il4i1 |
| 9.87 | uc011wwu.1 | exon sense | Pyhin1 |
| −9.61 | uc007ecc.1 | exon sense | Batf3 |
| −9.25 | AK086022 | intergenic | |
| −9.25 | AK086961 | intergenic | |
| −8.02 | NR_040271 | intergenic | |
| −7.74 | AK136179 | intergenic | |
| −7.61 | AK035470 | natural antisense | Six1 |
If applicable, associated mRNAs and their relationship to the lncRNA are shown in Columns 3 and 4.
We also note that 17 putative lncRNAs that were regulated by infection mapped to ultraconserved regions of the genome25, suggesting that they may function across species (Table 2). This result is potentially significant because lncRNA are generally regarded as poorly conserved through evolution. Using qRT-PCR, we validated expression for one ultraconserved region uc.70 (Supplementary Fig. S2). We note that these ultraconserved regions were identified through genomic sequence comparisons and are generally not based on transcriptomic data. Therefore, the transcripts for these ultraconserved regions would need to be mapped out before further study.
Table 2.
Differentially regulated lncRNAs that map to ultraconserved genomic regions.
| Fold Change | Gene Symbol | Relationship | Associated gene name | Co-regulated |
|---|---|---|---|---|
| 11.2 | uc.170 | intron sense | Fam172a | No |
| 8.79 | uc.47 | intergenic | ||
| 6.31 | uc.462 | intronic antisense | Pola1 | Yes |
| 5.76 | uc.113 | intergenic | ||
| 5.37 | uc.84 | intergenic | ||
| 3.93 | uc.340 | intergenic | ||
| 3.85 | uc.3 | intron sense | Casz1 | No |
| 3.52 | uc.412 | intron sense | Aatf | No |
| 2.26 | uc.50 | intron sense | Srsf7 | Yes |
| 2.09 | uc.419 | exon sense | Srsf1 | Yes |
| −4.00 | uc.70 | intron sense | Arhgap15 | Yes |
| −3.63 | uc.239 | intronic antisense | Tox | No |
| 2.84 | uc.294 | intergenic | ||
| −8.51 | uc.275 | intronic antisense | Pbx3 | No |
| −7.14 | uc.402 | intronic antisense | Rpgrip1l | No |
| −3.07 | uc.30 | intergenic | ||
| −2.08 | uc.473 | exon sense | Nlgn3 | No |
The “Relationship” column denotes the relationship between the lncRNA and its associated (overlapping or nearby) gene. The “Co-regulated” column denotes differential regulation of both the lncRNA and its associated mRNA.
Hundreds of mRNAs are differentially regulated during infection with Toxoplasma
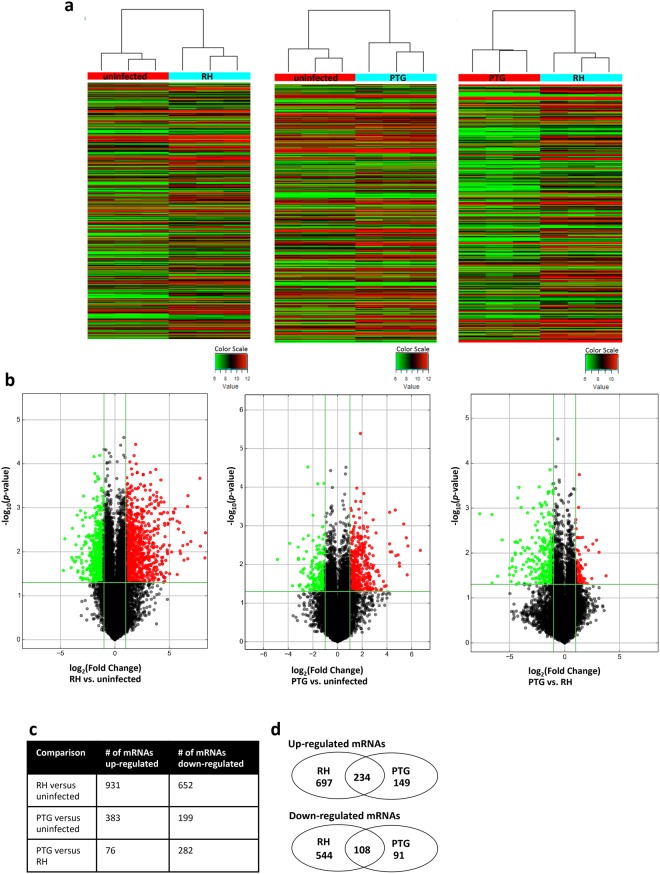
In addition to lncRNAs, the microarray contains probes for 24,881 mRNAs, enabling the measurement of changes in expression of protein-encoding transcripts in response to infection. Consistent with results from Fig. 1, hierarchical clustering (Fig. 2a) and volcano plot filtering (Fig. 2b) revealed parasite-strain-specific control of mRNA expression. 1,583 mRNAs were differentially regulated during infection with the high-virulence RH strain compared to an uninfected control (Fig. 2c; the complete data set is shown in Supplementary Data S3). 582 mRNAs were differentially expressed during infection with the less-virulent PTG strain compared to an uninfected control. 358 mRNAs were differentially regulated between RH and PTG. Venn diagrams (Fig. 2d) show that there is overlap (342 total) in the up- or down-regulated mRNAs by RH and PTG infection. A compiled list of mRNAs regulated during both RH and PTG infection is included in Supplementary Data S4. In addition, and particularly for RH, there was substantial parasite-strain-specific regulation of mRNA expression. Altogether, 1,927 unique mRNAs were identified in the microarray as significantly differentially regulated across all three comparisons (RH vs. uninfected, PTG vs. uninfected, and PTG vs. RH). Table 3 shows the top 20 differentially regulated mRNAs for pairwise comparisons of RH vs. uninfected, PTG vs. uninfected, and PTG vs. RH.
Figure 2.
Differential expression patterns of BMDM mRNA during infection with Toxoplasma. Changes in mRNA expression were assessed 6 hr after infection of BMDM. (a) Hierarchical clustering of pairwise combinations of uninfected, RH-infected, and PTG-infected samples. Values in the color scale are normalized intensities. Red bands indicate high relative expression, and green bands indicate low relative expression. (b) Volcano plot filtering to reveal statistically significantly (p-value < 0.05) up (red) or down (green) regulated (>2-fold) expression changes in pairwise comparisons of uninfected, RH-infected, and PTG-infected samples. (c) Total number of mRNAs up- or down-regulated by infection. (d) Venn diagrams of up- and down-regulated mRNA expression reveal unique and shared mRNA species regulated by RH and PTG infection. Experiments were performed in triplicate with BMDM from three separate mice.
Table 3.
Magnitude of the 20 largest mRNA fold changes during RH and PTG infection in each indicated comparison: RH vs. uninfected, PTG vs. uninfected, and PTG vs. RH.
| Fold Change | Sequence Name | Gene Symbol | Description |
|---|---|---|---|
| RH versus uninfected | |||
| 318.78 | NM_011332 | Ccl17 | chemokine (C-C motif) ligand 17 |
| 306.46 | NM_020596 | Egr4 | early growth response 4 |
| 230.38 | NM_178591 | Nrg1 | neuregulin 1 |
| 223.03 | NM_019577 | Ccl24 | chemokine (C-C motif) ligand 24 |
| 125.54 | NM_001077508 | Tnfrsf9 | tumor necrosis factor receptor superfamily, member 9 |
| 104.53 | NM_009504 | Vdr | vitamin D receptor |
| 103.57 | NM_001044740 | Slc7a2 | solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 |
| 102.04 | NM_007759 | Crabp2 | cellular retinoic acid binding protein II |
| 101.10 | NM_130905 | Cd209e | CD209e antigen |
| 81.07 | NM_010276 | Gem | GTP binding protein (gene overexpressed in skeletal muscle) |
| 79.04 | NM_008479 | Lag3 | lymphocyte-activation gene 3 |
| 75.30 | NM_009398 | Tnfaip6 | tumor necrosis factor alpha induced protein 6 |
| 65.23 | NM_001101443 | Prrt4 | proline-rich transmembrane protein 4 |
| 63.07 | NM_009864 | Cdh1 | cadherin 1 |
| 61.60 | NM_138648 | Olr1 | oxidized low density lipoprotein (lectin-like) receptor 1 |
| 56.87 | NM_010215 | Il4i1 | interleukin 4 induced 1 |
| 52.70 | NM_146174 | Fam115c | family with sequence similarity 115, member C |
| 52.47 | NM_008125 | Gjb2 | gap junction protein, beta 2 |
| 45.92 | NM_001167996 | 1110032F04Rik | RIKEN cDNA 1110032F04 gene |
| 42.19 | NM_001033248 | Gm266 | predicted gene 266 |
| PTG versus uninfected | |||
| 104.88 | NM_178591 | Nrg1 | neuregulin 1 |
| 52.15 | NM_011851 | Nt5e | 5′ nucleotidase, ecto |
| 49.93 | NM_010809 | Mmp3 | matrix metallopeptidase 3 |
| 47.65 | NM_019389 | Vcan | versican |
| 41.21 | NM_001081249 | Vcan | versican |
| 32.62 | NM_001080815 | Gipr | gastric inhibitory polypeptide receptor |
| 32.09 | NM_008361 | Il1b | interleukin 1 beta |
| 29.95 | NM_001172160 | Flrt3 | fibronectin leucine rich transmembrane protein 3 |
| −29.38 | NM_146511 | Olfr107 | olfactory receptor 107 |
| 26.51 | NM_172955 | Vcan | versican |
| 25.67 | NM_001134475 | Vcan | versican |
| 21.13 | NM_001134474 | Vcan | versican |
| 19.33 | NM_001033228 | Itga1 | integrin alpha 1 |
| 18.63 | NM_009705 | Arg2 | arginase type II |
| 18.62 | NM_133219 | Gcnt2 | glucosaminyl (N-acetyl) transferase 2, I-branching enzyme |
| 15.44 | NM_001167996 | 1110032F04Rik | RIKEN cDNA 1110032F04 gene |
| −14.62 | NM_027363 | Chp2 | calcineurin-like EF hand protein 2 |
| 14.01 | NM_009398 | Tnfaip6 | tumor necrosis factor alpha induced protein 6 |
| 13.14 | NM_212444 | Gyk | glycerol kinase |
| −12.57 | NM_001113481 | Sema4f | sema domain, immunoglobulin domain (Ig), TM domain, and short cytoplasmic domain |
| PTG versus RH | |||
| 208.03 | NM_011332 | Ccl17 | chemokine (C-C motif) ligand 17 |
| 96.40 | NM_007759 | Crabp2 | cellular retinoic acid binding protein II |
| 95.86 | NM_020596 | Egr4 | early growth response 4 |
| 67.36 | NM_146174 | Fam115c | family with sequence similarity 115, member C |
| 50.95 | NM_008125 | Gjb2 | gap junction protein, beta 2 |
| 31.91 | NM_175178 | Aifm3 | apoptosis-inducing factor, mitochondrion-associated 3 |
| 31.78 | NM_019577 | Ccl24 | chemokine (C-C motif) ligand 24 |
| 29.07 | NM_010415 | Hbegf | heparin-binding EGF-like growth factor |
| 27.59 | NM_008479 | Lag3 | lymphocyte-activation gene 3 |
| 26.73 | NM_001045526 | Scimp | SLP adaptor and CSK interacting membrane protein |
| 26.63 | NM_175122 | Rab39b | RAB39B, member RAS oncogene family |
| 24.98 | NM_009864 | Cdh1 | cadherin 1 |
| 22.72 | NM_010296 | Gli1 | GLI-Kruppel family member GLI1 |
| 22.59 | NM_009252 | Serpina3n | serine (or cysteine) peptidase inhibitor, clade A, member 3N |
| 22.20 | NM_001101483 | Slc35g2 | solute carrier family 35, member G2 |
| 21.82 | NM_145829 | Nags | N-acetylglutamate synthase |
| 21.50 | NM_130905 | Cd209e | CD209e antigen |
| 21.36 | NM_001199940 | Serpina3i | serine (or cysteine) peptidase inhibitor, clade A, member 3I |
| 20.77 | NM_001101443 | Prrt4 | proline-rich transmembrane protein 4 |
| 19.12 | NM_001033248 | Gm266 | predicted gene 266 |
Gene ontology (GO) analysis identified many immune-related biological processes as differentially regulated in the microarray, including regulation of T-helper cell differentiation, interleukin 2 production, macrophage differentiation, JAK-STAT cascade, and cytokine secretion (Supplementary Fig. S3). Several of the biological pathways identified were regulated in a parasite strain-specific manner. For example, infection with RH was associated with regulation of macrophage differentiation and negative regulation of the JAK-STAT cascade (Supplementary Fig. S3e and f). KEGG Pathway analysis identified many immune-related and infection pathways as differentially regulated in the microarray, including cytokine-cytokine receptor interaction, TGF-beta signaling pathway, Toll-like receptor signaling pathway, RIG-I-like receptor signaling pathway, JAK-STAT signaling pathway, chemokine signaling pathway, Wnt signaling pathway, RapI signaling pathway, measles, malaria, HTLV-1 infection, Hepatitis B, Hepatitis C, and tuberculosis (Supplementary Fig. S4). KEGG analysis also revealed parasite strain-specific pathway enrichment. For example, RH infection was associated with selective enrichment of pathways involved in cytokine-receptor interaction, the JAK-STAT pathway and the p53 signaling pathway (Supplementary Fig. S4e and f).
Four co-regulated lncRNAs and mRNAs were validated by qRT-PCR
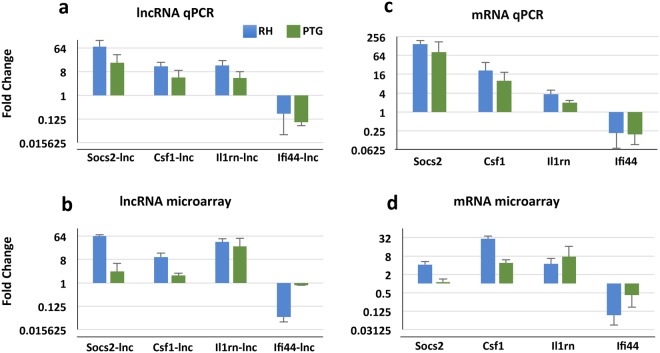
Many known lncRNAs regulate adjacent or overlapping protein-coding genes in a cis-regulatory manner26,27. For this reason, we examined the 282 lncRNAs that were co-regulated with a nearby protein-coding gene (Supplementary Data S5). Of these 282, we identified ~60 lncRNAs that were co-regulated with infection- or immune-related protein-coding genes (Supplementary Data S5), suggesting a potential regulatory role for these lncRNAs in the immune response. From this list, we chose 4 lncRNAs to validate by qRT-PCR: Socs2-lnc, Csf1-lnc, Il1rn-lnc, and Ifi44-lnc. Socs2-lnc (uc007gwf.2) is associated with the protein-coding gene Socs2, a suppressor of cytokine synthesis possibly involved in the anti-inflammatory response during T. gondii infection28. Csf1-lnc (ENSMUST00000155557) is associated with the protein-coding gene csf1, a cytokine that drives stem cell differentiation into the macrophage lineage29. Ifi44-lnc (ENSMUST00000133888) is associated with Ifi44, an interferon-alfa inducible protein connected with viral infection30. Il1rn-lnc (ENSMUST00000143423) is associated with Il1rn, an antagonist cytokine that inhibits activities of interleukin 1 and modulates the interleukin 1 immune and inflammatory responses31.
We designed primers to bind a unique region of each lncRNA and co-regulated mRNA. qRT-PCR largely confirmed that lncRNA transcripts were up- or down-regulated (≥or ≤2-fold) during infection with either the RH or PTG in a manner similar to the microarray (Fig. 3a and b). The microarray was considered valid if the directionality (up, down, or not regulated) of transcription changes compared to uninfected samples was consistent between the qRT-PCR data and the microarray data. Likewise, mRNA transcripts were similarly regulated in both the microarray and qRT-PCR (Fig. 3c and d). Nevertheless, during infection with the PTG strain, we identified two loci, Ifi44-lnc and Socs2, that were not expressed in the microarray but did appear to be regulated in the qRT-PCR.
Figure 3.
Validation of lncRNA and mRNA microarray data by qRT-PCR. RNA from mouse BMDM were collected 6 hr after infection with either RH or PTG strains of T. gondii, and qRT-PCR was subsequently performed. Fold changes represent the comparison of infected samples to uninfected samples. Regulation of representative lncRNAs as determined by qPCR (a) and microarray (b) analysis. Regulation of immune-related mRNA is shown by qPCR (c) and microarray (d) analysis. Microarray fold change values differ slightly from those listed in Supplementary Spreadsheets 1 and 2 (which are the geometric means) because arithmetic means were calculated from the microarray data to directly compare to qRT-PCR data. Experiments were completed a minimum of three times with BMDM from three separate mice and were obtained independently of experiments used for microarray analysis.
Toxoplasma rhoptry kinase ROP16 manipulates expression of host lncRNA
During intracellular infection, T. gondii secretes a subset of effector molecules whose activities modify host responses to infection32. One example is the rhoptry protein ROP16, a kinase that directly activates host cell STAT3 and STAT617,21,33,34. While Type I ROP16 possesses strong STAT kinase activity, Type II ROP16 is catalytically inactive. Previous studies in BMDM indicate that 538 protein-coding genes are regulated by Type I (RH) ROP1618.
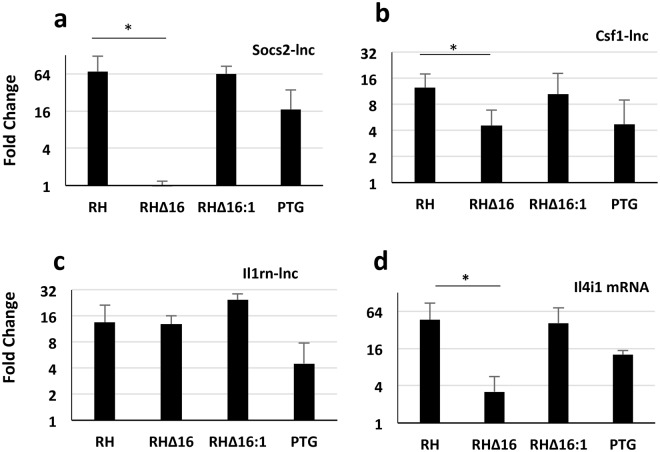
We hypothesized that the activity of ROP16 extends to control of lncRNAs, particularly those strongly up-regulated by the Type I RH strain relative to the Type II PTG. By using engineered ROP16 deletion and complementation mutants created on the RH background17, we examined expression of three lncRNAs that showed evidence of Type I strain-specific up-regulation: Socs2-lnc, Csf1-lnc, and Il1rn-lnc. Expression of Csf1-lnc and Socs2-lnc was significantly reduced during infection with the ROP16 deletion mutant (RH∆16) compared to RH (Fig. 4a and b). Complementation of RH∆16 with a functional copy of ROP16 (RH∆16:1) restored the up-regulation phenotype. In contrast, Il1rn-lnc was not controlled by ROP16, and the expression of Il1rn-lnc RH∆16 in Fig. 4c is similar to that of RH and RH∆16:1. As a positive control for a gene controlled by ROP16, we examined the expression of an mRNA (Il4i1) that we suspected was also controlled by ROP16 based upon the strain-specific expression pattern. Il4i1 protein-coding mRNA expression was also significantly lower in the RH∆16 strain (Fig. 4d). Our data demonstrate that the secreted parasite effector molecule ROP16 regulates several putative host lncRNAs.
Figure 4.
lncRNAs are targeted for manipulation by T. gondii rhoptry kinase ROP16. BMDM were infected with RH, a ROP16 deletion mutant on the RH background (RHΔ16), a ROP16 complementation strain (RHΔ16:1), and PTG. Then, RNA was isolated 6 hr later for qRT-PCR analysis. Expression patterns of Socs2-lnc (a), Csf1-lnc (b), Il1rn-lnc (c) and Il4i1 mRNA (d) were determined. Fold changes represent the comparison of infected samples to uninfected samples. p < 0.05 was considered significant when comparing RH and RH∆16. The experiments were completed a minimum of three independent times.
Additionally, to isolate effects of live infection from other potential sources of gene regulation (such as the presence of dead parasites or soluble parasite protein), we examined expression of Socs2-lnc after addition of live, dead, and soluble tachyzoite antigen (STAg). Addition of dead parasites and STAg did not result in increased expression of Socs2-lnc, suggesting that Socs2-lnc is induced by live T. gondii infection (Supplementary Fig. S5).
Discussion
In this study we employed a microarray approach to comprehensively survey host lncRNAs that are regulated during intracellular infection of macrophages with T. gondii. We identified ~900 putative lncRNAs that were up-regulated by infection, and a similar number whose expression was down-regulated. A subset of the parasite-regulated lncRNAs was associated with mRNA transcripts that encode immune response genes. We selected four of these lncRNA molecules for validation by qRT-PCR: Socs2-lnc, Csf1-lnc, Il1rn-lnc, and Ifi44-lnc. We determined that expression of Socs2-lnc and Csf1-lnc depended on the parasite rhoptry kinase ROP16. Our lncRNA screen was performed in vitro using a single host cell type, and therefore it is possible that we are missing some important lncRNAs that are induced in vivo but not in our BMDM cultures. Nevertheless, the fact that we have identified several lncRNAs that are regulated by Toxoplasma in our study of macrophages (which are a major host reservoir during in vivo infection) provides rich ground for further analysis. We also cannot rule out that some of our observed expression effects may not be due to live infection and could be due to the presence of dead parasites, the presence of soluble parasite extract, or the phagocytosis of live parasites. While some of our identified lncRNAs may be induced by these other factors, they still may be important for the immune response to T. gondii infection.
The microarray simultaneously enabled us to examine the expression of 24,881 mRNAs, and we identified 1,927 protein-coding transcripts that were differentially regulated during T. gondii infection. Our results are consistent with prior studies on BMDM responses to Toxoplasma. For example, expression levels of ccl17, csf1, ccl24, ccl7, cxcl2, and Socs2 in response to infection agree with previous publications18,35. While the focus of the present study was on lncRNA expression patterns, the mRNA expression analysis allows the placement of this study in the context of previous work.
We examined lncRNA responses to infections with Type I RH and Type II PTG parasite strains, which are commonly found in European and North American host populations. The Type I strains exhibit a highly virulent phenotype in mice in that one tachyzoite is sufficient to cause death before encystment36–38. There is also epidemiological evidence that Type I strains cause more severe pathology in humans39,40. The Type II strains display decreased virulence in mice and can establish long-term infection through formation of quiescent cysts. Previous experiments using BMDM show that Type I tachyzoites drive an M2 phenotype, whereas Type II parasites drive an M1 phenotype. Therefore, some of the strain-specific effects on lncRNA expression may reflect generalized M1/M2 responses17,18. Infection with Type I RH induces a massive pro-inflammatory cytokine response that likely contributes to death41,42. Paradoxically, in vitro studies show that Type II strains elicit stronger IL-12 responses from macrophages, a response that results from NFκB activation by the parasite secretory molecule GRA1543–45. It is also well known that Toxoplasma Type I strains evade host IFN-γ-induced immunity-related GTPase destruction of the parasitophorous vacuole through the activities of secretory rhoptry proteins ROP5, ROP17 and ROP1846–50. The findings in the present study indicate that the impact of parasite strain type also extends to host lncRNA expression. For example, we observed a substantial difference in the number of differentially regulated lncRNAs between Type I RH and Type II PTG (1522 and 528, respectively). Given the emerging understanding of the importance of lncRNAs in infection and immunity3, we hypothesize that noncoding RNA responses will prove to be important determinants of virulence.
During invasion, tachyzoites inject the rhoptry protein ROP16 into the host cell cytoplasm. Type I ROP16 is a host-directed kinase that phosphorylates STAT3 and STAT6. Type II parasite strains express a form of ROP16 that lacks kinase activity. In addition to cytoplasmic STAT activation, ROP16 contains a nuclear localization sequence21. This characteristic raises the possibility that ROP16 possesses other activities that may require localization to the host cell nucleus. In this regard, a yeast 2 hybrid system was recently used to identify host proteins Dnaja1 (DnaJ heat shock protein family member A1) and Gabra4 (gamma-aminobutyric acid A receptor, subunit alpha 4) as non-STAT proteins that interact with ROP1651.
Here, we identify two lncRNA molecules whose expression depends upon ROP16, namely Csf1-lnc and Socs2-lnc. Csf1-lnc is a lncRNA associated with the gene that encodes the cytokine Csf1, which drives hematopoietic stem differentiation into macrophage lineage cells. Socs2-lnc is a lncRNA that overlaps with the Socs2 gene. Socs2 has previously been implicated in anti-inflammatory responses to T. gondii28. In the case of Socs2-lnc, deletion of the entire ROP16 gene on the Type I background had a much more profound impact than loss of ROP16 kinase activity in Type II PTG. One explanation of this result is that on a Type II genetic background the dense granule protein GRA15 controls Socs2-lnc, as has been shown for the Socs2 gene itself18. Another explanation of this result is that Socs2-lnc expression is controlled by kinase domain-independent portions of the ROP16 protein.
The role of lncRNA in the immune system, particularly in the context of infection, is not well understood, but its importance is becoming increasingly clear3,52,53. During viral infection, for example, lncRNAs NEAT1, lncRNA-CMPK2, and lncBST2 control production of IFN and other antiviral cytokines54–56. Other lncRNAs, including GAS5, interfere with assembly by direct binding to viral structural components57. In mouse macrophages, both stimulation with bacterial lipopolysaccharide and infection with Listeria monocytogenes induces expression of lncRNA AS-IL1α and intergenic lncRNAs lincRNA-Cox2 and lincRNA-Tnfaip3. Through distinct mechanisms, each of these noncoding RNAs regulates expression of pro-inflammatory cytokines6,58,59.
While our work was in progress, another group used a similar microarray approach to identify lncRNA expression in human foreskin fibroblast cells infected with only Type II T. gondii60. In that study, a lncRNA designated NONSHAT022487 was up-regulated during infection of macrophages. Based on correlation analysis, it was suggested to play a role in suppression of UNC93B1, a molecule involved in TLR11/12-mediated recognition of Toxoplasma61,62. Our study extends this work by showing that parasite strain exerts a strong influence on lncRNA expression patterns. We also show that the secretory kinase ROP16 controls expression of a subset of lncRNAs that are linked to immune response genes. Given the abundance of lncRNAs encoded within the genome as well as the increasing number of studies indicating their regulatory function, we hypothesize that lncRNAs are major regulators of the host response to Toxoplasma and other microbial pathogens.
Materials and Methods
Parasites and infections
Wildtype Toxoplasma strains RH and PTG, as well as engineered strains RH∆16 and RH∆16:1, were used in this study. The latter two strains were constructed and kindly provided by D. Bzik and B. Fox (Dartmouth Geisel School of Medicine). The construction of both mutant strains has been described previously17. In brief, the ROP16 coding region was deleted in the Type I RH strain KU80 knockout background by homologous recombination of a HXGPRT marker. The RH∆16 strain was then complemented with a single copy of a functional allele of ROP16 from Type I RH by replacement of HXGPRT at the ROP16-deleted locus to generate strain RH∆16:1. RH was heat-treated for 30 minutes at 65 °C to kill T.gondii for experiments with inactivated parasites. Soluble tachyzoite antigen (STAg) was prepared according to a previously published protocol63. STAg was added to BMDM at a concentration of 20 µg per 106 BMDM.
Tachyzoites of all strains were maintained by approximately twice-weekly passage on human foreskin fibroblast monolayers in human fibroblast medium (HFM) consisting of DMEM (Life Technologies) supplemented with 1% heat-inactivated bovine growth serum (Thermo Fisher Scientific), 100 U/mL penicillin (Life Technologies), and 0.1 mg/mL streptomycin (Life Technologies). Infections were accomplished by addition of tachyzoites to mouse BMDM (4:1 ratio of parasites to cells) on 12-well plates (Falcon, non-tissue culture treated). Plates were briefly centrifuged (3 min, 200 × g) to initiate contact between tachyzoites and macrophages. Cultures were incubated 6 hrs (37 °C, 5% CO2), then cells were harvested for RNA extraction. Percent infection and number of tachyzoites per infected cell were calculated using an Olympus BX51 immunofluorescence microscope. DAPI was used to stain the nucleus. Toxoplasma-specific polyclonal antibody conjugated to FITC (ThermoFisher Scientific) was used to stain the parasites.
Generation of BMDM
Femur and tibia from female C57BL/6 mice (6–8 wks of age; The Jackson Laboratory) were used as a source of bone marrow cells. Macrophages were generated from single cell suspensions of bone marrow cells by 5-day culture in L929-containing supernatants, as previously described64. One day prior to infection, BMDM were harvested, counted, and plated on 12-well tissue culture plates in HFM at a concentration of 1–2 × 106 cells per well.
Microarray and data analysis
Total RNA was prepared from BMDM by RNeasy Mini Kit purification (Qiagen). Total RNA from each sample was quantified using a NanoDrop ND-1000 (ThermoFisher Scientific), and the RNA integrity was assessed using standard denaturing agarose gel electrophoresis. Microarray analysis was carried out by Arraystar, Inc. using the Mouse LncRNA v3.0, an Agilent Array platform (Agilent Technologies, Inc.). The sample preparation and microarray hybridization were performed based on Agilent standard protocols with minor modifications. Briefly, mRNA was purified from total RNA after removal of rRNA (mRNA-ONLY™ Eukaryotic mRNA Isolation Kit, Epicentre). Then, each sample was amplified and transcribed into fluorescent cRNA along the entire length of the transcripts without 3′ bias utilizing a mixture of oligo(dT) and random primers (Arraystar Flash RNA Labeling Kit, Arraystar). The labeled cRNAs were hybridized onto the Mouse LncRNA Array v3.0 (8 × 60 K, Arraystar). After washing slides, the arrays were scanned by the Agilent Scanner G2505C.
Agilent Feature Extraction software (version 11.0.1.1) was used to analyze the acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v12.1 software package (Agilent Technologies). After quantile normalization of the raw data, lncRNA and mRNA for which at least 3 out of 9 samples had flags in Present or Marginal (“All Targets Value”) were chosen for further data analysis. Differentially expressed lncRNA and mRNA with statistical significance were identified through Volcano Plot filtering between two groups using the R software package. Pathway analysis and GO analysis were applied to determine the roles that these differentially expressed mRNA played in these biological pathways or GO terms (https://www.genome.jp/kegg/; http://www.geneontology.org/). Hierarchical clustering, using R heatmap.2, was performed to display the distinguishable lncRNA and mRNA expression patterns among samples. Normalized intensities were used as the values. The distance metric was Euclidean and the linkage criterion was average. (https://www.rdocumentation.org/packages/gplots/versions/3.0.1/topics/heatmap.2) (https://www.rdocumentation.org/packages/stats/versions/3.5.1/topics/hclust)
Additional data analysis was performed using the Microsoft Excel software.
Quantitative RT-PCR
Total RNA was prepared using a RNeasy Mini Kit (Qiagen), and samples were subjected to Turbo DNase treatment (Life Technologies). RNA was converted to cDNA using the SuperScript IV VILO Master Mix (ThermoFisher Scientific). Quantitative PCR was performed on target genes and normalized to the expression of the housekeeping gene Ppia using the SYBR green method (SsoAdvanced Universal SYBR Green Supermix, Bio-Rad) and the Bio-Rad CFX96 RT-PCR machine. Expression relative to uninfected control samples was calculated using the ∆∆Ct method. A control with no added reverse transcriptase was included for each sample. Primer sequences used are shown in Table 4. For our Socs2-lnc primers, some of our samples (such as uninfected and RHΔ16) were occasionally at the limit detection of our qRT-PCR assay. In those cases, we used 35 cycles as the Cq.
Table 4.
Primer sequences used for qRT-PCR analysis.
| Il4i1 mRNA | F | CAACAGGGAAAGGGGCCATTC |
| R | GGCCTTGAGGTCTTTGAAGGC | |
| Ppia mRNA | F | GCATGTGGTCTTTGGGAAGGTG |
| R | GGGTAAAATGCCCGCAAGTCAA | |
| Ifi44 mRNA | F | CCAGGGGAAAAGCACACAAAACA |
| R | TGCGATTGGTCCTTGTAGCTGA | |
| Csf1 mRNA | F | TTGCTAAGTGCTCTAGCCGAGG |
| R | AGGTGGAAGACAGACTCAGGGA | |
| Il1rn mRNA | F | GCAACCACCTTGAGCCTGAAAT |
| R | TTAGGTGACTGTAGGGTCCCCA | |
| Socs2 mRNA | F | CAAGATCCCTTGTGCCCGGAG |
| R | GCAGAATGGTGTGGCAAAGTCT | |
| Csf1 lncRNA | F | CGGTAGTGATGGAGTGTGGCTT |
| R | CTGCCTGTACCTCTGGATTGCT | |
| Socs2 lncRNA | F | AGATGGCTCAGTTACGTGCCTT |
| R | ACCTGACCAATTCTGCTCAGCT | |
| Il1rn lncRNA | F | TGTGTGCCTTACAGGGTGAACA |
| R | TTGATGGCATCTCCCAAGGCTT | |
| Ifi44 lncRNA | F | GGTGGGCTGTGATGAAGATGGA |
| R | AAGTTGAACCGAGAAGCCTGGG | |
| uc.70 lncRNA | F | ATCAGGGAGAGATCTCAGGCCA |
| R | CCCCTGGCCTTCTGTGAGAATG |
Primers were designed to encompass unique regions of each lncRNA and mRNA examined.
Statistical Analyses
Microarray expression results from Arraystar were transformed from logarithmic values to linear values. This step enabled the direct comparison of arithmetic means and standard deviations of the microarray and qRT-PCR data. Error bars in Figs 3 and 4 are shown as the standard deviation of 3–6 biological experiments. Grubbs’ test, with a significance level of 0.05, was used to determine if a replicate was an outlier and discard it. Dixon’s Q test gave comparable results. Expression differences in lncRNAs and in mRNAs were tested for significance using an unpaired Student t-test. Two tailed tests were performed, with p < 0.05 considered statistically significant.
Ethical Approval
All experimental protocols were approved by the University of New Mexico Institutional Animal Care and Use Committee (IACUC). The methods were carried out in accordance with the Panel on Euthanasia of the American Veterinary Medical Association.
Electronic supplementary material
Acknowledgements
We thank S. Carpenter (UC-Santa Cruz) for discussion and advice. This work was supported by National Institute of General Medical Sciences of the National Institutes of Health award P30 GM110907 and GM06021. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International Inc., for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525. This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the U.S. Department of Energy or the United States Government.
Author Contributions
E.Y.D. conceived of the study and submitted samples for microarray analysis. K.L.M. analyzed the microarray data. K.L.M. and B.E.H. performed experiments and analyzed qRT-PCR data. K.L.M. and E.Y.D. drafted the manuscript. A.P.C. assisted with statistical analyses and implemented a tool for calculating in-well qPCR efficiencies. All authors read and approved the final manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article (and Supplementary Information files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33274-5.
References
- 1.Atianand MK, Caffrey DR, Fitzgerald KA. Immunobiology of Long Noncoding RNAs. Annual review of immunology. 2017;35:177–198. doi: 10.1146/annurev-immunol-041015-055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spurlock CF, III., Crooke PS, III., Aune TM. Biogenesis and Transcriptional Regulation of Long Noncoding RNAs in the Human Immune System. J Immunol. 2016;197:4509–4517. doi: 10.4049/jimmunol.1600970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YG, Satpathy AT, Chang HY. Gene regulation in the immune system by long noncoding RNAs. Nature immunology. 2017;18:962–972. doi: 10.1038/ni.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer MK, et al. The landscape of long noncoding RNAs in the human transcriptome. Nature genetics. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szczesniak MW, Makalowska I. lncRNA-RNA Interactions across the Human Transcriptome. PloS one. 2016;11:e0150353. doi: 10.1371/journal.pone.0150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter S, et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald KA, Caffrey DR. Long noncoding RNAs in innate and adaptive immunity. Current opinion in immunology. 2014;26:140–146. doi: 10.1016/j.coi.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey, J. P. In Toxoplasma gondii. The model apicomplexan: Perspective and methods (eds Weiss, L. M. & Kim, K.) 1–17 (Academic Press, 2013).
- 9.McLeod, R. M., van Tubbergen, C., Montoya, J. G. & Petersen, E. In Toxoplasma gondii. The Model Apicomplexan: Perspectives and Methods (eds L.M. Weiss & K. Kim) 100-159 (Academic Press, 2013).
- 10.Cohen SB, Denkers EY. Border maneuvers: deployment of mucosal immune defenses against Toxoplasma gondii. Mucosal immunology. 2014;7:744–752. doi: 10.1038/mi.2014.25. [DOI] [PubMed] [Google Scholar]
- 11.Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin Immunopathol. 2012;34:793–813. doi: 10.1007/s00281-012-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaddi PJ, Yap GS. Cytokine regulation of immunopathology in toxoplasmosis. Immunology and cell biology. 2007;85:155–159. doi: 10.1038/sj.icb.7100038. [DOI] [PubMed] [Google Scholar]
- 13.Bierly AL, Shufesky WJ, Sukhumavasi W, Morelli A, Denkers EY. Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J. Immunol. 2008;181:8445–8491. doi: 10.4049/jimmunol.181.12.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen SB, Denkers EY. Impact of Toxoplasma gondii on Dendritic Cell Subset Function in the Intestinal Mucosa. J Immunol. 2015;195:2754–2762. doi: 10.4049/jimmunol.1501137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courret N, et al. CD11c and CD11b expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nature reviews. Microbiology. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butcher BA, et al. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS pathogens. 2011;7:e1002236. doi: 10.1371/journal.ppat.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen KD, et al. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell host & microbe. 2011;9:472–483. doi: 10.1016/j.chom.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim L, et al. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J. Immunol. 2005;174:4178–4184. doi: 10.4049/jimmunol.174.7.4178. [DOI] [PubMed] [Google Scholar]
- 20.Olias P, Etheridge RD, Zhang Y, Holtzman MJ, Sibley LD. Toxoplasma Effector Recruits the Mi-2/NuRD Complex to Repress STAT1 Transcription and Block IFN-gamma-Dependent Gene Expression. Cell host & microbe. 2016;20:72–82. doi: 10.1016/j.chom.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeij JP, et al. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider AG, Abi Abdallah DS, Butcher BA, Denkers EY. Toxoplasma gondii triggers phosphorylation and nuclear translocation of dendritic cell STAT1 while simultaneously blocking IFNg-induced STAT1 transcriptional activity. PloS one. 2013;8:e60215. doi: 10.1371/journal.pone.0060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, et al. Prediction of novel long non-coding RNAs based on RNA-Seq data of mouse Klf1 knockout study. BMC Bioinformatics. 2012;13:331. doi: 10.1186/1471-2105-13-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejerano G, et al. Ultraconserved elements in the human genome. Science. 2004;304:1321–1325. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- 26.Engreitz JM, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539:452–455. doi: 10.1038/nature20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotzin JJ, et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537:239–243. doi: 10.1038/nature19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machado FS, et al. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nature medicine. 2006;12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 29.Hume DA, Freeman TC. Transcriptomic analysis of mononuclear phagocyte differentiation and activation. Immunological reviews. 2014;262:74–84. doi: 10.1111/imr.12211. [DOI] [PubMed] [Google Scholar]
- 30.Haralambieva IH, et al. Genome-wide associations of CD46 and IFI44L genetic variants with neutralizing antibody response to measles vaccine. Human genetics. 2017;136:421–435. doi: 10.1007/s00439-017-1768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine & growth factor reviews. 2002;13:323–340. doi: 10.1016/S1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 32.Hakimi MA, Olias P, Sibley LD. Toxoplasma Effectors Targeting Host Signaling and Transcription. Clinical microbiology reviews. 2017;30:615–645. doi: 10.1128/CMR.00005-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denkers EY, Bzik DJ, Fox BA, Butcher BA. An inside job: hacking into Janus kinase/signal transducer and activator of transcription signaling cascades by the intracellular protozoan Toxoplasma gondii. Infection and immunity. 2012;80:476–482. doi: 10.1128/IAI.05974-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto M, et al. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. The Journal of experimental medicine. 2009;206:2747–2760. doi: 10.1084/jem.20091703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CW, Bennouna S, Denkers EY. Screening for Toxoplasma gondii regulated transcriptional responses in LPS-activated macrophages. Infect. Immun. 2006;74:1916–1923. doi: 10.1128/IAI.74.3.1916-1923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howe DK, Summers BC, Sibley LD. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii. Infect. Immun. 1996;64:5193–5198. doi: 10.1128/iai.64.12.5193-5198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 38.Su C, Howe DK, Dubey JP, Ajioka JW, Sibley LD. Identification of quantitative trait loci controlling acute virulence in Toxoplasma gondii. Proc. Natl. Acad. Sci. 2002;99:10753–10758. doi: 10.1073/pnas.172117099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behnke MS, Dubey JP, Sibley LD. Genetic Mapping of Pathogenesis Determinants in Toxoplasma gondii. Annual review of microbiology. 2016;70:63–81. doi: 10.1146/annurev-micro-091014-104353. [DOI] [PubMed] [Google Scholar]
- 40.Xiao J, Yolken RH. Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiol (Oxf) 2015;213:828–845. doi: 10.1111/apha.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavrilescu LC, Denkers EY. IFN-g overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J. Immunol. 2001;167:902–909. doi: 10.4049/jimmunol.167.2.902. [DOI] [PubMed] [Google Scholar]
- 42.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 2001;167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 43.Kim L, et al. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J. Immunol. 2006;177:2584–2591. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- 44.Robben PM, et al. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol. 2004;172:3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 45.Rosowski EE, et al. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. The Journal of experimental medicine. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behnke MS, et al. The Polymorphic Pseudokinase ROP5 Controls Virulence in Toxoplasma gondii by Regulating the Active Kinase ROP18. PLoS pathogens. 2012;8:e1002992. doi: 10.1371/journal.ppat.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behnke MS, et al. Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of Toxoplasma gondii. PLoS genetics. 2015;11:e1005434. doi: 10.1371/journal.pgen.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behnke MS, et al. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9631–9636. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etheridge RD, et al. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell host & microbe. 2014;15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niedelman W, et al. The Rhoptry Proteins ROP18 and ROP5 Mediate Toxoplasma gondii Evasion of the Murine, But Not the Human, Interferon-Gamma Response. PLoS pathogens. 2012;8:e1002784. doi: 10.1371/journal.ppat.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan M, et al. Screening and Identification of the Host Proteins Interacting with Toxoplasma gondii Rhoptry Protein ROP16. Frontiers in microbiology. 2017;8:2408. doi: 10.3389/fmicb.2017.02408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu W, Ding C. Roles of LncRNAs in Viral Infections. Frontiers in cellular and infection microbiology. 2017;7:205. doi: 10.3389/fcimb.2017.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zur Bruegge J, Einspanier R, Sharbati S. A Long Journey Ahead: Long Non-coding RNAs in Bacterial Infections. Frontiers in cellular and infection microbiology. 2017;7:95. doi: 10.3389/fcimb.2017.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barriocanal M, Carnero E, Segura V, Fortes P. Long Non-Coding RNA BST2/BISPR is Induced by IFN and Regulates the Expression of the Antiviral Factor Tetherin. Frontiers in immunology. 2014;5:655. doi: 10.3389/fimmu.2014.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imamura K, et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Molecular cell. 2014;53:393–406. doi: 10.1016/j.molcel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Kambara H, et al. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic acids research. 2014;42:10668–10680. doi: 10.1093/nar/gku713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian X, Xu C, Zhao P, Qi Z. Long non-coding RNA GAS5 inhibited hepatitis C virus replication by binding viral NS3 protein. Virology. 2016;492:155–165. doi: 10.1016/j.virol.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Chan J, et al. Cutting Edge: A Natural Antisense Transcript, AS-IL1alpha, Controls Inducible Transcription of the Proinflammatory Cytokine IL-1alpha. J Immunol. 2015;195:1359–1363. doi: 10.4049/jimmunol.1500264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma S, et al. A long noncoding RNA, lincRNA-Tnfaip3, acts as a coregulator of NF-kappaB to modulate inflammatory gene transcription in mouse macrophages. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2017;31:1215–1225. doi: 10.1096/fj.201601056R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu W, et al. Microarray analysis of long non-coding RNA expression profiles uncovers a Toxoplasma-induced negative regulation of host immune signaling. Parasites & vectors. 2018;11:174. doi: 10.1186/s13071-018-2697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melo Mariane B., Kasperkovitz Pia, Cerny Anna, Könen-Waisman Stephanie, Kurt-Jones Evelyn A., Lien Egil, Beutler Bruce, Howard Jonathan C., Golenbock Douglas T., Gazzinelli Ricardo T. UNC93B1 Mediates Host Resistance to Infection with Toxoplasma gondii. PLoS Pathogens. 2010;6(8):e1001071. doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pifer R, Benson A, Sturge CR, Yarovinsky F. UNC93B1 is essential for TLR11 activation and IL-12 dependent host resistance to Toxoplasma Gondii. The Journal of biological chemistry. 2011;286:3307–3314. doi: 10.1074/jbc.M110.171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-a, and the chemokines macrophage-inflammatory protein-1a and -1b in response to Toxoplasma gondii antigens. J. Immunol. 1999;162:7369–7375. [PubMed] [Google Scholar]
- 64.Kim L, Butcher BA, Denkers EY. Toxoplasma gondii interferes with lipopolysaccharide-induced mitogen-activated protein kinase activation by mechanisms distinct from endotoxin tolerance. J. Immunol. 2004;172:3003–3010. doi: 10.4049/jimmunol.172.5.3003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and Supplementary Information files).