Abstract
The “Cavendish” and “Prata” subgroups represent respectively 47% and 24% of the world banana production. Compared to world average progressing from 10.6 to 20.6 t ha−1 between 1961 and 2016, and despite sustained domestic demand and the introduction of new cultivars, banana yield in Brazil has stagnated around 14.5 t ha−1 mainly due to nutrient and water mismanagement. “Prata” is now the dominant subgroup in N-E Brazil and is fertigated at high costs. Nutrient balances computed as isometric log-ratios (ilr) provide a comprehensive understanding of nutrient relationships in the diagnostic leaf at high yield level by combining raw concentration data. Although the most appropriate method for multivariate analysis of compositional balances may be less efficient due to non-normal data distribution and limited nutrient mobility in the plant, robustness of the nutrient balance approach could be improved using Box-Cox exponents assigned to raw foliar concentrations. Our objective was to evaluate the accuracy of nutrient balances to diagnose fertigated “Prata” orchards. The dataset comprised 609 observations on fruit yields and leaf tissue compositions collected from 2010 to 2016 in Ceará state, N-E Brazil. Raw nutrient concentration ranges were ineffective as diagnostic tool due to considerable overlapping of concentration ranges for low- and high-yielding subpopulations at cutoff yield of 40 Mg ha−1. Nutrient concentrations were combined into isometric log-ratios (ilr) and normalized by Box-Cox corrections between 0 and 1 which may also account for restricted nutrient transfer from leaf to fruit. Despite reduced ilr skewness, Box-Cox coefficients did not improve model robustness measured as the accuracy of the Cate-Nelson partition between yield and the multivariate distance across ilr values. Sensitivity was 94%, indicating that low yields are attributable primarily to nutrient imbalance. There were 148 false-positive specimens (high yield despite nutrient imbalance) likely due to suboptimal nutrition, contamination, or luxury consumption. The profitability of “Prata” orchards could be enhanced by rebalancing nutrients using ilr standards with no need for Box-Cox correction.
Introduction
The “Cavendish” and “Prata” subgroups represent 47% and 24% of the world banana production, respectively1. The current cultivars of bananas originate from the hybridization of diploid subspecies of Musa acuminata Colla (A genome) and Musa balbisiana Colla (B genome), and they exhibit various levels of ploidy and genomic constitution, such as diploid (AA; BB; or AB; 2n = 2x = 22); triploid (AAA; AAB; or ABB; 2n = 3x = 33); and tetraploid (AAAA; AAAB; AABB; or ABBB; 2n = 4x = 44)2. The main banana cultivars in Brazil have genomes AA, AAA, AAB, ABB, and AAAB2. Brazil produces 6.9 × 106 tons of banana fruits annually on 480 × 103 hectares3. Although banana production in Brazil is supported by strong domestic demand representing 98% of total production4, the average yield of 14.5 tons ha−1 year−1 remains lower than the world average5 of 20.6 tons ha−1 year−1.
Banana nutrient requirements depend on yield potential, plant density, soil fertility, and root development6. Low fruit yields are generally attributed to nutrient mismanagement and water shortage7–10. The K is generally the main limiting nutrient and interacts with N, Ca and Mg11. Because K is also of public health concern due to too low daily intake12–15, it is applied in relatively large amounts to boost banana yield and quality, potentially leading to luxury consumption. “Prata” is the dominant banana subgroup in N-E Brazil and is fertigated. Fertilization represents 16–22% of production costs compared to 14–27% for irrigation16.
Nutrient acquisition by the banana fruit relies on plant’s ability to translocate nutrients. N, P, K, Mg, Cu, and Zn have the greatest potential for nutrient translocation from leaf to fruit17–19. S, Cu, Zn, Mn and Fe have variable mobility, and Ca and B are relatively immobile20,21. The indirect relationship between relatively phloem-immobile leaf nutrients and fruit yield depends on soil test and water supply regulating xylem transport to the fruit22. As source of variability and heteroscedasticity nutrient mobility from leaf to fruit could be constrained by Box-Cox exponents23 between zero (immobile nutrients unrelated to yield) and 1 (mobile nutrients related to yield).
Banana diagnostic leaf tissue is collected close to blooming stage11. There are several methods to interpret the results of tissue analysis. The most common method is the critical concentration range diagnosing nutrients separately24, assuming that all other factors are close to their optima25, an over-optimistic assumption. Because components of a system are intrinsically interactive and multivariate26, nutrients can be combined into balances using isometric log-ratios (ilr)27. The ilr transformation is the most suitable method to run multivariate analyses on compositional data28 such as leaf composition29–31. However, the ilr transformation may not return normality or homoscedasticity.
Box-Cox coefficients assigned to raw concentration data could improve ilr data distribution and model accuracy. Exponents may be varied between 0 and 1, zero not contributing to ilr () and 1, fully contributing to it (); intermediate Box-Cox coefficients reflect partial contribution. Model accuracy is commonly determined after partitioning data into true-negative (TN), false-negative (FN), true-positive (TP) and false-positive (FP) specimens using the Cate-Nelson procedure, the receiving operating characteristic (ROC) or the confusion matrix29,30. Accuracy is computed as (TN+TP)/(TN+FN+TP+FP). However, nutrient excess at high yield level (FP specimens) is diagnosed as sub-optimal concentration, luxury consumption of contamination. Accuracy defined in terms of diagnostic power could thus be computed as (TN+TP+FP)/(TN+FN+TP+FP). The conventional ilr transformation is robust if Box-Cox coefficients of one across concentrations return greatest model accuracy and diagnostic power after varying coefficients between zero and one.
Our objective was to measure the robustness, accuracy and diagnostic power of nutrient balance designs for fertigated “Prata” banana. We hypothesized that high yields of fertigated “Prata” are reached within narrow foliar nutrient combinations into balances after optimizing Box-Cox coefficients assigned to raw concentration data.
Results
Yield partitioning and Box-Cox transformation
Due to irrigation, fruit yield did not differ significantly between the wet (18.35 ± 4.39 Mg ha−1) and dry (18.35 ± 3.77 Mg ha−1) seasons. Fruit yields were normally distributed in both seasons and varied between 9 and 53 tons ha−1 year−1. The critical Mahalanobis distance across the whole dataset was 3.9 at a cutoff yield of 40 Mg fruit ha−1 (Fig. 1). The partition showed 8.4% TN, 3.9% FN, 63.4% TP, and 24.3% FP specimens. Combining nutrient concentrations into ilr values returned an accuracy of 72%, negative predictive value of 68%, positive predictive value of 72%, and sensitivity of 94%. The diagnostic power was 96%. Specificity was 26% due to the high number of FP specimens. The ilr of the TN subgroup (n = 51 specimens) used to compute the Mahalanobis distance are presented as (Supplementary Material Table 1). Using the traditional critical value approach, it was not possible to establish “adequate” nutrient concentration ranges due to considerable overlap between high- and low-yielding subpopulations (Table 1). Imbalanced nutrition was the main cause of low yields in these intensively managed production systems. The proportion of imbalanced crops, determined as (FP+TP)/total, was 88%. Thus, fertilization regimes were suboptimal in most orchards.
Figure 1.
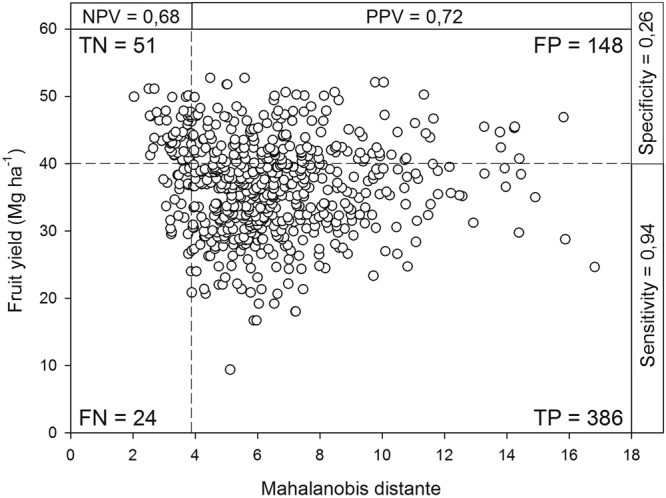
Cate–Nelson partitioning of the relationship between Mahalanobis distance and banana yield (critical distance = 3.9 at cutoff yield of 40 Mg ha−1). TN = true-negative, FN = false-negative, TP = true-positive, FP = false-positive.
Table 1.
Ranges of concentration values for two yield classes.
| Nutrient | Minimum | Median | Maximum | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|
| High yield ≥ 40 tons ha−1 | Low yield < 40 tons ha−1 | |||||
| g kg−1 | ||||||
| N | 18.2 | 21.7 | 26.8 | 16.4 | 21.9 | 27.0 |
| P | 1.0 | 1.7 | 2.9 | 0.7 | 1.6 | 2.7 |
| K | 13.8 | 33.5 | 59.5 | 14.4 | 33.5 | 48.6 |
| Ca | 2.9 | 6.5 | 11.1 | 2.6 | 6.3 | 10.3 |
| Mg | 1.6 | 2.4 | 3.9 | 0.8 | 2.4 | 4.1 |
| S | 1.0 | 1.5 | 2.2 | 0.7 | 1.5 | 2.0 |
| mg kg−1 | ||||||
| B | 3 | 11 | 21 | 1 | 10 | 11 |
| Cu | 3 | 6 | 13 | 2 | 5 | 21 |
| Fe | 41 | 65 | 105 | 29 | 66 | 100 |
| Mn | 22 | 175 | 403 | 18 | 132 | 539 |
| Zn | 8 | 16 | 26 | 7 | 16 | 32 |
| Na | 10 | 44 | 100 | 10 | 48 | 100 |
| Al | 2 | 27 | 77 | 2 | 28 | 80 |
Discussion
Although nutrient imbalance appeared to be the main yield-limiting factor in Ceará state, there were 24 FN specimens in which yields were limited by other factors. There are several classes of soil that enable the soil to sustain banana production relative to soil properties32. High-yielding crops in Group 1 were grown in level or gently undulating well-drained soils with solum thickness exceeding 100 cm and with loam to clayey texture. Group 1 soils were well-structured, fertile, and neutral to slightly acidic, with no saline limitations. Medium-yielding crops in Group 2 were limited by natural fertility, slope, solum thickness, or drainage, and required investment to reach high yield. Low-yielding crops in Group 3 soils were limited by low soil fertility level, sandy texture, or solum thickness (30–35 to 75 cm). Very low-yielding crops in Group 4 soils had several major limitations, such as shallow (<25 cm) solum and high salinity. Soil bulk density, pH, and electrical conductivity did not limit yield in Ceará33, leaving solum thickness as the probable yield-limiting factor. Quartzispamments require major investments in fertilization and irrigation due to low nutrient and water reserves in Ceará soils (Group 3).
Levels of banana leaf nutrients such as Ca, Mg, and Mn were relatively stable at daytime temperatures between 17 and 30 °C34, such as those typical of Missão Velha. Mg did not appear to be yield-limiting at Missão Velha despite high K levels that are potentially antagonistic to Mg6,17,18. However, Ca, B, and S may accumulate as a result of local soil properties, such as high pH and organic matter content35. The S level likely reflected water quality due to the presence of gypsum layers in the sedimentary basin at Missão Velha36. The relative shortage of Mn can be generally attributed to high soil pH37. Banana plants are highly responsive to added Mn38, but foliar Mn diagnostic standards are elusive9. Although often shown to be in relative shortage in banana orchards9,39, Zn levels were adequate or in apparent excess at Missão Velha. The [B | Ca] balance among TP specimens was often high, which indicated potential Ca excess or B shortage.
The status of Na and Al in banana leaf has been little documented. Accumulation of Na in banana seedlings may reduce biomass production33,40. Exponential increase from 0.6 to 3.1 g Na kg−1 was observed in the shoots of banana seedlings33, well below the Na level of 25 g kg−1 in leaf margins suffering from necrosis40. Any beneficial role of Na could occur below around 1 g Na kg−1, as is the case in Missão Velha. Foliar Al concentration41 normally ranges between 0.050 and 0.400 g Al kg−1. Foliar Al may accumulate in high-pH soils due to the attack of soil-borne pathogens on banana roots42. Roots react by exuding oxalic, malic, and fumaric acids43, which may chelate Al ions44,45. As a result, tissue Al varied widely between orchards but did not reach toxic levels.
The cutoff yield to establish nutrient standards was 40 tons ha−1. For AAB banana a cutoff yield of 30 tons ha−1 was suggested in Minas Gerais, Brazil46, while for AAA banana a cutoff yield close to 40 tons ha−1 was stipulated in São Paulo state47. In Ceará state, commercial yields of AAB “Prata” are generally classified as low (<30 tons ha−1), medium (30–35 tons ha−1), or high (≥35 tons ha−1). In the Ceará dataset, 106 orchards produced <30 tons ha−1, 137 orchards produced 30–35 tons ha−1, 317 orchards produced 35–45 tons ha−1, and 49 orchards produced >45 tons ha−1. Furthermore, 199 orchards (33%) produced ≥40 tons ha−1. Obviously, there is a large potential to increase yield in Ceará orchards by rebalancing nutrients.
Whereas nutrients diagnosed separately may return low diagnostic performance31, ternary diagrams and multivariate analyses allow the entire nutrient status to be captured48,49. Additionally, criticism of the misuse of multivariate analysis to analyze compositional data is reported in the literature26. Log-ratio transformations address resonance in the compositional space (if one concentration increases, one or more concentration values must decrease), subcompositional incoherence, and the intrinsically non-normal distribution in the constrained compositional space compared to the unconstrained real space required to conduct statistical analyses. The clr transformation50 is the most common log-ratio used to diagnose nutrient limitations in crops. To facilitate interpretation of the diagnosis from the Mahalanobis distance, clr indices can be ordered in ascending order from the most negative (relative shortage) to the most positive (relative excess), as in DRIS51.
The diagnostic accuracy of 72% reported here was lower than the generally obtained value of 80%, whereas the critical Mahalanobis distance of 3.9 was close to the range of 4.1–5.9 that has been reported for other crops29,31,52,53. Low accuracy was due to the high proportion of FP specimens (24%), indicating suboptimal fertilization, luxury consumption of nutrients, or leaf contamination. Fertigation appeared to be adequately run in 12% of orchards (TN+FN). Pests or unfavorable soil factors may have limited the yield of FN specimens. The fertigation regime should be rebalanced in 88% of orchards (FP+TP). Thus, nutrient mismanagement appeared to be the main yield-limiting factor at Missão Velha. Regional nutrient standards appeared to be more reliable than so-called “universal” nutrient ratio standards35.
Conclusion
Nutrient imbalance limited yield in Ceará’s “Prata” banana orchards. Nutrient concentration ranges did not discriminate between low- and high-yielding crops but combining nutrients into ilr and clr made possible performing nutrient standards. The Cate–Nelson partitioning about cutoff yield of 40 tons ha−1 and the multivariate distance across ilr values returned a critical Mahalanobis distance of 3.9, below which nutrient balances were adequate. Although appealing to improve data distribution and constrain nutrient mobility in plants, the Box-Cox coefficients assigned to raw nutrient concentrations failed to improve model accuracy.
The order of nutrient limitation to yield shown by clr indices should be further validated by using nutrient tests for specimens showing the Mahalanobis distance above the critical value. While 4% of orchards (FN specimens), solum thickness or other factors limited fruit yield, 96% of orchards were classified as nutritionally balanced (TN specimens) or imbalanced (FP and TP specimens). The most commonly deficient nutrient was boron, and the most commonly excessive was nitrogen. Although fertigation can enhance banana yield, the dosage of nutrients requires adjustment based on reliable diagnostic tools. Banana is the fruit crop showing yet the highest proportion of false positive specimens and the smallest number of false negative specimens. Hence nutrient imbalance is a crucial problem that could be addressed using tools of compositional data analysis.
Methods
Climatic and soil conditions
Data were collected from 2010 to 2016 in 6- to 18-year-old banana stands at Missão Velha, Ceará state, Brazil (7° 35′ S and 39° 21′ W, 442 m in altitude). Climate in this part of Brazil is semi-arid tropical (Aw in the Köppen-Geiger classification), with a dry winter season and rainfall concentrated in the summer season. The warmest months in the area extend from September to December (Supplementary Table 2)54. Maximum and minimum temperature ranges were 31–35 °C and 19–21 °C, respectively. For comparison, optimal mean temperatures for banana crops are as follows: 22 °C for floral initiation, 31 °C for leaf growth and development, and 28 °C (range: 15–35 °C) for high commercial yields32. Total rainfall averaged 1022.6 mm, compared to 1200–1800 mm as an effective precipitation regime for banana production32.
Soils are sandy and classified as Neossolo Quartzarênico55 or Quartzipsamment56. Soil properties in the top layer (0–20 cm) are presented as (Supplementary Table 3).
Orchard management
Commercial orchards averaging 3 ha in size were surveyed for yield and foliar composition. Plant density averaged 1332 plants ha−1 (4.0 m × 2.0 m double row, 2.5 m between plants). Plants were sprinkler-irrigated at a crop evapotranspiration (ETc) rate proportional to potential evapotranspiration ET0 (ETc = KCET0, where KC varied during the season)6,57. To sustain high yield and quality, the banana clump was restricted to a few fruiting plants by yearly pruning. Banana yields could be affected not only by nutrient imbalance but also by diseases and pests, including nematodes58. The most frequent pest is the banana weevil (Cosmopolites sordidus), and the most frequent disease is yellow Sigatoka (Mycosphaerella musicola).
In fertigated orchards, nitrogen is generally supplied as urea (45% N) every 3 to 15 days up to 440–600 kg N ha−1 year−1, as follows: 10% during the first 3 months, 75% between the fourth month and blooming (seventh to ninth month), and 15% up to harvest. Depending on the soil test, potassium is applied as potassium chloride (60% of K2O) every 3–15 days at total rate of 1300 to 1700 kg K2O ha−1 year−1 as follows: 0% during the first 3 months, 90% from the fourth month to blooming, and 10% toward harvest. Phosphorus is applied as reactive natural phosphate (27% of P2O5) at planting and via fertigation as monoammonium phosphate (48% of P2O5) at total rate of 160 kg P2O5 ha−1 year−1. Calcium is supplied to maintain the soil K: Ca: Mg molar ratio between 0.5: 3.5: 1.0 and 0.3: 2.0: 1.0. Manganese, zinc, and boron are applied at rates of 60 kg manganese sulfate (26% Mn) ha−1 year−1, 96 kg zinc sulfate (20% Zn) ha−1 year−1, and 20–30 kg boric acid (17% B) ha−1 year−1, respectively.
Data collection and tissue analysis
The dataset comprised 609 observations of cv. ‘Prata’, AAB “Prata” subgroup. The period between budding and harvest varied from 8 to 12 months. Yield data were reported for the dry and wet seasons. In July and December of each year, the third most fully expanded leaf of banana plants was collected at blooming stage twice a year during the wet and dry seasons, and 10-cm-wide pieces were cut from inner halves on both sides of the midrib and at the midpoint of the lamina59. Four samples made of 10 subsamples were composited in each orchard. Samples were oven-dried at 72 °C for 48–96 hours and ground to less than 1 mm. N was determined by the micro-Kjeldahl method. After sample digestion in a mixture of nitric and perchloric acids60, the elements Ca, Mg, Fe Zn, Cu, Al and Mn were quantified by atomic absorption spectrophotometry, P by colorimetry, S by turbidimetry, and K and Na by emission flame photometry61. B was quantified by colorimetry62.
Log-ratio and Box-Cox transformations
The tissue compositional simplex comprised N, P, K, Ca, Mg, S, Cu, Zn, Mn, Fe, B, Na, and Al, as well as a filling value computed as the difference between the measurement unit and the sum of nutrients. The orthonormal balance was computed as the ilr, as follows63,64:
where nj+ and nj− are numbers of components at the numerator and denominator, respectively, and are geometric means across components at the numerator and the denominator, respectively, p and q are Box-Cox coefficients assigned to components at numerator and denominator, respectively, and is a normalization coefficient. The ilr is designated as [components at denominator|components at numerator] because the log-ratio becomes more negative as values at the denominator increase. More negative numbers are located on the left-hand side of the array, as in algebra. The ilr values were used to compute the Mahalanobis distance from a reference subpopulation65. Box-Cox coefficients varying between 0 and 1 are assigned to raw concentrations at numerator and denominator, hence transforming ilr values to reach additivity, normality or homoscedasticity and possibly controlling nutrient mobility, a coefficient near zero making the nutrient immobile and a coefficient close to one making the nutrient mobile.
The balance design was elaborated following a sequential binary partition (Supplementary Table 4). N, P, K, and Mg are mobile nutrients, S, Cu, Zn, Mn, and Fe are of variable mobility, and Ca and B are relatively immobile20,21. K and Mg are antagonistic to each other22. N and P reflect protein synthesis and energy transport, respectively66. Among nutrients of limited mobility, S is involved in protein synthesis, whereas Cu, Zn, and Mn are involved in metabolism22 and fungicide formulations. Fe and Mn are involved in soil genesis55. Sodium (Na) acts as a functional element as osmoticium for cell enlargement and an accompanying cation for long-distance transport67. Plant tolerance to Al toxicity depends on Al interactions with other minerals, such as B, P, Ca, and Mg41.
Statistical analysis
Computations were performed by using the R statistical package version 3.4.168. The dataset was separated into calibration (2010–2014) and validation (2015–2016) datasets that were merged in case of similar accuracy. The Cate–Nelson procedure partitions data into true-negative (TN), false-negative (FN), true-positive (TP), and false-positive (FP) quadrants from the relationship between crop yield and Mahalanobis distance65. Specificity is computed as TN/(TN+FP), sensitivity as TP/(TP+FN), accuracy as (TN+TP)(TN+FN+TP+FP), negative predictive value as TN/(TN+FN), and positive predictive value as TP/(TP+FP). The centered log-ratio (clr) values of the TN subpopulation were compared with published data after adjusting the geometric mean for the number of components (Table 2).
Table 2.
Comparison of centered log-ratio standards (mean and standard deviation [SD]) for the banana diagnostic leaf in the present study (6–14 components), India (10 components), and Uganda (6 components).
| clr | Ceará standards | India‡ | Uganda§ | ||
|---|---|---|---|---|---|
| Mean ± SD (14) | Mean ± SD (10) | Mean ± SD (6) | Mean ± SD (10) | Mean ± SD (6) | |
| VN | 3.670 ± 0.098 | 2.259 ± 0.102 | 0.315 ± 0.065 | 2.226 ± 0.199 ns | 0.302 ± 0.153 ns |
| VP | 1.073 ± 0.100 | −0.339 ± 0.100 | −2.282 ± 0.076 | −0.505 ± 0.289** | −2.312 ± 0.176 ns |
| VK | 4.159 ± 0.117 | 2.747 ± 0.119 | 0.804 ± 0.102 | 2.485 ± 0.231** | 0.607 ± 0.168** |
| VMg | 1.396 ± 0.116 | −0.015 ± 0.115 | −1.959 ± 0.093 | 0.318 ± 0.449** | −1.546 ± 0.199** |
| VS | 0.997 ± 0.076 | −0.415 ± 0.076 | — | −1.057 ± 0.361** | na |
| VCu | −4.632 ± 0.109 | — | — | na | na |
| VZn | −3.613 ± 0.100 | −5.024 ± 0.095 | — | −4.815 ± 0.359** | na |
| VMn | −1.208 ± 0.525 | −2.619 ± 0.515 | — | −2.246 ± 0.412** | na |
| VFe | −2.191 ± 0.093 | −3.602 ± 0.098 | — | −3.212 ± 0.384** | na |
| VCa | 2.412 ± 0.096 | 1.001 ± 0.093 | −0.943 ± 0.098 | 1.121 ± 0.432 ns | −0.859 ± 0.289 ns |
| VB | −3.834 ± 0.291 | — | — | na | na |
| VNa | −2.451 ± 0.361 | — | — | na | na |
| VAl | −3.196 ± 0.225 | — | — | na | na |
| VFv† | 7.419 ± 0.095 | 6.007 ± 0.092 | 4.066 ± 0.058 | 5.685 ± na** | 3.809 ± 0.087** |
Electronic supplementary material
Acknowledgements
We are grateful to Coordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq) (#141578/2014-9) for a doctoral scholarship, to Sítio Barreiras Fruticultura Ltda. for research support, and to the Natural Sciences and Engineering Research Council of Canada (NSERC-DG-2254) and Centre SEVE-FRQNT for financial support.
Author Contributions
Conceived and designed the experiments: J.A.L.D., J.C.L.N., M.C.M.C., performed the experiments: J.A.L.D., analyzed the data: J.A.L.D., S.E.P., L.E.P., wrote the manuscript: J.A.L.D., W.N., L.E.P. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32328-y.
References
- 1.Soto, M. Situación y avances tecnologicos en la producción bananera. Rev. Bras. Frut. Special Volume, 13–28 (2011).
- 2.Jesus ON, et al. Genetic diversity and population structure of Musa accessions in ex situ conservation. BMC Plant Biol. 2013;13:41. doi: 10.1186/1471-2229-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Instituto Brasileiro de Geografia e Estatística – IBGE. Produção agrícola municipal: culturas temporárias e permanentes. Preprint at http://biblioteca.ibge.gov.br/visualizacao/periodicos/66/pam_2015_v42_br.pdf (2015).
- 4.Kist, B. B, Carvalho, C., Treichel, M. & Santos, C. E. Brazilian Fruit Yearbook 2018 (Editora Gazeta Santa Cruz, 2018)
- 5.Food and Agriculture Organization of the United Nations – FAO. Statistics Division (FAOSTAT). figsharehttp://www.fao.org/faostat/en/#data (2018).
- 6.Borges, A. L., Coelho, E. F., Costa, E. L. & Teixeira, A. H. C. Irrigação e fertirrigação na cultura da banana in Irrigação e fertirrigação em fruteiras e hortaliças (eds Sousa, V. F., Marouelli, W. A., Coelho, E. F., Pinto, J. M. & Coelho Filho, M. A.) 369–397 (Embrapa, 2011).
- 7.Twyford IT, Walmsley D. The mineral composition of the Robusta banana plant. I. Methods and plant growth studies. Plant Soil. 1973;39:227–243. doi: 10.1007/BF00014790. [DOI] [Google Scholar]
- 8.Moreira A, Fageria NK. Yield, Uptake, and Retranslocation of Nutrients in Banana Plants Cultivated in Upland Soil of Central Amazonian. J. Plant Nutr. 2009;32:443–457. doi: 10.1080/01904160802660750. [DOI] [Google Scholar]
- 9.Raghupathi HB, Reddy BMC, Srinivas K. Multivariate diagnosis of nutrient imbalance in banana. Commun. Soil Sci. Plant Anal. 2002;33:2131–2143. doi: 10.1081/CSS-120005753. [DOI] [Google Scholar]
- 10.Wairegi L, Asten Pvan. Norms for multivariate diagnosis of nutrient imbalance in the Esat African Highland Bananas (Musa spp. AAA) J. Plant Nutr. 2011;34:1453–1472. doi: 10.1080/01904167.2011.585203. [DOI] [Google Scholar]
- 11.Martin-Prével P. Échantillonnage du bananier pour l’analyse foliaire: conséquences des différences techniques. Fruits. 1977;32:151–166. [Google Scholar]
- 12.Welch RM. The impact of mineral nutrients in food crops on global human health. Plant Soil. 2002;247:83–90. doi: 10.1023/A:1021140122921. [DOI] [Google Scholar]
- 13.Zuo Y, Zhang F. Iron and zinc biofortification of dicot plantsd with gramineous species. A review. Agron. Sustain. Dev. 2008;29:63–71. doi: 10.1051/agro:2008055. [DOI] [Google Scholar]
- 14.International Food Information Council Foundation – IFICF. Potassium & heart health. Fact sheet. https://www.foodinsight.org/ (2011).
- 15.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary. Guidelines for Americans. 8thEd. (HHS-USDA, 2015)
- 16.Almeida, C. O. & Souza, J. S. Custos e rentabilidade in Banana Produção: Aspectos Técnicos (ed. Cordeiro, Z. J. M.) 136–139 (Embrapa, 2000).
- 17.Twyford IT, Walmsley D. The mineral composition of the Robusta banana plant. III. Uptake and distribution of mineral constituents. Plant Soil. 1974;41:471–491. [Google Scholar]
- 18.Twyford IT, Walmsley D. The mineral composition of the Robusta banana plant. IV. The application of fertilizers for high yields. Plant Soil. 1974;41:493–508. [Google Scholar]
- 19.Moreira A, Moraes LAC, Pereira JCR, Castro C. Retranslocation of nutrients and zinc sulphate fertilization of banana plants in central Amazon. J. Appl. Hort. 2007;9:91–96. [Google Scholar]
- 20.Hill. J. The remobilization of nutrients from leaves. J. Plant Nutr. 1980;2:407–444. doi: 10.1080/01904168009362788. [DOI] [Google Scholar]
- 21.Barker, A.V. & Pilbeam, D. J. Handbook of Plant Nutrition. 2ndEd. (CRC Press, 2015).
- 22.Marschner, P. Marschner’s Mineral Nutrition of Higher Plants 3rd Ed. (Elsevier, 2011).
- 23.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Series B Stat Methodol. 1964;26:211–252. [Google Scholar]
- 24.Ulrich, A. & Hills. F. J. Principles and practices of plant analysis in Soil Testing and Plant Analysis. Part II. (eds. Stelly, M. & Hamilton, H.) 11–24 (Soil Science Society of America, 1967).
- 25.Wit CT. Resource use in agriculture. Agric. Syst. 1992;40:125–151. doi: 10.1016/0308-521X(92)90018-J. [DOI] [Google Scholar]
- 26.Aitchison, J. The single principle of compositional data analysis, continuing fallacies, confusions and misunderstandings and some suggested remedies. Preprint at http://dugi-doc.udg.edu/bitstream/handle/10256/706/Aitchison_CoDaWork08_keynote_final_19_MAIG.pdf?sequence=7 (2008).
- 27.Parent LE. Diagnosis of the nutrient compositional space of fruit crops. Rev. Bras. Frut. 2011;33:321–334. doi: 10.1590/S0100-29452011000100041. [DOI] [Google Scholar]
- 28.Filzmoser P, Hron K, Reimann C. Univariate statistical analysis of environmental (compositional) data: problems and possibilities. Sci. Total Environ. 2009;407:6100–6108. doi: 10.1016/j.scitotenv.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Parent LE, Parent SÉ, Hébert-Gentile V, Naess K, Lapointe L. Mineral balance plasticity of cloudberry (rubus chamaemorus) in Quebec-Labrador. Am. J. Plant Sci. 2013;4:1508–1520. doi: 10.4236/ajps.2013.47183. [DOI] [Google Scholar]
- 30.Parent SÉ, et al. The plant ionome revisited by the nutrient balance concept. Front. Plant Sci. 2013;4:1–10. doi: 10.3389/fpls.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowaki RHD, et al. Phosphorus Over-Fertilization and Nutrient Misbalance of Irrigated Tomato Crops in Brazil. Front. Plant Sci. 2017;8:825. doi: 10.3389/fpls.2017.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borges, A. L. & Oliveira, A. M. G. Nutrição, Calagem e Adubação in Banana Produção: Aspectos Técnicos (ed. Cordeiro, Z. J. M.) 47–59 (Embrapa, 2000).
- 33.Almeida AMM, Gomes VFF, Mendes Filho PF, Lacerda CF, Freitas ED. Influence of salinity on the development of the banana colonised by arbuscular mycorrhizal fungi. Rev. Ciênc. Agron. 2016;47:421–428. doi: 10.5935/1806-6690.20160051. [DOI] [Google Scholar]
- 34.Lahav E, Turner DW. Temperature influences the composition of diagnostic samples used to assess the nutrient status of banana plants. Scient. Hortic. 1985;27:275–283. doi: 10.1016/0304-4238(85)90032-9. [DOI] [Google Scholar]
- 35.Walworth JL, Sumner ME. The Diagnosis and Recommendation Integrated System (DRIS) Adv. Soil Sci. 1987;6:149–188. doi: 10.1007/978-1-4612-4682-4_4. [DOI] [Google Scholar]
- 36.Silva MAM. Lower cretaceous sedimentary sequences in the basin, Northeastern Brazil: A revision. Braz. J. Geol. 1986;16:311–319. [Google Scholar]
- 37.Barber, S. A. Manganese in Soil nutrient bioavailability: a mechanistic approach. 338-344 (John Wiley & Sons, 1995).
- 38.Turner DW, Barkus B. The uptake and distribution of mineral nutrients in the banana in response to supply of K, Mg and Mn. Fert. Res. 1983;4:89–99. doi: 10.1007/BF01049669. [DOI] [Google Scholar]
- 39.Moreira SG, Prochnow LI, Kiehl JC, Martin Neto L, Pauletti V. Formas químicas, disponibilidade de manganês e produtividade de soja em solos sob semeadura direta. Rev. Bras. Ciênc. Solo. 2006;30:121–136. doi: 10.1590/S0100-06832006000100013. [DOI] [Google Scholar]
- 40.Shapira O, Khadka S, Israeli Y, Shani U, Schwartz A. Functional anatomy controls ion distribution in banana leaves: significance of Na+ seclusion at the leaf margins. Plant Cell Envir. 2009;32:476–485. doi: 10.1111/j.1365-3040.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 41.Vardar F, Ünal M. Aluminum toxicity and resistance in higher plants. Adv, Mol. Biol. 2007;1:1–12. [Google Scholar]
- 42.Blamey, F. P. C., Kopittke. P. M., Wehr. J. B. & Menzies, N. W. Aluminium in Handbook of Plant Nutrition. 2nded. (eds Barker, A. V. & Pilbeam D. J.) 567–605 (CRC Press, 2015).
- 43.Yuan J, et al. Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens NJN-6. Sci. Rep. 2015;5:13438. doi: 10.1038/srep13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drábek O, Kiplagat IK, Komárek M, Tejnecký V, Borůvka L. Study of interactions between Relevant Organic Acids and Aluminium in Model Solutions Using HPLC and IC. Soil Water Res. 2015;10:172–180. doi: 10.17221/256/2014-SWR. [DOI] [Google Scholar]
- 45.Van Hees PAW, Lundström US, Starr M, Giesler R. Factors influencing aluminium concentrations in soil solution from podzols. Geoderma. 2000;94:289–310. doi: 10.1016/S0016-7061(98)00138-4. [DOI] [Google Scholar]
- 46.Silva JTA, Carvalho JG. Establishment of DRIS norms for ‘Prata Anã’ banana (AAB) under irrigation. Cienc. Agrotec. 2006;30:43–51. doi: 10.1590/S1413-70542006000100006. [DOI] [Google Scholar]
- 47.Teixeira LAJ, Zambrosi FCB, Bettiol Neto JE. Avaliação do estado nutritional de bananeiras do subgrupo Cavendish no estado de São Paulo: Normas DRIS e níveis críticos de nutrients. Rev. Bras. Frut. 2007;29:613–620. doi: 10.1590/S0100-29452007000300037. [DOI] [Google Scholar]
- 48.Holland DA. The interpretation of leaf analysis. J. Hort. Sci. 1966;41:311–329. doi: 10.1080/00221589.1966.11514179. [DOI] [Google Scholar]
- 49.Lagatu H, Maume L. Le diagnostic foliaire de la pomme de terre. Montpellier École Natl. d’Agr. Ann. 1934;22:50–158. [Google Scholar]
- 50.Parent. LE, Dafir. MA. Theoretical Concept of Compositional Nutrient Diagnosis. J. Am. Soc. Hort. Sci. 1992;117:239–242. [Google Scholar]
- 51.Beaufils ER. Diagnosis and recommendation integrated system (DRIS). A general scheme for experimentation and calibration based on principles developed from research in plant nutrition. Soil Sci. 1973;1:1–132. [Google Scholar]
- 52.Parent, S. E. et al. Nutrient balance as paradigm of plant and soil chemometrics in Soil Fertility (ed. Issaka, R. N.) 83–114 (InTech Publ., 2012).
- 53.Parent SÉ, Barlow P, Parent LE. Nutrient balance of New Zealand kiwifruit (Actinidia deliciosa) at high yield level. Commun. Soil Sci. Plant Anal. 2015;46:256–271. doi: 10.1080/00103624.2014.989031. [DOI] [Google Scholar]
- 54.Instituto Nacional de Meteorologia – INMET. Banco de Dados meteorológicos para Ensino e Pesquisa – Série Histórica – Dados Mensais. figshare, http://www.inmet.gov.br/projetos/rede/pesquisa/form_mapas_mensal.php (2017).
- 55.Santos, H. G. et al. Sistema brasileiro de classificação de solo 3rd Ed. (Embrapa, 2013)
- 56.Soil Survey Staff. Keys to Soil taxonomy (USDA-NRCS, 2014).
- 57.Santos MR, Donato SLR, Lourenço LL, Silva TS, Coelho Filho MA. Irrigation management strategy for Prata-type banana. Rev. Bras. Eng. Agríc. Ambient. 2016;20:817–822. doi: 10.1590/1807-1929/agriambi.v20n9p817-822. [DOI] [Google Scholar]
- 58.Su L, et al. Suppression on plant-parasitic nematodes using a soil fumigation strategy based on ammonium bicarbonate and its effects on the nematode Community. Sci. Rep. 2015;5:17597. doi: 10.1038/srep17597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin-Prével, P. Bananier in L’analyse végétale dans le contrôle de l’alimentation des plantes tempérées et tropicales (eds. Martin-Prével, P., Gagnard, J. & Gautier, P.) 715–751 (Tec & Doc, 1984).
- 60.Jones. J. B. Jr. & Case, V. W. Sampling handling and analyzing plant tissue samples in Soil Testing and Plant Analysis 3rded. (ed. Westerman, R. L.) 389–427 (Soil Science Society of America, 1990).
- 61.Raij, B. V, Andrade, J. C., Cantarella, H. & Quaggio, J. A. Analise química para avaliação de fertilidade de solos tropicais (Instituto Agronômico de Campinas, 2001).
- 62.Wolf B. Improvement in the Azometine-H method for determination of boron. Commun. Soil Sci. Plant Anal. 1974;5:39–44. doi: 10.1080/00103627409366478. [DOI] [Google Scholar]
- 63.Egozcue JJ, Pawlowsky-Glahn V, Mateu-Figueras G, Barceló-Vidal C. Isometric log-ratio transformations for compositional data analysis. Math. Geol. 2003;35:279–300. doi: 10.1023/A:1023818214614. [DOI] [Google Scholar]
- 64.Egozcue JJ, Pawlowsky-Glahn V. Groups of parts and their balances in compositional data analysis. Math. Geol. 2005;37:795–828. doi: 10.1007/s11004-005-7381-9. [DOI] [Google Scholar]
- 65.Parent SÉ, Parent. LE, Rozane DE, Natale. W. Plant ionome diagnosis using sound balance: case study with mango (Mangifera indica) Front. Plant Sci. 2013;4:449. doi: 10.3389/fpls.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Güsewell S. N: Pratios in terrestrial plants: variation and functional significance. New Phytologist. 2004;164:243–266. doi: 10.1111/j.1469-8137.2004.01192.x. [DOI] [PubMed] [Google Scholar]
- 67.Subbarao GV, Ito O, Berry WL, Wheeler RM. Sodium – A functional plant nutrient. Crit. Rev. Plant Sci. 2003;22:391–416. [Google Scholar]
- 68.R Core Team. The R Project for Statistical Computing. https://www.r-project.org/ (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


