Abstract
Purinergic receptor signaling is increasingly recognized as an important regulator of inflammation. The P2X family purinergic receptors P2X5 and P2X7 have both been implicated in bone biology, and it has been suggested recently that P2X5 may be a significant regulator of inflammatory bone loss. However, a role for P2X5 in periodontitis is unknown. The present study aimed to evaluate the functional role of P2X5 in ligature-induced periodontitis in mice. Five days after placement of ligature, analysis of alveolar bone revealed decreased bone loss in P2rx5−/− mice compared to P2rx7−/− and WT control mice. Gene expression analysis of the gingival tissue of ligated mice showed that IL1b, IL6, IL17a and Tnfsf11 expression levels were significantly reduced in P2rx5−/− compared to WT mice. These results suggest the P2X5 receptor may regulate bone loss related to periodontitis and it may thus be a novel therapeutic target in this oral disease.
Keywords: Bone loss, Inflammation, Osteoclast, Periodontitis, Purinergic receptor
INTRODUCTION
Periodontitis is a common chronic inflammatory disease that affects the periodontium surrounding and supporting the teeth and represents the most prevalent form of bone pathology in humans. Specifically, almost 50% of adults suffer from some form of periodontitis with approximately 10% of the population being affected by generalized severe periodontitis (1). This disease is initiated by dysbiotic microbial communities that colonize subgingival tooth surfaces and cause gingival inflammation that leads to destruction of periodontal connective tissue and bone (2). The gram-negative bacterium Porphyromonas gingivalis is a major factor in the pathogenesis of periodontal disease where it functions as a keystone pathogen (3). The LPS of this pathogen is an important virulence factor and has been implicated in both periodontal disease pathogenesis and associated systemic conditions (4). The immunopathologic lesions in periodontitis are characterized by breakdown of the collagen fibers of the periodontal ligament and the loss of gingival tissue and alveolar bone, which is mediated locally by host-produced inflammatory factors, such as interleukin-1β (IL-1β), tumor necrosis factor (TNF), IL-6, IL-17 and receptor activator of nuclear factor kappa-B ligand (RANKL). Expression of these factors occurs in response to the dysbiotic microbiota and associated molecular components, including LPS. It is known that cytokines, chemokines, arachidonic acid metabolites, and proteolytic enzymes are collectively pivotal in promoting periodontal inflammation, and in periodontitis are elevated in both the gingival crevicular fluid (5–7) and the gingival tissue (8). Several studies suggest that pro-inflammatory cytokines, including IL-1, IL-4, IL-17, and TNF-α, that are induced during T cell-mediated immune responses, play crucial roles in regulating RANKL expression on osteoblasts, which is associated with inflammation that can affect bone metabolism (9).
The purinergic signaling system has a wide range of physiological and pathological roles (10, 11), including inflammation and bone remodeling, and transmits signals from extracellular nucleotides via cell surface P2 purinergic receptors, which consist of two sub-families, ionotropic receptors (P2X) and metabotropic receptors (P2Y), that are expressed on various cell types (12). Functional expression of multiple P2X and P2Y receptor subtypes has been demonstrated on both osteoblasts and osteoclasts (13, 14). Activation of these receptors by endogenous ligands regulates various cellular functions, including proliferation and apoptosis, that are crucial role for bone remodeling in bone microenvironments (15). In addition, purinergic receptors are involved in the activation of the inflammasome, which is responsible for post-translational processing of IL-1β and IL-18 cytokines to mature forms.
Previous work has reported roles for P2X7 in both osteoblast and osteoclast physiology. However, P2rx7-deficient mice and precursor cells maintain capacity to form multinucleated osteoclasts in vivo and in vitro (16, 17), respectively, suggesting the need for additional investigation into the role of purinergic signaling in bone. Recently, a study from our group showed that P2X5 is required for optimal inflammasome-induced IL-1β production by osteoclasts, and for osteoclast maturation and hyper-multinucleation (18). Interestingly, inflammasomes have been reported to enhance osteoclast differentiation and bone resorption (19). Inflammation and bone loss are tightly connected with periodontal disease. However, although the actions of purinergic receptors during inflammation are appreciated, nothing is currently known about how P2X5 functions in the context of periodontal disease, or whether P2X5 exerts specific function that is independent of previously reported functions of P2X7 (20). In this study, we used a ligature-induced experimental periodontitis model and found that P2X5 deficiency significantly decreased ligature-induced periodontal bone loss. P2X5 deficiency was also found to inhibit local osteoclast differentiation and activation. These findings suggest that P2X5 (and/or its associated signaling components) may be worth investigating as potential therapeutic targets in the context of periodontal disease and other bone loss disorders.
RESULTS
P2X5-dependent induction of IL-1β and osteoclast multinucleation by P. gingivalis LPS
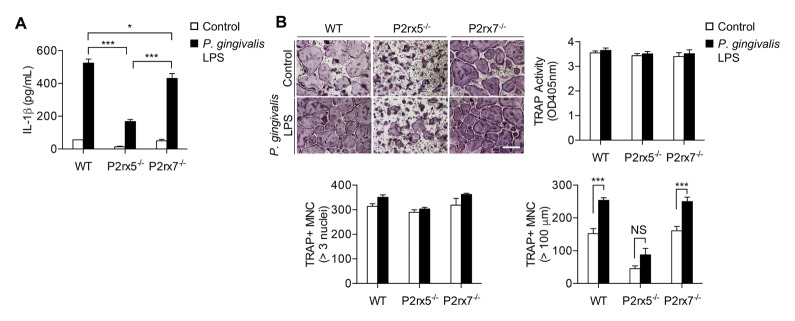
We recently reported that in a model of inflammatory bone loss, in which LPS from Escherichia coli O111:B4 is injected above the calvarial bone, P2rx5−/− mice exhibit comparatively low osteoclast numbers and localized bone resorption (18). However, whether P2X5 plays a similar role in the setting of periodontitis is uncertain and was investigated in this study. To test whether P. gingivalis LPS-mediated signaling is similarly dependent on P2X5, we cultured BMMs from control and P2rx5−/− mice, as well as from mice deficient in P2X7, the P2X member best characterized as an inflammatory activator (21), for two days with M-CSF and RANKL, then cultured for an additional day in the presence or absence of 100 ng/ml P. gingivalis LPS. ELISA of culture supernatants revealed defective LPS-induced IL-1β secretion by both P2rx5−/− and P2rx7−/− cells compared to control cells (Fig. 1A). P. gingivalis LPS treatment did not affect osteoclast differentiation as measured by TRAP staining (Fig. 1B, upper panels) or numbers of osteoclasts with greater than three nuclei (Fig. 1B, lower left plot). In contrast, P. gingivalis LPS treatment induced osteoclast hyper-multinucleation, a marker of inflammation-related osteoclast formation defined by the number of cells of greater than 100 μm diameter; this activity was observed in control or P2rx7−/− cells but was specifically unaffected in P2rx5−/− cells (Fig. 1B, lower right plot). Together these results suggest a potential role for P2X5 in inflammatory bone loss conditions associated with P. gingivalis.
Fig. 1.
P2X5-dependent induction of IL-1β and osteoclast multinucleation by P. gingivalis LPS. BMMs were cultured for 2 days in the presence of M-CSF and RANKL, then cultured for 1 day with or without P. gingivalis LPS (100 ng/ml). (A) IL-1β secretion into the culture medium was analyzed by ELISA. (B) Cells were fixed and stained for TRAP (upper panels). TRAP+ MNCs were counted both by the presence of more than 3 nuclei (lower left plot) and cell size larger (lower right plot) than 100 μm in diameter. Scale bar represents 200 μm. Data are means ± SD. Data represent three independent experiments, each performed in three replicas. *P < 0.05. ***P < 0.001. NS: not significant.
P2X5 deficiency decreases alveolar bone loss in ligature-induced periodontitis
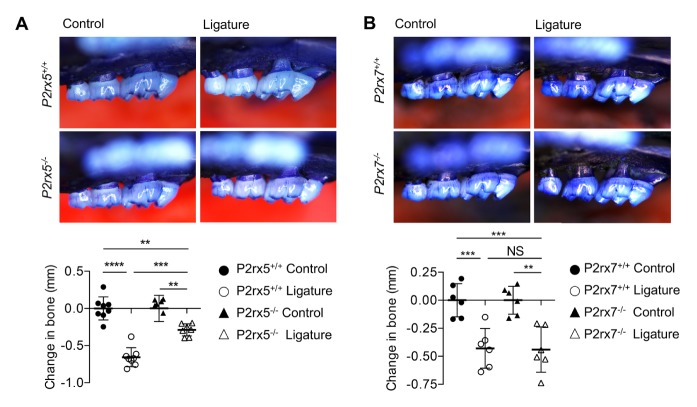
To investigate the role of P2X5 in periodontal disease, ligature-induced periodontitis was performed using littermate WT and P2rx5−/− mice. P2rx7−/− mice were also tested to determine whether potential effects observed in P2rx5−/− mice were specific to disruption of a particular P2X receptor, or to P2X signaling more generally. Alveolar bone loss was measured using CEJ-ABC distance. Significant differences in alveolar bone loss between unligated control and corresponding ligated teeth 5 days after surgery were observed for WT and P2rx7−/− groups, showing that the ligatures around the teeth were able to promote bone loss (Fig. 2B). However, alveolar bone loss decreased by 44% in P2rx5−/− (P < 0.001) mice compared with WT mice after ligation (Fig. 2A). These data suggest that P2X5 receptor, but not P2X7 receptor, regulate alveolar bone loss in the ligature-induced periodontitis model.
Fig. 2.
Induction of periodontal bone loss in P2rx5−/− and P2rx7−/− mice. Representative images of maxillae (upper jaws) illustrating the morphometric findings of P2rx5−/− (A) and P2rx7−/− (B). Determination of bone changes in the jaws was calculated by measuring the CEJ-ABC distance for each mouse and transforming the data to directly indicate bone loss (lower panel on each). Data are means ± SD. Data pooled from two independent experiments with n = 6–8 mice per group. **P < 0.01. ***P < 0.001. P < 0.0001. NS: Not significant.
Decreased cytokine expression in P2X5-deficient gingival tissues post-ligation
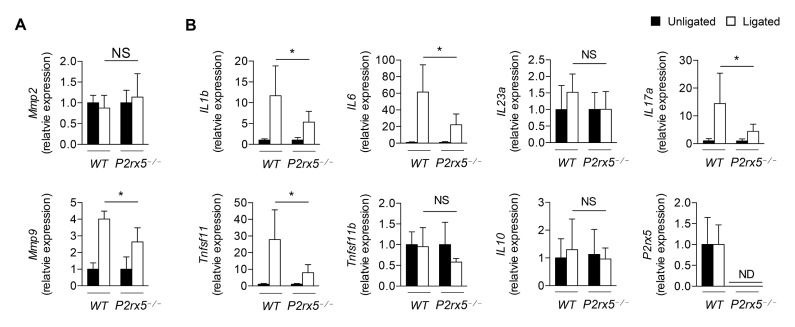
The matrix metalloproteinases Mmp2 and Mmp9 are reported biomarkers for periodontitis (22). We examined Mmp2 and Mmp9 expression in the context of our model, and found that while expression of Mmp2 was not significantly induced during periodontitis progression, Mmp9 expression in ligated gingival tissue was lower in P2rx5−/− samples compared to those from WT controls (Fig. 3A). The elevation of pro-inflammatory cytokines, such as IL1b, IL6, and IL17, in periodontitis has been found to stimulate alveolar bone loss (23, 24). To examine whether the production of pro-inflammatory cytokines was decreased in P2rx5−/− mice, we performed qPCR in gingival tissues of ligature-induced periodontitis from WT and P2rx5−/− mice. We observed that induction of IL1b, IL6, IL17a and Tnfsf11 was substantially higher in ligated WT gingival tissue than in ligated P2rx5−/− tissue (P < 0.05). In contrast, there were no significant differences in IL23a, IL10 and Tnfsf11b expression in the unligated versus ligated gingiva in WT and P2rx5−/− mice (Fig. 3B). These data suggest that in addition to regulating osteoclast function, the P2X5 receptor also regulates the host inflammatory response in the setting of experimental periodontitis.
Fig. 3.
Matrix metalloproteinases (Mmp) and cytokine mRNA expression levels at the ligated vs. unligated gingival tissues between WT and P2rx5−/− mice. (A) Expression of Mmp2 and Mmp9 in gingival tissues. (B) Expression of IL1b, IL6, IL23a, IL17a, Tnfsf11, Tnfsf11b, IL10 and P2rx5 in gingival tissues, as determined by qPCR. Data are normalized to Gapdh and are presented as fold change in the transcript levels in ligated sites relative to those of unligated sites, set as 1. Data are means ± SD. Data pooled from two independent experiments with n = 6–8 replicates per group. *P < 0.05. ND: Not determined, NS: Not significant.
DISCUSSION
In this study, we examined the role of P2X5 in inflammatory events and bone loss associated with periodontal disease. We used a well-established experimental model of ligature-induced periodontitis in mice, wherein ligature placement leads to rapid accumulation of bacteria in the adjacent dentogingival areas that allows for intense host-bacterial interactions. These interactions cause inflammation that compromises connective and bone tissue integrity, which is evident by day 5 of periodontal disease induction (25). These processes give rise to inflammatory cell influx and destruction of the alveolar bone and other periodontal connective tissues, leading to pathologic destruction of the periodontal tissues and those similarly observed in human periodontitis.
Expression of P2X purinergic receptor family members expression is widespread, and activation by extracellular ATP has variously immunomodulatory effects (21). P2X7 receptor, in particular, has been characterized as an upstream activator of inflammasomes, where, for example, potassium efflux through the P2X7 ion channel triggers the activation of NALP3 inflammasome, resulting in activation of caspase 1 and subsequent cleavage of pro-IL-1β into releasable mature IL-1β (26). P2rx7-deficient mice were generated by two independent groups (16, 17) and their bone phenotypes analyzed with somewhat conflicting findings. In one case, P2rx7 deficiency showed a reduction in total and cortical bone content in the femur, reduced periosteal bone formation, increased trabecular bone resorption in the tibia and a reduced sensitivity to mechanical loading (17). The other model of P2rx7 deficiency only showed that cortical thickness was increased compared to WT controls (16). In this study, we used the first P2rx7-deficient model to examine potential roles in periodontal disease, but found that P2rx7 deficiency exhibited no significant differences in periodontal bone loss compared to WT controls (Fig. 2B). By contrast, it was observed that 65% of periodontitis-associated bone loss was ameliorated in P2rx5-deficient mice (Fig. 2A).
Excessive production of MMPs leads to accelerated periodontal matrix degradation, and we observed Mmp9 levels are lower in P2rx5−/− mice compared with WT, while in P2rx7−/− mice levels were similar to WT (data not shown). Interestingly, we did not detect any difference in Mmp2 expression during periodontitis progression. Mmp2 is secreted by gingival fibroblasts and/or osteoblasts and Mmp9 is mainly secreted by polymorphonuclear leukocytes including osteoclasts. For the qPCR analysis, we peeled out soft tissues which contained very low osteoblasts cells from bone surface.
Under inflammatory conditions, the response of the host is primarily mediated by innate immune system activation, causing inflammatory cells to migrate, proliferate, differentiate, or produce diverse inflammatory mediators (27). Cytokines such as TNF, IL-1, and IL-17 promote substantially the pathological bone loss that frequently occurs with periodontal disease (23, 24). Moreover, IL-1 stimulates osteoclast differentiation and bone resorption, largely through up-regulation of the RANK and RANKL, whereas TNF stimulates osteoclast differentiation and activation through RANKL-dependent or independent mechanisms. To investigate the role of P2X5 in periodontitis we used the murine ligature-induced periodontitis model. This is an established model of experimental periodontitis, where placement of a silk suture around molar teeth leads to local accumulation of bacteria and rapid inflammation in surrounding gingival tissues resulting in destruction of the underlying alveolar bone (25). Using this model, we found that the expression of pro-inflammatory cytokines (including IL1b, IL6 and IL17a) in the gingival tissue was inhibited by P2X5 deficiency. These cytokines play critical roles in the development of the periodontitis, which is initiated by microbial dysbiosis (2). IL-17 is important for the recruitment of neutrophils, which are causative in periodontitis as they are responsible for a substantial portion of inflammatory tissue destruction and their numbers correlate positively with the severity of the disease (28, 29). However, a conclusive role of Th17 cells, a major source of IL-17, is uncertain. In this context, a recent study showed that there is significantly increased number of Th17 cells in the gingival tissue of older mice (24 weeks) as compared to 8 weeks old mice (30). Because 24-week-old mice exhibit more bone loss than 8-week-old mice, it appears that the presence of Th17 cells correlates with increased bone loss. In vitro studies show that IL-17 stimulates osteoclast differentiation most likely indirectly through induction of RANKL (31). Moreover, IL-17 pathways have been also shown to have a potentially critical role in the pathogenesis of rheumatoid arthritis, notably in the regulation of bone devastation (31, 32). Our findings that both IL-17 and RANKL were significantly inhibited by P2X5 deficiency suggests that the expression of these cytokines might be associated with P2X5 signaling pathways in periodontal disease progression.
Osteoclasts differentiate from hematopoietic precursors in the monocyte-macrophage lineage at various stage including proliferation, migration, fusion, and activation. Osteoclasts are specialized multinucleated cells involved in bone resorption and increased osteoclast differentiation functionally leads to devastating bone loss-related diseases, such as osteoporosis, periodontitis, rheumatoid arthritis, osteosarcoma, and cancer metastasis to the bone. Recently, we have shown that the purinergic receptor P2X5 plays a pivotal role in regulating osteoclast maturation and hyper-multinucleation (18). Furthermore, P2rx5-deficient mice are protected from LPS-induced inflammatory bone loss in an in vivo model that targets calvarial bone (18). IL-1β is involved in various cellular and tissue functions including osteoclast differentiation (33), and is regulated by purinergic signaling through inflammasome-mediated conversion of pro-IL-1β into active IL-1β (34). Under physiological conditions, osteoclast differentiation is dependent on RANKL stimulation, but IL-1β can induce RANKL-independent osteoclast differentiation (35). However, it is uncertain whether the P2X5 specifically regulates production of IL-1β in the context of periodontal disease.
Taken together, although a full understanding of how P2X5 signaling contributes to inflammatory bone loss in vivo remains unclear, we provide evidence that this pathway drives experimental periodontitis via promotion of inflammation and direct regulation of osteoclast maturation, thereby suggesting a potential candidate for therapeutic targeting in periodontal disease.
MATERIALS AND METHODS
Mice
Experiments were performed on 9 week-old female P2rx5−/− (18) and P2rx7−/− (The Jackson Laboratory, stock #005576) mice (both on the C57BL/6 background). The experimental protocol for surgical procedures and animal treatments was approved by Institutional Animal Care and Use Committee of the University of Pennsylvania (Animal protocols #805528 and #804178).
ELISA assay
The ELISA for IL-1β was performed following the manufacturer’s instructions (BD Biosciences). Briefly, collected culture media were filtered through a 0.45 μm syringe filter, added to a well coated with anti-IL-1β capture antibody, and incubated overnight at 4°C. Samples from each well were then removed and the wells washed, and 100 μl of biotin-antibody working solution was then added to each well for 1 h. The mixture was removed and the wells washed before HRP-avidin working solution was added to each well for 1 h incubation. After washing, TMB substrate was added and incubated for 30 min, and the reaction was stopped by adding 50 μl of stop solution (1M H3PO4) to each well. The optical density of each well was determined using a microplate reader (Molecular Devices Vmax, Menlo Park, CA, USA) set to 450 nm.
Osteoclast differentiation and tartrate-resistant acid phosphatase (TRAP) staining
Analysis of osteoclast differentiation was performed as previously reported (36, 37). Briefly, BMMs were generated from cultures of bone marrow cells and re-plated at 2 × 104 per well in 96-well cell culture plates. Cells were cultured for 3 days with M-CSF (60 ng/ml) and RANKL (150 ng/ml). Cells were then fixed with 3.7% formalin for 30 min and stained using the Acid, Phosphatase, Leukocyte (tartrate-resistant acid phosphatase) Kit (387A-1KT, Sigma). TRAP-positive multi-nuclear cells (MNCs) were counted under light microscopy (Nikon).
TRAP activity assay
TRAP assays were carried out as previously described (38). Briefly, osteoclasts in a 96 well plate were fixed in 3.7% (v/v) formalin for 10 min, washed with DDW, then incubated with 150 μl of TRAP buffer (120 mM Na-acetate and 52 mM Na-tartrate, pH 5.2) containing 1 mg/ml p-nitrophenylphosphate (Sigma) for 1 h at 37°C. The reaction mixtures were then transferred to a new plate containing 50 μl of 1N NaOH. Absorbance was measured at 405 nm using a microplate reader (Molecular Devices Vmax, Menlo Park, CA, USA).
Ligature-induced periodontal disease
P2rx5−/− and P2rx7−/− mice and their littermate controls (P2rx5+/+ or P2rx7+/+) were co-housed for at least for 4 weeks prior to experiments. Anesthesia was induced in mice by intraperitoneal injection of ketamine and xylazine. A 5-0 silk ligature was tied around the maxillary left second molar and the mice were euthanized five days after placement of the ligatures. The contralateral molar tooth in each mouse was left unligated as a baseline control for bone loss measurements. Periodontal bone loss was assessed morphometrically in defleshed and stained maxillae. Images were taken using a dissecting microscope (40×) fitted with a video image measurement system (Nikon Instruments). Specifically, the distance between cementoenamel junction (CEJ) and alveolar bone crest (ABC) was measured on six predetermined points on the ligated second molar and the affected adjacent regions (25). Bone loss was calculated by subtracting the 6-site total CEJ-ABC distance for the ligated side of each mouse from the 6-site total CEJ-ABC distance of the contralateral unligated side. Negative values (in mm) indicated bone loss relative to the baseline (unligated control).
Real time-PCR (qPCR)
Isolation of total RNA, and cDNA synthesis, were performed from cells with the use of Trizol reagent (Invitrogen) or from gingival tissues with the use of the RNeasy Mini Kit (QIAGEN), and RNA was reversed transcribed by using random hexamer primers and Superscript III reverse transcriptase (Invitrogen). In brief, quantitative qPCR was performed as described previously (39). The synthesized cDNA was analyzed by qPCR using an ABI PRISM 7300 or 7500 real time PCR instrument (Applied Biosystems) and specific Taqman™ probes as follows: Mmp2 (Mm00439498_m1), Mmp9 (Mm00442991_m1), IL1b (Mm00434228_m1), IL17a (Mm00439618_m1), IL23a (Mm00518984_m1), IL6 (Mm00446190_m1), Tnfsf11 (Mm00441906_m1), Tnfsf11b (Mm00435454_m1), IL10 (Mm01288386_m1), P2rx5 (Mm00473677_m1), and Gapdh (Mm99999915_g1). Three technical replicates per sample and gene were performed. Amplification parameters were 5 min of denaturation and activation at 95°C and 50 cycles of 30 s at 95°C and 1 min at 60°C. The CT method of relative quantification was used to determine the fold change in expression.
Statistics
Statistical analysis was performed using GraphPad Prism Ver. 6.0e (GraphPad, San Diego, CA). For determining statistical significance, a two-tailed unpaired t-test (for comparing two groups) or one-way ANOVA with a Tukey’s multiple comparisons test (for comparing three or more groups) was used. Values of P < 0.05 were considered significant.
ACKNOWLEDGEMENTS
This study was supported in part by NIH grants (AR067726 and AR069546 to YC., and DE015254 and DE024716 to GH).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. J Clin Periodontol. 2017;44(Suppl 18):S94–S105. doi: 10.1111/jcpe.12677. [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zenobia C, Hasturk H, Nguyen D, Van Dyke TE, Kantarci A, Darveau RP. Porphyromonas gingivalis lipid A phosphatase activity is critical for colonization and increasing the commensal load in the rabbit ligature model. Infect Immun. 2014;82:650–659. doi: 10.1128/IAI.01136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thunell DH, Tymkiw KD, Johnson GK, et al. A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J Periodontal Res. 2010;45:148–152. doi: 10.1111/j.1600-0765.2009.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita Y, Ito H, Sekino S, Numabe Y. Correlations between pentraxin 3 or cytokine levels in gingival crevicular fluid and clinical parameters of chronic periodontitis. Odontology. 2012;100:215–221. doi: 10.1007/s10266-011-0042-1. [DOI] [PubMed] [Google Scholar]
- 7.Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 8.Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol. 2017;18:612–621. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- 9.Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013;9:491–540. doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8:629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- 13.Orriss IR, Key ML, Brandao-Burch A, Patel JJ, Burnstock G, Arnett TR. The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: The role of p2× receptors. Bone. 2012;51:389–400. doi: 10.1016/j.bone.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Su X, Floyd DH, Hughes A, et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 2012;122:3579–3592. doi: 10.1172/JCI38576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gartland A, Skarratt KK, Hocking LJ, et al. Polymorphisms in the P2X7 receptor gene are associated with low lumbar spine bone mineral density and accelerated bone loss in post-menopausal women. Eur J Hum Genet. 2012;20:559–564. doi: 10.1038/ejhg.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartland A, Buckley KA, Hipskind RA, et al. Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice. Crit Rev Eukaryot Gene Expr. 2003;13:243–253. doi: 10.1615/CritRevEukaryotGeneExpr.v13.i24.160. [DOI] [PubMed] [Google Scholar]
- 17.Ke HZ, Qi H, Weidema AF, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Walsh MC, Takegahara N, et al. The purinergic receptor P2X5 regulates inflammasome activity and hyper-multinucleation of murine osteoclasts. Sci Rep. 2017;7:196. doi: 10.1038/s41598-017-00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu C, Bonar SL, Hickman-Brecks CL, et al. NLRP3 mediates osteolysis through inflammation-dependent and -independent mechanisms. FASEB J. 2015;29:1269–1279. doi: 10.1096/fj.14-264804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos-Junior ES, Morandini AC, Almeida-da-Silva CL, et al. A Dual Role for P2X7 Receptor during Porphyromonas gingivalis Infection. J Dent Res. 2015;94:1233–1242. doi: 10.1177/0022034515593465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lister MF, Sharkey J, Sawatzky DA, et al. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franco C, Patricia HR, Timo S, Claudia B, Marcela H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int J Mol Sci. 2017;18:440. doi: 10.3390/ijms18020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 25.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob F, Perez Novo C, Bachert C, Van Crombruggen K. Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal. 2013;9:285–306. doi: 10.1007/s11302-013-9357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016;9:1163–1172. doi: 10.1038/mi.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajishengallis G, Moutsopoulos NM, Hajishengallis E, Chavakis T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin Immunol. 2016;28:146–158. doi: 10.1016/j.smim.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutzan N, Abusleme L, Bridgeman H, et al. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity. 2017;46:133–147. doi: 10.1016/j.immuni.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robaszkiewicz A, Qu C, Wisnik E, et al. ARTD1 regulates osteoclastogenesis and bone homeostasis by dampening NF-kappaB-dependent transcription of IL-1beta. Sci Rep. 2016;6:21131. doi: 10.1038/srep21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 35.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI200523394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takegahara N, Kim H, Mizuno H, et al. Involvement of Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL)-induced Incomplete Cytokinesis in the Polyploidization of Osteoclasts. J Biol Chem. 2016;291:3439–3454. doi: 10.1074/jbc.M115.677427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Choi HK, Shin JH, et al. Selective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in mice. J Clin Invest. 2009;119:813–825. doi: 10.1172/JCI36809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Kim T, Jeong BC, et al. Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell Metab. 2013;17:249–260. doi: 10.1016/j.cmet.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song H, Kim H, Lee K, et al. Ablation of Rassf2 induces bone defects and subsequent haematopoietic anomalies in mice. EMBO J. 2012;31:1147–1159. doi: 10.1038/emboj.2011.480. [DOI] [PMC free article] [PubMed] [Google Scholar]